Abstract
Background and aims
Changes in supplies of resources will modify plant functional traits. However, few experimental studies have addressed the effects of nitrogen and water variations, either singly or in combination, on functional traits.
Methods
A 2-year field experiment was conducted to test the effects of nitrogen and water addition on leaf longevity and other functional traits of the two dominant (Agropyron cristatum and Stipa krylovii) and three most common species (Cleistogenes squarrosa, Melilotoides ruthenica and Potentilla tanacetifolia) in a temperate steppe in northern China.
Key Results
Additional nitrogen and water increased leaf nitrogen content and net photosynthetic rate, and changed other measured functional traits. Leaf longevity decreased significantly with both nitrogen addition (–6 days in 2007 and –5·4 days in 2008; both P < 0·001) and watering (–13 days in 2007 and –9·9 days in 2008; both P < 0·001), and significant differences in leaf longevity were also found among species. Nitrogen and water interacted to affect leaf longevity and other functional traits. Soil water content explained approx. 70 % of the shifts in leaf longevity. Biomass at both species and community level increased under water and nitrogen addition because of the increase in leaf biomass production per individual plant.
Conclusions
The results suggest that additional nitrogen and water supplies reduce plant leaf longevity. Soil water availability might play a fundamental role in determining leaf longevity and other leaf functional traits, and its effects can be modified by soil nitrogen availability in semi-arid areas. The different responses of species to resource alterations may cause different global change ramifications under future climate change scenarios.
Keywords: Agropyron cristatum, Cleistogenes squarrosa, Melilotoides ruthenica, Potentilla tanacetifolia, Stipa krylovii, grassland, Inner Mongolia, leaf functional traits, leaf longevity, nitrogen, steppe, water
INTRODUCTION
Nitrogen (N) deposition and changes in precipitation are two important components of global change. It has been demonstrated that N supply is enhancing globally due to ever-increasing N deposition caused by increasing use of N fertilizers and combustion of fossil fuels (Vitousek et al., 1997). Precipitation patterns are predicted to change at both the regional and the global scales (Dore, 2005; Cui et al., 2009), which will have fundamental significance in arid or semi-arid areas where water supply is limited (Hooper and Johnson, 1999). Changes in N and water supplies in the context of global change can profoundly affect ecosystem processes and functioning.
Increasing N and water availability can change plant uptake of nutrients, and cause further changes in leaf longevity and other plant functional traits (Chapin et al., 2002; Lambers et al., 2008). Leaf longevity largely determines the duration of photosynthesis of an individual leaf (Chabot and Hicks, 1982), and thus plays an important role in linking ecophysiology of each individual leaf to the growth of the whole plant (Kikuzawa and Ackerly, 1999; Craine and Reich, 2001). Leaf longevity has been documented to correlate with other leaf functional traits, such as photosynthetic rate, leaf N content and leaf mass per area (LMA) (Reich et al., 1997, 1999; Cordell et al., 2001; Shipley et al., 2006; Abrahamson, 2007). It also reflects the strategies of plants in their adaptation to variable resource conditions and plant resource use (Chapin, 1980; Eckstein et al., 1999). Alterations in resource use strategies of species indicated by changed leaf longevity could cause further shifts in community composition under changing resource availability. However, it remains unclear how variations in N and water supplies and their interaction affect leaf longevity and other leaf functional traits, and how different species respond to resource variations.
The determinant of the longevity of an individual leaf is largely considered to be the result of a balance between leaf construction and maintenance costs and carbon gain (Chabot and Hicks, 1982; Kikuzawa, 1995; Cordell et al., 2001). Longer leaf longevity has been observed under conditions of N and water shortages (Sandquist and Ehleringer, 1998; Wright et al., 2002). But most of these studies focused on meta-data analysis, and there are only a few manipulatively experimental studies on the effects of either adding N (Shaver, 1981, 1983; Craine and Reich, 2001) or drought treatment (Casper et al., 2001) on leaf longevity and other plant leaf functional traits, and especially fewer on the combined effects of the two factors.
The typical steppe of Inner Mongolia in northern China is the dominant vegetation type in semi-arid regions of Eurasia. It is also extremely sensitive to climate change (Christensen et al., 2004; Kang et al., 2007) because N and water are each limiting factors for plant growth in this area (Xia et al., 2009; Xu et al., 2010). As summer precipitation in northern China is predicted to increase in the future (Cholaw et al., 2003) as is N deposition, understanding how increased supplies of N and water, and their combination, alter leaf longevity and other leaf functional traits in this extensive grassland ecosystem is urgently required. As increasing N availability may enhance leaf N content, stimulate photosynthetic activity (Field and Mooney, 1983; Pons and Anten, 2004) and then lead to rapid leaf turnover, we hypothesize that leaf longevity will decline with increasing N supply. As water addition may also promote leaf metabolic activity (Lajtha and Whitford, 1989; Patrick et al., 2007) and hence accelerate leaf ageing, we also hypothesize that leaf longevity will be reduced under increased water supply.
MATERIALS AND METHODS
Study site and experimental design
This study was carried out in a typical steppe ecosystem in Duolun county (116°17′E, 42°02′N, elevation 1324 m a.s.l.), Inner Mongolia, China. It has a temperate continental monsoon climate with cold winters and warm summers (mean January and July temperatures of –17·5 and 18·9 °C, respectively). Mean annual temperature is 2·1 °C and mean annual precipitation is around 380 mm. More than 80 % of the precipitation occurs between June and September. Chestnut soil is a predominant type in this area with mean soil bulk density of 1·21 g cm−3 and pH of 7·29. The plant community at the study site is dominated by two grasses (Stipa krylovii and Agropyron cristatum) and one forb species (Artemisia frigida).
The experiment used a randomized block design, with five replicate blocks, each containing four 8 × 8-m2 plots. Within each block, each of the four plots was randomly assigned to one of the following four treatments: control, N addition, water addition, and combined N and water addition. The plots with N addition received 10 g N m−2 yr−1 in the form of urea applied twice, half in early May and the other half in late June. From June to August, the watered plots received 15 mm of precipitation weekly by sprinkling irrigation. A total 180 mm of precipitation was added in the growing season (12 weeks of 15 mm week−1), which was approx. 50 % of mean annual precipitation in this study site.
Five perennial herbs (A. cristatum, S. krylovii, Cleistogenes squarrosa, Melilotoides ruthenica and Potentilla tanacetifolia), which occurred in each plot, were selected to determine the responses of leaf longevity and other leaf functional traits to N and water addition. The two co-dominant species, S. krylovii and A. cristatum, are C3 grasses that are widespread in the studied ecosystem. C. squarrosa is the exclusive C4 perennial grass in the research area. M. ruthenica is a typical C3 legume, and P. tanacetifolia is a common perennial forb in the study site.
