Abstract
Background and Aims
Most lichens form associations with Trebouxia phycobionts and some of them simultaneously include genetically different algal lineages. In other symbiotic systems involving algae (e.g. reef corals), the relative abundances of different endosymbiotic algal clades may change over time. This process seems to provide a mechanism allowing the organism to respond to environmental stress. A similar mechanism may operate in lichens with more than one algal lineage, likewise protecting them against environmental stresses. Here, the physiological responses to oxidative stress of two distinct Trebouxia phycobionts (provisionally named TR1 and TR9) that coexist within the lichen Ramalina farinacea were analysed.
Methods
Isolated phycobionts were exposed to oxidative stress through the reactive oxygen species propagator cumene hydroperoxide (CuHP). Photosynthetic pigments and proteins, photosynthesis (through modulated chlorophyll fluorescence), the antioxidant enzymes superoxide dismutase (SOD) and glutathione reductase (GR), and the stress-related protein HSP70 were analysed.
Key Results
Photosynthetic performance was severely impaired by CuHP in phycobionts, as indicated by decreases in the maximal PSII photochemical efficiency (Fv/Fm), the quantum efficiency of PSII (ΦPSII) and the non-photochemical dissipation of energy (NPQ). However, the CuHP-dependent decay in photosynthesis was significantly more severe in TR1, which also showed a lower NPQ and a reduced ability to preserve chlorophyll a, carotenoids and D1 protein. Additionally, differences were observed in the capacities of the two phycobionts to modulate antioxidant activities and HPS70 levels when exposed to oxidative stress. In TR1, CuHP significantly diminished HSP70 and GR but did not change SOD activities. In contrast, in TR9 the levels of both antioxidant enzymes and those of HSP70 increased in response to CuHP.
Conclusions
The better physiological performance of TR9 under oxidative conditions may reflect its greater capacity to undertake key metabolic adjustments, including increased non-photochemical quenching, higher antioxidant protection and the induction of repair mechanisms.
Keywords: Oxidative stress, phycobiont, lichen, Ramalina farinacea, stress response, Trebouxia
INTRODUCTION
Approximately 20 % of fungal species develop obligate mutualistic associations with green algae and/or cyanobacteria. Lichens are the result of such associations; they consist of a nutritionally specialized fungus (mycobiont) that acquires fixed carbon from its photosynthetic partner(s) (photobiont). During development of the lichen thallus, both partners undergo a complex series of morphological, biochemical and physiological changes, resulting in a new organism with novel features (Chapman and Margullis, 1998; Barreno, 2004). Lichenization is a clearly successful symbiosis as evidenced by the fact that lichens are found in almost all terrestrial habitats, allowing both the photobiont and the mycobiont to expand into many geographical regions where separately they would be rare or non-existent.
Ramalina farinacea is an epiphytic fruticose, pendulose lichen that propagates via vegetative propagules (soredia). It is commonly present in Mediterranean areas on different shrubs and trees, especially in sclerophyllous oak forests. In these natural habitats it is subjected to long summer days of desiccation and rehydration by dew during the night or, during the rainy periods of the spring and autumn seasons, by rain. However, this lichen is also found in other, less restrictive and humid ecosystems as well as in more stressful environments such as high mountains. The diversity of ecological contexts in which R. farinacea proliferates suggests a wide ecophysiological plasticity of the lichen association to cope with changing and often stressful environmental conditions.
Water deficit, suboptimal temperatures, air pollution and other types of stress disrupt the metabolic balance of cells, resulting in the enhanced production of reactive oxygen species (ROS) (Miller et al., 2010) such as 1O2 (singlet oxygen), O2·− (superoxide anion radical), H2O2 and .OH (hydroxyl radical). In chloroplasts, water stress, excessive light intensity, suboptimal temperatures, xenobiotics and heavy metals interfere with photosynthesis and induce a higher electron transfer from photosynthetic electron carriers to O2, increasing the generation of ROS (Galvez-Valdivieso and Mullineaux, 2010). These ROS trigger deleterious reactions, including degradative processes that involve key chloroplastic components, for example thylakoid proteins (Casano et al., 1994; Song et al., 2006), Calvin cycle enzymes (Asada, 1999), and pigments and membrane lipids (Asada, 1999; Calatayud et al., 1999). To prevent oxidative damage, plants have evolved a complex antioxidant defence system made up of non-enzymatic and enzymatic constituents. Among the former, reduced glutathione (GSH) and ascorbic acid are the most important soluble antioxidants (Noctor and Foyer, 1998). The enzymatic constituents include superoxide dismutase (EC 1·15·1·1) (SOD), ascorbate peroxidase (EC 1·11·1·11) (APX) and glutathione reductase (EC 1·6·4·2) (GR) as key antioxidant enzymes that scavenge O2·− and H2O2, thus preventing formation of the highly toxic .OH (Foyer et al., 1994; Scandalios, 2001). Several studies have examined the possible causal relationships in lichens between stress tolerance and the ability to remove and/or prevent ROS formation (Deltoro et al., 1998; Kranner et al., 2003, 2005; Weissman et al., 2005 and references therein). In general, it was observed that, under stress, tolerant lichens modulate the status and activity of their antioxidant system(s) in response to increased levels of ROS. According to Kranner et al. (2006), failure of the antioxidant system during stressful conditions could trigger programmed cell death, i.e. ageing and eventual death of the organism. This suggests a potent antioxidant machinery as one of the underlying mechanisms of desiccation tolerance. NPQ (non-photochemical dissipation of energy) is another important process operating under stress conditions, as it transforms into heat the excess light energy that cannot be used in photosynthesis and which could lead to ROS formation. In vascular plants, this dissipation of excess light energy is associated with acidification of the thylakoidal luminal space, the xanthophylls cycle and the activity of the pSBS protein associated with photosystem II (Niyogi et al., 2004). Lichens, however, exhibit different response and defence mechanisms under adverse conditions but as yet they have been rarely characterized. Evidence for this difference between lichens and vascular plants comes from a recent report in which non-photochemical dissipation processes in lichen photobionts were shown to differ from those of vascular plants (Kopecky et al., 2005). Thus, in homeohydric vascular plants dissipation processes are dependent on the incident light and quickly disappear in response to darkness (Müller et al., 2001; Szabó et al., 2005). In contrast, we found that energy dissipation in Trebouxia erici continues after the organism has been subjected to 24 h of darkness and is independent of the xanthophyll cycle (Gasulla et al., 2009). Additional elements involved in the protection of cells from the deleterious effects of different stress conditions are heat shock proteins (HSPs). Among them, the chaperone HSP70 participates in protein folding and in maintaining the correct conformation and function of a number of proteins. Moreover, there is abundant experimental evidence demonstrating the induction of HSP70 under several types of stress (Duan et al., 2010, and references therein).
