Abstract
Background and Aims
Functional trait differences and trait adjustment in response to influences of the biotic environment could reflect niche partitioning among species. In this study, we tested how variation in above-ground plant traits, chosen as indicators for light and nitrogen acquisition and use, differs among taxonomically closely related species (Poaceae) to assess their potential for niche segregation at increasing plant diversity.
Methods
Traits of 12 grass species were measured in experimental grasslands (Jena Experiment) of varying species richness (from 1 to 60) and presence of particular functional groups (grasses, legumes, tall herbs and small herbs).
Key Results
Grass species increased shoot and leaf length, investment into supporting tissue (stem mass fraction) and specific leaf area as well as reduced foliar δ13C values with increasing species richness, indicating higher efforts for light acquisition. These species-richness effects could in part be explained by a higher probability of legume presence in more diverse communities. Leaf nitrogen concentrations increased and biomas s : N ratios in shoots decreased when grasses grew with legumes, indicating an improved nitrogen nutrition. Foliar δ15N values of grasses decreased when growing with legumes suggesting the use of depleted legume-derived N, while decreasing δ15N values with increasing species richness indicated a shift in the uptake of different N sources. However, efforts to optimize light and nitrogen acquisition by plastic adjustment of traits in response to species richness and legume presence, varied significantly among grass species. It was possible to show further that trait adjustment of grass species increased niche segregation in more diverse plant communities but that complementarity through niche separation may differ between light and nutrient acquisition.
Conclusions
The results suggest that even among closely related species such as grasses different strategies are used to cope with neighbours. This lack in redundancy in turn may facilitate complementary resource use and coexistence.
Keywords: Biodiversity, grasses, functional traits, Jena Experiment, legumes, redundancy, species richness, trait variation
INTRODUCTION
Plant functional traits are morphological, physiological or life-history characteristics which directly or indirectly influence plant fitness via their effects on survival, growth and reproduction (Violle et al., 2007). The set of trait values of a species results from trade-offs among different functional requirements (Díaz and Cabido, 1997). Such a set of trait values may reflect strategies which species use to respond to their abiotic and biotic environment (Suding et al., 2003). Differences among species in such strategies may allow complementary resource use and thus local coexistence of a number of plant species in a small area (Silvertown, 2004). Consequently, functional differences among species which compete for the same major resources are a prerequisite for complementary strategies of resource acquisition and use (e.g. Tilman et al., 1997).
Complementary resource use among species can promote positive effects of increasing species richness on primary productivity of plant communities. This has been observed in a number of biodiversity experiments (Hooper et al., 2005; Balvanera et al., 2006). Capture of above- and below-ground resources have been shown to increase at increasing plant diversity (e.g. Spehn et al., 2005; Oelmann et al., 2007; Lorentzen et al., 2008). Maximization of growth demands for an optimal allocation of resources (Bloom et al., 1985; Hirose, 1987). Trait plasticity allows plant species to compensate for growth-limiting constraints and to cope with a wider range of environmental conditions (Schlichting, 1989). Functional traits are increasingly used to describe and group plant species according to their functions, to understand and to predict the assembly and stability of plant communities and ecosystem functioning, but so far, in particular, the effects of plant diversity on the expression of plant functional traits have not been studied in detail.
Multiple changes in the biotic and abiotic environment of a plant at increasing community diversity require a co-ordinated response in numerous traits to achieve a balance among different functions, which could finally affect niche partitioning among co-existing plant species. For instance, smaller plants growing in the canopy shade of taller neighbours, may adjust morphological traits to avoid or tolerate low light availability through the formation of longer and thinner leaves or an increase in stem length (Valladares and Niinemets, 2008). Variation in leaf nitrogen content per unit leaf mass, which positively correlates with rates of light-saturated photosynthesis, may serve as a physiological adjustment to improve photosynthetic carbon gain (Anten and Hirose, 2003).
Foliar isotopic signatures can be used as integrative measures providing information about plant species interactions or environmental changes. Foliar N isotope ratios (δ15N) of non-legume species may indicate shifts in the uptake of different N sources at increasing competition for soil nitrogen or the supply of depleted legume-derived N in communities with legumes (Högberg, 1997; Craine et al., 2009). Declining foliar carbon isotope ratios (δ13C) may indicate higher photosynthetic activity or stomatal conductance (Farquhar et al., 1989; Dawson et al., 2002) if δ13C of source CO2 remains stable (Buchmann et al., 2002).