Measurements of soil water content, inorganic N and total N contents
In 2007 and 2008, soil water content (SWC, fresh weight basis) was determined by taking a 10-cm-deep soil core randomly from each plot every week from late June to late September and then dried for 48 h at 105 °C. Soil samples were collected by mixing three 3-cm-diameter cores from 10-cm-deep soil randomly taken in each plot at the peak of growing season for measuring total N (KjektecTM 2300, Hillerød, Denmark) and inorganic N (FIAstar 5000 Analyzer, Foss Tecator, Denmark).
Added water significantly raised SWC by 39·8 % (P < 0·001) while N addition weakly enhanced SWC by 2·3 % (P > 0·05) across the two study years. Soil inorganic N content (SIN) increased by 85 % in N added plots compared with no N added plots (P < 0·001), but decreased by 19·4 % in water-added plots compared with no water added plots (P < 0·05). Soil total N content (STN) showed no notable responses to N and water addition.
Measurements of leaf longevity
We employed the census protocol and calculations of Craine et al. (1999) and Craine and Reich (2001) with small modifications to determine the average leaf longevity of the five steppe species. Three randomly chosen individuals per species in each plot were monitored. Three approximately fully expanded leaves per individual plant were marked by tying a small tag to the leaf base. Thus, 180 leaves per plant species was monitored each year. Censuses of the tagged leaves were made every 10 d from late June to mid-October in each year. When a leaf was judged to be fully expanded, the time was defined as its birth date. When a leaf or at least two-thirds of its area turned yellow or brown, it was considered senesced.
At each census date, for three grasses (C. squarrosa, A. cristatum and S. krylovii), length of total leaf and the senesced portion of the leaf were measured. For two forbs (M. ruthenica and P. tanacetifolia), the maximum width, and total and senesced length of the compound leaf were recorded and leaf area was predicted by establishing regression equations. For M. ruthenica, the equation was: Leaf area = 0·17 + 0·38 Length × Width (r2 = 0·82 in 2007, P < 0·001); Leaf area = 0·06 + 0·52 Length × Width (r2 = 0·95 in 2008, P < 0·001). For P. tanacetifolia, the equation was: Leaf area = 1·29 + 0·46 Length × Width (r2 = 0·93 in 2007, P < 0·001); Leaf area = 0·68 + 0·41 Length × Width (r2 = 0·94 in 2008, P < 0·001).
The average longevity of the leaves was estimated from division of the leaf-days of all the leaves sampled in a plot by the length or area of these leaves that were born and senesced over the census period using the following model:
Leaf longevity = (A – B – C – D)/(Sj – Li) or (A – B – C – D)/(Sj – Ai)
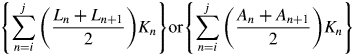 |
(1a) |
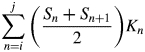 |
(1b) |
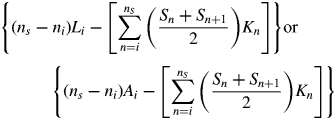 |
(1c) |
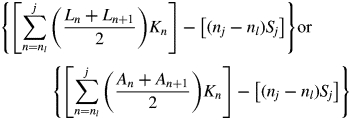 |
(1d) |
where i is the starting census date; j is the ending census date; Ln and An are cumulative length or area of leaves produced by the census date n, and Sn is the corresponding cumulative length or area of leaves senesced by the census date; Kn is the time interval between census n and n + 1; ns is the date at which the amount of leaf length or leaf area senesced is equivalent to the amount of leaf length or leaf area at the first census (S = Li, or S = Ai); and nl is the date at which the cumulative leaf length or leaf area produced is equal to the amount of leaf length or leaf area senesced by the last census date (L = Sj, or A = Sj); nl and ns were calculated by linear interpolation.
Equations (1a) and (1b) represent the cumulative amount of leaf-days for both green and senesced leaves and for senesced leaves, respectively, and their difference is the total unsenesced leaf-days over the entire census period. Equation (1c) represents the amount of leaf-days for leaves present at the beginning of the census period, and eqn (1d) represents the leaf-days of leaves that had not senesced by the end of the census period.
Measurements of leaf functional traits
Leaf net photosynthetic rate based on area (Pnet) and stomatal conductance (gs) were measured every 2 weeks from early July to late September, by using a Li-Cor 6400 photosynthesis system (Li-Cor, Lincoln, NE, USA) at a saturated photosynthetic photon flux density of 1500 µmol m−2 s−1 using an LED light source, for the two dominant species (S. krylovii and A. cristatum) in this study site. The SPAD value, which indicates leaf chlorophyll concentration, was determined with a portable SPAD-502 meter (Minolta, Tokyo, Japan).
Area, weight and nutrient contents of leaves were measured biweekly for the five species during the period from mid-June to mid-October. For each species at each sampling time, 10–60 representative leaves were harvested from at least three individual plants in each plot and then kept in a cooler. After immediate measurements of leaf fresh weight and leaf area in the laboratory, leaves were dried at 65 °C for 48 h and then weighed to determine leaf dry mass. The dried leaf samples were used to determine total N (LNC), phosphorus (LPC) and carbon concentrations (C) with the Auto-Kjeldahl method (Kjektec System 1026 distilling unit, Sweden), molybdenum stibium anti-spectrophotometry and K2CrO7–H2SO4 oxidation, respectively. Leaf mass per area was determined as the leaf dry mass per unit area. Dry matter content (DMC) was the ratio of dry mass to fresh mass. Nitrogen use efficiency (NUE) was estimated from the inverse of N content in dead leaves.
Leaf samples collected at the peak of the growing season were used to analyse carbon isotopic composition (δ13C), via a Thermo Finnigan MAT DeltaPlus XP isotope ratio mass spectrometer coupled to a Flash elemental analyser (Thermo Finnigan, Bremen, Germany).
Measurements of biomass at leaf, species and community levels
In late August 2007 and 2008, above-ground living tissues were harvested by species in a 0·15 × 2-m randomly sampled quadrat in each plot. Plant tissues of each species were weighed after drying to constant weight to determine the species biomass and community above-ground net primary productivity (ANPP). Leaf biomass per individual was also measured by dividing leaves from each individual so as to determine leaf biomass production in 2008. We employed the root in-growth core method to measure below-ground net primary productivity (BNPP) (Xu and Wan, 2008).
Data analysis
Data for SWC and leaf functional traits except leaf longevity for statistical analysis were mean values taken across all the measurement dates. Multiple-way ANOVA was performed to test the effects of year, species, N and water addition on leaf longevity, other leaf functional traits and soil attributes. Pearson's correlation coefficient was used to investigate correlations between variables. Stepwise multiple regression analysis (forward) was conducted to examine the contribution of leaf functional traits and environmental factors to the variation of leaf longevity. Standardized major axis (SMA) was run to test the differences in SMA slopes and intercepts among groups (Falster et al., 2006). The SMA relationships between leaf longevity and net photosynthetic rate based on mass (Pnet-mass), LNC and LMA in the present study were analysed in comparison with the Cedar Creek data set (Tjoelker et al., 2005) and Glopnet data set (Wright et al., 2004). Furthermore, the species in this study were categorized intoi four plant functional groups, i.e. C3 grass, C4 grass, forb and legume, to compare with grasses or sedges and herbs chosen from the Glopnet data set and the corresponding plant functional groups of the Cedar Creek data set, respectively. All statistical analyses were performed using SAS software (version 8·2).
