Abstract
Episodes of hypoxia in utero present a potentially serious challenge to the fetus, but are counteracted by defence responses including marked redistribution of blood flow from peripheral circulations to the brain. Here, we report the novel observation that the oxidant tone is an important modulator of this cardiovascular defence. Using pregnant Welsh Mountain sheep surgically prepared for long-term recording, we investigated in vivo the effects on the fetal cardiovascular defence to acute hypoxaemia of fetal treatment with the antioxidant vitamin C. The mechanisms via which vitamin C may affect the vascular oxidant tone were investigated by monitoring fetal plasma concentrations of nitrates and nitrites, by determining changes in the activity of superoxide dismutase (SOD) in fetal plasma, and by investigating the effect of vitamin C treatment on the fetal cardiovascular defence to hypoxaemia following nitric oxide (NO) synthase blockade. Fetal treatment with vitamin C markedly depressed the normal femoral constrictor response to acute hypoxaemia in the fetus (5.2 ± 1.0 vs. 1.1 ± 0.3 mmHg (ml min−1)−1, mean ±s.e.m.; P < 0.05) an effect which was completely restored following NO synthase blockade (6.2 ± 1.3 mmHg (ml min−1)−1). Compared to saline infusion, fetal treatment with vitamin C during acute hypoxaemia also significantly increased fetal plasma SOD activity from normoxic baseline (−8.9 ± 6.5 vs. 15.0 ± 6.6% inhibition, P < 0.05) and decreased the plasma concentration ratio of nitrate:nitrite from normoxic baseline (ΔNO3−:NO2−; 0.15 ± 0.30 vs. −0.29 ± 0.11, P < 0.05). The data provide in vivo evidence of redox modulation of redistribution of blood flow in the fetus, part of the fetal brain sparing during acute hypoxaemic stress.
Introduction
Hypoxaemia is one of the most common challenges that the fetus experiences during gestation and, in particular, during the processes of labour and delivery (Beard & Rivers, 1979). An inadequate fetal defence to hypoxaemia renders the fetal brain susceptible to injury leading to hypoxic-ischaemic encephalopathy (Low et al. 1985). The latter is predictive of developing cerebral palsy and cognitive disability later in life (Hall, 1989). Therefore, the physiology underlying the fetal defence to hypoxaemia remains at the forefront of basic science and clinical interest.
The fetal defence to hypoxaemia is contingent on its cardiovascular response, which involves a transient bradycardia and peripheral vasoconstriction (Rudolph, 1984; Giussani et al. 1994). The increase in peripheral vascular resistance aids the redistribution of the fetal cardiac output, prioritising blood flow away from peripheral and towards essential vascular beds, such as the cerebral circulations. This innate brain-sparing response has been conserved across all species studied to date, including the sheep, non-human primate and human fetus (Cohn et al. 1974; Jackson et al. 1987; Akalin-Sel & Campbell, 1992). The mechanisms mediating the fetal peripheral vasoconstrictor response to hypoxaemia are well characterised. It is triggered by a carotid chemoreflex (Bartelds et al. 1993; Giussani et al. 1993) and maintained by the release of vasoconstrictor agents into the fetal circulation, such as catecholamines, neuropeptide Y (NPY) and vasopressin (Jones & Robinson, 1975; Fletcher et al. 2000; Giussani et al. 2001). There is also a local component due to the direct effects of hypoxia at the tissue level. In the fetus, as in the adult, the endothelium may act as a hypoxic sensor and effector system that releases vasoactive agents to act locally on the vascular smooth muscle, such as NO and endothelin (Vane et al. 1990; Green et al. 1998). The net fetal peripheral vasomotor response to hypoxaemia thus represents the balance between neural, endocrine and local paracrine vasoactive mechanisms. While endothelin does not appear to contribute to the fetal cardiovascular response to acute hypoxaemia (Green et al. 1998), we have previously reported that fetal exposure to acute hypoxaemia during fetal NO synthase blockade leads to a significant enhancement of the femoral constrictor response (Morrison et al. 2003). In the fetus, hypoxia-induced increases in NO therefore oppose and diminish the peripheral constrictor neural and endocrine influences on the fetal peripheral circulation (Morrison et al. 2003).
More recently, it has become evident that the cellular oxidant milieu is also an important modulator of vascular resistance (Chen & Keaney, 2004). Vascular cells generate reactive oxygen species (ROS), such as the superoxide anion (•O2−) (Droge, 2002). Superoxide readily combines with NO, limiting its bioavailability (Kissner et al. 1997). Hence, under physiological conditions, manipulation of the vascular NO:•O2− ratio is also an important determinant of vascular tone. Several studies have investigated the effects of manipulation of the vascular NO:•O2− ratio in the lung circulation with regard to pulmonary hypertension in the adult (Irodova et al. 2002) and in the placenta with regard to preeclampsia (Davidge, 1998). What remains completely unknown is whether manipulation of the vascular NO:•O2− ratio can also affect cardiovascular function in the fetus. One mechanism of manipulating the fetal vascular NO:•O2 ratio in vivo may be to expose the fetal circulation to established antioxidants, such as vitamin C, during stimulated conditions, such as during acute hypoxaemia. Exposure to vitamin C has been reported to increase NO bioavailability by several pathways, including quenching ROS, stimulating NO synthase and increasing the expression of antioxidant enzymes (Geetha et al. 1989; Jackson et al. 1998; Heller et al. 1999; Wilson, 2009).
This study used the chronically instrumented, unanaesthetised fetal sheep preparation to test the hypothesis that the cellular oxidant milieu is an important modulator of vascular resistance in the fetus. The hypothesis was tested by in vivo manipulation of the NO:•O2− ratio with fetal treatment with vitamin C during acute hypoxaemia. It was expected that fetal treatment with vitamin C would increase NO bioavailability which, in turn, would depress the femoral vasoconstrictor response to acute hypoxaemia. Therefore, the mechanism via which vitamin C would increase NO bioavailability in the fetus was investigated in three ways: (1) by measuring the concentration of nitrite [NO2−] and nitrate [NO3−] in plasma during acute hypoxaemia in fetuses with and without vitamin C treatment; (2) by investigating the effect of fetal treatment with vitamin C on the plasma activity of the antioxidant enzyme superoxide dismutase (SOD) during acute hypoxaemia; and (3) by determining whether suppression of the fetal peripheral vasoconstrictor response to hypoxaemia during vitamin C treatment could be reversed following NO synthase blockade.