Previous morphological and molecular analyses indicated a highly specific pattern of association between the lichen-forming fungus R. farinacea and the same two phycobionts [provisionally named TR1 and TR9 phycobionts in del Campo et al. (2010) and L. M. Casano et al., unpubl. res.] in all studied lichen thalli, independent of their geographical location. These findings and the diversity of ecological contexts in which R. farinacea proliferates suggest a wide eco-physiological plasticity of lichen associations, acquired without major changes in phycobiont composition but probably by modulating the relative abundance of each phycobiont (del Campo et al., 2010). An initial physiological characterization of isolated TR1 and TR9 algae based on their growth and photosynthetic response to light and temperature regimes suggested a better physiological performance of TR9 under relatively high temperature and irradiances than TR1. It can thus be hypothesized that the constant presence of both Trebouxia phycobionts in R. farinacea thalli is favoured by the different and probably complementary physiological behaviour of each alga, thus improving the ecological fitness of the holobiont. To test this hypothesis, the aim of the present study was to search for physiological differences between TR1 and TR9 by studying their response to stress conditions. We therefore focused our attention on the effects of oxidative stress as it is a common internal response to externally generated abiotic and biotic stresses. In the experiments described herein, oxidative stress was imposed by treating the phycobionts with the ROS propagator cumene hydroperoxide (CuHP). CuHP has been used as an intracellular source of reactive oxygen intermediates in studies assessing the effect of oxidative stress on various biological systems, especially animal and human cells (Ayala et al., 1996, and references therein). Previous confocal microscopy analysis employing specific fluorochromes demonstrated that CuHP induces ROS formation in isolated Trebouxia cells, with the chloroplast as the main target (Catalá et al., 2010). After CuHP treatments, photosynthesis, photosynthetic pigments and proteins were analysed in isolated phycobionts. In addition, the defence response to CuHP was determined with respect to the levels of several important antioxidant enzymes, such as SOD and GR, as well as the stress-related protein HSP70.
MATERIALS AND METHODS
Phycobiont isolation and culture
TR1 and TR9 were isolated in our laboratories from a population of the lichen Ramalina farinacea (L.) Ach. according to Gasulla et al. (2010). Thalli were collected in the air-dried state on Quercus rotundifolia Lam. at Sa El Toro (Castellón, Spain; 39°54′16″N, 0°48′22″W). Samples were frozen at –20 °C for 3 months, at which time they were cultured in either liquid or semisolid Bold 3N medium (Bold and Parker, 1962) in a growth chamber at 15 °C, under a 14-h/10-h light/dark cycle (lighting conditions: 25 µmol m−2 s−1).
CuHP treatments
Unless stated otherwise, 3-week-old liquid cultures of TR1 and TR9 phycobionts were diluted to 2 × 106 cells mL−1 and incubated for up to 24 h with concentrations of CuHP (Sigma-Aldrich Co., St. Louis, MO, USA) ranging from 0 to 1 mm. At the indicated times, the algae were centrifuged and then rinsed twice with ultrapure cold water. These water-rinsed phycobiont cells were used in subsequent analyses. Typically, 50 mL of treated cultures rendered approx. 250–300 mg of phycobiont cells.
Photosynthetic pigment determination
Treated and control phycobiont cells in amounts of approx. 20 mg were resuspended in 100 % dimethyl sulfoxide and incubated for 15 min at 65 °C. This treatment solubilized at least 98 % of the photosynthetic pigments (data not shown). After centrifugation at 5000g for 5 min, chloroplyll a, chloroplyll b and carotenoids were measured spectrophotometrically according to Lichtenthaller (1987).
Soluble and membrane protein extracts
Treated and control phycobiont cells (approx. 200–250 mg) were homogenized using a mortar and pestle with a minimal amount of siliceous particles and 0·5 mL of the following grinding medium: 50 mm Tris-Cl (pH 7·5), 2 mm EDTA, 1 mm ascorbic acid, 10 % (v/v) glycerol and 1 % (w/v) insoluble polyvinyl pyrrolidone (PVPP). The homogenates were centrifuged for 2 min at 200g to remove the siliceous particles, PVPP, unbroken cells and cell-wall debris. Supernatants were fractionated by centrifugation at 20 000g for 20 min, resulting in ‘soluble proteins’ and ‘membrane proteins’. Pellets (membrane proteins) were rinsed twice with 50 mm Tris-Cl (pH 7·5), 2 mm EDTA and 100 mm NaCl, and then resuspended in 0·1 mL of grinding medium without PVPP. All of the steps were carried out at 4 °C.