RESULTS
Patterns of leaf longevity with varying N and water availabilities
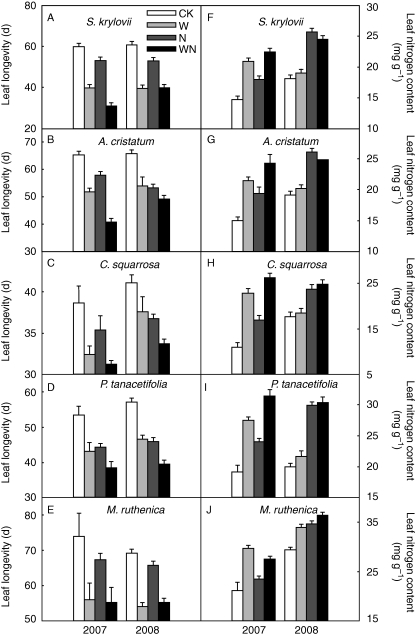
Nitrogen addition decreased leaf longevity by 6 d in 2007 and by 5·4 d in 2008 across all the studied species (P < 0·001, Table 1). Water addition also reduced leaf longevity by 13·0 d in 2007 and by 9·9 d in 2008 (P < 0·001, Table 1). There was a significant interaction between species and N in 2008 on leaf longevity, with the highest decrease in M. ruthenica (17·7 %), followed by A. cristatum (14·4 %), C. squarrosa (10·4 %), S. krylovii (7·6 %) and the lowest decrease in P. tanacetifolia (only 1·8 %) (Table 1, Fig. 1A–E). Interaction between species and water was also prominent in both 2007 and 2008 with S. krylovii exhibiting the highest reduction (37·4 and 30·0 %, respectively), C. squarrosa the lowest reduction (14·1 and 8·2 %), and A. cristatum, M. ruthenica and P. tanacetifolia moderate reductions (24·9 and 13·3 %, 21·4 and 19·1 %, and 16·6 and 16·5 %, respectively) in leaf longevity (Table 1, Fig. 1A–E). Significant interactive effects of N and water addition on leaf longevity were also found in 2008 (P < 0·001, Table 1). Compared with the ambient N control, N addition decreased leaf longevity by 13·3 % in the unwatered plots but only by 5·6 % in the watered plots across all the studied species in 2008 (Fig. 1A–E). Water addition caused a 20·3 % decrease in leaf longevity without N addition but a 13·8 % reduction with N addition in 2008 (Fig. 1A–E).
Table 1.
Results (F values) of three-way ANOVA of responses of leaf longevity and biomass production to N and water addition
| Leaf longevity |
Leaf biomass production | ||
|---|---|---|---|
| 2007 | 2008 | 2008 | |
| Species (Sp) | 70·5*** | 173·65*** | 43·63*** |
| Water (W) | 128·93*** | 263·21*** | 55·69*** |
| Nitrogen (N) | 27·11*** | 77·22*** | 39·32*** |
| W × N | 0·33 | 16·87*** | 0·21 |
| Sp × W | 6·13*** | 14·73*** | 2·32 |
| Sp × N | 1·31 | 6·38*** | 5·18** |
| Sp × W × N | 0·66 | 1·31 | 3·45* |
Asterisks indicate statistically significant differences at *P < 0·05, **0·01 and ***0·001.
Fig. 1.
Effects of N and water addition on longevity and N content in leaves for the five steppe species. CK, control; W, water addition; N, N addition; WN, combined addition of N and water.
Responses of leaf photosynthetic parameters, LNC, DMC, C/N ratio, δ13C value to N and water addition
Marginally significant differences were found between the two dominant species, S. krylovii and A. cristatum, for Pnet and gs across the two sampling years (P < 0·10, Table 2). Nitrogen addition significantly increased Pnet by 8·4 % (P < 0·05, Table 2), but had no significant effects on gs. Water addition enhanced Pnet and gs by 51·5 and 69·5 %, respectively (P < 0·001, Table 2). The interactive effects of N with water addition were also significant on Pnet (P < 0·05), but not significant on gs. Pnet and gs decreased, respectively, by 119·2 and 161·4 % in 2008 than in 2007.
Table 2.
ANOVA results (F values) of leaf functional traits in response to N and water addition
| Pnet | gs | SPAD | LNC | DMC | C/N ratio | δ13C | |
|---|---|---|---|---|---|---|---|
| Year (Yr) | 450·27**** | 125·14**** | 93·25**** | 173·32**** | 1942·24**** | 124·36**** | 27·56**** |
| Species (Sp) | 2·27* | 3·94* | 1307·72**** | 238·24**** | 822·01**** | 49·72**** | 2050**** |
| Water (W) | 135·21**** | 41·94**** | 248·82**** | 300·15**** | 234**** | 131·22**** | 6·37*** |
| Nitrogen (N) | 5·27** | 1·14 | 37·54**** | 400·6**** | 114·96**** | 126·55**** | 2·63* |
| W × N | 6·17** | 1·66 | 39·21**** | 17·41**** | 29·34**** | 26·14**** | 0·03 |
| Yr × N | 1·41 | 1·24 | 0·47 | 50·77**** | 11·26**** | 0·44 | 0·86 |
| Yr × W | 10·15*** | 1·24 | 43·77**** | 176·07**** | 48·64**** | 99·15**** | 0·34 |
| Yr × Sp | 4·43** | 5·24** | 7·59**** | 34·2**** | 25·64**** | 8·91**** | 1·52 |
| Sp × W | 0·05 | 1·54 | 4·08*** | 6·54**** | 8·11**** | 1·4 | 1·24 |
| Sp × N | 0 | 0·04 | 1·57 | 11·33**** | 4·73*** | 5·24**** | 1·5 |
| Sp × W × N | 0·53 | 0 | 2·87** | 0·96 | 0·87 | 0·46 | 1·09 |
| Yr × Sp × W × N | 0·50 | 1·03 | 1·53* | 2·36*** | 1·43 | 3·23**** | 0·77 |
Pnet, net photosynthetic rate based on area; gs, stomatal conductance; SPAD, estimated leaf chlorophyll concentration; LNC, leaf nitrogen content; DMC, leaf dry matter content; C/N ratio, ratio of leaf carbon to nitrogen contents; δ13C, leaf carbon isotopic composition.