Methods
Surgical preparation
All procedures were performed under the UK Animals (Scientific Procedures) Act 1986 and were approved by the Ethical Review Committee of the University of Cambridge. Seven Welsh Mountain sheep fetuses were surgically instrumented for long-term recording at 125 ± 1 days (±s.d.) of gestation (term is ca. 145 days) using strict aseptic conditions as previously described in detail (Giussani et al. 2001). In brief, food, but not water, was withheld from the pregnant ewes for 24 h prior to surgery. Following induction with 20 mg kg−1i.v. sodium thiopentone (Intraval Sodium; Merial Animal Health Ltd, Rhone Mérieux, Dublin, Ireland), general anaesthesia (1.5–2.0% halothane in 50:50 O2:N2O) was maintained using positive pressure ventilation. Midline abdominal and uterine incisions were made, the fetal hind limbs were exteriorised and, on one side, femoral arterial (i.d., 0.86 mm; o.d., 1.52 mm; Critchly Electrical Products, NSW, Australia) and venous (i.d., 0.56 mm; o.d., 0.96 mm) catheters were inserted. The catheter tips were advanced carefully to the descending aorta and inferior vena cava, respectively. Another catheter was anchored onto the fetal hind limb for recording of the reference amniotic pressure, and a Transonic flow transducer was positioned around the contra-lateral femoral artery (2R or 3S). The uterine incisions were closed in layers, the dead space of the catheters was filled with heparinised saline (80 i.u. heparin ml−1 in 0.9% NaCl) and the catheter ends were plugged with sterile brass pins. All catheters and the flow probe lead were exteriorised via a keyhole incision in the maternal flank and kept inside a plastic pouch sewn onto the maternal skin.
Postoperative care
During recovery, ewes were housed in individual pens in rooms with a 12 h–12 h light–dark cycle where they had free access to hay and water and were fed concentrates twice daily (100 g sheep nuts no. 6; H & C Beart Ltd, Kings Lynn, UK). Antibiotics were administered daily to the ewe (0.20–0.25 mg kg−1i.m. Depocillin; Mycofarm, Cambridge, UK) and fetus i.v. and into the amniotic cavity (150 mg kg−1 Penbritin; SmithKline Beecham Animal Health, Welwyn Garden City, UK). The ewes also received 2 days of postoperative analgesia (10–20 mg kg−1 oral phenylbutazone; Equipalozone paste, Dechra Veterinary Products, Stoke-on-Trent, UK). Generally, normal feeding patterns were restored within 48 h of recovery. Following 72 h of postoperative recovery, ewes were transferred to metabolic crates where they were housed for the remainder of the protocol. The fetal arterial and amniotic catheters were connected to sterile pressure transducers (COBE; Argon Division, Maxim Medical, Athens, TX, USA) and calibrated mean fetal arterial blood pressure (corrected for amniotic pressure) and fetal heart rate (triggered via a tachometer from the pulsatility in the arterial blood pressure signal) were recorded continually using a computerized Data Acquisition System (DAS; Department of Physiology, Cambridge University, UK). Whilst on the metabolic crates, the patency of the fetal catheters was maintained by a slow continuous infusion of heparinized saline (80 i.u. heparin ml−1 at 0.1 ml h−1 in 0.9% NaCl) containing antibiotic (1 mg ml−1 benzylpenicillin; Crystapen, Schering-Plough Animal Health Division, Welwyn Garden City, UK).
Experimental protocol
Following at least 5 days of postoperative recovery, all fetuses were subjected to hypoxaemic experiments, carried out on consecutive days in a randomised order (Fig. 1). Each protocol consisted of a 3 h period divided into 1.5 h normoxia, 0.5 h hypoxaemia and 1 h recovery, during a slow i.v. infusion of heparinised saline vehicle (80 i.u. heparin ml−1 in 0.9% NaCl), treatment with vitamin C alone (ascorbate; A-5960; Sigma Chemicals, UK; 8.9 ± 0.4 mg kg−1 min−1i.v.) or treatment with vitamin C during NO synthase blockade with the NO clamp (Fig. 1). In brief, a bolus dose (100 mg kg−1 dissolved in 2 ml heparinised saline) of NG-nitro-l-arginine methyl ester (l-NAME; Sigma-Aldrich, Poole, UK) was injected via the femoral artery, immediately followed by fetal i.v. infusion with sodium nitroprusside (SNP; Sigma-Aldrich; 5.1 ± 2.0 μg kg−1 min−1: mean ± 1 s.d.; dissolved in heparinised saline) the concentration of which was titrated to avoid any perturbation in basal arterial blood pressure. While fetal treatment with l-NAME alone leads to pronounced systemic vasoconstriction and hypertension, combined treatment of the fetus with both l-NAME and SNP compensates for the tonic production of the gas, maintains basal cardiovascular function and blocks de novo synthesis of NO during stimulated conditions. This technique has been established in our laboratory and previously validated (Gardner et al. 2001, 2002; Thakor & Giussani, 2005). At the end of the experimental protocol, the effectiveness of NO synthase blockade by the NO-clamp and the persistence of l-NAME in the system were tested by withdrawal of the SNP infusion.
Figure 1. Diagrammatic representation of the acute hypoxaemia protocol.
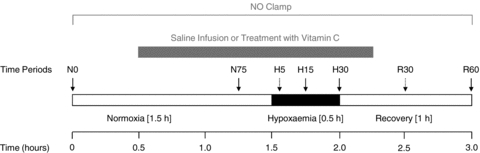
The experimental protocol consisted of a 3 h period divided into: 1.5 h normoxia, 0.5 h hypoxaemia (black bar) and 1 h recovery, during saline infusion (n = 7), treatment with vitamin C (8.9 ± 0.4 mg kg−1 min−1) alone (n = 7) or treatment with vitamin C during the NO clamp (n = 7; grey bar). Arrows represent times at which arterial blood samples were collected. Dotted arrows were for blood gas analysis only.
Acute hypoxaemia in the fetus was induced by maternal inhalational hypoxia. In brief, a large transparent respiratory hood was placed over the ewes’ head into which air was passed at a rate of ca 50 l min−1 for the 1.5 h period of normoxia. Following this control period, acute fetal hypoxaemia was induced for 30 min by changing the concentrations of gases breathed by the ewe to 6% O2 in N2 with small amounts of CO2 (15 l min−1 air–35 l min−1 N2–1.5–2.5 l min−1 CO2). This mixture was designed to reduce fetal 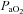 to ca 10 mmHg while maintaining
to ca 10 mmHg while maintaining 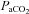 . Following the 0.5 h period of hypoxaemia, the ewe was returned to breathing air for the 1 h recovery period. At the end of the experimental protocol, the ewes and fetuses were killed with a lethal dose of sodium pentobarbitone (200 mg kg−1i.v. Pentoject; Animal Care Ltd, York, UK). A post-mortem was carried out at 132 ± 1 days of gestation. Each fetus was weighed and the positions of the implanted catheters and the flow probes were confirmed.
. Following the 0.5 h period of hypoxaemia, the ewe was returned to breathing air for the 1 h recovery period. At the end of the experimental protocol, the ewes and fetuses were killed with a lethal dose of sodium pentobarbitone (200 mg kg−1i.v. Pentoject; Animal Care Ltd, York, UK). A post-mortem was carried out at 132 ± 1 days of gestation. Each fetus was weighed and the positions of the implanted catheters and the flow probes were confirmed.