Measurements of chlorophyll a fluorescence
Chlorophyll a fluorescence was measured at room temperature using a pulse modulation fluorometer (PAM-2000; Walz, Effeltrich, Germany). Isolated algae that had been allowed to grow for 3 weeks on cellulose acetate discs placed on semisolid culture medium were incubated for 1 h with concentrations of CuHP of up to 10 mm, after which the discs were returned to control conditions for 24 h. Thereafter, the cells were maintained in a fully hydrated state by layering the discs on filter paper wetted with distilled water and kept in the dark for 15 min prior to chlorophyll a fluorescence measurements. The minimal fluorescence yield (F0) was obtained by excitation of the phycobionts with a weak measuring beam from a light-emitting diode. A saturating pulse (600 ms) of white light (at a photosynthetic photon fluence rate of 4000 µmol m−2 s−1 over the waveband 400–700 nm), closing all reaction centres, was applied to obtain the maximal fluorescence (Fm). Variable fluorescence in dark-adapted samples (Fv) was calculated as Fm – F0. Following 2 min of dark readaptation, actinic white light (260 µmol photons m−2 s−1) was switched on, and 600-ms saturating pulses were applied at 60-s intervals to determine the maximum fluorescence yield during actinic illumination (F′m), the level of modulated fluorescence during a brief interruption of actinic illumination in the presence of far-red light (F′o) and the chlorophyll fluorescence yield during actinic illumination (Fs). Quenching as a result of non-photochemical dissipation of absorbed light energy (NPQ) was determined at each saturating pulse according to the equation NPQ = (Fm – F′m)/ F′m (Bilger and Björkman, 1991). The coefficient for photochemical quenching, qp, was calculated as (F′m – Fs)/ (F′m – F′o) (Schreiber et al., 1986). The quantum efficiency of PSII photochemistry, ΦPSII, closely associated with the quantum yield of non-cyclic electron transport, was estimated from (F′m – Fs)/F′m (Genty et al., 1989).
Activity of antioxidant enzymes
Antioxidant enzyme activities were assayed in the soluble protein fraction by spectrophotometric and zymogram analyses. SOD activity was determined spectrophotometrically at 560 nm as described by Casano et al. (1999). GR activity was assayed at 25 °C according to Casano et al. (1999), following the decrease in A340 due to NADPH oxidation.
For zymograms, soluble proteins (usually 20 µg) were separated by electrophoresis in native PAGE (ND-PAGE). GR and SOD activities were developed as described previously (Lascano et al., 2001). Different SOD isoforms were distinguished based on their sensitivity to inhibition by 2 mm KCN and 5 mm H2O2. Mn-SOD is resistant to CN- and H2O2, Fe-SOD is resistant to CN- but inhibited by H2O2 and both inhibitors inactivate CuZn-SOD (Fridovich, 1986).
Immunoblot analysis of photosynthetic proteins, HSP70 and GR
PSI (PsaC) and PSII (D1) proteins were assayed in the ‘membrane protein’ fraction, and HSP70 and GR were assayed in the ‘soluble protein’ fraction. After SDS–PAGE, the separated proteins (usually 10 µg) were transferred to PVDF membranes (Millipore, Billerica, MA, USA). Immunocomplexes with commercially available antibodies against PsaC, D1 and HSP70 (Agrisera, Vännäs, Sweden) or prepared previously against wheat chloroplastic GR (Lascano et al., 2001) were detected with an alkaline phosphatase Western blotting system (Sigma-Aldrich Co., St Louis, MO, USA).
RESULTS
Effects of CuHP on photosynthetic pigments and proteins, and fluorescence parameters
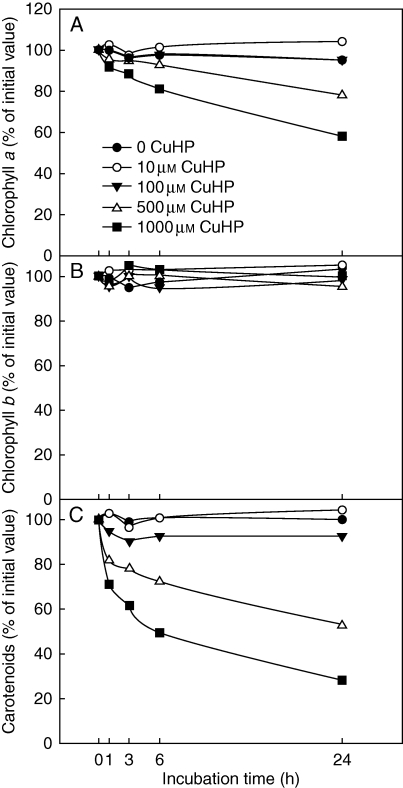
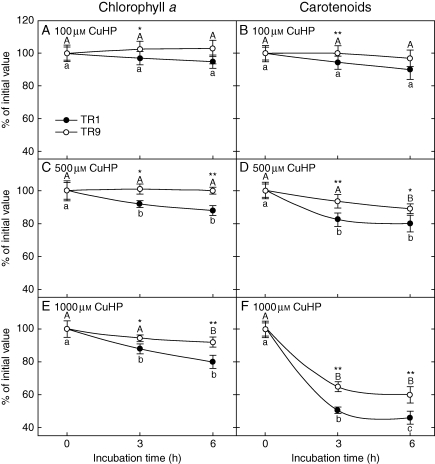
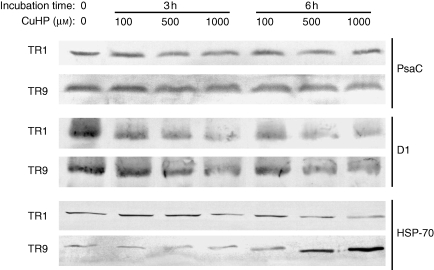
The physiological response to oxidative stress of R. farinacea phycobionts was studied by analysing the changes in photosynthetic pigments and proteins in liquid cultures of isolated TR1 and TR9 subjected to different concentrations of CuHP. In a first approach we determined the time course of chlorophyll a, chlorophyll b and carotenoid levels in CuHP-treated TR1 cells over a period of 24 h (Fig. 1). Carotenoid content was the most sensitive to oxidative stress, decreasing by approx. 70 % after a 24-h incubation with 1 mm CuHP, while chlorophyll b was not significantly affected by CuHP treatments and chlorophyll a showed an intermediate degradation rate. Pigment changes were more intense in the first hour of incubation, especially in carotenoids (Fig. 1). Accordingly, we decided to measure the changes in chlorophyll a and carotenoid content in TR1 and TR9 cells incubated with CuHP for 6 h. As shown in Fig. 2, pigment levels decreased as CuHP and incubation time increased. However, CuHP-induced oxidative stress had a markedly higher impact on TR1 than on TR9. Indeed, no significant chlorophyll a decay was observed in TR9 even following incubation with 1 mm CuHP for 6 h, as this phycobiont maintained carotenoid at higher levels than TR1 under all assayed conditions. The effects of CuHP on the steady-state levels of the proteins PsaC and D1 were analysed by Western blot in isolated TR1 and TR9 cells (Fig. 3). Whereas there was no apparent change in PsaC, D1 decayed progressively in both phycobionts as oxidative stress increased; however, the decrease was markedly more intense in TR1 than in TR9.