Asterisks indicate statistically significant differences at *P < 0·10, **0·05, ***0·01, and ****0·001.
Nitrogen and water addition increased SPAD values by 3·7 and 9·8 %, respectively, and significant interactions of N with water addition on SPAD were observed (P < 0·001, Table 2). SPAD in 2008 was 5·9 % higher than in 2007 (P < 0·001).
Leaf N content showed marked increases of 22·3 and 19·0 %, respectively, in response to N and water addition, and responded significantly to the interaction between N and water addition (P < 0·001, Table 2, Fig. 1F–J). In addition, leaf N content was 14·1 % higher in 2008 than in 2007 (P < 0·001).
Nitrogen addition and supplemental water decreased DMC by 4·9 and 6·9 %, respectively, and their interaction had a marked effect on DMC (P < 0·001, Table 2). DMC was 18·6 % lower in 2008 than in 2007 (P < 0·001).
C/N ratio decreased with N and water addition by 20·7 and 21·0 %, respectively, and there was a notable response to the interaction of N with water addition (P < 0·001, Table 2). C/N ratio in 2008 was 20·5 % lower than in 2007 (P < 0·001).
Leaf δ13C value was lower in plots with N addition than in those without N addition (P < 0·10), and was significantly lower in watered plots than in non-watered plots (P < 0·01) (Table 2). Interactive effects of N and water addition on δ13C were not significant. Leaf δ13C was 2·3 % lower in 2008 than in 2007 (P < 0·001).
There were strong species differences for the above-mentioned functional traits (P < 0·001, Table 2). Leaf N content and SPAD were higher but leaf DMC, C/N ratio and δ13C were lower for M. ruthenica and P. tanacetifolia than for S. krylovii, A. cristatum and C. squarrosa.
Effects of N and water addition on biomass at the leaf, species and community levels
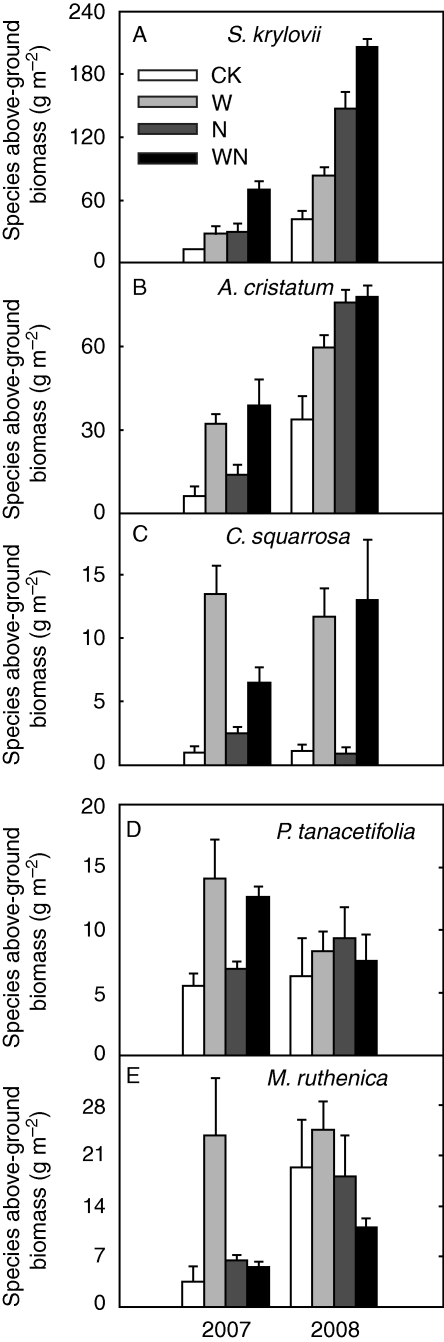
Nitrogen addition and water addition enhanced leaf biomass production by 60·6 and 76·5 %, respectively (both P < 0·001, Table 1). Interaction between species and N was also significant in 2008 on leaf biomass production (P < 0·01, Table 1), with P. tanacetifolia showing the highest increase (164·9 %), followed by S. krylovii (99·3 %), A. cristatum (80·0 %) and C. squarrosa (62·2 %); M. ruthenica showed the lowest increase (4·2 %). There was a marginally significant interaction between species and water in 2008 on leaf biomass production (P < 0·10, Table 1).
As the dominant species of the community, the above-ground biomass of S. krylovii and A. cristatum increased by 171·9 and 56·5 %, respectively, with N addition and by 66·7 and 61·4 %, respectively, with watering over the two years (P < 0·001, Fig. 2A, B). Water addition notably increased the above-ground biomass of C. squarrosa and P. tanacetifolia (P < 0·05, Fig. 2C, D), whereas N addition showed no such significant effects (P > 0·10, Fig. 2C, D). For M. ruthenica, N addition decreased its biomass by 42·2 % (P < 0·05), while supplemental water enhanced its biomass by 199·9 % in 2007 (P < 0·05), but had no significant effects in 2008 (P > 0·10) (Fig. 2E). The above-ground biomass of S. krylovii, A. cristatum and M. ruthenica was significantly higher in 2008 than in 2007 (P < 0·05, Fig. 2A, B, E), while insignificant interannual differences existed for C. squarrosa and P. tanacetifolia (P > 0·10, Fig. 2C, D).
Fig. 2.
Impacts of N and water addition on species above-ground biomass. CK, control; W, water addition; N, N addition; WN, combined addition of N and water.
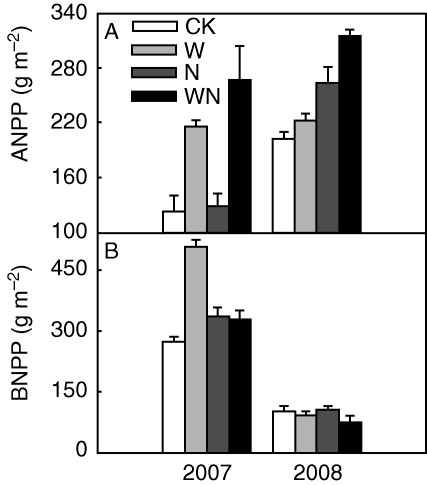
Additional N and water supplies notably increased community ANPP by 27·8 and 42·2 % (P < 0·001, Fig. 3A), respectively, over the two years. BNPP was 36·9 % higher under watering than the control in natural and increased N plots, but elevated N significantly decreased BNPP by 14·9 % across the natural and increased water plots in 2007 (both P < 0·01, Fig. 3B). Neither N nor water addition, however, resulted in marked changes in BNPP in 2008 (Fig. 3B). Nevertheless, ANPP was significantly higher in 2008 than in 2007, whereas BNPP was lower in 2008 than in 2007 (both P < 0·001, Fig. 3).
Fig. 3.