Blood sampling regimen and assays
During the experimental protocol, descending aortic blood samples were taken using sterile techniques from the fetus at set time intervals to determine arterial blood gases and pH (0.3 ml). Additional plasma samples were taken for measurement of plasma SOD activity, and plasma concentrations of NO2−, NO3− and ascorbate (1.5 ml; Fig. 1). Arterial blood gas and pH measurements were made using an ABL5 blood gas analyser (Radiometer; Copenhagen, Denmark; measurements corrected to 39.5°C). Values for percentage saturation of haemoglobin with oxygen (Sat Hb) were determined using a haemoximeter (OSM3; Radiometer, Copenhagen, Denmark). Plasma SOD activity was assayed using a SOD Assay Kit from BioVision (Mountain View, CA, USA) according to the instructions of the manufacturer. This assay utilizes WST-1, a tetrazolium salt, which produces a water soluble formazan dye upon reduction with superoxide anion. The rate of the reduction with a superoxide anion is linearly related with the amount to the xanthine oxidase activity and is inhibited by SOD. The SOD activity was expressed as the percentage of inhibition of the WST-1 reduction rate (Andres et al. 2008). Plasma concentrations of NO2− and NO3− were determined by a commercially available Nitrate/Nitrite Colorimetric Assay Kit (Caymen Chemical Co., Ann Arbor, MI, USA, cat no. 780001) working on the principles of the Griess reaction (Green et al. 1982). In brief, total NO2− and NO3− (NOx) was measured from plasma samples which were ultra-filtrated to reduce the background interference due to any haemoglobin present. Assay buffer, nitrate standards and 40 μl of plasma samples were then loaded in duplicate into a 96-well microplate with an enzyme cofactor and nitrate reductase before a 60 min incubation at room temperature. Following further addition of the Griess reagents, the plates were allowed to incubate for 10 min before reading absorbance at 540 nm (Bioteck ELx800 Absorbance Microplate Reader, Potton, Bedfordshire, United Kingdom). Plasma NO2− concentrations were measured by the same method but omitting the nitrate reductase step. Plasma NO3− was calculated as total NOx minus NO2−. The inter- and intra-assay coefficients of variation were 3.4% and 2.7%, respectively, and the lower limit of detection of the assay is 0.24 μm. Plasma ascorbate concentrations were measured using HPLC with electrochemical detection (Iriyama et al. 1984). Total ascorbate was measured following the treatment of the pre-acidified plasma sample with Tris-[2-carboxyethyl]-phosphine (TCEP) HCl (Lykkesfeldt, 2000). Aliquots of 20 μl were injected into the HPLC unit with a chromatography column (250 × 4.6 mm, C18, 5 μm; Apex II column with guard; Jones Chromatography, Glamorgan, UK) and eluted with a mobile phase containing K2HPO4–H3PO4 (200 mmol l−1; pH 2.1) and octane sulphonic acid (250 μmol l−1; pH 2.56) at a flow rate of 1.0 ml min−1. An electrochemical detector (EG & G Instruments; Wokingham, UK) with a working electrode (set at 400 mV and sensitivity of 0.5 μA) was used for detection. Final concentrations for ascorbate were calculated with external standards, which were run simultaneously. The limit of sensitivity for the assay was 0.5 μmol l−1 and the inter-assay coefficient of variation was less than 5%.
Data and statistical analyses
All variables are expressed as means ±s.e.m. Summary measure analysis was applied to the serial cardiovascular data to focus the number of comparisons (Matthews et al. 1990). Femoral vascular resistance was calculated by using Ohm's principle and dividing fetal arterial blood pressure (corrected for amniotic pressure) by femoral blood flow. Similarly, femoral vascular conductance was calculated by dividing femoral blood flow by corrected fetal arterial blood pressure. Area under the curve was calculated at 30 min intervals (N1, N2, N3, H, R1, R2) for the absolute data describing the fetal cardiovascular responses. Plasma SOD activity and plasma concentrations of ascorbate, NO2− and NO3− were expressed as absolute values and/or as the mean ±s.e.m. change or percentage change from normoxic baseline, as appropriate. All variables were assessed statistically using the Student's t test for paired data, one-way or two-way analysis of variance (ANOVA) with repeated measures (RM) comparing the effect of time, treatment and interactions between time and treatment, as appropriate. Where a significant effect of time or treatment was indicated, Tukey's post hoc test was used to isolate the statistical differences. For all comparisons, statistical significance was accepted when P < 0.05.
Results
Fetal arterial blood gas status
Basal values for fetal arterial blood gases and pH were similar in all fetuses and were within the normal range for the Welsh Mountain sheep fetus at ca 130 days of gestation (Table 1). Infusion with saline had no effect on basal arterial blood gas status. In contrast, treatment with vitamin C alone (−0.06 ± 0.01) and treatment with vitamin C during NO synthase blockade (−0.06 ± 0.01) produced similar decrements in fetal pHa (P < 0.05; Table 1). In all fetuses, acute hypoxaemia induced significant falls of similar magnitude in 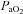 and Sat Hb, without any alteration to
and Sat Hb, without any alteration to 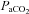 (Table 1). In addition, acute hypoxaemia during saline infusion (−0.08 ± 0.01), treatment with vitamin C alone (−0.09 ± 0.01) or treatment with vitamin C during NOS blockade (−0.09 ± 0.01) all produced similar decrements in fetal pHa (P < 0.05) relative to normoxia (Table 1). During recovery, pHa remained significantly depressed in all fetuses, whereas
(Table 1). In addition, acute hypoxaemia during saline infusion (−0.08 ± 0.01), treatment with vitamin C alone (−0.09 ± 0.01) or treatment with vitamin C during NOS blockade (−0.09 ± 0.01) all produced similar decrements in fetal pHa (P < 0.05) relative to normoxia (Table 1). During recovery, pHa remained significantly depressed in all fetuses, whereas 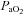 and Sat Hb returned towards basal values (Table 1).
and Sat Hb returned towards basal values (Table 1).
Table 1.
