Abstract
The intermediate zone of the spinal grey matter contains premotor interneurons mediating reflex actions of group I and II muscle afferents. However, limited information is available on how activity of inhibitory versus excitatory interneurons in this population are modulated and how they contribute to motor networks. There were three aims of this study: (1) to characterize excitatory axonal contacts on interneurons; (2) to determine if contact patterns on excitatory and inhibitory interneurons are different; (3) to determine if there are differences in presynaptic inhibitory control of excitatory and inhibitory interneurons. We used intracellular labelling of electrophysiologically identified cells along with immunochemistry to characterise contacts formed by axons that contain vesicular glutamate transporters (VGLUT1 and VGLUT2) and contacts formed by VGLUT1 terminals which in turn were contacted by GABAergic terminals on cells that were characterised according to their transmitter phenotype. All 17 cells investigated were associated with numerous VGLUT1 contacts originating from primary afferents, and similar contact densities were found on excitatory and inhibitory cells, but VGLUT2-immunoreactive terminals originating from intraspinal neurons were less frequent, or were practically absent, especially on excitatory cells. Similar numbers of VGLUT1 contacts with associated GABAergic terminals were found on excitatory and inhibitory cells indicating a similar extent of presynaptic GABAergic control. However, scarce VGLUT2 terminals on intermediate zone excitatory premotor interneurons with input from muscle afferents suggest that they are not significantly excited by other spinal neurons but are under direct excitatory control of supraspinal neurons and, principally inhibitory, control of spinal neurons.
Introduction
The actions of both primary afferents and supraspinal neurons on motoneurons are principally mediated through spinal interneurons. The consequences of these actions are complex but they result in coordinated movements expressed by various patterns of muscle contraction. Spinal interneurons interposed in pathways from muscle afferents have an important role in relaying information on muscle tension and length, but may also influence centrally initiated movements and contribute to a variety of movements depending on the behavioural context. Selection and modulation of actions of these interneurons is therefore essential to achieve appropriate movement coordination and a variety of modulatory mechanisms are available to this end. It has been established that these include both excitatory and inhibitory postsynaptic actions, presynaptic inhibition and facilitation of synaptic transmission and modulation of membrane or intrinsic properties of the neurons (for references see Jankowska, 2001, 2008). However, only preliminary observations have been made on how modulation of activity of various functional interneuronal populations is actually achieved.
The present study addressed this question with respect to a population of intermediate zone interneurons that mediate reflex actions of group I and II muscle afferents which is distinct from Ia inhibitory interneurons (with input from group Ia but not group II afferents) located in the ventral horn and interneurons with input from group II but not group I afferents located in the dorsal horn and in lamina VIII (for references see Jankowska, 1992; Bannatyne et al. 2003, 2006; Jankowska et al. 2009). Intermediate zone interneurons were traditionally subdivided into populations of cells in pathways from tendon organs (group Ib afferents) or from secondary endings of muscle spindles (group II afferents). However, the available evidence indicates that they belong to one population of interneurons with distributed input from both group I and II afferents, with intra- rather than inter-population variations (Jankowska & Edgley, 2010).
In our recent study, we made a comparison of axonal projections of and input to representative samples of these interneurons which included both glutamatergic and glycinergic interneurons (Bannatyne et al. 2009). The study revealed that these cells had a number of common features in addition to their location in laminae V–VII of Rexed and monosynaptic input from group I and II muscle afferents. One of these features was that all well-labelled interneurons projected both to motor nuclei and areas outside motor nuclei, i.e. they operated as premotor interneurons (confirming previous electrophysiological results; Cavallari et al. 1987; Edgley & Jankowska, 1987b) but were also involved in coordinating activity of other interneurons. The study also revealed a considerable variability in terminal projection areas and in disynaptic excitatory and inhibitory input from primary afferents and descending tract neurons to individual interneurons; this indicates that the most suitable subpopulations of these interneurons may be selected under different behavioural conditions. However, only preliminary observations have been made on the relative contribution of different sources of input to these neurons to the selection of appropriate interneurons, e.g. of direct monosynaptic input from afferents compared with input via other spinal or supraspinal neurons. In the present study we addressed this question by examining excitatory terminals in contact with interneurons, and related contact properties to the input and transmitter phenotype of individual interneurons. This also provided an opportunity to examine the occurrence of putative GABAergic axoaxonic contacts on glutamatergic terminals in apposition to these neurons, which would be expected to be found in association with contacts formed by primary afferents (Riddell et al. 1995; Maxwell et al. 1997; Maxwell & Riddell, 1999) but not of other types of fibre (for a review see Rudomín & Schmidt, 1999). Many interneurons, including those examined here, are activated by several classes of primary afferent (albeit often one class is dominant) and may be considered to have a ‘multifunctional’ character as they perform a variety of functions in several motor networks (Jankowska, 2001, 2008). Presynaptic inhibition of transmission from a particular class of primary afferent therefore has the capacity to alter such functions and hence select particular motor pathways. For example, group II input to intermediate zone neurons is under strong presynaptic inhibition from other group II afferents which favours polysynaptic group II pathways via dorsal horn interneurons to motoneurons over disynaptic ones (Riddell et al. 1995; Jankowska et al. 2002).
There were three principal aims of the study: (1) to characterize the excitatory axonal contacts on intracellularly labelled intermediate zone interneurons with electrophysiologically established monosynaptic input from group I and/or II muscle afferents; (2) to determine whether the distribution of monosynaptic contacts from primary afferents differs on excitatory and inhibitory interneurons and could be related to their different axonal projections and involvement in different networks (Bannatyne et al. 2009); and (3) to examine the anatomical substrate of GABAergic presynaptic control of primary afferent input to excitatory and inhibitory interneurons. It is well established that myelinated primary afferent terminals in the grey matter of lumbar spinal cord, with the exception of those in lamina I, are immunoreactive for the vesicular glutamate transporter 1 (VGLUT1), while immunoreactivity for the vesicular glutamate transporter 2 (VGLUT2) is present predominantly in axon terminals derived from intrinsic spinal interneurons (Varoqui et al. 2002; Todd et al. 2003). Hence we were able to make a comparison between electrophysiological input characteristics and neurochemical properties of axonal contacts on labelled cells by characterizing them with these two markers.
The study was made on a sample of 17 neurons selected from a larger sample of cells investigated by Bannatyne et al. (2009). We selected about half of the cells characterised as glutamatergic and half of those characterised as glycinergic, including neurons that were or were not monosynaptically excited by reticulospinal neurons which are the main source of monosynaptic descending input to this population of cells. This sample may be considered to be representative of the whole population of intermediate zone premotor interneurons mediating disynaptic reflex actions of group I and II afferents on motoneurons as their properties fully conform with the location, input, general morphology, axonal projections and actions of larger samples of this type of neuron previously investigated physiologically (for references see Jankowska & Edgley, 2010), morphologically and immunohistochemically (Bannatyne et al. 2009).
Methods
We used combinations of intracellular labelling of electrophysiologically identified cells with confocal microscopic and immunochemical labelling to examine contacts with interneurons.
Experiments were performed on seven adult cats (weighing 2.5–3.5 kg). All experiments were approved by the Ethics Committee for Animal Research at the University of Göteborg (Göteborgs Djurförsöksetiska Nämnd) and comply with the ethical policies and regulations of The Journal of Physiology (Drummond, 2009) and with National Institutes of Health and European Union guidelines. The animals were bred and housed under veterinary supervision at the Experimental Biomedicine Unit at Sahlgrenska Academy where the experiments were carried out.
Surgical procedures and labelling of spinal interneurons
General anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg kg−1 administered every 1–2 h, up to 55 mg kg−1, i.v.) as required to maintain full anaesthesia. The level of anaesthesia was monitored by continuous recording of blood pressure and heart rate and by observing pupil dilatation. Additional doses of α-chloralose were given when increases in the blood pressure or heart rate were evoked by any experimental procedures, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1, i.v.) and animals were artificially ventilated. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml h−1 kg−1). Core body temperature was kept at about 37.5°C by servo-controlled infrared lamps.