Reponses of above-ground net primary productivity (ANPP) and below-ground net primary productivity (BNPP) at community level to N and water addition. CK, control; W, water addition; N, N addition; WN, combined addition of N and water.
Relationships between leaf longevity and environmental factors
For all the species studied, SWC exhibited a close negative correlation with leaf longevity in 2008 (P < 0·10 for C. squarrosa, P < 0·01 for the other species) (Table 3). There was no significant relationship between STN and leaf longevity (P > 0·10), except a weak negative correlation for M. ruthenica in 2007 (P < 0·10) (Table 3). The negative relationship between SIN and leaf longevity was significant for A. cristatum in 2008 (P < 0·05), and marginally significant for P. tanacetifolia (P < 0·10) (Table 3).
Table 3.
Correlations between leaf longevity and environmental factors and other leaf functional traits for the five steppe species
| Species | Environmental factors |
Other leaf functional traits |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWC | SIN | STN | LNC | LPC | C/N | DMC | LMA | δ13C | NUE | SPAD | Pnet | gs | |
| 2007 | |||||||||||||
| S. krylovii | –0·85**** | 0·09 | –0·33 | –0·89**** | –0·57** | 0·86*** | 0·89**** | 0·65** | 0·60** | 0·75*** | –0·69*** | –0·90**** | –0·78*** |
| A.cristatum | –0·86**** | –0·02 | –0·17 | –0·88**** | –0·3 | 0·85**** | 0·84**** | 0·65** | 0·07 | 0·77*** | –0·82**** | –0·84**** | –0·64** |
| C. squarrosa | –0·57*** | 0·13 | 0·04 | –0·89**** | –0·28 | 0·82**** | 0·82**** | 0·35 | 0·44* | –0·88**** | – | – | |
| P. tanacetifolia | –0·69**** | –0·17 | –0·23 | –0·83**** | –0·03 | 0·74*** | 0·75*** | 0·75*** | 0·27 | 0·76*** | –0·84**** | – | – |
| M. ruthenica | –0·62*** | 0·15 | –0·39* | –0·88**** | 0·14 | 0·84**** | 0·72*** | 0·55* | 0·52* | 0·71*** | –0·91**** | – | – |
| 2008 | |||||||||||||
| S. krylovii | –0·76**** | –0·01 | 0·03 | –0·06 | –0·21 | 0·16 | 0·44 | –0·13 | 0·43 | 0·1 | –0·46* | –0·83**** | –0·75*** |
| A. cristatum | –0·57*** | –0·44** | –0·16 | –0·82**** | 0·07 | 0·85**** | 0·81** | 0·78*** | –0·1 | 0·69*** | –0·61** | –0·48* | –0·25 |
| C. squarrosa | –0·42* | –0·26 | 0·25 | –0·92**** | 0·47* | 0·88**** | 0·86**** | 0·83**** | –0·18 | 0·39 | –0·48* | – | – |
| P. tanacetifolia | –0·64*** | –0·42* | 0·01 | –0·79*** | –0·63** | 0·83**** | 0·82**** | 0·67** | 0·61* | 0·54* | –0·84**** | – | – |
| M. ruthenica | –0·93**** | 0·12 | 0·14 | –0·71*** | –0·48* | 0·66** | 0·74*** | 0·58** | 0·75** | 0·45* | –0·72*** | – | – |
SWC, soil water content; SIN, soil inorganic nitrogen; STN, soil total nitrogen; LNC, leaf nitrogen content; LPC, leaf phosphorus content; C/N ratio, ratio of leaf carbon to nitrogen contents; DMC, leaf dry matter content; LMA, leaf mass per area; δ13C, leaf carbon isotopic composition; NUE, nitrogen use efficiency; SPAD, estimated leaf chlorophyll concentration; Pnet, net photosynthetic rate based on area; gs, stomatal conductance.
Asterisks indicate statistically significant differences at *P < 0·10, **0·05, ***0·01 and ****0·001.
Stepwise multiple regression analysis indicated that SWC explained 77·4 and 63·4 % of the shifts in leaf longevity (LL) in 2007 (LL = –3·55SWC + 77·84) and 2008 (LL = –3·05SWC – 0·12SIN + 85·84), respectively, across all the studied species (both P < 0·001, n = 20). In addition, SIN accounted for 7·9 % of the variation in leaf longevity in 2008 (P < 0·05). Soil total N content was excluded from the regression model.
Relationships between leaf longevity and other leaf functional traits
Leaf longevity showed significantly negative correlations with Pnet and gs for S. krylovii over the two years (P < 0·01, Table 3). For A. cristatum, leaf longevity was significantly negatively correlated with Pnet (P < 0·001) and gs (P < 0·05) in 2007, and marginally significantly correlated with Pnet (P < 0·10) but insignificantly with gs (P > 0·10) in 2008 (Table 3). Negative correlations between leaf longevity and SPAD values were marginally significant at least for all the species over the two years (P < 0·10 for S. krylovii and C. squarrosa in 2008; P < 0·05 for the other species, Table 3). There were markedly negative relationships between LNC and leaf longevity for the studied species (P < 0·01) except S. krylovii in 2008 (P > 0·10) (Table 3). The negative correlations between LPC and leaf longevity were significant for S. krylovii in 2007 and P. tanacetifolia in 2008 (both P < 0·05, Table 3), and marginally significant for C. squarrosa and M. ruthenica in 2008 (both P < 0·10, Table 3). Leaf C/N ratio and DMC were both highly positively associated with leaf longevity for all the studied species (P < 0·05) except for S. krylovii in 2008 (P > 0·10) (Table 3). Positive correlations of leaf longevity with leaf mass per area were found (for M. ruthenica, P < 0·10 in 2007; for the other species, P < 0·05), although the effects were not statistically significant for C. squarrosa in 2007 (P > 0·10) or for S. krylovii in 2008 (P > 0·10) (Table 3). There were marginally significant positive correlations between δ13C value and leaf longevity for C. squarrosa and M. ruthenica in 2007 (P < 0·10), and significantly positive correlations for S. krylovii in 2007 (P < 0·05), P. tanacetifolia in 2008 (P < 0·05) and M. ruthenica in 2008 (P < 0·01) (Table 3). NUE was positively correlated with leaf longevity for the studied species (P < 0·10) except for S. krylovii and C. squarrosa in 2008 (P > 0·10) (Table 3).
Stepwise multiple regression analysis revealed that leaf photosynthetic capacity including Pnet, gs and SPAD, and LNC could explain between 17·1 and 94·3 % of the variances in leaf longevity for the different species (all P < 0·01, Appendix). Leaf C/N ratio, DMC and δ13C value were also strongly positively associated with leaf longevity (all P < 0·01, Appendix). Other leaf functional traits were rejected from the regression equation.