Fig. 1.
Effect of oxidative stress on photosynthetic pigments in TR1 R. farinacea phycobiont. Liquid cultures of isolated TR1 algae were subjected to oxidative stress through incubations with the ROS propagator CuHP at the indicated concentrations. At the indicated times, carotenoids and chloroplylls a and b were determined spectrophotometrically as described in Materials and Methods. Initial (incubation time = 0) values of chlorophyll a, chlorophyll b and carotenoids were 1·98 (± 0·10), 0·35 (± 0·013) and 0·86 (± 0·039) μg per 106 cells, respectively. Values represent mean results of 4–5 independent experiments; s.d. <5 % in all cases.
Fig. 2.
Changes in chlorophyll a and carotenoids in TR1 and TR9 R. farinacea phycobionts exposed to oxidative stress. Liquid cultures of isolated TR1 or TR9 algae were subjected to oxidative stress through incubation with (A, B) 100 µm, (C, D) 500 µm or (E, F) 1000 µm CuHP. At the indicated times, chloroplyll a (A, C, E) and carotenoids (B, D, F) were determined spectrophotometrically as described in Materials and methods. Initial (incubation time = 0) values of chlorophyll a were 2·05 (± 0·09) and 2·23 (± 0·11) μg per 106 cells for TR1 and TR9, respectively. Initial values of carotenoids were 0·80 (± 0·092) and 0·94 (± 0·086) μg per 106 cells for TR1 and TR9, respectively. Control cultures incubated without CuHP for 3 and 6 h did not show significant changes with respect to the initial values for all the analysed pigments. Depicted values represent mean results (±s.d.) from four independent experiments (only s.d. values larger than the symbol size are represented). Data were statistically analysed by a two-way ANOVA, in which the phycobiont species and incubation time with the indicated CuHP concentration were considered as factors. Differences among phycobiont and treatment means were evaluated using Student's t-test. Different lower-case or upper-case letters indicate significant differences (P > 0·05) among 0, 3 and 6 h incubation with CuHP for each TR1 and TR9, respectively, and asterisks indicate significant differences between TR1 and TR9 at each CuHP treatment at *P > 0·05 or **P > 0·01.
Fig. 3.
Changes in PSI, PSII and HSP70 proteins in TR1 and TR9 R. farinacea phycobionts exposed to oxidative stress. Liquid cultures of isolated TR1 or TR9 algae were subjected to oxidative stress through incubations with the ROS propagator CuHP at the indicated concentrations. At the indicated times, soluble and membrane proteins were extracted and separated by differential centrifugation as described in Materials and methods. PSI (PsaC) and PSII (D1) proteins encoded by psaC and psbA genes, respectively, were immunodetected by Western blotting of the membrane protein fraction and HSP70 by Western blotting of the soluble protein fraction.
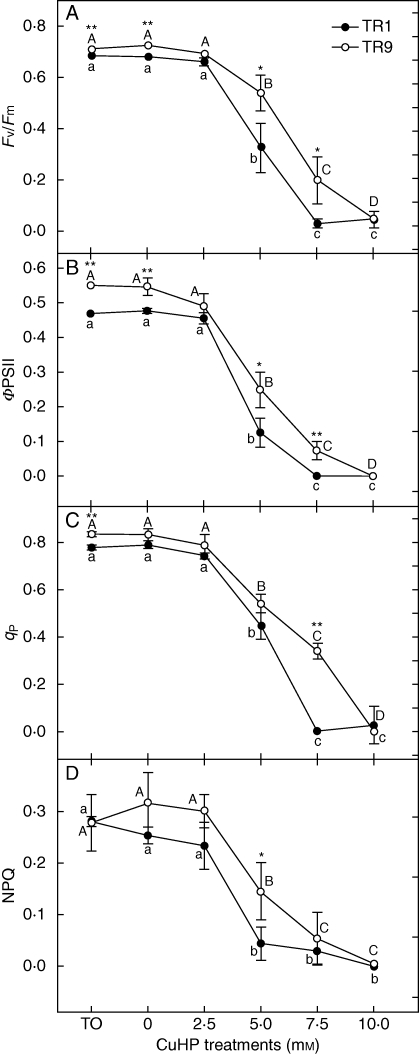
In order to study whether the observed differences in the photosynthetic pigments and proteins of TR1 and TR9 accounted for the differences in photosynthetic behaviour under oxidative stress, chlorophyll fluorescence parameters were analysed in isolated phycobionts exposed to CuHP. Treatments were carried out in solid (cellulose acetate discs) cultures incubated for 1 h with CuHP concentrations of up to 10 mm, after which the discs were returned to control conditions for 24 h and fluorescence parameters were subsequently measured. In general, photosynthesis was negatively affected by the presence of CuHP. Both TR1 and TR9 tolerated 2·5 mm CuHP, but photosynthetic activity decreased linearly as the CuHP concentration rose to 10 mm, at which virtually no photosynthesis was detected using the chlorophyll fluorescence technique. Figure 4A shows the effect of CuHP on the maximum efficiency of PSII photochemistry after dark adaptation (Fv/Fm). Exposure of TR1 and TR9 to CuHP concentrations higher than 2·5 mm produced a decrease in Fv/Fm, demonstrating that the oxidative stress generated by CuHP caused PSII photoinhibition. However, whereas at 5 mm CuHP the Fv/Fm ratio decreased by approx. 52 % in TR1, in TR9 it diminished by only 25 % with respect to the corresponding control values. At 7·5 mm CuHP, TR1 completely lost photosynthetic activity whereas TR9 retained 27 % of the initial activity. PSII photochemical efficiency during actinic light exposure (ΦPSII, Fig. 4B) declined in the presence of 2·5 mm CuHP in the two algal species, albeit to differing extents. Specifically, ΦPSII was less affected in TR9 than in TR1, with significant decreases of 54·4 and 74·7 %, respectively. At 7·5 mm CuHP, ΦPSII was completely suppressed in TR1 whereas in TR9 13·5 % of the initial level was maintained. Figure 4C depicts the effect of CuHP treatment on the proportion of ‘open’ PSII centres, estimated as qP. At 5 mm CuHP, qP decreased to similar extents in TR1 and TR9 (43·3 and 35·2 %, respectively). However, at 7·5 mm CuHP, all TR1 reaction centres were closed whereas TR9 retained 40·9 % of its initially open centres. The degree of NPQ (Fig. 4D) was adversely affected by exposure to CuHP in both species. Although the differences were not statistically significant due to the high variability of the data, NPQ values were consistently higher in TR9 than in TR1.