A laminectomy exposed the fourth to seventh lumbar (L4–L7) and low thoracic (Th11–Th13) segments of the spinal cord. Neurons in the lumbar segments were approached through small holes (about 1–2 mm2) made in the dura mater. At the Th12–13 level, pairs of stimulating electrodes were put in contact with the lateral funicule to identify and exclude neurons with axons ascending either ipsilaterally or contralaterally beyond this level by their antidromic activation. Two contralateral hindlimb nerves (the quadriceps, Q, and sartorius, Sart) and a number of ipsilateral nerves were transected and mounted on stimulating electrodes. The latter included Q, Sart, the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius and soleus (GS), plantaris (PI) and deep peroneal (DP) nerves. In most experiments the caudal part of the cerebellum was exposed by craniotomy and a tungsten electrode (impedance 40–250 kΩ) was placed in the ipsilateral medial longitudinal fascicle (MLF) to stimulate fibres of reticulospinal neurons. The electrode was inserted at an angle of 30 deg (with the tip directed rostrally). The initial target was at Horsley–Clarke co-ordinates P9, L0.6, H–5 but its final position was adjusted on the basis of records of descending volleys recorded from the surface of the lateral funiculus at the Th11 level.
Interneurons were searched for in the intermediate zone and ventral horn grey matter of the L4–L6 segments at a depth where field potentials evoked by group I and/or group II afferents were maximal (Edgley & Jankowska, 1987a,b; Bannatyne et al. 2009), indicating the highest concentration of neurons with input from these afferents. They were usually recorded first extracellularly and only those that were not projecting rostral to the lumbosacral enlargement and that were monosynaptically activated after stimulation of peripheral muscle nerves at sufficient intensities to recruit group I and/or group II muscle afferent fibres, or after stimulation of the MLF, were tracked for until they were penetrated. Peripheral nerves and MLF fibres were stimulated with constant voltage stimuli (0.2 ms duration, with intensity expressed in multiples of threshold for the most sensitive fibres in the given nerve) and constant current stimuli (0.2 ms, 100 μA), respectively.
After penetration and after their identification had been confirmed, cells were labelled intracellularly with a mixture of equal parts of 5% tetramethylrhodamine-dextran (Molecular Probes, Inc., Eugene, OR, USA) and 5% Neurobiotin (Vector, UK) in saline (pH 6.5). The marker was introduced to micropipettes with tips broken to about 2 μm (‘sharp’), with the resulting impedances of 12–20 MΩ, and was ejected by passing depolarizing constant current up to 5 nA for 5–8 min. New injection sites were a minimum of 500 μm away from previous sites and the distance between each injected cell and its location was carefully recorded on a diagram of the lumbar segments. At the conclusion of the experiments, the animals were given a lethal dose of pentobarbital and perfused initially with physiological saline and subsequently with paraformaldehyde (4%) in 0.1 m phosphate buffer (PB; pH 7.4). Lumbar spinal cord segments containing labelled cells (L4, L5 or L6) were removed and placed in the same fixative for 8 h, and then were cut into blocks which were rinsed several times in 0.1 m PB and were cut into 50-μm-thick transverse sections with a Vibratome (Oxford Instruments, Technical products international Inc., USA) and collected in strict serial order to enable reconstruction of labelled cells. All sections were placed immediately in an aqueous solution of 50% ethanol for 30 min to enhance antibody penetration. Following this treatment, sections were mounted in serial order on glass slides with Vectashield (Vector Laboratories, Peterborough, UK) and examined with a fluorescence microscope. Sections containing labelled interneurons were reacted with avidin–rhodamine (1:1000; Jackson Immunoresearch, Luton, UK) and re-examined with a fluorescence microscope.
Immunocytochemical processing of tissue
Sections were incubated in the combinations of primary antibodies listed in Table 1. All antibodies were diluted in Tris phosphate-buffered saline (TPBS) and sections were incubated for 72 h. They were rinsed in phosphate-buffered saline (PBS) and incubated for 3 h in solutions of secondary antibodies coupled to fluorophores before mounting with a glycerol-based anti-fade medium (see Table 1 for details).
Table 1.
Summary of primary and secondary antibody combinations and concentrations used in the current study
| Sections containing | Primary antibody combination | Primary antibody concentration | Supplier | Secondary antibodies | Sequential immunoreaction | Secondary antibodies |
|---|---|---|---|---|---|---|
| Axon terminals | mo. gephyrin | 1:100 | Connex, Martinsried, Germany | Alexa488 | ||
| gp. VGLUT1 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | |||
| Axon terminals | rbt GAD | 1:2000 | Sigma, Poole, UK | Alexa488 | ||
| gp VGLUT2 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | |||
| Soma and dendrite | rbt GAD | 1:2000 | Alexa488 | gp VGLUT2 | Rh.Red | |
| gp VGLUT1 | 1:5000 | Cy-5 |
All secondary antibodies were raised in donkey and conjugated to Rhodamine Red (Rh.Red; 1:100), or Cyanine 5.18 (Cy-5; 1:100; both supplied by Jackson ImmunoResearch, West Grove, USA), or Alexa-fluor 488 (Alexa488; 1:500; Molecular Probes). GAD, glutamic acid decarboxylase; VGLUT vesicular glutamate transporter; mo., mouse; rbt, rabbit; gp, guinea pig.
Selected sections containing axon terminals from each labelled cell were reacted with one of the following antibodies: mouse anti-gephyrin and guinea pig anti-vesicular glutamate transporter 1 (VGLUT1); or rabbit anti-glutamic acid decarboxylase (GAD) and guinea pig anti-vesicular glutamate transporter 2 (VGLUT2; see Table 1). Gephyrin was used to identify glycinergic cells because antibodies for the glycine transporter 2 do not work well in cat tissue. All terminals that apposed gephyrin were not immunoreactive for GAD and therefore cells with these properties were considered to be purely glycinergic. Once the transmitter phenotype of a cell was established, its axonal projections and (where possible) target neurons were documented in detail. Further details are provided in Bannatyne et al. (2009) and Jankowska et al. (2009).
Sections containing labelled cell bodies and dendrites were reacted with rabbit anti-glutamic acid decarboxylase (GAD) and guinea-pig anti-VGLUT1 firstly in order to examine primary afferent contacts on labelled cells and determine their relationship with GABAergic terminals. Secondary antibodies coupled to Alexa 488 and Cyanine 1.58 were used to identify GAD and VGLUT1, respectively. When the initial reactions of both primary and secondary antibodies were complete, a sequential incubation was performed (e.g. see Olave & Maxwell, 2003); sections were re-incubated with an anti-guinea pig VGLUT2 antibody, followed by a secondary antibody coupled to Rhodamine Red (see Table 1), in order to study contacts formed by excitatory interneurons. As a consequence, VGLUT1 terminals became purple because of the red over the original blue labelling, while VGLUT2 terminals were red only. Although patterns of VGLUT1 and 2 expression were investigated originally in the rat spinal cord (Varoqui et al. 2002; Todd et al. 2003), the distribution of these transporters in the grey matter of cat is identical. Furthermore, all the interneurons we studied previously expressed VGLUT2 but not VGLUT1 (e.g. Bannatyne et al. 2009) which is consistent with the rat findings. Sections were mounted and examined with a Bio-Rad MRC 1024 confocal laser scanning microscope. A contact was defined as an apposition between an immunoreactive terminal and a labelled cell where no intervening dark pixels were present.
Confocal microscopy, reconstruction and analysis
Sections containing labelled cells and their dendrites were initially imaged at a low magnification (×10 lens; zoom factor 2). These images were used as a frame of reference for the location of labelled processes within each section and to make preliminary reconstructions of the cells. Each cell was then examined systematically at a higher magnification by using a ×40 oil immersion lens at zoom factor of 2. Series of three-colour confocal images were gathered at intervals of 0.5 μm and their locations were determined by using the corresponding low-power reference image so that a montage was constructed for each section. Usually 10 to 20 series of images were collected from each section and processes of cells were contained within four or five sections. Sections containing axonal terminals of labelled cells were scanned by using a ×60 oil-immersion lens, zoom factor 3 or 4 at 0.5 μm intervals.