DISCUSSION
The results of the present study indicate that additional N and water supply can, either singly or in combination, reduce the leaf longevity of all the studied perennials in the temperate typical steppe ecosystem, although significant differences exist among the different plant species.
Leaf longevity of all the five herbaceous perennials declined significantly with addition of N, in agreement with the results of previous studies which showed that leaf longevity decreased with N fertilization (Shaver, 1983; Cordell et al., 2001; Craine and Reich, 2001). We suggested that additional N supply resulted in high LNC, high chlorophyll content and consequently high Pnet (Table 2), and further led to high metabolic activity and leaf turnover, and therefore shortened leaf longevity. Such negative relationships between photosynthetic rates and leaf longevity have also been reported previously (Mooney and Gulmon, 1982; Reich et al., 1992; Wright et al., 2004; Escudero et al., 2008).
Increasing water supply also shortened leaf longevity. This was in agreement with results of a previous study which showed that plants that lived in sites where water was abundant had shorter leaf longevities as photosynthetic rates can be high at such sites (Kikuzawa, 1995). Results from regression analysis indicated that SWC could explain a large proportion of this variation in leaf longevity. We thus consider that water addition can trigger an increase of LNC (Table 2, Fig 1F–J) as high water availability can enhance both the transformation from organic to inorganic N and the mobility of inorganic N in soils. Thus, increased LNC as a result of water addition could promote plant photosynthetic activity and leaf senescence, and accordingly reduce leaf longevity. However, we also noted that the higher natural precipitation in 2008 (357 mm, close to the long-term mean) than in 2007 (208 mm, 46·7 % lower than the mean) did not change leaf longevity in most studied species, although the manipulative water addition did shorten leaf longevity of all studied species (Fig. 1A–E). We speculate that artificial increases of water supply in the growing season may exert stronger effects on leaf longevity than any natural increase in precipitation, which is distributed throughout the year and may not be efficient for plant use especially in the non-growing season.
Few studies have addressed the interactive effects of N and water addition on leaf longevity. In the present study, a significant interaction between N and water addition was found in 2008, but not in 2007. This interannual difference in interactive effects of the two factors could mainly be attributed to the remarkable interannual variation in natural precipitation in the study area. Leaf N content significantly increased with additional water supply in 2007 but changed little in 2008 except for the legume M. ruthenica (Fig. 1F–J). As a consequence, the effect of N on leaf longevity might be influenced significantly by water availability in this semi-arid typical steppe.
Most previous studies reported close relationships between leaf longevity and other leaf functional traits (Reich et al., 1997, 1999; Cordell et al., 2001; Shipley et al., 2006; Abrahamson, 2007). Leaf δ13C may reflect the water use efficiency and metabolism of plants (Livingston et al., 1999). C/N ratio can indicate total carbon fixed per unit N and carbon use efficiency (Chapin and Van Cleve, 1989; Patterson et al., 1997). Dry matter content also implies a trade-off between rapid biomass production and efficient nutrient conservation (Poorter and De Jong, 1999; Garnier et al., 2001; Saura-Mas and Lloret, 2007). There are close inherent relationships among these leaf functional traits. Shorter leaf longevity under increased N and water supplies could imply lower use efficiency of both N and water by plants. Different strategies of resource use by different species under changing resource conditions may reflect how a community might respond to variable resource supplies, and will thus contribute greatly to ecosystem stability in both structure and function. Furthermore, the different responses of species to alterations in water and N availability may have different global change ramifications as different areas are dominated by different species and global change drivers.
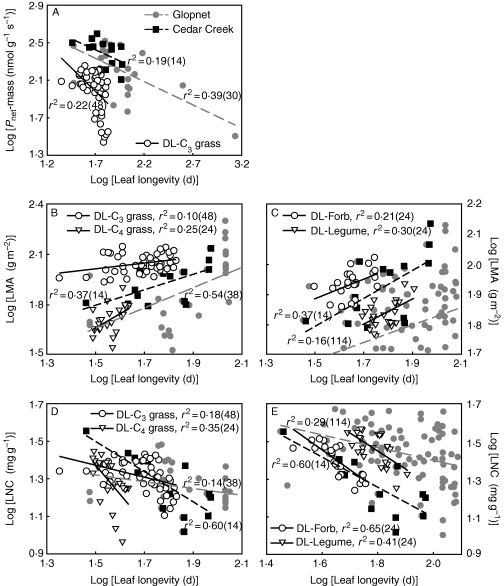
Placing our results into a quantitative context with regard to previous studies, we found that mean values of leaf longevity in this study (48·71 d) were close to the results for Cedar Creek (63·36 d, P > 0·05) but different from those for Glopnet (133·39 d, P < 0·05). This indicated that higher discrepancies might occur among different biomes as the Glopnet data set included more species from a variety of ecosystems while the Cedar Creek data set included species only from grassland, similar to those of the present study. LMA might be a relatively stable leaf trait as its values in this study (86·11 g m−2) were close to the means from both the Glopnet (83·78 g m−2) and the Cedar Creek (86·63 g m−2) data sets. The higher LNC values in this study (23·34 mg g−1) than in Cedar Creek (19·81 mg g−1) might be explained by the N addition in this study (10 g m−2 yr−1) whereas no N was applied in Cedar Creek. The differences in Pnet-mass values (Glopnet, 192·24 nmol g−1 s−1; Cedar Creek, 258·14 nmol g−1 s−1; present study, 110·36 nmol g−1 s−1) among the three data sets were presumably caused by the different resource availability, especially for water. At Cedar Creek, precipitation was around 660 mm, much higher than the 380 mm for Duolun county, and water stress was largely ignored by watering all the plots during the growing season (Tjoelker et al., 2005). In the present study, water was the major limiting factor for plant growth (Xu et al., 2010), although it was added in half of the experimental plots.
The overall relationships between leaf longevity and Pnet-mass, LMA and LNC in the present study are largely in agreement with previous findings although the coefficients of determination might be different between studies; in particular, the relationships between leaf longevity and LMA have identical slopes in these three data sets (common slopes of 0·70 for C3 grasses, 0·73 for forbs and 0·75 for legumes; Fig. 4A–E). This suggests there are globally consistent patterns of relationships among leaf functional traits despite the fact that different biomes and environmental factors among study sites may influence their slopes.
Fig. 4.
Standardized major axis (SMA) relationships between leaf longevity (LL) and mass-based net photosynthetic rate (Pnet-mass), leaf mass per area (LMA) and leaf nitrogen content (LNC) for Duolun (DL) steppe species in comparison with the Cedar Creek data set (CC) and the Glopnet data set (G). LL versus Pnet-mass; (A) slopes: DL –2·22, CC –1·02, G –0·80. LL versus LMA: (B) common fitted slope among three sites was 0·70 for C3 grasses; slopes: DL 1·71, CC 0·77, G 0·82 for C4 grasses. (C) Common slopes fitted within each functional group: forb 0·73, legume 0·75. LL versus LNC; (D) slopes: DL for C3 grasses –0·75, DL for C4 grasses –2·57, CC –1·03, G –0·49. (E) Slopes: DL for forb –1·31, DL for legume –1·50, CC –1·03, G –0·62. All relationships were significant, P < 0·05, except the correlation between longevity and Pnet-mass in Cedar Creek (P = 0·191). Sample sizes are given in parentheses. The Glopnet data set is not shown in totality, but the statistical results were obtained via the whole data set.