Fetal arterial blood gas status
| Normoxia | Acute hypoxaemia | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| N0 | N75 | H5 | H15 | H30 | R30 | R60 | ||
| Saline infusion or Treatment with vitamin C (± NO clamp) | ||||||||
| pHa | Saline Infusion | 7.36 ± 0.01 | 7.36 ± 0.01 | 7.36 ± 0.01 | 7.34 ± 0.01 | 7.29 ± 0.01a | 7.32 ± 0.01a | 7.34 ± 0.01 |
| Vitamin C infusion | 7.35 ± 0.01 | 7.29 ± 0.01a,b | 7.28 ± 0.01a,b | 7.25 ± 0.01a,b | 7.21 ± 0.01a,b | 7.25 ± 0.02a,b | 7.28 ± 0.01a,b | |
| Vitamin C infusion and NO clamp | 7.35 ± 0.01 | 7.28 ± 0.01a,b | 7.26 ± 0.01a,b | 7.23 ± 0.01a,b | 7.19 ± 0.01a,b | 7.21 ± 0.01a,b | 7.24 ± 0.01a,b | |
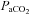 (mmHg) (mmHg) |
Saline infusion | 54.7 ± 0.7 | 54.8 ± 0.6 | 53.7 ± 0.6 | 54.1 ± 0.5 | 54.2 ± 0.6 | 54.3 ± 0.7 | 55.1 ± 0.5 |
| Vitamin C infusion | 56.0 ± 0.5 | 56.3 ± 0.8 | 55.8 ± 0.7 | 56.0 ± 0.6 | 54.7 ± 0.7 | 54.6 ± 0.6 | 55.3 ± 0.5 | |
| Vitamin C infusion and NO clamp | 55.3 ± 0.8 | 56.2 ± 0.5 | 54.5 ± 0.6 | 54.5 ± 0.7 | 55.2 ± 0.3 | 55.7 ± 0.6 | 55.0 ± 0.9 | |
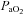 (mmHg) (mmHg) |
Saline infusion | 22.0 ± 0.8 | 21.3 ± 0.6 | 10.8 ± 0.3a | 11.4 ± 0.2a | 11.4 ± 0.2a | 21.2 ± 0.8 | 21.1 ± 0.8 |
| Vitamin C infusion | 21.2 ± 1.0 | 21.2 ± 0.5 | 11.3 ± 0.3a | 11.3 ± 0.2a | 12.0 ± 0.3a | 21.2 ± 1.4 | 22.2 ± 1.5 | |
| Vitamin C infusion and NO clamp | 21.7 ± 0.9 | 21.0 ± 0.8 | 11.7 ± 0.4a | 11.7 ± 0.2a | 11.2 ± 0.2a | 20.7 ± 1.0 | 22.0 ± 1.5 | |
| Sat Hb (%) | Saline infusion | 56.3 ± 1.4 | 53.5 ± 1.4 | 25.4 ± 1.3a | 25.2 ± 1.6a | 25.8 ± 0.9a | 53.2 ± 2.1 | 52.4 ± 2.1 |
| Vitamin C infusion | 54.8 ± 1.9 | 53.3 ± 2.0 | 24.1 ± 1.2a | 23.2 ± 1.3a | 23.9 ± 1.4a | 53.0 ± 2.1 | 53.8 ± 2.3 | |
| Vitamin C infusion and NO clamp | 52.8 ± 3.4 | 51.6 ± 2.9 | 24.2 ± 1.3a | 23.9 ± 1.4a | 23.8 ± 1.5a | 50.7 ± 4.3 | 51.4 ± 4.6 | |
Values are means ±s.e.m. at 0 (N0) and 75 min (N75) of normoxia, at 5 (H5), 15 (H15) and 30 min (H30) of hypoxemia, and at 30 (R30) and 60 min (R60) of recovery for fetuses exposed to 0.5 h of hypoxaemia during saline infusion (n = 7), treatment with vitamin C alone (n = 7) or treatment with vitamin C during the NO clamp (n = 7). Significant differences:
P < 0.05, vs. time period N0
P < 0.05, vs. saline infusion (two-way RM-ANOVA with Tukey's post hoc test).
pHa, arterial pH; 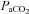 , arterial partial pressure of CO2;
, arterial partial pressure of CO2; 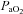 , arterial partial pressure of O2; Sat Hb, percentage saturation of haemoglobin with oxygen.
, arterial partial pressure of O2; Sat Hb, percentage saturation of haemoglobin with oxygen.
Fetal plasma ascorbate concentrations
Basal values for plasma concentrations of ascorbate were 24.2 ± 3.0 μmol l−1. Fetal treatment with vitamin C induced a progressive elevation in fetal ascorbate concentrations before reaching a plateau. Following the onset of treatment, fetal ascorbate plasma concentrations were elevated to 4.3 ± 0.4 mmol l−1 by the end of normoxia and to 7.3 ± 0.6 mmol l−1 by the end of the acute hypoxaemic challenge (Fig. 2).
Figure 2. Fetal plasma concentrations of ascorbate.
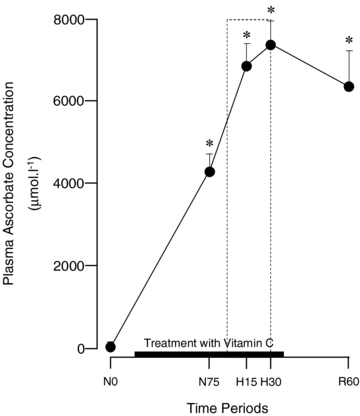
Values are means ±s.e.m. for plasma concentrations of ascorbate at 0 (N0) and 75 min (N75) of normoxia, at 15 (H15) and 30 min (H30) of hypoxaemia, and at 60 min (R60) of recovery for fetuses exposed to 0.5 h hypoxaemia (dashed box) during treatment with vitamin C (8.9 ± 0.4 mg kg−1 min−1; n = 6). *Significant difference (P < 0.05) vs. time period N0 (one-way RM-ANOVA with Tukey's post hoc test).
Fetal plasma SOD activity and plasma concentrations of NO2− and NO3−
Basal values for fetal arterial plasma SOD activity and plasma concentrations of NO2− and NO3− were 33.2 ± 2.6% inhibition, 0.68 ± 0.10 μm and 34.6 ± 6.4 μm, respectively. Fetal plasma SOD activity and fetal plasma NO2− and NO3− concentrations remained unaltered from baseline following fetal treatment with vitamin C during normoxia (P > 0.05). However, fetal treatment with vitamin C during acute hypoxaemia led to a significant increment from baseline in fetal plasma SOD activity, a significant decrement in fetal plasma concentrations of NO3−, a maintenance of fetal plasma concentrations of NO2−, and, thereby, a decrease from baseline in the plasma [NO3−]:[NO2−] concentration ratio in the fetal circulation (Fig. 3A–D).
Figure 3. Fetal plasma SOD activity and plasma concentrations of NO2− and NO3−.
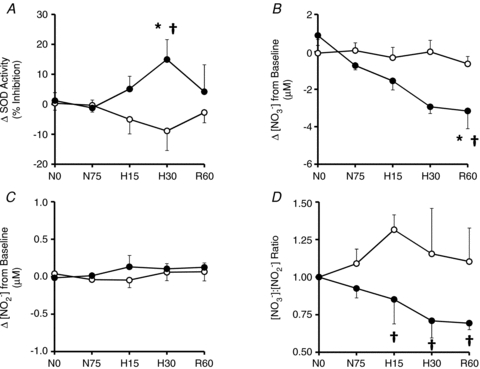
Values are means ±s.e.m. for changes from baseline in fetal plasma SOD activity (A), plasma concentrations of NO3− and NO2− (B and C, respectively) and the plasma concentration ratio of [NO3−]:[NO2−] (D) at 0 (N0) and 75 min (N75) of normoxia, at 15 (H15) and 30 min (H30) of hypoxaemia and 60 min (R60) of recovery for fetuses exposed to 0.5 h of hypoxaemia during saline infusion (○, n = 6) or treatment with vitamin C (•, n = 6). Significant differences are *P < 0.05 vs. normoxia, †P < 0.05 vs. saline (two-way RM ANOVA with Tukey's post hoc test).