Cells were reconstructed three-dimensionally, and VGLUT1, VGLUT2 and presynaptic GAD contacts on labelled cells were recorded with appropriate markers by using Neurolucida for confocal software (MicroBrightField, Colchester, VT, USA). Reconstructions always started from stacks of single optical sections containing the cell body, and then the dendritic processes were systematically added from the next stacks until reconstruction of the dendritic tree was complete. Putative contacts were viewed in single optical sections in merged image stacks showing all three channels and were assessed by switching through different channels (if necessary). Individual contacts were plotted on reconstructions. The Neurolucida program provides information about the distribution of contacts over the dendritic tree; therefore, a three-dimensional Sholl analysis was performed to show absolute numbers of contacts and numbers of contacts per 100 μm of dendritic length contained within concentric spheres at standard distances from the centre of the cell body of each cell. By using this program, the density of contacts per unit area (100 μm2) of cell membrane was also estimated. The equivalent diameter of each labelled cell body was measured from projected confocal images obtained from all seven cats by using Image J software (National Institutes of Health, USA).
Two-group comparisons were made by using a Student's t test, and multi-group comparisons were made by ANOVA followed by a post hoc Tukey's analysis. A P < 0.05 was considered to be statistically significant.
Results
A total of 20 interneurons (2–5 cells from each of the 7 adult animals) were selected for analysis from the total sample of neurons labelled by Bannatyne et al. (2009) and Jankowska et al. (2009). We selected 17 interneurons that were monosynaptically excited by stimulation of peripheral nerves. These interneurons were previously characterised by Bannatyne et al. (2009) as excitatory (n = 7) or inhibitory (n = 10) (see Table 2). Both the excitatory and inhibitory interneurons included those in which monosynaptic EPSPs were evoked from group I or group II, or both group I and II afferents and were followed by disynaptic IPSPs from either one or both of these groups of afferents, as illustrated in Fig. 1. This was indicated by latencies of 0.6–1.1 ms from the afferent volleys of EPSPs of group I origin (illustrated for cell 9 in Fig. 1; indicated by the second vertical line), by latencies of 1.4–2.8 ms for EPSPs of group II origin (illustrated for cell 12 in Fig. 1; indicated by the second vertical line but evoked also in cell 9, in which it is indicated by the third vertical line) and by latencies of 1.3–1.8 and 2.2–3.5 ms for the IPSPs from group I and II afferents, respectively. The IPSPs were evoked either separately, as in cell 18 in Fig. 1 or followed EPSPs, either of group I or II origin, as indicated by steep declining phases of the EPSPs in cells 9 and 12. Hence forward these cells will be referred to as group I/II cells. Any excitatory input from the MLF to them was monosynaptic or disynaptic, as indicated by segmental latencies of EPSPs evoked in them (0.4–1.0 ms or >1 ms from the first component of the descending volleys from the MLF indicated by the first dotted lines in the right panels of records from cells 12, 18 and 20). Monosynaptic EPSPs followed all successive stimuli without increments, as in cells 18 and 20, while disynaptic EPSPs were only evoked by the 2nd or 3rd stimulus in a train (as in cell 12) and were usually temporally facilitated. MLF stimuli evoked either EPSPs (as in cell 20) or EPSPs preceded (as in cell 12) or followed (as in cell 18) by IPSPs. For more details see Bannatyne et al. (2009). All the interneurons were located within the L4 to L6 segments. As shown in Fig. 2 the location of the cell bodies of these interneurons was distributed within the intermediate zone (laminae VI–VII). The diameters of the equivalent circles of their cell bodies ranged from 22 to 64 μm. Excitatory cells included both ipsilaterally and contralaterally or bilaterally projecting neurons whereas all inhibitory cells, with the exception of cell 12, had ipsilateral projections only (see Table 2). Cell 12 was unusual in that it was located within the border region between laminae VII and VIII and its axonal projection was like group II activated commissural lamina VIII interneurons rather than intermediate zone interneurons (see Jankowska et al. 2009). All 17 cells had radial dendritic arbors that spread extensively up to 1000 μm from the soma. Immunocytochemical properties of terminals contacting labelled cells are shown in Fig. 3, and reconstructions of representative cells and the distribution of contacts on them are shown in Figs 4 and 5. Their morphological and neurochemical properties are summarised in Table 2.
Table 2.
Summary of morphological, neurochemical and electrophysiological data
| Morphology |
Monosynaptic input |
|||||
|---|---|---|---|---|---|---|
| Segmental location | Soma position | Axonal projection | Afferents | MLF | Immunocytchemical identity of terminals | |
| Cell 1 | L4 | Mid VII | Bilateral | I (II) | No | VGLUT2 |
| Cell 2 | L6 | Mid VII | Ipsilateral | I II | MLF | VGLUT2 |
| Cell 3 | L6 | Medial VII | Contralateral | I II | MLF | VGLUT2 |
| Cell 4 | L5 | VII | Bilateral | I (II) | nt | VGLUT2 |
| Cell 5 | L4 | Lat VII | Bilateral | I (II) | MLF | VGLUT2 |
| Cell 6 | L6 | VII | Ipsilateral | I II | MLF | VGLUT2 |
| Cell 7 | L4 | Lat VII | Contralateral | I II | no | VGLUT2 |
| Cell 8 | L4 | Medial VII | Ipsilateral | I II | MLF | Gephyrin |
| Cell 9 | L6 | Lat VI | Ipsilateral | I II | no | Gephyrin |
| Cell 10 | L6 | Mid VII | Ipsilateral | I II | MLF | Gephyrin |
| Cell 11 | L6 | Mid VII | Ipsilateral | II | no | Gephyrin |
| Cell 12 | L5 | VII/VIII | Contralateral | II | no | Gephyrin |
| Cell 13 | L4 | Mid VII | Ipsilateral | II | MLF | Gephyrin |
| Cell 14 | L6 | Medial VII | Ipsilateral | I II | no | Gephyrin |
| Cell 15 | L6 | Mid VII | Ipsilateral | II | no | Gephyrin |
| Cell 16 | L4 | Mid VI | Ipsilateral | I (II) | no | Gephyrin |
| Cell 17 | L4 | Mid VII | Ipsilateral | I (II) | no | Gephyrin |
Lat, lateral; Mid, central; I, group I afferent; II, group II afferent; MLF, medial longitudinal fasciculus; no, no input found; nt, not tested; (II), very weak group II afferent input.
Figure 1. Examples of PSPs used to identify the 20 interneurons analysed morphologically.
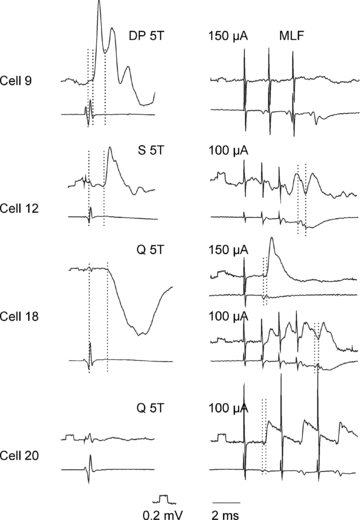
In each panel, the upper trace is an intracellular record from the cell indicated (with negativity downward) and the lower trace is from the cord dorsum (with negativity upward). The stimuli were applied to the deep peroneal (DP), sartorius (S) and quadriceps (Q) nerves. The intensity of the stimuli is expressed in multiples of the threshold (T) for the most sensitive afferents in a given nerve. Stimuli applied to the medial longitudinal fasciculus (MLF) were at intensity of 100 or 150 μA. In the left panels the first dotted lines indicate the afferent volleys while the second and the third dotted lines indicate the onset of PSPs evoked by these afferents; monosynaptic EPSPs from group I and II afferents (as defined by both their threshold and segmental latency) in cell 9, monosynaptic EPSPs from group II afferents in cell 12 and disynaptic IPSPs from group II afferents in cell 18. In right panels the first dotted lines indicate the descending volleys from the MLF and the second dotted lines indcate the onset of disynaptic IPSPs evoked by these volleys in cell 12 and of monosynaptic EPSPs evoked in cells 18 and 20. Calibration pulses at the beginning of all microelectrode records are 0.2 mV, time calibration: 2 ms. The records illustrate monosynaptic EPSPs from group I and II afferents and lack of synaptic actions from the MLF in cell 9, and monosynaptic EPSPs from the MLF evoked in parallel with disynaptic IPSPs from group II afferents in cell 18 or without PSPs from the dissected muscle nerves in cell 20.