Fig. 4.
Effect of oxidative stress on photosynthetic performance of TR1 and TR9 R. farinacea phycobionts. Chlorophyll a fluorescence was measured at room temperature using a pulse modulation fluorometer. Isolated algae on cellulose acetate discs were incubated for 1 h with CuHP at the indicated concentrations and then returned to control conditions for 24 h. Fv/Fm provides an estimate of the maximal quantum efficiency: ΦPSII, the effective quantum yield of photochemical conversion in PSII; qP, the photochemical quenching; and NPQ, the quantum yield of regulated energy dissipation in PSII. Depicted values represent mean results (± s.d.) from five independent experiments (only s.d. values larger than the symbol size are represented). Data were analysed by a two-way ANOVA, in which the phycobiont species and CuHP treatment were considered as factors. Differences among phycobiont and treatment means were evaluated using Student's t-test. Different lower-case or upper-case letters indicate significant differences (P > 0·05) among CuHP treatments for each TR1 and TR9, respectively, and asterisks indicate significant differences between TR1 and TR9 at each CuHP treatment at *P > 0·05 or **P > 0·01.
Antioxidant enzymes and HSP70 protein under CuHP treatment
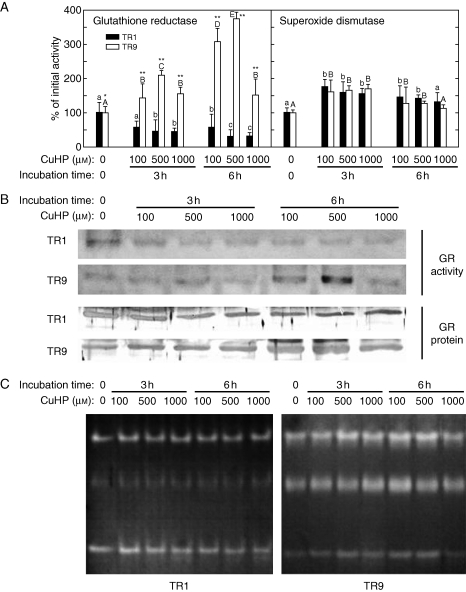
To elucidate the possible cause(s) of the observed differences in photosynthetic behaviour of TR1 and TR9 phycobionts under oxidative stress, we looked for differences in their capacity to modulate the activity of antioxidant enzymes in response to increased levels of ROS. Accordingly, the effects of different CuHP concentrations on the activity and protein levels of GR and the activity of SOD were investigated in liquid cultures of isolated TR1 and TR9 phycobionts through spectrophometric assays, zymograms and Western blotting. In general, GR and SOD were differentially modulated by oxidative stress in each phycobiont (Fig. 5). Total activity in spectrophotometric assays (Fig. 5A) as well as zymograms (Fig. 5B) showed that initial GR activity was lower in TR9 than in TR1 (50 and 21 nmol NADPH min−1 mg−1, respectively). However, GR activity in TR1 significantly decreased with incubation time and CuHP concentration whereas in TR9 this antioxidant enzyme was dramatically induced by oxidative treatments. As expected, zymograms confirmed the results of total activity spectrophotometric assays, as both phycobionts contain only one GR isoform (as determined by one-dimensional ND-PAGE). Moreover, the results of Western blots probed with anti-wheat GR polyclonal antibodies (Lascano et al., 2001) suggested that changes in GR activity were due, at least in part, to parallel variations in the amount of GR protein (Fig. 5B). Based on the extent of total SOD activity (Fig. 5A), this enzyme does not appear to play a particularly relevant role in the different behaviours demonstrated by the TR1 and TR9 phycobionts. However, a more complex picture emerges by analysing SOD isoform patterns (Fig. 5C). The two phycobionts showed the same three main groups of SOD bands, seen as slow, intermediate and high migration rates. However, whereas SOD activity in TR9 was approximately equally distributed among the three groups, in TR1 the contribution of the intermediate bands to global activity was less (compare TR1 and TR9 T0 lines in Fig. 5C). In addition, in TR1 cells the relatively slight increase (10–45 %) in total SOD activity induced by CuHP treatment (Fig. 5A) was almost completely due to the slowly and highly mobile bands, but in TR9 cells the increases were mainly accounted for by the intermediate bands. Interestingly, the intermediate band group corresponds to Cu–Zn-SOD isoform(s), probably located within the chloroplast (Supplementary Data, available online). Possible changes in the steady-state level of HSP70 were analysed in TR1 and TR9 cells exposed to oxidative stress through CuHP exposure. As shown in Fig. 3, the initial level of HSP70 was slightly lower in TR9 than in TR1. However, in TR1, HSP70 significantly decreased with incubation time and CuHP concentration while in TR9 oxidative treatment caused a strong increase in the amounts of this defence protein.
Fig. 5.