Fetal cardiovascular function
Basal values for fetal arterial blood pressure, heart rate and femoral haemodynamic variables were similar in all fetuses (Figs 4 and 5). Infusion with saline had no effect on basal cardiovascular function (Figs 4 and 5). In contrast, treatment with vitamin C during normoxic conditions resulted in significant increments from baseline in femoral blood flow (9.8 ± 4.5%) and femoral vascular conductance (9.2 ± 4.3%). Acute hypoxaemia during saline infusion induced significant increments in fetal arterial blood pressure (8.6 ± 0.6 mmHg) and femoral vascular resistance (5.21 ± 1.02 mmHg (ml min−1)−1) and significant falls in fetal heart rate (−27 ± 5 beats min−1) and femoral blood flow (−27 ± 2 ml min−1; Figs 4 and 5). In contrast, treatment with vitamin C alone significantly diminished the magnitude of the pressor and femoral vasoconstrictor responses to acute hypoxaemia (Figs 4 and 5). However, treatment with vitamin C during NO synthase blockade recovered both the pressor and the femoral vasopressor responses to acute hypoxaemia, but it did not affect the femoral vasodilator response during baseline measured in fetuses during saline infusion (Figs 4 and 5). The bradycardic response to acute hypoxaemia was not affected by treatment with vitamin C alone. However, the area under the fetal heart rate curve was significantly enhanced with treatment with vitamin C during NO synthase blockade compared to saline infused controls (Fig. 5). During recovery, fetal arterial blood pressure remained significantly elevated in all fetuses, while femoral vascular resistance returned towards basal values. Values for femoral blood flow and heart rate returned towards baseline in saline infused and vitamin C treated fetuses, but remained significantly depressed from baseline in fetuses treated with vitamin C during NO synthase blockade (Figs 4 and 5).
Figure 4. Fetal cardiovascular responses to acute hypoxaemia.
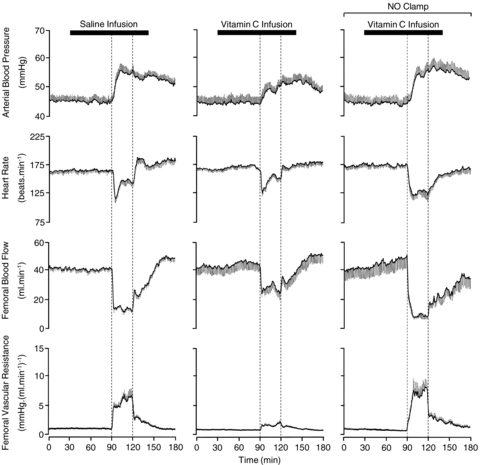
Values are means ±s.e.m. calculated every minute for arterial blood pressure, heart rate, femoral blood flow and femoral vascular resistance during 1.5 h of normoxia, 0.5 h of hypoxaemia (dashed box) and 1 h of recovery for fetuses during saline infusion (n = 7), treatment with vitamin C alone (n = 7) or treatment with vitamin C during the NO clamp (n = 7).
Figure 5. Statistical summary of the fetal cardiovascular responses to acute hypoxaemia.
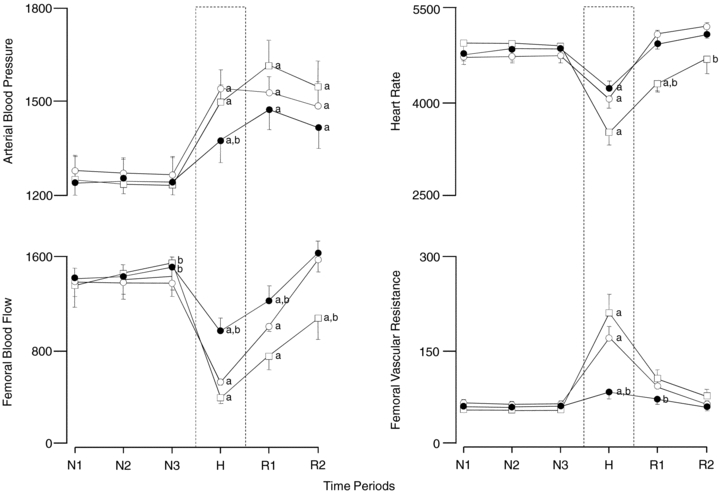
Values are means ±s.e.m for the area under the curve every 30 min during normoxia (N), hypoxemia (H) and recovery (R) for fetuses during saline infusion (○; n = 7), treatment with vitamin C alone (•; n = 7) or treatment with vitamin C during the NO clamp (□; n = 7). Significant differences: aP < 0.05 vs. time period N1, bP < 0.05 vs. saline infusion (two-way RM-ANOVA with Tukey's post hoc test).
Following the end of the recovery period of the acute hypoxaemia protocol, withdrawal of saline infusion had no effect on any measured fetal cardiovascular variable (data not shown). In contrast, withdrawal of the SNP infusion in fetuses undergoing the NO clamp led to significant hypertension, bradycardia and femoral vasoconstriction (Fig. 6).
Figure 6. The effect on the cardiovascular variables of removal of the NO clamp.
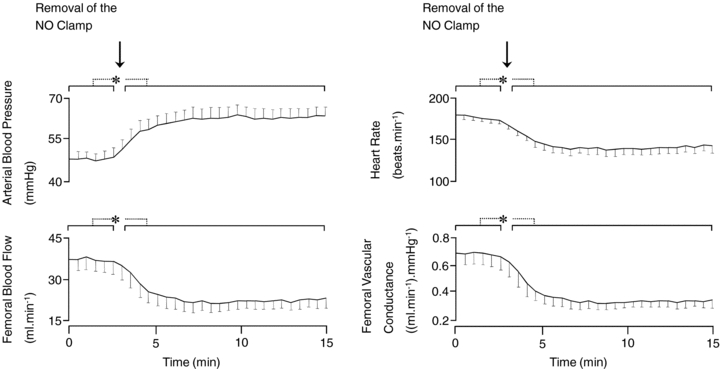
Values are means ±s.e.m calculated every 30 s following the end of the recovery period of the acute hypoxaemia protocol for fetal arterial blood pressure, heart rate, femoral blood flow and femoral vascular conductance before and after withdrawal of sodium nitroprusside infusion in fetuses undergoing treatment with the NO clamp. *Significant differences (P < 0.05) are before vs. after withdrawal of sodium nitroprusside infusion (Student's t test for paired data).
Discussion
The data show that fetal treatment with vitamin C had a marked inhibitory effect on the normal fetal peripheral vasoconstrictor response to acute hypoxaemia. Fetal treatment with vitamin C also led to significant elevations in fetal plasma SOD activity and a decrease in the fetal plasma concentration ratio of NO3−:NO2−. Fetal treatment with vitamin C in the presence of NO synthase blockade normalised the magnitude of the femoral vasoconstrictor response. Therefore, these data support the hypothesis tested and suggest that manipulation of the NO:•O2− ratio can have a profound effect on fetal cardiovascular function during the stimulated condition of acute hypoxaemic stress.
Vitamin C was the antioxidant of choice in this study for several reasons. During pregnancy, vitamin C is detectable in the placenta and the fetal plasma, the brain, the liver, the kidney and the adrenal glands (Kratzing & Kelly, 1986; Zalani et al. 1987; Kolb et al. 1991). Fetal plasma vitamin C levels also increase throughout gestation in human (Baydas et al. 2002) and ovine (Kolb et al. 1991) pregnancy, and preterm human infants have lower serum levels of vitamin C than term infants (Baydas et al. 2002), findings which are all consistent with a functional role for vitamin C in prenatal life. In addition, vitamin C is one of the most important water soluble antioxidants in mammalian tissues (Levine, 1986; Rose & Bode, 1993; Meister, 1994; Winkler et al. 1994) and Frei et al. (1989) demonstrated that vitamin C was the most effective aqueous-phase antioxidant in plasma. Finally, since vitamin C enhances vasodilator responses to endothelium-dependent agonists, such as arginine and acetylcholine (Heitzer et al. 1996; Ting et al. 1997; Kugiyama et al. 1998; Taddei et al. 1998; Tousoulis et al. 1999) but not to endothelium-independent agonists, such as papaverine (Solzbach et al. 1997; Ting et al. 1997; Taddei et al. 1998), its protective antioxidant effects on the cardiovascular system are likely to be mediated at the level of the endothelium in the circulation, the target organ of this study.