In the present study, 12 grass species were used as a model to study functional traits of above-ground plant organs related to light and nitrogen acquisition and use in a long-term biodiversity experiment based on a pool of 60 temperate grassland species divided into four functional groups: grasses, legumes, small herbs and tall herbs (Jena Experiment; Roscher et al., 2004). Grasses represent a group of taxonomically and phylogenetically closely related species in grasslands and are often regarded as a single plant functional group. At the same time, they usually form the matrix in grassland vegetation and dominate biomass abundance distributions. Thus, the question arises how different species within this functional group may coexist if they are so closely related and thus prone to compete strongly for the same resources. One possibility is that these species have evolved different strategies that allow them to occupy different resource niches (Silvertown et al., 2001). However, it is also possible that these grass species do not differ in their resource niches, but that neutral processes such a demographic stochasticity or dispersal allow for their coexistence (Hubbell, 2001). To assess whether niche differentiation contributes to explain the coexistence of grass species in plant communities of increasing diversity, the following were tested: (a) whether functional traits of grass species indicate different strategies for light and nitrogen acquisition and use; (b) how these grass species adjust their traits by plasticity in response to increasing species richness and presence of other plant functional groups; and (c) whether the strategies found in (a) and (b) will allow species to avoid competition by increased niche segregation at increasing plant diversity.
MATERIAL AND METHODS
Field site and experimental design
The Jena Experiment was established in spring 2002 on former highly fertilized agricultural land. The field site is located in the floodplain of the River Saale close to Jena (Thuringia, Germany; 50°55'N, 11°35'E, 130 m a.s.l.). The soil is an Eutric Fluvisol developed from up to 2-m-thick fluvial sediments. Soil texture ranges from sandy loam near the river to silty clay with increasing distance from the riverside. The region around Jena is characterized by a mean annual air temperature of 9·3 °C and a mean annual precipitation of 587 mm (Kluge and Müller-Westermeier, 2000).
The experimental species pool consisted of 60 grassland species (central European mesophilic grasslands of the Molinio-Arrhenatheretea type; Ellenberg, 1988) which typically occur in meadows of the study region. Based on a cluster analysis of morphological, phenological and physiological traits compiled from literature (Roscher et al., 2004), species were assigned to four plant functional groups: 16 grasses, 12 small herbs, 20 tall herbs and 12 legumes. In total, 82 large plots, 20 × 20 m in size, were established crossing the experimental factors species richness (1, 2, 4, 8, 16 and 60) and functional group composition (grass × small herb × tall herb × legume presence) in a near-orthogonal design. Additionally, two monoculture plots per species, 3·5 × 3·5 m in size, were sown. At each richness level, different species compositions were determined by random draws from the species pool with replacement, thus allowing partial overlap of species compositions.
Species were sown with an initial density of 1000 viable seeds per square metre equally distributed among species in mixtures. The experimental site was divided into four blocks parallel to the river to account for the gradient in soil characteristics, and each block contained an equal number of plots per richness level (for further details, see Roscher et al., 2004). All plots were regularly weeded. Plots were mown twice a year in early June and September to mimic the traditional management of extensive hay meadows in the region. No fertilizer was added during the course of the experiment.
Data collection
Twelve grass species were chosen for the current study and investigated in all large plots in which they were present (totalling 37 out of the 82 large plots) and in two small monocultures per species (see Table 1 for an overview of species occurrences). Four years after sowing, the experiment data were recorded in two campaigns at estimated peak biomass shortly before mowing in May and August 2006. At this time plant communities were adequately developed to exclude establishment effects.
Table 1.