Figure 2. Location of the 17 interneurons with monosynaptic primary afferent input.
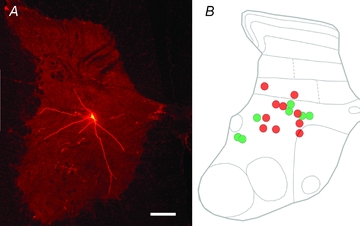
A, a low-power image showing the location of one of the cells (cell 2) in a transverse section of the L6 spinal cord. Note the cell body in lamina VII and dendrites which extend dorsally into lamina VI and ventrally into lamina VIII. B, a diagram illustrating the locations of somata of all cells located in the L4–6 segments projected on a schematic contour of the L6 segment, taking into account their distances from the central canal and medial and lateral borders of the grey matter. The green and red circles represent excitatory and inhibitory cells, respectively. Scale bar = 300 μm.
Figure 3. Morphology and immunocytochemical characteristics of VGLUT1 and VGLUT2 terminals in contact with labelled interneurons.
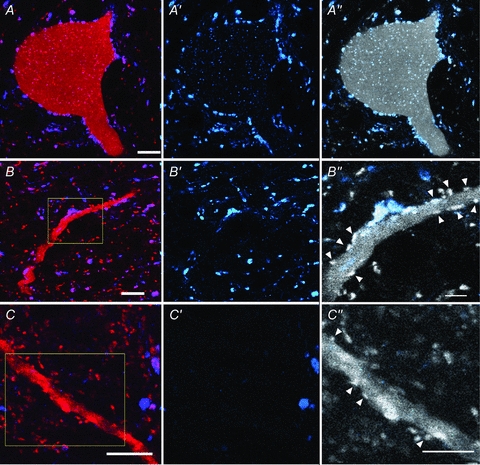
Series of single optical sections of confocal microscope images illustrating VGLUT1 and VGLUT2 immunoreactive axon terminals in contact with the cell body of cell 1 (A) and a dendrite of cell 8 (B) which were both monosynaptically activated by primary afferent input. A dendrite of a further cell (cell 20) in which monosynaptic EPSPs were evoked by stimulation of the MLF but not primary afferents is shown in C. Labelled cells are shown in red, immunoreactivity for VGLUT1 is blue and for VGLUT2 is red. The first panel of each series shows a merged image of the red and blue channels. The second series shows the blue channel only and illustrates the pattern of VGLUT1 immunoreactivity and the third panel shows a monochrome version of the red channel (showing the cell and VGLUT1 and 2) with a blue overlay which reveals which contacts are VGLUT1 immunoreactive. VGLUT2 contacts are indicated by arrowheads in B″ and C″ which are the areas indicated by the boxes in B and C. Note that VGLUT2 terminals are more intensely labelled than the dendrites of interneurons. Scale bars: A, B, C = 10 μm; B″, C″ = 5 μm.
Figure 4. Reconstructions of excitatory cells with (A) and without (B) monosynaptic primary afferent input showing the distribution of VGLUT1 and VGLUT2 contacts.
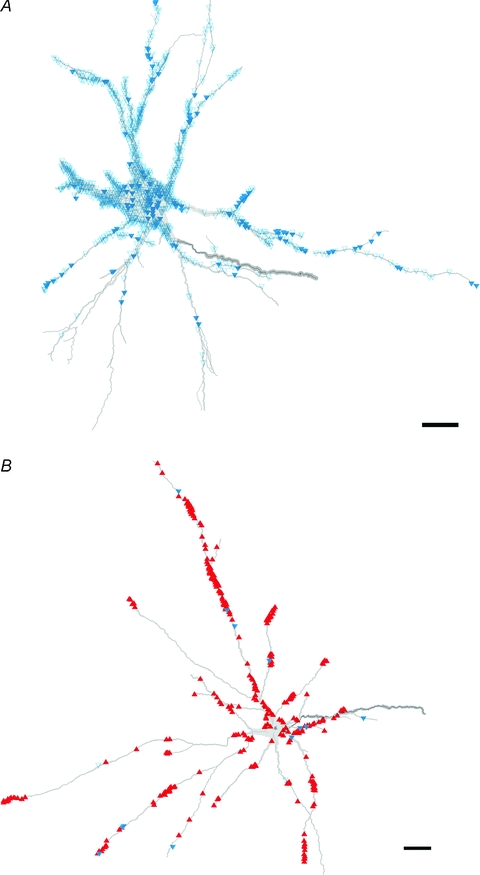
A and B show reconstructions of cell 1 and cell 20, respectively. The reconstructions show the distribution of contacts on the two cells. The blue open triangles and blue filled triangles represent VGLUT1 terminals with and without associated presynaptic GAD terminals, respectively. The red triangles represent VGLUT2 terminals. Scale bar = 50 μm.
Figure 5. Reconstructions of cells with monosynaptic primary afferent input showing the distribution of VGLUT1 and VGLUT2 contacts.
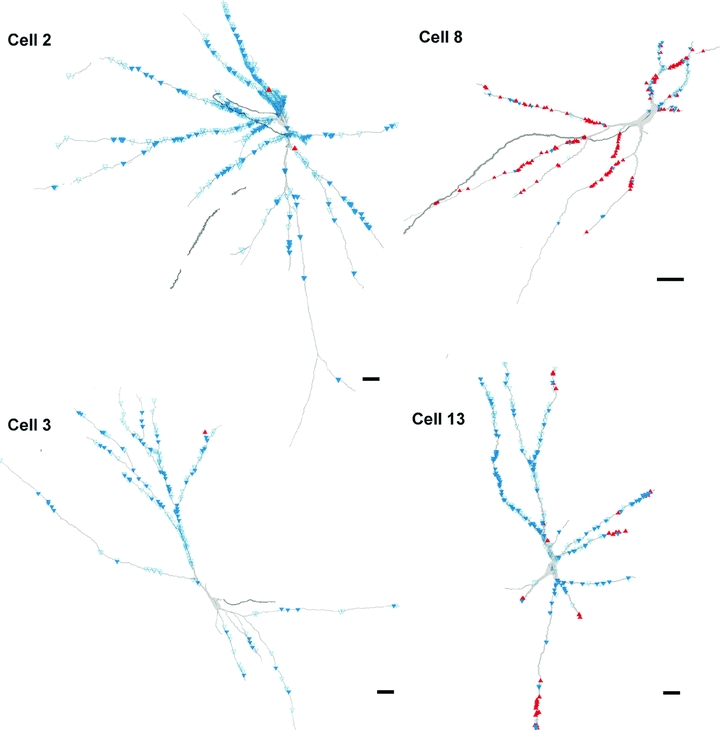
The reconstructions show contacts on two excitatory (left, cells 2 and 3) and two inhibitory (right, cells 8 and 13) interneurons. The blue open triangles and filled triangles represent VGLUT1 terminals with and without associated presynaptic GAD terminals, respectively. The red triangles represent VGLUT2 terminals. Scale bars = 50 μm.
In addition, we analysed three cells that were not monosynaptically activated by primary afferents but had monosynaptic input from the MLF. Cell bodies of all of these cells were located in lamina VIII and they gave rise to contralateral axonal projections. In neurons of this group, EPSPs were evoked at latencies <1 ms from the MLF descending volleys and the first components of these EPSPs did not show temporal facilitation after successive stimuli (illustrated for cells 18 and 20 in Fig. 1), which defines them as monosynaptically evoked. Most were followed by disynaptic IPSPs. In keeping with previously reported characteristics of commissural interneurons (Bannatyne et al. 2003, 2009; Jankowska et al. 2005), these cells belonged to a population of commissural interneurons with excitatory input predominantly from descending fibres and it would be predicted that they would have no or few contacts from VGLUT1 axon terminals which originate predominantly from myelinated primary afferent fibres (Varoqui et al. 2002; Todd et al. 2003). They could therefore provide a ‘control’ group to test the validity of our immunocytochemical labelling associated with cells monosynaptically activated from primary afferent axons.