Changes in glutathione reductase and superoxide dismutase in TR1 and TR9 R. farinacea phycobionts exposed to oxidative stress. Liquid cultures of isolated TR1 or TR9 algae were subjected to oxidative stress through incubations with the ROS propagator CuHP at the indicated concentrations. At the indicated times, soluble proteins were extracted by homogenization and separated by differential centrifugation. Total GR and SOD activities (A) were determined in soluble protein extracts by spectrophotometric assays as described in Materials and methods. Initial (incubation time = 0) values of GR activity were 50 (± 3·61) and 21 (± 1·85) nmol NADPH min−1 mg−1 for TR1 and TR9, respectively. Initial values of SOD activity were 31·95 (± 17·55) and 30·30 (± 12·08) U mg−1 for TR1 and TR9, respectively. Enzyme activities in control cultures incubated without CuHP for 3 and 6 h did not show significant changes with respect to the values at 0 h. Values depicted represent the means of 3–4 independent experiments ±s.d. Data were statistically analysed by a two-way ANOVA, in which the phycobiont species and CuHP treatment (CuHP concentration and incubation time) were considered as factors. Differences among phycobiont and treatment means were evaluated using Student's t-test. Different lower-case or upper-case letters indicate significant differences (P > 0·05) among CuHP treatments for each TR1 and TR9, respectively, and asterisks indicate significant differences between TR1 and TR9 at each CuHP treatment at *P > 0·05 or **P > 0·01, respectively. (B) Zymograms of GR (GR activity) after separation of the putative isozymes by ND-PAGE and activity staining (only one GR band was observed), and steady-state levels of GR protein (GR protein) as revealed by immunobloting after SDS–PAGE. (C) Zymograms of SOD after isozyme separation by ND-PAGE and activity staining.
DISCUSSION
The present study shows that isolated TR1 and TR9 R. farinacea phycobionts respond differently to oxidative stress generated by the ROS propagator CuHP. Compared with TR1, TR9 (Figs 2 and 3) seemed to possess an increased ability to preserve key chloroplast components, such as chlorophyll a, carotenoids and D1 protein (a component of the PSII core complex). The effect of CuHP on the algae was also analysed by modulated chlorophyll a fluorescence. This technique has been broadly used to monitor lichen health and the effects of a broad range of stresses, such as heavy metals (Branquinho et al., 1997), SO2 (Deltoro et al., 1998), dehydration (Beckett et al., 2005; Gasulla et al., 2009) and ozone (Calatayud et al., 2000), as it is non-invasive and allows changes in photosynthetic activity to be detected before the onset of other signs of injury. In general, photosynthetic performance was severely impaired by oxidative stress in TR1 and TR9, as indicated especially in the former by decreases in maximal PSII photochemical efficiency (Fv/Fm) and the quantum efficiency of PSII (ΦPSII), along with a higher proportion of closed PSII centres (Fig. 4). The decline in Fv/Fm could have been due to an increase in protective NPQ, to photodamage of the PSII centres or to both (Osmond, 1994). Our results suggest that the lower Fv/Fm in CuHP-treated algae was the result of severe stress at the PSII level, because NPQ was likewise adversely affected. Furthermore, the decrease of qP in the CuHP-treated algae indicated that oxidative stress decreased their capacity for reoxidizing QA during actinic illumination. Consequently, CuHP may have increased the excitation pressure on PSII, thus contributing to the closure of PSII reaction centres. Closed PSII centres have fully reduced QA, eliminating the possibility of electron transport to PSI and beyond (Seaton and Walker, 1990). In agreement with this finding, ΦPSII, which closely correlates with the quantum yield of non-cyclic electron transport (Genty et al., 1989), was clearly reduced by CuHP. However, it should be noted that the impact of oxidative treatments was consistently and significantly more severe in TR1 than in TR9. This probably reflects the greater capacity of TR9 to preserve key chloroplast components, which in turn enables it to maintain a better photosynthetic performance under relatively strong oxidative stress.
Under natural conditions, the chloroplast is a potent source of ROS, levels of which may increase dramatically in response to stresses involving temperature, irradiance and hydration state (Weissman et al., 2005; Catalá et al., 2010). Therefore, survival of the chloroplast and of the entire phycobiont will depend, at least in part, on their capacity to modulate steady-state levels of ROS. This regulation is complex and still poorly understood in lichen algae, but probably involves at least two important strategies: the prevention of excess ROS formation by increasing mechanisms of light dissipation, and ROS degradation and/or scavenging through the antioxidant defence system. Accordingly, and with the aim of identifying major metabolic distinctions that explain the differences in the oxidative stress tolerance observed in the phosynthetic machinery of TR1 and TR9, we determined the non-photochemical energy dissipation associated with photosynthetic electron transfer (NPQ) and the activity of the two main antioxidant enzymes, SOD and GR. NPQ, as calculated by a Stern–Volmer approach, is a useful measure of the allocation of absorbed light to thermal energy dissipation (Logan et al., 2007). In vascular plants it is assumed that NPQ affords a protection system for the photosynthetic mechanism, through the light-dependent formation of a proton gradient across the thylakoid membrane activating the xanthophyll cycle (Adams and Demmig-Adams, 2004). However, it is currently assumed that the non-photochemical dissipation processes in lichen photobionts differ from those in vascular plants (Kopecky et al., 2005). Indeed, in homeohydric vascular plants NPQ is dependent on light and quickly disappears when it is dark (Müller et al., 2001; Szabó et al., 2005), whereas energy dissipation in the lichen phycobiont Trebouxia erici can continue after spending 24 h in darkness and is independent of the xanthophylls cycle (Gasulla et al., 2009). Whatever the metabolic basis of NPQ in lichen algae, oxidative stress diminished NPQ in both phycobionts. TR1 seemed to be more sensitive than TR9 to CuHP treatments, as evidenced by a greater decrease in NPQ. In addition to non-photochemical energy dissipation, enzymatic and non-enzymatic antioxidants offer protection against the oxidative stress resulting from the increases in ROS that occur in response to diverse environmental stresses (Minibayeva and Beckett, 2001; Weissman et al., 2005; Kosugi et al., 2009). Antioxidant mechanisms common in vascular plants have been analysed in lichens, such as SOD (Weissman et al., 2005), peroxidases (Silberstein et al., 1996; Kranner et al., 2005), enzymes of the ascorbate–glutathione cycle (Kranner, 2002; Kranner et al., 2005) and low-molecular-weight antioxidants (Deltoro et al., 1998; Kranner, 2002; Kranner et al., 2005). In a study of atmospheric pollutants on photosynthetic performance of Parmelia quercina, Calatayud et al. (1999) reported that ascorbate feeding decreased photon excess in thalli from polluted sites owing to the stimulation of linear electron flow and non-radiative energy dissipation. However, it has not yet been possible to establish a clear relationship between the different levels of stress tolerance exhibited by most lichens and antioxidant mechanisms. Some of the conflicting results reported in the literature could be due to the fact that spectrophotometric assays of most antioxidant enzymes in crude extracts only yield an approximate ‘average’ cellular activity as most of these enzymes are expressed as different isoforms associated with different cellular compartments. Therefore, in our study SOD and GR activities were determined by spectrophotometric assay of crude extracts as well as by zymogram analysis. This approach confirmed the different abilities of the two phycobionts to modulate their antioxidant enzymes in response to oxidative stress (Fig. 5). In TR1, CuHP treatments significantly diminished GR protein levels and enzymatic activity and only slightly increased overall SOD activity without detectable changes in the activity of its different isozymes. Contrastingly, in TR9 the levels of GR and the activity of the putative chloroplast SOD isozyme increased in response to CuHP treatment.