Actual number of plots per species-richness level without/with legumes in which the studied grass species were sampled
| Species richness, and total number of plots |
||||||
|---|---|---|---|---|---|---|
| 1, 24/0 | 2, 7/0 | 4, 4/5 | 8, 4/5 | 16, 4/6 | 60, 0/3 | |
| Alopecurus pratensis | 2/0 (2/0) | 1/0 (1/0) | 1/0 (1/0) | 2/0 (2/0) | 3/2 (3/2) | 0/3 (0/3) |
| Anthoxanthum odoratum | 2/0 (2/0) | 0/0 (0/0) | 1/1 (1/1) | 1/0 (1/1) | 3/1 (3/3) | 0/2 (0/3) |
| Arrhenatherum elatius | 2/0 (2/0) | 0/0 (0/0) | 1/1 (1/1) | 1/0 (1/0) | 3/1 (3/1) | 0/3 (0/3) |
| Avenula pubescens | 2/0 (2/0) | 0/0 (0/0) | 0/0 (0/0) | 1/0 (1/0) | 2/2 (2/2) | 0/3 (0/3) |
| Bromus erectus | 2/0 (2/0) | 0/0 (0/0) | 2/0 (2/0) | 2/1 (2/1) | 2/1 (2/1) | 0/2 (0/3) |
| Dactylis glomerata | 2/0 (2/0) | 1/0 (1/0) | 0/0 (0/0) | 1/0 (1/0) | 1/0 (1/0) | 0/3 (0/3) |
| Festuca pratensis | 2/0 (2/0) | 2/0 (2/0) | 1/1 (1/1) | 1/0 (1/0) | 2/2 (2/2) | 0/3 (0/3) |
| Festuca rubra | 2/0 (2/0) | 1/0 (1/0) | 0/0 (0/0) | 3/0 (3/0) | 1/1 (1/1) | 0/3 (0/3) |
| Phleum pratense | 2/0 (2/0) | 0/0 (0/0) | 0/1 (0/1) | 1/3 (1/3) | 3/2 (3/2) | 0/3 (0/3) |
| Poa pratensis | 2/0 (2/0) | 1/0 (1/0) | 0/0 (0/0) | 0/0 (0/0) | 4/2 (4/2) | 0/3 (0/3) |
| Poa trivialis | 2/0 (2/0) | 0/0 (0/0) | 2/0 (2/0) | 1/2 (1/2) | 2/2 (2/2) | 0/3 (0/3) |
| Trisetum flavescens | 2/0 (2/0) | 2/0 (2/0) | 0/1 (0/1) | 1/2 (1/2) | 2/1 (2/1) | 0/3 (0/3) |
In total, all grass species were grown in two replicated monocultures per species and occurred in 37 experimental mixtures.
Values in parentheses indicate the original number of plots where the seed mixtures contained the respective grass species and differ from the number of sampled plots when species abundance was too low for sampling.
Modules, i.e. single shoots (Harper and White, 1974), were the basic unit for all measurements because the ability of some grass species to grow with below-ground runners does not allow for the identification of plant individuals (genets) of these species in dense vegetation. Five to seven modules per species and plot were randomly selected along a line transect divided into fixed sections, excluding the outer 50 cm from the plot margin. To avoid sampling from a single genet twice, the minimum distance between sampled modules was 1 m. In the field, module height and canopy height of the surrounding vegetation within a radius of 20 cm were measured. Canopy height was used to calculate relative module height (module height divided by canopy height) and served as a surrogate for the experienced light regime.
Modules were cut off at ground-level, put in sealed plastic bags to prevent dehydration and transported in a cool box into the laboratory. In the laboratory, stem length and length of three internodes in the upper shoot part were measured. A leaf area meter (LI-3000A portable area meter; Li-COR, Lincoln, NE, USA) was used to determine the length and area of three or four fully developed leaf blades per module. Plant material was separated into leaf blades (later referred to as leaves), supporting tissue (leaf sheaths and stems; later referred to as stems), inflorescences and dead tissue, dried for 48 h at 70 °C and weighed. For subsequent chemical analyses, the samples of each species were pooled per plot and ground to a fine powder. Nitrogen concentrations as well as nitrogen and carbon isotope ratios (δ15N and δ13C, respectively) were determined from the measured leaf blades with an isotope-ratio mass spectrometer (Finnegan MAT Deltaplus XP, Bremen, Germany). The residual plant material was analysed for nitrogen concentrations with an elemental analyser (Flash EA 1112; Thermo Italy, Rhodano, Italy) to assess total nitrogen concentrations per module. Traits derived from field and laboratory measurements are summarized in Table 2.
Table 2.