What are the characteristics of excitatory axonal contact patterns on interneurons with monosynaptic primary afferent input?
Confocal microscope analysis revealed that the majority of terminals contacting group I/II cells were VGLUT1 positive and that some cells appeared to lack VGLUT2 immunoreactive terminals. Figure 3A shows confocal microscope images of VGLUT1 immunoreactive axon terminals in contact with the cell body of an interneuron (cell 1) where no convincing examples of VGLUT2-positive contacts could be identified, while Fig. 3B illustrates both VGLUT1 and VGLUT2 immunoreactive axon terminals in contact with a dendrite of another interneuron (cell 8). For comparison, a dendrite of an MLF excited interneuron (cell 18) contacted predominantly by VGLUT2-positive terminals is shown in Fig. 3C. The average density (±s.d.) of VGLUT1 and VGLUT2 contacts per 100 μm2 of total surface area of group I/II cells was 1.04 ± 0.66 and 0.09 ± 0.16, respectively, and this difference is highly significant (Student's t test, P < 0.001). The reconstructions in Figs 4 and 5 show examples of the distribution of these contacts on representative cells. The vast majority of VGLUT1 contacts associated with group I/II cells were located on dendritic trees. However, some cells (e.g. cell 1; Fig. 4) also had numerous axosomatic contacts whereas others (e.g. cell 8; Fig. 5) were virtually devoid of this type of contact.
Proportions of VGLUT1 and VGLUT2 contacts on the three MLF-excited cells were found to be the opposite of those associated with group I/II cells; the average density (±s.d.) of VGLUT1 and VGLUT2 contacts on these cells was 0.08 ± 0.06 and 0.43 ± 0.2 per 100 μm2, respectively. Details of the density of VGLUT1 and VGLUT2 contacts per 100 μm2 and the total surface area (μm2) for each of the cells are given in Table 3.
Table 3.
The density of VGLUT1 and VGLUT2 contacts per 100 μm2 for each cell
| Total surface of dendrite (μm2) | Density of VGLUT1 terminals per 100 μm2 | Density of VGLUT2 terminals per 100 μm2 | Density of VGLUT1 terminals associated with GAD terminals per 100 μm2 | Percentage of VGLUT1 terminals associated with GAD terminals of total VGLUT1 terminals | |
|---|---|---|---|---|---|
| Cell 1 | 66,628 | 1.90 | 0.00 | 1.56 | 82.1% |
| Cell 2 | 63,565 | 0.86 | 0.00 | 0.64 | 74.4% |
| Cell 3 | 35,957 | 0.64 | 0.00 | 0.44 | 68.8% |
| Cell 4 | 63,263 | 0.66 | 0.00 | 0.55 | 83.3% |
| Cell 5 | 48,830 | 0.74 | 0.03 | 0.40 | 54.1% |
| Cell 6 | 68,064 | 1.87 | 0.00 | 1.21 | 64.7% |
| Cell 7 | 41,549 | 2.43 | 0.18 | 2.25 | 92.6% |
| Cell 8 | 23,975 | 0.51 | 0.65 | 0.33 | 64.7% |
| Cell 9 | 34,631 | 0.89 | 0.10 | 0.53 | 59.6% |
| Cell 10 | 42,852 | 0.46 | 0.05 | 0.31 | 67.4% |
| Cell 11 | 25,751 | 0.54 | 0.00 | 0.33 | 61.1% |
| Cell 12 | 33,569 | 0.55 | 0.00 | 0.43 | 78.2% |
| Cell 13 | 35,055 | 0.81 | 0.10 | 0.47 | 58.0% |
| Cell 14 | 10,413 | 1.09 | 0.02 | 0.77 | 70.6% |
| Cell 15 | 44,856 | 0.38 | 0.02 | 0.25 | 65.8% |
| Cell 16 | 31,988 | 2.29 | 0.26 | 1.97 | 86.0% |
| Cell 17 | 34,204 | 1.08 | 0.08 | 0.77 | 71.3% |
| Cell 18 | 72,897 | 0.15 | 0.20 | nt | nt |
| Cell 19 | 34,965 | 0.07 | 0.53 | nt | nt |
| Cell 20 | 54,518 | 0.03 | 0.55 | nt | nt |
The numbers of VGLUT1 terminals on group I/II cells per unit length of dendritic arbor, decreased with distance from the cell body. This was confirmed by performing a Sholl analysis for numbers of contacts per unit (100 μm) dendritic length contained within concentric shells with radii which increase in 25 μm units from the centre of the cell body and calculating the average number of contacts per unit length within each radius (Fig. 6). The average number of VGLUT1 contacts per 100 μm of dendritic length of all 17 cells contained in the first two 25 μm concentric units exceeded 20 contacts (24.1 and 24.8 contacts per 100 μm, respectively), but it decreased significantly to less than 15 contacts and 10 contacts when the radius of concentric shells increased to 100 μm and to 200 μm, respectively.
Figure 6. Histograms derived from Sholl analysis.
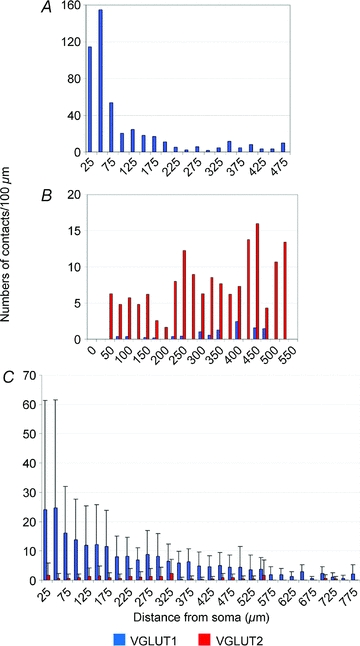
The histograms show the distribution of numbers of contacts per 100 μm of dendritic length within 25 μm shells from the cell body of cell 1 which is a cell that was monosynaptically activated by group I and II afferents (A), cell 20 which is a cell that was monosynaptically activated from the MLF with no primary afferent input (B) and the distribution of average numbers of contacts per unit length (±s.d.) for all 17 cells that were monosynaptically activated by group I and II primary afferents (C).
Are there differences in contacts associated with excitatory and inhibitory interneurons?
Of the 17 group I/II interneurons investigated, the axon terminals of seven neurons (cells 1 to 7) were VGLUT2 immunoreactive and the remaining ones (cells 8 to 17) possessed axon terminals that were apposed to gephyrin and were therefore glycinergic (for details see Bannatyne et al. 2009). Confocal microscopic analysis revealed that glutamatergic cells tended to receive more VGLUT1 contacts than glycinergic cells. The average number (±s.d.) of VGLUT1 contacts on excitatory and inhibitory cells was 729 ± 442.4 and 262 ± 185.8, respectively, and the average packing densities of VGLUT1 terminals in contact with the excitatory and inhibitory cells were 1.24 ± 0.70 per 100 μm2 and 0.85 ± 0.57 per 100 μm2, respectively. However, the distribution of VGLUT1 contacts on individual dendritic branches of an interneuron was not always even. The average density of VGLUT2 terminals was higher on inhibitory cells (0.13 ± 0.2 per 100 μm2) when compared with excitatory cells (0.03 ± 0.07 per 100 μm2). In addition, we were unable to detect convincing examples of contacts between VGLUT2 terminals and five of the seven excitatory cells but only two out of ten inhibitory cells. Nonetheless, none of these differences were found to be statistically significant, most probably because of the relatively small size of our sample.
Are there differences in presynaptic inhibitory control of excitatory versus inhibitory interneurons?