In vascular endothelial cells, NO is produced constitutively in vivo, but it is rapidly inactivated by •O2− to produce •ONOO− (rate constant = 2 × 1010 mol l−1 s−1) (Kissner et al. 1997). Although the availability of •O2− in tissues is strictly limited by the abundance of SOD, which is able to dismutate •O2− at a similar rate constant (2 × 109 mol l−1 s−1) (Fridovich, 1978), NO can still compete effectively with SOD for •O2−. In an elegant study, Jackson and colleagues (1998) reported that vitamin C could scavenge •O2− at concentrations as low as 100 μmol l−1, but it could only prevent the impairment by •O2− of endothelium derived NO-mediated arterial relaxation at the much higher physiological concentrations of 10 mmol l−1. Therefore, the capacity of vitamin C to scavenge •O2− and its ability to prevent the interaction between NO and •O2− appear to occur at very different concentrations in vivo. Accordingly, the rate constant for ascorbic acid to scavenge •O2− is ca 3 × 105 mol l−1 s−1 (Nishikimi, 1975; Gotoh & Niki, 1992), which is approximately 100,000-fold less than the rate at which SOD or NO react with •O2−. Therefore, for vitamin C to compete effectively with NO at any given concentration of •O2−, its concentrations must exceed that of NO by a factor of 100,000. Since NO concentrations are approximately 0.1 μmol l−1 adjacent to endothelial cells (Malinski et al. 1993; Blatter et al. 1995), the concentrations of vitamin C would therefore need to be ca 10 mmol l−1 to prevent the in vivo interaction between •O2− and NO (Jackson et al. 1998; Sherman et al. 2000). In the present study, treatment with vitamin C elevated fetal plasma concentrations of ascorbate to 7.3 ± 0.6 mmol l−1, which is within the concentration range required for it to act in vivo as an antioxidant in plasma, justifying the dosing regimen.
There are several possible mechanisms via which vitamin C may modulate the NO:•O2− ratio in the fetal circulation. In addition to quenching ROS directly (Jackson et al. 1998), ascorbate can act through tetrahydrobiopterin (BH4) to stimulate NO production by endothelial NO synthase (Wilson, 2009). Vitamin C can also prevent the S-nitrosylation of sensitive thiol groups on endothelial NO synthase, thereby maintaining their stability and the affinity of NO synthase for BH4 (Heller et al. 1999). In addition, it has been reported that vitamin C may enhance the expression and/or action of potent antioxidant enzymes (Geetha et al. 1989). Because of this, potential mechanisms via which vitamin C may have enhanced NO bioavailability in the fetal circulation in the present study were determined in three ways: (1) by measuring the concentration of nitrite [NO2−] and nitrate [NO3−] in fetal plasma; (2) by investigating the effect of fetal treatment with vitamin C on the plasma activity of the antioxidant enzyme SOD; and (3) by determining whether suppression the fetal peripheral vasoconstrictor response to hypoxaemia during vitamin C treatment could be reversed following NO synthase blockade.
In the present experiment, it would have been desirable to investigate the effects of acute hypoxaemia with and without fetal treatment with vitamin C on the in vivo absolute circulating concentrations of NO and of •O2−. However, the rate of NO reaction with oxyHb and deoxy-Hb is exceptionally rapid so that the half-life of free NO in blood is only fractions of a second (Butler et al. 1998; Huang et al. 2001). As a result, physiological concentrations of free NO in blood are maintained in the subnanomolar range, making the in vivo measurement of free NO in fetal blood impractical by currently available methods (Blood et al. 2009). Similarly, it is by no means a mystery that the measurement of the evanescent reactive oxygen species is difficult, particularly in vivo and in the fetus. Trapping methods are useful in biochemical studies in vitro. Likewise, fingerprinting methods, those that measure the damage that they cause, rather than the species themselves, are best applied to isolated tissues. Nitrite (NO2−) and nitrate (NO3−) concentrations are often measured together (NOx) and used as an index of NO in biological fluids. However, because nitrate concentrations are many-fold higher than nitrite and they are influenced by many factors including diet and liver enzymes, the ratio of NO3−:NO2− concentration in fetal plasma rather than their absolute concentrations may provide a more informative index of the relative NO and •O2− bioavailability (Kleinbongard et al. 2003, 2006). It is accepted that the interaction between NO and •O2− promotes NO3− formation and that absolute levels of NO2− rather than NO3− are a better index of NO bioavailability (see Ignarro et al. 1993; Kleinbongard et al. 2003, 2006). Therefore, changes in the ratio of NO3−:NO2− concentration provide an indirect index of •O2− bioavailability. Data in the present study show that fetal treatment with vitamin C during acute hypoxaemia led to a decrease in the concentration ratio of NO3−:NO2− in fetal plasma. These data suggest that one mechanism via which fetal treatment with vitamin C influenced the NO:•O2− ratio in the fetal circulation during acute hypoxaemia is by decreasing the bioavailability of •O2−.
Superoxide dismutase is one of the most important antioxidant enzymes in the vasculature. It is established that non-selective pharmacological inhibition of SOD isoforms increases vascular oxidative stress and attenuated endothelium-dependent relaxation (Mügge et al. 1991). An elegant study using SOD-deficient mice has also demonstrated that endogenous extracellular SOD is a major enzyme antagonising •O2− formation, and thereby indispensable in regulating the bioavailability of NO and in the control of vascular function and arterial blood pressure (Jung et al. 2003). Data in the present study show that fetal treatment with vitamin C during acute hypoxaemia led to significant elevations in SOD activity in the fetal circulation. Therefore, a second mechanism via which fetal treatment with vitamin C may have influenced the NO:•O2− ratio in the fetal circulation during acute hypoxaemia is by increasing antioxidant enzyme activity in fetal plasma.
In addition to episodes of ischaemia and reperfusion, hypoxaemia alone is a potent stimulator of free radical generation, such as the •O2− (Chandel, 2010). If hypoxaemia-induced increases in •O2− contribute to an increase in femoral vascular resistance in the fetus, then hypoxia-induced increases in fetal femoral vascular resistance should be diminished by fetal treatment with antioxidants. Further, if these actions of antioxidants are mediated by scavenging the •O2−, and thereby increasing the production ratio of NO:•O2− in the fetal vasculature, then fetal treatment with antioxidants during blockade of NO synthesis should restore the femoral vasoconstrictor response to acute hypoxaemia in the fetus. Additional data in the present study show that fetal treatment with vitamin C in the presence of NO synthase blockade did indeed restore the normal peripheral vasoconstrictor response to acute hypoxaemia measured in vivo. Withdrawal of the nitroprusside infusion at the end of the recovery period led to a significant increase in fetal arterial blood pressure, decrease in fetal heart rate and vasoconstriction in the femoral circulation. These changes provide evidence for the effectiveness of NO synthase blockade by the clamp and the persistence of the action of l-NAME within the system until the end of the experimental protocol. Therefore, a third mechanism via which fetal treatment with vitamin C may have influenced the NO:•O2− ratio in the fetal circulation during acute hypoxaemia is by stimulating NO synthase activity. That tonic production of •O2− contributes to vascular tone in peripheral circulations in the fetus is also supported by the finding that fetal treatment with vitamin C during basal conditions resulted in an increase in fetal peripheral vascular conductance.