Although increased N input and water supply shortened leaf longevity, species biomass production was nevertheless increased in the N-fertilized and watered plots. According to the cost–benefit model proposed by Kikuzawa (1991, 1995), leaf longevity was adjusted to maximize carbon gain, and plants would produce more new leaves to replace the old leaves when leaf longevity was reduced. Our results corroborated the model in that N and water addition increased leaf biomass production of individual plants for all the five studied species although their leaf longevity decreased (Table 1). Thus, plant above-ground biomass, especially for the two dominant species, S. krylovii and A. cristatum, increased with enhanced N and water supply (Fig. 2A, B), and consequently increased ANPP at the community level (Fig. 3A). Increasing N and water availability enhanced LNC and leaf photosynthetic capacity, and hence more carbon was transferred to the plant root. As a result, BNPP also showed a positive response to water addition (Fig. 3B).
In summary, future changes in N deposition and precipitation in temperate typical steppe and similar terrestrial ecosystems may significantly alter leaf longevity and other leaf functional traits. Soil water supply may be a central factor in controlling leaf longevity and other leaf functional traits, and its effects could be mediated by soil N supply. As molecular biological studies have shown that leaf senescence is programmed by cell death and is genetically controlled (Hensel et al., 1993; Jiang et al., 1993; Chandlee, 2001; Buchanan-Wollaston et al., 2003; Lambers et al., 2008), further work, either genetically, physiologically or ecologically, is required to determine in more detail the mechanism of variations in plant functional traits.
ACKNOWLEDGEMENTS
We thank Xiaoyong Cui and Guohong Wang for helpful suggestions regarding the experiment; Zhenqing Li, Haishan Niu and Jianxiong Huang for statistical assistance; Wenming Bai and Osvaldo Sala for constructive comments on a previous version of the manuscript; and Bill Shipley and two anonymous referees for their valuable comments on previous versions of the manuscript. This research was financially supported by the National Basic Research Program of China (973 Program) (2007CB106801) and the National Natural Science Foundation of China (30821062). We also thank the Duolun Restoration Ecology Research Station for access to the study site and technical assistance.
APPENDIX
Results of stepwise multiple regression analysis between leaf longevity and other leaf functional traits in five steppe species
| Species | Regression equations | Step no. | Variable entered | R2 | Change in R2 (%) | P value |
|---|---|---|---|---|---|---|
| 2007 | ||||||
| S. krylovii | LL = –3·39Pnet + 70·89 | 1 | Pnet | 0·805 | 80·5 | <0·001 |
| A. cristatum | LL = –1·11LNC – 3·12Pnet + 103·65gs + 86·69 | 1 | LNC | 0·779 | 77·9 | <0·001 |
| 2 | Pnet | 0·883 | 10·4 | <0·001 | ||
| 3 | gs | 0·943 | 6·0 | <0·001 | ||
| C. squarrosa | LL = –0·49LNC + 43·67 | 1 | LNC | 0·790 | 79·0 | <0·001 |
| P. tanacetifolia | LL = –1·84SPAD + 121·27 | 1 | SPAD | 0·713 | 71·3 | <0·001 |
| M. ruthenica | LL = –2·63SPAD + 191·18 | 1 | SPAD | 0·830 | 83·0 | <0·001 |
| 2008 | ||||||
| S. krylovii | LL = –3·43Pnet + 109·27 | 1 | Pnet | 0·683 | 68·3 | <0·001 |
| A. cristatum | LL = 1·64C/N – 24·84gs + 0·19DMC – 54·59 | 1 | C/N | 0·722 | 72·2 | <0·001 |
| 2 | gs | 0·913 | 19·1 | <0·001 | ||
| 3 | DMC | 0·955 | 4·2 | <0·001 | ||
| C. squarrosa | LL = –0·74LNC + 0·06DMC + 28·58 | 1 | LNC | 0·855 | 85·5 | <0·001 |
| 2 | DMC | 0·927 | 7·2 | <0·001 | ||
| P. tanacetifolia | LL = –1·76SPAD + 0·15DMC + 73·84 | 1 | SPAD | 0·701 | 70·1 | <0·001 |
| 2 | DMC | 0·835 | 13·4 | <0·001 | ||
| M. ruthenica | LL = 7·08δ13C – 1·19SPAD + 314·61 | 1 | δ13C | 0·564 | 56·4 | <0·01 |
| 2 | SPAD | 0·735 | 17·1 | <0·01 |
LL, leaf longevity; Pnet, net photosynthetic rate based on area; gs, stomatal conductance; LNC, leaf nitrogen content; SPAD, estimated leaf chlorophyll concentration; C/N ratio, ratio of leaf carbon to nitrogen contents; DMC, leaf dry matter content; δ13C, leaf carbon isotopic composition.