As noted in the Introduction, R. farinacea has a highly specific and selective pattern of symbiotic association in which a single mycobiont associates with the same two Trebouxia phycobionts (TR1 and TR9) in all analysed populations, independent of this lichen's geographical location (del Campo et al., 2010; L. M. Casano et al., unpubl. res.). Additionally, an initial physiological characterization of TR1 and TR9 algae suggested a better physiological performance of TR9 under relatively high temperature and irradiances while TR1 seems to prefer more temperate and shaded conditions. Interestingly, TR1 predominates in R. farinacea populations from the Iberian Peninsula and California, whereas TR9 is more abundant in Canary Islands populations located at high altitudes (L. M. Casano et al., unpubl. res.), which are exposed to relatively harsher environmental conditions. Based on the results of the present study, we propose that the relative abundance of each phycobiont in each thallus might vary among different populations as a consequence of different environmental conditions along with different physiological capacities of the two algal species, similar to the ‘symbiont shuffling’ described in reef corals (Goulet, 2006). Studies on the latter suggested that the thermal tolerance of zooxanthellae relies on the stability of thylakoid membranes. The more stable the thylakoid membranes, the less vulnerable host tissues are to attack by ROS (Berkelmans and van Oppen, 2006). In the present study, comparative analysis of the responses of TR1 and TR9 phycobionts to CuHP with respect to key chloroplast constituents and photosynthetic traits suggests a better physiological performance of TR9 under oxidative conditions. This higher stress tolerance could be the consequence of a higher capacity to undertake key metabolic adjustments, including increased non-photochemical quenching, higher antioxidant protection and induced repair mechanisms.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Spanish Ministry of Science and Innovation (CGL2009-13429-C02-01/02) and the Generalitat Valenciana (PROMETEO 174/2008 GVA).
LITERATURE CITED
- Adams WW, III, Demmig-Adams B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In: Papageorgiou C, Govindjee J, editors. Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration. Vol. 19. Dordrecht: Springer; 2004. pp. 583–604. [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Ayala A, Parrado J, Bougria M, Machado A. Effect of oxidative stress produced by cumene hydroperoxide on the various steps of protein synthesis. Journal of Biological Chemistry. 1996;38:23105–23110. doi: 10.1074/jbc.271.38.23105. [DOI] [PubMed] [Google Scholar]
- Barreno E. Hongos simbiontes: Líquenes, micoficobiosis y micorrizas. In: Izco J, editor. Botánica. Madrid: MacGraw-Hill Interamericana; 2004. pp. 309–340. [Google Scholar]
- Beckett RP, Mayaba N, Minibayeva FV, Alyabyev AJ. Hardening by partial dehydration and ABA increase desiccation tolerance in the cyanobacterial lichen Peltigera polydactylon. Annals of Botany. 2005;96:109–115. doi: 10.1093/aob/mci156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Temperature dependence of violaxanthin deepoxidation and non- photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta. 1991;184:226–234. doi: 10.1007/BF00197951. [DOI] [PubMed] [Google Scholar]
- Bold HC, Parker BC. Some supplementary attributes in the classification of Chlorococcum species. Archives of Microbiology. 1962;42:267–288. doi: 10.1007/BF00422045. [DOI] [PubMed] [Google Scholar]
- Branquinho C, Brown DH, Máguas C, Catarino F. Pb uptake and its effects on membrane integrity and chlorophyll fluorescence in different lichen species. Environmental and Experimental Botany. 1997;37:95–105. [Google Scholar]
- Calatayud A, Deltoro VI, Abadía A, Abadía J, Barreno E. Effects of ascorbate feeding on chlorophyll fluorescence and xanthophyllcycle components in the lichen Parmelia quercina (Willd.) Vainio exposed to atmospheric pollutants. Physiologia Plantarum. 1999;105:679–684. [Google Scholar]
- Calatayud A, Temple P, Barreno E. Chlorophyll a fluorescence emission, xanthophylls cycle activity and net photosynthesis responses to ozone in some foliose and fruticose lichen species. Photosynthetica. 2000;38:281–286. [Google Scholar]
- Casano LM, Lascano HR, Trippi VS. Hydroxil radicals and a thylakoid-bound endopeptidase are involved in the light- and oxygen-induced proteolysis in oat chloroplasts. Plant and Cell Physiology. 1994;35:145–152. [Google Scholar]
- Casano LM, Martín M, Zapata JM, Sabater B. Leaf age- and paraquat-dependent effects on the levels of enzymes protecting against photooxidative stress. Plant Science. 1999;149:13–22. [Google Scholar]
- Catalá M, Gasulla F, Pradas del Real A, García-Breijo F, Reig-Armiñana J, Barreno E. Nitric oxide is involved in oxidative stress during rehydration of Ramalina farinacea (L.) Ach. in the presence of the oxidative air pollutant cumene hydroperoxide. In: Nash T, editor. Bibliotheca Lichenologica. Vol. 105. Stuttgart: Schweizerbart science publishers; 2010. pp. 87–92. [Google Scholar]
- Chapman MJ, Margulis L. Morphogenesis by symbiogenesis. International Microbiology. 1998;1:319–326. [PubMed] [Google Scholar]
- del Campo EM, Gimeno J, Casano LM, et al. South European populations of Ramalina farinacea (L.) Ach. share different Trebouxia algae. In: Nash T, editor. Bibliotheca Lichenolgica. Vol. 105. Stuttgart: Schweizerbart science publishers; 2010. pp. 247–256. [Google Scholar]
- Deltoro VI, Calatayud A, Gimeno C, Abadía A, Barreno E. Changes in chlorophyll a fluorescent, photosynthetic CO2 assimilation and xanthophyll cycle interconversions during dehydration in desiccation-tolerant and intolerant liverworts. Planta. 1998;207:224–228. [Google Scholar]