It could be argued that the effects of vitamin C on the fetal peripheral vasoconstrictor response to acute hypoxaemia could be at the level of the carotid body since both NO and ROS have been implicated in peripheral chemo-transduction mechanisms (Dinger et al. 2007; Kumar & Phil, 2007). It could further be argued that the effects of vitamin C on the fetal haemodynamic response to hypoxia may be secondary to HIF inactivation. The hypoxia inducible factor (HIF) is down-regulated in oxygenated cells by a series of Fe(II)- and 2-oxoglutarate-dependent dioxygenases that hydroxylate specific residues in the regulatory HIF α-subunits. Because these enzymes require ascorbate for activity, it has been reported that ascorbate can suppress HIF-1α protein levels and HIF transcriptional targets, for instance in human cancer cell lines (Knowles et al. 2003). The effect of vitamin C on the femoral vasoconstrictor response to acute hypoxia in the fetus may therefore be due to silencing of HIF by ascorbate. In the fetus, both the bradycardia and the femoral vasoconstrictor responses to acute hypoxaemia are triggered exclusively by the same carotid chemoreflex since two independent studies have shown that bilateral section of the carotid sinus nerves abolishes both responses during acute hypoxaemia (Bartelds et al. 1993; Giussani et al. 1993). In the present study, fetal treatment with vitamin C diminished the fetal peripheral vasoconstrictor response to acute hypoxaemia without affecting the fetal bradycardia. Dissociation of the effects of vitamin C on the fetal cardiac and vasomotor responses to acute hypoxaemia does not support an effect of vitamin C at the level of the carotid body chemoreflex or an effect by silencing HIF. Rather, the differential effect further supports an action of vitamin C at the level of the peripheral vasculature.
A final consideration is to address whether the effects of vitamin C on the femoral vascular response to acute hypoxaemia may have been secondary to the mild fetal acidaemia induced by the ascorbic acid treatment. However, mild fetal acidaemia, of the magnitude induced by fetal treatment with vitamin C in the present study, significantly enhances rather than depresses the fetal femoral vasopressor responses to acute hypoxaemia (Thakor & Giussani, 2009). In fact, mild acidaemia doubled the magnitude of the femoral vasoconstrictor response to acute hypoxaemia (Thakor & Giussani, 2009). This highlights that the depressor effects of vitamin C on the femoral haemodynamic response to hypoxaemia appear to overwhelm even a sensitised peripheral vasoconstrictor response, and that the effects of vitamin C on the peripheral haemodynamic response to hypoxaemia in the current study may be diluted.
In conclusion, data in the present study advance our understanding of the physiological basis underlying the fetal cardiovascular defence to acute hypoxaemia. Here, we show in vivo that the cellular oxidant milieu is an important modulator of vascular tone in the fetal circulation and that it can be manipulated by antioxidant treatment during acute hypoxaemic stress.
Acknowledgments
This work was supported by the International Journal of Experimental Pathology, the Lister Institute for Preventive Medicine, the BBSRC and the British Heart Foundation. D.A.G. is a Royal Society Wolfson Research Merit Award holder. We are thankful to Mr S. Gentle and Mrs S. Nicholls for their invaluable help with the maintenance of the animals. The authors declare no conflicts of interest.
Glossary
Abbreviations
- l-NAME
NG-nitro-l-arginine methyl ester
- NO3−
nitrate
- NO2−
nitrite
- NOx
total nitrate and nitrite
- NPY
neuropeptide Y
- •O2−
superoxide anion
- pHa
arterial pH
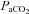
arterial partial pressure of CO2
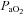
arterial partial pressure of O2
- ROS
reactive oxygen species
- Sat Hb
percentage saturation of haemoglobin with oxygen
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
Author contributions
The experiments in this study were performed in the Department of Physiology, Development and Neuroscience, University of Cambridge, UK and in the Environmental Research Group, Division of Pharmaceutical Science, King's College London. A.S.T. and D.A.G. conceived and designed the experiments. A.S.T., H.G.R., A.D.K., C.D., F.J.K., L.P. and D.A.G. collected, analysed and interpreted the experimental data. A.S.T., H.G.R., A.D.K., L.P., F.J.P. and D.A.G. drafted the article and revised it critically for important intellectual content. All authors approved the final version for publication.
References
- Akalin-Sel T, Campbell S. Understanding the pathophysiology of intra-uterine growth retardation: the role of the ‘lower limb reflex’ in redistribution of blood flow. Eur J Obstet Gynecol Reprod Biol. 1992;46:79–86. doi: 10.1016/0028-2243(92)90250-3. [DOI] [PubMed] [Google Scholar]
- Andres MT, Viejo-Diaz M, Fierro JF. Human lactoferrin induces apoptosis-like cell death in Candida albicans: critical role of K+-channel-mediated K+ efflux. Antimicrob Agents Chemother. 2008;52:4081–4088. doi: 10.1128/AAC.01597-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelds B, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Baydas G, Karatas F, Gursu MF, Bozkurt HA, Ilhan N, Yasar A, Canatan H. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Arch Med Res. 2002;33:276–280. doi: 10.1016/s0188-4409(02)00356-9. [DOI] [PubMed] [Google Scholar]
- Beard RW, Rivers RP. Fetal asphyxia in labour. Lancet. 1979;2:1117–1119. doi: 10.1016/s0140-6736(79)92514-5. [DOI] [PubMed] [Google Scholar]
- Blatter LA, Taha Z, Mesaros S, Shacklock PS, Wier WG, Malinski T. Simultaneous measurements of Ca2+ and nitric oxide in bradykinin-stimulated vascular endothelial cells. Circ Res. 1995;76:922–924. doi: 10.1161/01.res.76.5.922. [DOI] [PubMed] [Google Scholar]
- Blood AB, Tiso M, Verma ST, Lo J, Joshi MS, Azarov I, Longo LD, Gladwin MT, Kim-Shapiro DB, Power GG. Increased nitrite reductase activity of fetal versus adult ovine hemoglobin. Am J Physiol Heart Circ Physiol. 2009;296:H237–H246. doi: 10.1152/ajpheart.00601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- Chandel NS. Mitochondrial regulation of oxygen sensing. Adv Exp Med Biol. 2010;661:339–354. doi: 10.1007/978-1-60761-500-2_22. [DOI] [PubMed] [Google Scholar]
- Chen K, Keaney J. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11:109–121. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Semin Reprod Endocrinol. 1998;16:65–73. doi: 10.1055/s-2007-1016254. [DOI] [PubMed] [Google Scholar]
- Dinger B, He L, Chen J, Liu X, Gonzalez C, Obeso A, Sanders K, Hoidal J, Stensaas L, Fidone S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007;157:45–54. doi: 10.1016/j.resp.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Edwards CM, Gardner DS, Fowden AL, Giussani DA. Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology. 2000;141:3976–3982. doi: 10.1210/endo.141.11.7770. [DOI] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Oxygen radicals, hydrogen peroxide and oxygen toxicity. In: Pryor WA, editor. Free radicals in Biology. Vol. 1. New York: Academic press; 1978. pp. 239–277. [Google Scholar]