LITERATURE CITED
- Abrahamson WG. Leaf traits and leaf life spans of two xeric-adapted palmettos. American Journal of Botany. 2007;94:1297–1308. doi: 10.3732/ajb.94.8.1297. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, et al. The molecular analysis of leaf senescence–a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Casper BB, Forseth IN, Kempenich H, Seltzer S, Xavier K. Drought prolongs leaf life span in the herbaceous desert perennial Cryptantha flava. Functional Ecology. 2001;15:740–747. [Google Scholar]
- Chabot BF, Hicks DJ. The ecology of leaf life spans. Annual Review of Ecology and Systematics. 1982;13:229–259. [Google Scholar]
- Chandlee JM. Current molecular understanding of the genetically programmed process of leaf senescence. Physiologia Plantarum. 2001;113:1–8. [Google Scholar]
- Chapin FS. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics. 1980;11:233–260. [Google Scholar]
- Chapin FS, Matson PA, Mooney HA. Principles of terrestrial ecosystem ecology. New York: Springer; 2002. [Google Scholar]
- Chapin FS, III, Van Cleve K. Approaches to studying nutrient uptake, use and loss in plants. In: Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW, editors. Plant physiological ecology: field methods and instrumentation. New York: Chapman and Hall; 1989. pp. 185–207. [Google Scholar]
- Cholaw B, Cubasch U, Lin YH, Ji LR. The change of North China climate in transient simulations using the IPCCSRES A2 and B2 scenarios with a coupled atmosphere–ocean general circulation model. Advances in Atmospheric Sciences. 2003;20:755–766. [Google Scholar]
- Christensen L, Coughenour MB, Ellis JE, Chen ZZ. Vulnerability of the Asian typical steppe to grazing and climate change. Climatic Change. 2004;63:351–368. [Google Scholar]
- Cordell S, Goldstein G, Meinzer FC, Vitousek PM. Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia. 2001;127:198–206. doi: 10.1007/s004420000588. [DOI] [PubMed] [Google Scholar]
- Craine JM, Reich PB. Elevated CO2 and nitrogen supply alter leaf longevity of grassland species. New Phytologist. 2001;150:397–403. [Google Scholar]
- Craine JM, Berin DM, Reich PB, Tilman GD, Knops JMH. Measurement of leaf longevity of 14 species of grasses and forbs using a novel approach. New Phytologist. 1999;142:475–481. [Google Scholar]
- Cui X, Huang G, Chen W, Morse A. Threatening of climate change on water resources and supply: case study of North China. Desalination. 2009;248:476–478. [Google Scholar]
- Dore MH. Climate change and changes in global precipitation patterns: what do we know? Environment International. 2005;31:1167–1181. doi: 10.1016/j.envint.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Eckstein RL, Karlsson PS, Weih M. Leaf life span and nutrient resorption as determinants of plant nutrient conservation in temperate–arctic regions. New Phytologist. 1999;143:177–189. [Google Scholar]
- Escudero A, Mediavilla S, Heilmeier H. Leaf longevity and drought: avoidance of the costs and risks of early leaf abscission as inferred from the leaf carbon isotopic composition. Functional Plant Biology. 2008;35:705–713. doi: 10.1071/FP08037. [DOI] [PubMed] [Google Scholar]
- Falster DS, Warton DI, Wright IJ. SMATR: standardised major axis tests and routines. 2006 http://www.bio.mq.edu.au/ecology/SMATR/ [Google Scholar]
- Field C, Mooney HA. Leaf age and seasonal effects on light, water, and nitrogen use efficiency in a California shrub. Oecologia. 1983;56:348–355. doi: 10.1007/BF00379711. [DOI] [PubMed] [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Hensel LL, Grbic V, Baumgarten DA, Bleecker AB. Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidoposis. Plant Cell. 1993;5:553–564. doi: 10.1105/tpc.5.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DU, Johnson L. Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry. 1999;46:247–293. [Google Scholar]
- Jiang CZ, Rodermel SR, Shibles RM. Photosynthesis, rubisco activity and amount, and their regulation by transcription in senescing soybean leaves. Plant Physiology. 1993;101:105–112. doi: 10.1104/pp.101.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Han XG, Zhang ZB, Sun OJ. Grassland ecosystems in China: review of current knowledge and research advancement. Philosophical Transactions of the Royal Society B-Biological Sciences. 2007;362:997–1008. doi: 10.1098/rstb.2007.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuzawa K. A cost–benefit-analysis of leaf habit and leaf longevity of trees and their geographical pattern. American Naturalist. 1991;138:1250–1263. [Google Scholar]
- Kikuzawa K. The basis for variation in leaf longevity of plants. Vegetatio. 1995;121:89–100. [Google Scholar]
- Kikuzawa K, Ackerly D. Significance of leaf longevity in plants. Plant Species Biology. 1999;14:39–45. [Google Scholar]
- Lajtha K, Whitford WG. The effect of water and nitrogen amendments on photosynthesis, leaf demography, and resource-use efficiency in Larrea tridentata, a desert evergreen shrub. Oecologia. 1989;80:341–348. doi: 10.1007/BF00379035. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapins FS, Pons TL. Plant physiological ecology. New York: Springer; 2008. [Google Scholar]
- Livingston NJ, Guy RD, Sun ZJ, Ethier GJ. The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell and Environment. 1999;22:281–289. [Google Scholar]
- Mooney HA, Gulmon SL. Constraints on leaf structure and function in reference to herbivory. Bioscience. 1982;32:198–206. [Google Scholar]
- Patrick L, Cable J, Potts D, et al. Effects of an increase in summer precipitation on leaf, soil, and ecosystem fluxes of CO2 and H2O in a sotol grassland in Big Bend National Park, Texas. Oecologia. 2007;151:704–718. doi: 10.1007/s00442-006-0621-y. [DOI] [PubMed] [Google Scholar]
- Patterson TB, Guy RD, Dang QL. Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia. 1997;110:160–168. doi: 10.1007/s004420050145. [DOI] [PubMed] [Google Scholar]
- Pons TL, Anten NPR. Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Functional Ecology. 2004;18:802–811. [Google Scholar]
- Poorter H, De Jong R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist. 1999;143:163–176. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecological Monographs. 1992;62:365–392. [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB, et al. Generality of leaf trait relationships: a test across six biomes. Ecology. 1999;80:1955–1969. [Google Scholar]
- Sandquist DR, Ehleringer JR. Intraspecific variation of drought adaptation in brittlebush: leaf pubescence and timing of leaf loss vary with rainfall. Oecologia. 1998;113:162–169. doi: 10.1007/s004420050364. [DOI] [PubMed] [Google Scholar]
- Saura-Mas S, Lloret F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Annals of Botany. 2007;99:545–554. doi: 10.1093/aob/mcl284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver GR. Mineral nutrition and leaf longevity in an evergreen shrub, Ledum palustre ssp. decumbens. Oecologia. 1981;49:362–365. doi: 10.1007/BF00347599. [DOI] [PubMed] [Google Scholar]
- Shaver GR. Mineral nutrition and leaf longevity in Ledum palustre: the role of individual nutrients and the timing of leaf mortality. Oecologia. 1983;56:160–165. doi: 10.1007/BF00379686. [DOI] [PubMed] [Google Scholar]
- Shipley B, Lechowicz MJ, Wright I, Reich PB. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology. 2006;87:535–541. doi: 10.1890/05-1051. [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytologist. 2005;167:493–508. doi: 10.1111/j.1469-8137.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Aber JD, Howarth RW, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications. 1997;7:737–750. [Google Scholar]
- Wright IJ, Westoby M, Reich PB. Convergence towards higher leaf mass per area in dry and nutrient-poor habitats has different consequences for leaf life span. Journal of Ecology. 2002;90:534–543. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Xia JY, Niu SL, Wan SQ. Response of ecosystem carbon exchange to warming and nitrogen addition during two hydrologically contrasting growing seasons in a temperate steppe. Global Change Biology. 2009;15:1544–1556. [Google Scholar]
- Xu WH, Wan SQ. Water- and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semiarid grassland in northern China. Soil Biology and Biochemistry. 2008;40:679–687. [Google Scholar]
- Xu ZW, Wan SQ, Zhu GL, Ren HY, Han XG. The influence of historical land use and water availability on grassland restoration. Restoration Ecology. 2010;18:217–225. [Google Scholar]