- Duan YH, Guo J, Ding K, et al. Characterization of a wheat HSP70 gene and its expression in response to stripe rust infection and abiotic stresses. Molecular Biology Reports. 2010 doi: 10.1007/s11033-010-0108-0. in press. doi:10.1007/s11033-010-0108-0. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiologia Plantarum. 1994;92:696–717. [Google Scholar]
- Fridovich I. Superoxide dismutases. In: Mekter A, editor. Advances in enzymology and related areas of molecular biology. Vol. 58. New York: John Wiley & Sons; 1986. pp. 61–97. [DOI] [PubMed] [Google Scholar]
- Galvez-Valdivieso G, Mullineaux PM. The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiologia Plantarum. 2010;138:430–439. doi: 10.1111/j.1399-3054.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- Gasulla F, de Nova PG, Esteban-Carrasco A, Zapata JM, Barreno E, Guéra A. Dehydration rate and time of desiccation affect recovery of the lichen alga Trebouxia erici: alternative and classical protective mechanisms. Planta. 2009;231:195–208. doi: 10.1007/s00425-009-1019-y. [DOI] [PubMed] [Google Scholar]
- Gasulla F, Guéra A, Barreno E. A simple micromethod for isolating lichen photobionts. Symbiosis. 2010;51:175–179. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Goulet TL. Most corals may not change their symbionts. Marine Ecology Progress Series. 2006;321:1–7. [Google Scholar]
- Kopecky J, Azarkovich M, Pfundel EE, Shuvalov VA, Heber U. Thermal dissipation of light energy is regulated differently and by different mechanisms in lichens and higher plants. Plant Biology (Stuttgart) 2005;7:156–167. doi: 10.1055/s-2005-837471. [DOI] [PubMed] [Google Scholar]
- Kosugi M, Arita M, Shizuma R, et al. Responses to desiccation stress in lichens are different from those in their symbionts. Plant Cell Physiology. 2009;50:879–888. doi: 10.1093/pcp/pcp043. [DOI] [PubMed] [Google Scholar]
- Kranner I. Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytologist. 2002;154:451–460. doi: 10.1046/j.1469-8137.2002.00376.x. [DOI] [PubMed] [Google Scholar]
- Kranner I, Zorn M, Turk B, Wornik S, Beckett RR, Batic F. Biochemical traits of lichens differing in relative desiccation tolerance. New Phytologist. 2003;160:167–176. doi: 10.1046/j.1469-8137.2003.00852.x. [DOI] [PubMed] [Google Scholar]
- Kranner I, Cram WJ, Zorn M, et al. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proceedings of the National Academy of Sciences of the USA. 2005;102:3141–3146. doi: 10.1073/pnas.0407716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Birtic S, Anderson KM, Pritchard HW. Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radicals in Biology and Medicine. 2006;40:2155–2165. doi: 10.1016/j.freeradbiomed.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Lascano HR, Antonicelli GE, Luna CM, et al. Antioxidative system response of different wheat cultivars under drought: field and in vitro studies. Functional Plant Biology. 2001;28:1095–1102. [Google Scholar]
- Lichtenthaller HK. Chlorophylls and carotenoids, pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Logan BA, Adams WW, III, Demmig-Adams B. Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Functional Plant Biology. 2007;34:853–859. doi: 10.1071/FP07113. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell and Environment. 2010;33:453–67. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Minibayeva F, Beckett RP. High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytologist. 2001;152:333–341. [Google Scholar]
- Müller P, Li X-P, Niyogi KK. Non-photochemical quenching: a response to excess light energy. Plant Physiology. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Li XP, Rosenberg V, Jung HS. Is psbS the site of non-photochemical quenching in photosynthesis? Journal of Experimental Botany. 2004;56:375–382. doi: 10.1093/jxb/eri056. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Osmond CB. What is photoinhibition? Some insights from comparison of shade and sun plants. In: Baker RR, Bowyer JR, editors. Photoinhibition of photosynthesis. Oxford: Bios Scientific Publishers Ltd; 1994. pp. 1–24. [Google Scholar]
- Scandalios JG. Molecular responses to oxidative stress. In: Hawkesford MJ, Buchner P, editors. Molecular analysis of plant adaptation to the environment. Dordrecht: Kluwer; 2001. pp. 181–208. [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photo-chemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Seaton GGR, Walker DA. Chlorophyll fluorescence as a measure of photosynthetic carbon assimilation. Proceedings of the Royal Society of London B. 1990;242:29–35. [Google Scholar]
- Szabó I, Bergantino E, Giacometti GM. Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Reports. 2005;6:629–634. doi: 10.1038/sj.embor.7400460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein L, Siegel BZ, Siegel SM, Mukhtar A, Galun M. Comparative studies on Xanthoria parietina, a pollution-resistant lichen, and Ramalina duriaei, a sensitive species. 1. Effects of air pollution on physiological processes. Lichenologist. 1996;28:355–365. [Google Scholar]
- Song YG, Liu B, Wang LF, Li MH, Liu Y. Damage to the oxygen-evolving complex by superoxide anion, hydrogen peroxide, and hydroxyl radical in photoinhibition of photosystem II. Photosynthesis Research. 2006;90:67–78. doi: 10.1007/s11120-006-9111-7. [DOI] [PubMed] [Google Scholar]
- Weissman L, Garty J, Hochman A. Characterization of enzymatic antioxidants in the lichen Ramalina lacera and their response to rehydration. Applied and Environmental Microbiology. 2005;71:6508–6514. doi: 10.1128/AEM.71.11.6508-6514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.