- Gardner DS, Fowden AL, Giussani DA. Adverse intrauterine conditions diminish the fetal defense against acute hypoxia by increasing nitric oxide activity. Circulation. 2002;106:2278–2283. doi: 10.1161/01.cir.0000033827.48974.c8. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Powlson AS, Giussani DA. An in vivo nitric oxide clamp to investigate the influence of nitric oxide on continuous umbilical blood flow during acute hypoxaemia in the sheep fetus. J Physiol. 2001;537:587–596. doi: 10.1111/j.1469-7793.2001.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha A, Catherine J, Shyamala Devi CS. Effect of alpha-tocopherol on the microsomal lipid peroxidation induced by doxorubicin: influence of ascorbic acid. Indian J Physiol Pharmacol. 1989;33:53–58. [PubMed] [Google Scholar]
- Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinergic contribution to circulatory, metabolic and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;280:R678–R685. doi: 10.1152/ajpregu.2001.280.3.R678. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Hanson MA. Fetal cardiovascular reflex responses to acute hypoxaemia. Fet Mat Med Rev. 1994;6:17–37. [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green LR, McGarrigle H, Bennet L, Hanson MA. The role of endothelin-A receptors in cardiovascular responses to acute hypoxaemia in the late gestation sheep fetus. J Physiol. 1998;509:297–304. doi: 10.1111/j.1469-7793.1998.297bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N, Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochim Biophys Acta. 1992;1115:201–207. doi: 10.1016/0304-4165(92)90054-x. [DOI] [PubMed] [Google Scholar]
- Hall DM. Birth asphyxia and cerebral palsy. BMJ. 1989;299:279–282. doi: 10.1136/bmj.299.6694.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- Heller R, Munscher-Paulig F, Grabner R, Till U. L-Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem. 1999;274:8254–8260. doi: 10.1074/jbc.274.12.8254. [DOI] [PubMed] [Google Scholar]
- Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang C, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci U S A. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: Comparison with the enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci U S A. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama K, Yoshiura M, Iwamoto T, Ozaki Y. Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984;141:238–243. doi: 10.1016/0003-2697(84)90451-2. [DOI] [PubMed] [Google Scholar]
- Irodova NL, Lankin VZ, Konovalova GK, Kochetov AG, Chazova IE. Oxidative stress in patients with primary pulmonary hypertension. Bull Exp Biol Med. 2002;133:580–582. doi: 10.1023/a:1020238026534. [DOI] [PubMed] [Google Scholar]
- Jackson BT, Piasecki GJ, Novy MJ. Fetal responses to altered maternal oxygenation in rhesus monkey. Am J Physiol Regul Integr Comp Physiol. 1987;252:R94–R101. doi: 10.1152/ajpregu.1987.252.1.R94. [DOI] [PubMed] [Google Scholar]
- Jackson TS, Xu A, Vita JA, Keaney JF. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in fetal and adult sheep. J Physiol. 1975;248:15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- Kolb E, Wahren M, Leo M, Siebert P, Erices J, Gollnitz L, Volker L. Ascorbic acid concentration in plasma, in amniotic and allantoic fluids, in the placenta and in 13 tissues of sheep fetuses and newborn lambs. Dtsch Tierarztl Wochenschr. 1991;98:424–427. [PubMed] [Google Scholar]
- Kratzing CC, Kelly JD. Ascorbic acid synthesis by the mammalian fetus. Int J Vitam Nutr Res. 1986;56:101–103. [PubMed] [Google Scholar]
- Kugiyama K, Motoyama T, Hirashima O, Ohgushi M, Soejima H, Misumi K, Kawano H, Miyao Y, Yoshimura M, Ogawa H, Matsumura T, Sugiyama S, Yasue H. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:103–109. doi: 10.1016/s0735-1097(98)00185-5. [DOI] [PubMed] [Google Scholar]
- Kumar P, Phil D. Translating blood-borne stimuli: chemotransduction in the carotid body. Sheng Li Xue Bao. 2007;59:128–132. [PubMed] [Google Scholar]
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892–902. doi: 10.1056/NEJM198604033141407. [DOI] [PubMed] [Google Scholar]
- Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA, Karchmar EJ. The relationship between perinatal hypoxia and newborn encephalopathy. Am J Obstet Gynecol. 1985;152:256–260. doi: 10.1016/s0002-9378(85)80205-2. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J. Determination of ascorbic acid and dehydroascorbic acid in biological samples by high-performance liquid chromatography using subtraction methods: reliable reduction with tris[2-carboxyethyl]phosphine hydrochloride. Anal Biochem. 2000;282:89–93. doi: 10.1006/abio.2000.4592. [DOI] [PubMed] [Google Scholar]
- Malinski T, Taha Z, Grunfeld S, Patton S, Kapturczak M, Tomboulian P. Diffusion of nitric oxide in the aorta wall monitored in situ by porphyrinic microsensors. Biochem Biophys Res Commun. 1993;193:1076–1082. doi: 10.1006/bbrc.1993.1735. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- Morrison S, Gardner DS, Fletcher AJ, Bloomfield MR, Giussani DA. Enhanced nitric oxide activity offsets peripheral vasoconstriction during acute hypoxaemia via chemoreflex and adrenomedullary actions in the sheep fetus. J Physiol. 2003;547:283–291. doi: 10.1113/jphysiol.2002.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mügge A, Elwell JH, Peterson TE, Harrison DG. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am J Physiol. 1991;260:C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975;63:463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7:1135–1142. [PubMed] [Google Scholar]
- Rudolph AM. The fetal circulation and its response to stress 1. J Dev Physiol. 1984;6:11–19. [PubMed] [Google Scholar]
- Sherman DL, Keaney JF, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936–941. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation. 1997;96:1513–1519. doi: 10.1161/01.cir.96.5.1513. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Giussani DA. Role of nitric oxide in mediating in vivo vascular responses to calcitonin gene-related peptide in essential and peripheral circulations in the fetus. Circulation. 2005;112:2510–2516. doi: 10.1161/CIRCULATIONAHA.105.562546. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Giussani DA. Effects of acute acidemia on the fetal cardiovascular defense to acute hypoxemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R90–R99. doi: 10.1152/ajpregu.90689.2008. [DOI] [PubMed] [Google Scholar]
- Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Davies G, Toutouzas P. Vitamin C increases nitric oxide availability in coronary atherosclerosis. Ann Intern Med. 1999;131:156–157. doi: 10.7326/0003-4819-131-2-199907200-00022. [DOI] [PubMed] [Google Scholar]
- Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35:5–13. doi: 10.1002/biof.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalani S, Bharaj BS, Rajalakshmi R. Ascorbic acid and reduced glutathione concentration of human fetal tissues in relation to gestational age, fetal size and maternal nutritional status. Int J Vitam Nutr Res. 1987;57:411–419. [PubMed] [Google Scholar]