Some of the VGLUT1 terminals forming contacts with labelled interneurons were themselves apposed by GAD-immunopositive axon terminals. This type of axoaxonic arrangement was associated with all 17 interneurons (i.e. both excitatory and inhibitory cells). In addition, it was very often the case that more than one presumed presynaptic terminal made contact with the same postsynaptic axon terminal. Figure 7 shows an example of several putative presynaptic GAD terminals (in green) that were in contact with three VGLUT1 terminals (in blue) which in turn formed contacts with a dendrite of the labelled neuron. GAD-immunoreactive terminals were also frequently seen in apposition to interneuron dendrites and cell bodies but were seldom associated with VGLUT2 terminals. The general distribution pattern of VGLUT1 terminals with (represented by blue open triangles) and without (represented by blue filled triangles) associated presynaptic GAD terminals is shown in Figs 4 and 5. The mean density (±s.d.) of VGLUT1 contacts associated with GAD terminals was 1.01 ± 0.70 per 100 μm2 for glutamatergic cells and 0.62 ± 0.51 per 100 μm2 for glycinergic cells. So, the average percentage (±s.d.) of the above densities expressed as a proportion of the density of the total number of VGLUT1 contacts were 74.3 ± 12.97% and 68.3 ± 8.69% for glutamatergic and glycinergic cells, respectively, and there was no statistical difference between these figures. Furthermore, the average numbers of total VGLUT1 contacts and VGLUT1 contacts associated with GAD terminals was calculated for both glutamatergic and glycinergic cells per 100 μm units of the dendritic length on proximal (<100 μm from the soma), intermediate (between 100 and 300 μm) and distal (>300 μm) dendrites. For excitatory neurons, these values were 30.0, 12.9 and 3.6 contacts per 100 μm for all VGLUT1 contacts and 26.8, 10.3 and 2.2 contacts per 100 μm for VGLUT1 contacts associated with apposed GAD terminals on proximal, intermediate and distal dendrites. For inhibitory neurons, these values were 12.5, 8.3 and 5.7 contacts per 100 μm for all VGLUT1 contacts and 10.1, 5.5 and 3.9 contacts per 100 μm for VGLUT1 contacts associated with presynaptic GAD terminals on proximal, intermediate and distal dendrites.
Figure 7. A series of confocal microscope images illustrating VGLUT1 terminals contacting a labelled interneuron and their relationship with GABA-containing terminals.
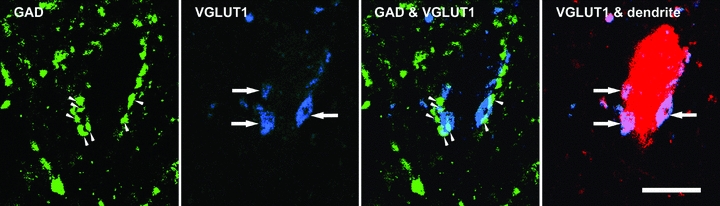
The left two panels show single optical sections through individual boutons (arrowheads and arrows). The dendrite of the postsynaptic interneuron is shown in red, immunoreactivity for GAD in green and VGLUT1 in blue. The right two panels are merged images confirming that the VGLUT1 terminals are associated with presynaptic GAD terminals and are in turn presynaptic to the labelled interneuronal dendrite. Scale bar = 10 μm.
Discussion
The results of the present study revealed that numerous VGLUT1 contacts were present on all interneurons of the sample to which monosynaptic input was provided by either group I and/or group II muscle afferents, while VGLUT2 terminals were less frequent, or were practically absent. As VGLUT1 terminals originate from primary afferents and VGLUT2 terminals from intraspinal neurons (Varoqui et al. 2002; Todd et al. 2003), intermediate zone interneurons mediating reflex actions of group I and II afferents appear to be under unexpectedly weak excitatory control by other spinal interneurons.
Classification of input to intracellularly labelled interneurons
The validity of classification of input to the labelled interneurons depends critically on the reliability of the criteria for EPSPs evoked mono- and disynaptically. Jankowska et al. (2005) have discussed these criteria in detail. Three features of EPSPs were used to differentiate monosynaptic from disynaptic EPSPs: (1) monosynaptic EPSPs had a fixed range of segmental latencies following the first positive peak of the afferent/descending volleys (0.6–1.1 ms, 1.4–2.8 ms and 0.4–1.0 ms for group I afferents, group II afferents and MLF, respectively), disynaptic EPSPs being evoked at longer latencies; (2) monosynaptic EPSPs were evoked successfully by each single stimulus while disynaptic EPSPs could be evoked by either single or only the second or third stimulus in a train; and (3) there was no or only minimal temporal facilitation of monosynaptic EPSPs but considerable facilitation of disynaptic EPSPs following successive stimuli in a train. Using these criteria it is easier to identify disynaptic EPSPs which are not preceded by monosynaptic ones, and long latency components of compound EPSPs evoked by stimulation of muscle nerves may indicate PSPs relayed by other spinal neurons (di- or tri-synaptically) as well as PSPs attributable to monosynaptic actions of slower conducting afferents. In view of the paucity of VGLUT2 terminals on intermediate zone interneurons, in particular the excitatory interneurons, the more plausible interpretation of such late components would therefore be that they are due to monosynaptic actions of slow afferents. Consequently they lead to the conclusion that these interneurons receive dominant excitatory input from primary afferents and are under more potent inhibitory rather than excitatory control by other spinal interneurons (see the last part of the Discussion for consequences of this kind of coupling).
The sample of neurons on which the analysis is based is relatively small (17 neurons with monosynaptic input from primary afferents and 3 cells that were monosynaptically activated by stimulation within the MLF) because as a consequence of a variety of technical limitations, it could only be carried on a small proportion of the neurons labelled originally. However, despite the size of the sample, all the results found were highly consistent and may therefore be considered as representative for the whole population of intermediate zone premotor interneurons mediating disynaptic actions of group I and II afferents on hindlimb motoneurons. As already indicated in the Introduction, the sample was selected as representative for this class of interneuron.
Patterns of contacts of excitatory terminals on intermediate zone interneurons
The vast majority of terminals contacting group I/II cells were found to be VGLUT1 positive and only relatively small numbers (or no) VGLUT2 contacts were found to form appositions with them. As pointed out above, most myelinated primary afferent terminals in the grey matter of lumbar spinal cord are VGLUT1 immunoreactive while VGLUT2 immunoreactivity characterises axon terminals that originate predominantly from spinal interneurons (Varoqui et al. 2002; Todd et al. 2003). The finding of a high proportion of VGLUT1-positive terminals is thus consistent with monosynaptic EPSPs from muscle afferents found in group I/II cells. The numbers of VGLUT2 contacts were expected to be larger as input to group I/II cells is not only from primary afferents but also from other spinal interneurons, including those mediating disynaptic actions of descending tract neurons (Grillner & Hongo, 1972; Hongo et al. 1972; Harrison & Jankowska, 1985; Edgley & Jankowska, 1987b; Davies & Edgley, 1994; Jankowska et al. 2002; Stecina et al. 2008a,b;). However, the small numbers of VGLUT2 contacts on intermediate zone interneurons is in keeping with the much lower density of VGLUT2 compared to VGLUT1-ir terminals in laminae V and VI reported in the study of Oliveira et al. (2003). In addition, VGLUT1-immunoreactive terminals in contact with labelled neurons might have originated not only from primary afferents but also represent terminals of corticospinal and rubrospinal neurons, because pyramidal cells and cells in the red nucleus show strong reactions for VGLUT1 mRNA (Fremeau et al. 2001). They also could originate from vestibulospinal neurons as some projections from vestibular nuclei contain either VGLUT1 or VGLUT2 (Cai et al. 2008). In contrast, terminals of reticulospinal neurons would more likely be VGLUT2 immunoreactive, at least in view of the predominance of VGLUT2 contacts on commissural interneurons with monosynaptic input from the MLF found in this study. One might also consider that not all classes of interneuron use the VGLUT2 transporter and that the cells we studied were also contacted by VGLUT1-containing interneurons. This suggestion might find support in reports of Kullander et al. (2003) and Oliveira et al. (2003), who found VGLUT1 (both mRNA and protein) in a small population of spinal neurons located in the dorsomedial part of the intermediate zone. It was concluded that these neurons were most likely to be interneurons (Oliveira et al. 2003) but they could also be dorsal spinocerebellar tract neurons located in either Clarke's column or in the dorsal horn (Edgley & Gallimore, 1988).
Another factor to consider might be an underestimation of the number of VGLUT2 contacts as a consequence of the technical limitations stated in the Methods section, as intracellularly labelled interneurons and the VGLUT2 terminals contacting them both appeared in the red channel of the confocal microscope. Nevertheless, VGLUT2 appositions on cells were usually obvious as the labelling associated with them was more intense than the labelling found within cells (see Fig. 3). In addition, MLF-activated cells were associated with significantly greater numbers of VGLUT2 terminals identified by using the same criteria and, as these cells were labelled in the same animals and processed along with group I/II cells, the limited numbers of VGLUT2 contacts found on group I/II cells cannot be attributed to problems of weak or inadequate immunostaining.
Unless other factors interfered with the detection of glutamatergic terminals of other interneurons on our sample of group I/II interneurons, the predominance of VGLUT1 contacts and the paucity of VGLUT2 contacts suggests that operation of these neurons is modulated to a greater extent by inhibitory rather than excitatory spinal interneurons. The electrophysiological records (Fig. 1) show that following the monosynaptic EPSP induced by stimulation of primary afferents, usually an IPSP appeared, representing disynaptic actions of stimulated afferents which were mediated via monosynaptically excited inhibitory group I/II cells while di- or poly-synaptic EPSPs were seldom recorded. No convincing examples of disynaptic EPSPs evoked from group I afferents were observed in this series of experiments. Previous evidence for disynaptic group II actions on unspecified intermediate zone interneurons via dorsal horn interneurons is strong (Jankowska et al. 2002) but di- or poly-synaptic EPSPs from group I and II afferents might be preferably evoked in inhibitory interneurons in which a tendency for a relatively larger proportion of VGLUT2 contacts was found, with the possibility that their actions would be strengthened by other interneurons, while activation of excitatory interneurons would be counteracted by di/polysynaptic IPSPs.
These possibilities are also consistent with recent anatomical studies. Excitatory dorsal horn interneurons with group II input and axon terminals containing VGLUT2 were found to project to the intermediate zone (Bannatyne et al. 2006) but transmitter phenotype of their target cells has not been defined. Even if disynaptic excitatory actions of group II afferents mediated by dorsal horn interneurons were evoked in a small proportion of excitatory intermediate zone group I/II interneurons, these might have not been represented in the present sample. Furthermore, Bannatyne et al. (2009) have shown that inhibitory intermediate zone interneurons in pathways from group I and II afferents contact cells in the intermediate grey matter with high concentrations of VGLUT1 terminals on their proximal dendrites. These postsynaptic cells thus would be likely to be group I/II cells and conform with the evidence for inhibitory interactions within the total population of these cells (Brink et al. 1983; Edgley & Jankowska, 1987b). In contrast, excitatory group I/II interneurons formed contacts with unidentified neurons, which were located in regions outside motor nuclei that had low concentrations of VGLUT1 contacts associated with them and thus were not likely to receive substantial input from primary afferents.
Postsynaptic excitation from and presynaptic inhibition of group I/II afferents monosynaptically contacting excitatory and inhibitory intermediate zone interneurons
Several studies have demonstrated that all the terminals that form axoaxonic synapses with group I and II afferents are GABAergic even though some of the presynaptic boutons also contain co-localised glycine (Maxwell et al. 1997; Maxwell & Riddell, 1999; Watson & Bazzaz, 2001). Although we did not extend the analysis to the ultrastructural level, it is likely that most of the appositions we observed between GAD and VGLUT1 terminals were synaptic (e.g. see Hughes et al. 2005). Therefore, by using GAD immunoreactivity to identify presynaptic terminals at axoaxonic contacts, virtually all putative axoaxonic presynaptic terminals should be labelled. Quantitative analysis indicates that most VGLUT1 terminals contacting excitatory and inhibitory group I/II cells are associated with putative presynaptic GAD terminals which provide a substrate for strong presynaptic inhibition (Riddell et al. 1995; Jankowska et al. 2002). An additional point is that all of the intracellularly labelled cells were glutamatergic or glycinergic and none of them contained GABA; therefore, it was unlikely that any of the cells themselves were involved in presynaptic inhibition. Watson and Bazzaz (2001) reported that the mean number of axoaxonic contacts received per group Ia afferent terminal in the rat ventral horn and deep dorsal horn was 2.7 and 1.6, respectively. Also a single group II afferent terminal may be associated with several GABAergic presynaptic terminals (Maxwell & Riddell, 1999). Consistent with these findings, it was quite often observed that more than one GABAergic bouton was in contact with the same VGLUT1 terminal which, in turn, formed a contact with an intermediate zone interneuron (Fig. 7).
As group II afferent terminals in the intermediate zone are subject to stronger presynaptic inhibition than those within the dorsal horn (Riddell et al. 1995; Jankowska et al. 2002), it was of interest to determine whether there are also differences in axoaxonic contacts formed with primary muscle afferents contacting excitatory and inhibitory interneurons within the same region of the grey matter of the spinal cord, the intermediate zone. Immunocytochemical results show that this was not the case because the proportion of VGLUT1 terminals associated with GAD presynaptic terminals contacting excitatory interneurons was not found to be statistically different from those contacting inhibitory interneurons. Nevertheless, this does not mean that the actions of group I/II afferents on their targeted excitatory and inhibitory interneurons are always equally affected by presynaptic inhibition. As discussed above, group I/II interneurons may be activated by primary afferents from different nerves. It is known that each type of afferent is differentially affected by presynaptic inhibition and their dominant evoking sources are also various. For example, presynaptic inhibition of Ia afferents is evoked by primarily flexor group Ib afferents while presynaptic inhibition of group Ib afferents is evoked by both flexor and extensor Ib afferents (Rudomin & Schmidt, 1999). Moreover, the degree of presynaptic inhibition is not invariant but may change according to the task to be performed. Duenas and Rudomin (1988) reported that the level of primary afferent depolarisation of group I afferents changes during the fictive locomotor cycle and it reaches maximal strength during the flexor phase. Therefore, presynaptic inhibition is very likely to be involved in selection of intermediate zone interneurons that are interposed in group I and II pathways to produce appropriate actions on motoneurons.
Functional consequences
The present findings provide a new basis for understanding the organization of interneurons mediating reflex actions from group I and II afferent fibres. As the majority of excitatory terminals on group I/II interneurons are VGLUT1 immunoreactive, this is consistent with monosynaptic actions of primary afferents along with some descending tract fibres (see above). However, relatively sparse or no contacts were formed with most cells by VGLUT2-containing axons. One interpretation of this observation is that cells with few or no VGLUT2 contacts are a subtype of (predominantly excitatory) intermediate zone interneurons which are activated by peripheral afferents as well as by direct actions of descending fibres and are under strong inhibitory control by other spinal interneurons, whereas those possessing VGLUT2 terminals may represent another subpopulation (most probably inhibitory) whose activation is either strengthened or weakened by other spinal interneurons. An alternative interpretation would be that any hypothetical spinal interneurons relaying excitatory input to excitatory intermediate zone interneurons are not glutamatergic. However, this suggestion is, with very few exceptions (e.g. Zagoraiou et al. 2009), at variance with known transmitter phenotypes of adult interneurons with input from peripheral afferents documented to date (e.g. see Bannatyne et al. 2009).
The diagram in Fig. 8 summarizes the putative connections between excitatory and inhibitory interneurons mediating actions of group I/II afferents to motoneurons based on our findings combined with data from previous studies of the axonal projections of intermediate zone interneurons (Bannatyne et al. 2003, 2009; Jankowska et al. 2005, 2009). The diagram indicates the unexpected asymmetry in these connections, inhibitory interneurons (represented by the red neuron) affecting the excitatory interneurons (represented by the green neuron) but the excitatory interneurons failing to provide disynaptic excitatory input to the inhibitory interneurons. The diagram indicates also that primary afferents contacting both excitatory and inhibitory interneurons are under control of GABAergic presynaptic inhibition. The network formed by these neurons would be consistent generally with a higher probability of evoking disynaptic inhibition rather than disynaptic excitation of hindlimb motoneurons while stimulating group I and/or II afferents. It would also proffer a new interpretation of the contribution of intermediate zone interneurons with group I/II input to centrally initiated movements. In particular, it would suggest that the excitation mediated by these neurons manifests itself most readily when activation of the inhibitory subpopulation is weakened. This might occur under various experimental conditions. For instance, in preparations in which the balance between the excitation and inhibition is changed in favour of excitation by a selective lesion of the brainstem (Holmquist & Lundberg 1961), by spinalization involving transection of the descending serotoninergic pathways (Aggelopoulos et al. 1996) or during particular phases of a locomotor step cycle (McCrea et al. 1995; Perreault et al. 1995; Angel et al. 1996. Stecina et al. 2005). Under these conditions, not only motoneurons but also excitatory intermediate zone interneurons would be disinhibited and more readily activated by primary afferents, with the resulting enhancement of disynaptic actions of group I and II afferents relayed by these interneurons and, consequentially, an increase in excitability of motoneurons. This may manifest itself as enhanced oligosynaptically evoked stretch reflexes (see Jankowska & Hammar, 2002), crossed reflexes (Aggelopoulos et al. 1996) or shortening in step cycle duration (Yakovenko et al. 2005) and be associated with the appearance of voltage-dependent persistent inward currents and plateau potentials (Bennett et al. 1998; Hultborn et al. 2004; Nielsen et al. 2007). Under conditions of negligible excitatory input from other interneurons, other means of modulating activity of intermediate zone interneurons must thus become of paramount importance, in particular their fine-tuning by descending tract neurons, modulation of the degree of their inhibition and modulation of input from primary afferents by presynaptic inhibition as well as other modulatory systems.
Figure 8. Synapses formed with the intermediate zone interneurons and by their axon collaterals.
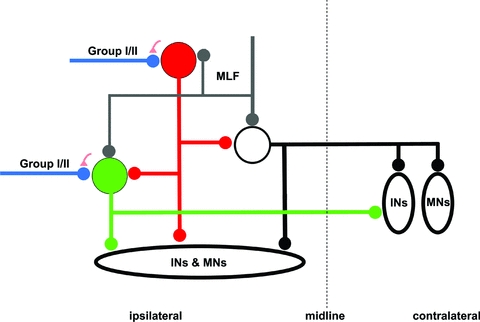
The axonal projections are partly based on the morphological findings reported by Bannatyne et al. (2003, 2009) and Jankowska et al. (2005, 2009). The green and red circles represent excitatory and inhibitory intermediate zone interneurons with monosynaptic group I and II afferent input, respectively. The white circle represents lamina VIII commissural interneurons with monosynaptic input from MLF but without monosynaptic primary afferent input. The green, red and black lines with small circles at the end represent the axon projections of the corresponding cells. The blue and grey lines with small circles at the end represent group I/II afferents and MLF inputs, respectively. The small red triangles indicate presynaptic terminals associated with the primary afferent terminals. INs, interneurons; MNs, motoneurons.
To discuss all these issues is outside the scope of the present paper but we may draw attention to two particularly interesting consequences of this arrangement. One is, that in contrast to inhibitory interneurons, excitatory intermediate zone interneurons with input from group I and II afferents may be last order interneurons of movements that are centrally initiated by direct actions of descending tract neurons with VGLUT1 terminals (e.g. corticospinal or rubrospinal tracts, see above and Jankowska, 1992; Davies & Edgley, 1994) but not by indirect actions relayed by spinal interneurons. The second consequence is closely related, in that excitatory intermediate zone interneurons can hardly operate as last order interneurons in neuronal networks of locomotion (often referred to as central pattern generators, CPG), even though the inhibitory interneurons could (see McCrea & Rybak, 2008). This may thus have implications for future models of CPG networks.
Acknowledgments
The study was supported by a grant from NINDS/NIH (R01 NS040863). T.T.L. was a University of Glasgow FBLS Scholar. We wish to thank Drs I. Hammar and K. Stecina for their permission to use some of the cells labelled with them for the present morphological study.
Glossary
Abbreviations
- ABSM
anterior biceps and semimembranosus
- DP
deep peroneal
- GAD
glutamic acid decarboxylase
- GS
gastrocnemius and soleus
- MLF
medial longitudinal fascicle
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PBST
posterior biceps and semitendinosus
- PI
plantaris
- Q
quadriceps
- Sart
sartorius
- TPBS
Tris phosphate-buffered saline
- VGLUT1
vesicular glutamate transporter 1
- VGLUT2
vesicular glutamate transporter 2
Author contributions
The experiments were conceived and designed by D.J.M. and E.J. The original material of Bannatyne et al. (2009) was collected and analysed electrophysiologically in the laboratory of E.J., University of Gothenburg. Morphological data were collated and analysed by T.T.L. under the supervision of D.J.M. and A.B. in the Spinal Cord Group Laboratories, University of Glasgow. The original draft was made by T.T.L. and revised by D.J.M. and E.J. All authors approved the final version.
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jimenez T, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol. 1996;494:851–861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol. 2009;587:379–399. doi: 10.1113/jphysiol.2008.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Brink E, Jankowska E, McCrea DA, Skoog B. Inhibitory interactions between interneurones in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol. 1983;343:361–373. doi: 10.1113/jphysiol.1983.sp014897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YL, Ma WL, Wang JQ, Li YQ, Li M. Excitatory pathways from the vestibular nuclei to the NTS and the PBN and indirect vestibulo-cardiovascular pathway from the vestibular nuclei to the RVLM relayed by the NTS. Brain Res. 2008;1240:96–104. doi: 10.1016/j.brainres.2008.08.093. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol. 1987;389:675–689. doi: 10.1113/jphysiol.1987.sp016677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res. 1988;70:15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Gallimore CM. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol. 1988;397:99–111. doi: 10.1113/jphysiol.1988.sp016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987a;385:393–413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol. 1987b;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hongo T. Vestibulospinal effects on motoneurones and interneurones in the lumbosacral cord. Prog Brain Res. 1972;37:243–262. doi: 10.1016/S0079-6123(08)63906-0. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Sources of input to interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol. 1985;361:379–401. doi: 10.1113/jphysiol.1985.sp015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurons. Acta Physiol Scand. 1961;54:1–51. [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15:54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci U S A. 2005;102:9038–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Bannatyne BA, Stecina K, Hammar I, Cabaj A, Maxwell DJ. Commissural interneurons with input from group I and II muscle afferents in feline lumbar segments: neurotransmitters, projections and target cells. J Physiol. 2009;587:401–418. doi: 10.1113/jphysiol.2008.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci. 2010;32:881–893. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurons; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Rev. 2002;40:19–28. doi: 10.1016/s0165-0173(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol. 2002;542:287–299. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DJ, Kerr R, Jankowska E, Riddell JS. Synaptic connections of dorsal horn group II spinal interneurons: synapses formed with the interneurons and by their axon collaterals. J Comp Neurol. 1997;380:51–69. [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS. Axoaxonic synapses on terminals of group II muscle spindle afferent axons in the spinal cord of the cat. Eur J Neurosci. 1999;11:2151–2159. doi: 10.1046/j.1460-9568.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity – from a basic science point of view. Acta Physiol (Oxf) 2007;189:171–180. doi: 10.1111/j.1748-1716.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Olave MJ, Maxwell DJ. Neurokinin-1 projection cells in the rat dorsal horn receive synaptic contacts from axons that possess α2C-adrenergic receptors. J Neurosci. 2003;23:6837–6846. doi: 10.1523/JNEUROSCI.23-17-06837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. J Physiol. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Stecina K, Jankowska E, Cabaj A, Pettersson L-G, Bannatyne BA, Maxwell DJ. Premotor interneurones contributing to actions of feline pyramidal tract neurones on ipsilateral hindlimb motoneurones. J Physiol. 2008a;586:557–574. doi: 10.1113/jphysiol.2007.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Quevedo J, McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. J Physiol. 2005;569:275–290. doi: 10.1113/jphysiol.2005.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecina K, Slawinska U, Jankowska E. Ipsilateral actions of feline rubrospinal tract neurones on hindlimb motoneurones. J Physiol. 2008b;586:5865–5884. doi: 10.1113/jphysiol.2008.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AH, Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol. 2001;433:335–348. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, McCrea DA, Stecina K, Prochazka A. Control of locomotor cycle durations. J Neurophysiol. 2005;94:1057–1065. doi: 10.1152/jn.00991.2004. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