Summary of functional traits investigated in this study
| Functional trait | Unit | Abbreviation | Description |
|---|---|---|---|
| Species positioning within the canopy | |||
| Relative height | cm cm−1 | Growth height divided by canopy height of the surrounding vegetation | |
| Light acquisition | |||
| Stem mass fraction | mgstem mg−1module | SMF | Stem dry mass (including leaf sheats) per module dry mass |
| Shoot length | cm | l.shoot | Stretched module length measured in the laboratory |
| Internode length | cm | l.int | Length of the longest internode per module |
| Leaf length | cm | l.leaf | Maximum leaf length |
| Specific leaf area | mm2leaf mg−1leaf | SLA | Leaf area per leaf dry mass |
| Nitrogen acquisition and use | |||
| Leaf nitrogen concentration | mg N g−1leaf | N.leaf | Nitrogen mass per leaf dry mass |
| Module biomas s : N ratio | gdw (g N−1) | Biom : N | Unit nitrogen per unit module biomass |
| Foliar isotopic signatures | |||
| Foliar δ15N | ‰ | δ15N | 15N isotopic signature of leaves |
| Foliar δ13C | ‰ | δ13C | 13C isotopic signature of leaves |
Data analyses
Trait values were averaged per species and plot for each harvest campaign. Because most shoots of grass species reached the reproductive stage before first mowing and grasses usually do not develop inflorescences after the first cut later in the season, seasonal differences equally refer to trait differences between reproductive and vegetative shoots in this study.
Because individual grass species were randomly assigned to mixtures, not all possible functional group combinations occurred for each species. To account for this design imbalance (see Table 1), data were analysed with mixed-effects models using the lme function in the package nlme of the statistical software R2·11·1 (R Development Core Team; http://www.R-project.org). Starting from a constant null model with block and plot within block as random factors, fixed effects were entered step-wise into the analyses. First, species richness (SR; log-linear term) and contrasts for the presence/absence of each particular plant functional group – legumes (LEG), small herbs (SHERB) and tall herbs (THERB) – were fitted in series of separate models. A term for grass species identity (ID) and its interaction terms with the previous terms (ID × SR and ID × LEG, ID × SHERB or ID × THERB) were then added to the models. Finally, terms were added for season (May vs. August harvest campaign) and interactions between season and the previously fitted terms. The maximum likelihood method and likelihood ratio tests (L ratio) were applied to assess the statistical significance of model improvement. To disentangle the effects of species richness and the presence/absence of plant functional groups, which do not vary completely independently, alternative models entering species richness after a term for the presence/absence of particular functional groups were fitted. If necessary, data were transformed to meet the assumptions of normality and homogeneity of variances. Variation in multiple traits of grass species was explored for reproductive (May) and vegetative (August) modules separately with standardized principal components analyses (PCA) using CANOCO 4·5 (ter Braak and Šmilauer, 2002). To assess the effects of species richness and the presence/absence of particular functional groups on trait ‘syndromes’ of grasses, the principle component scores on the two leading axes were analysed with mixed-effects models as described above.
To assess whether variance in trait values among different grass species is higher in diverse plant communities, separate models were fitted with the lmer function in the package lme4 of the statistical software R. Trait data recorded in monocultures and 60-species mixtures were used for these analyses, because all the grass species studied are equally represented at these levels of species richness (Table 1). Here, grass species identity was treated as random factor and the restricted maximum likelihood method was applied to estimate variance components. Niche separation among grass species was assessed from between-species variance components (square root of variance explained with the species identity term), and average niche width was estimated from within-species variance components (square root of residual variance) (Dimitrakopolous and Schmid, 2004). To assess whether trait adjustment of grass species led to increased niche segregation among grass species in diverse plant communities, convex hull volumes of trait space occupied by species in monocultures and in 60-species mixtures were computed using the FD package of the statistical software R (Laliberté and Shipley, 2010). The convex hull is defined as the minimum convex set enclosing species positioning in multiple-trait space (Barber et al., 1996; Cornwell et al., 2006).
RESULTS
All measured traits differed significantly among grass species (Table 3). Plant diversity in terms of species richness had significant effects on values of most traits, although the magnitude of trait plasticity in response to increasing species richness varied among grass species (significant interaction ‘ID × SR’; Table 3 and Fig. 1). Legume presence, fitted after species richness mostly explained a significant proportion of residual variation in trait values, while the presence of tall herbs or small herbs rarely had additional effects on trait values of grass species (Table 3). Species richness fitted after legume presence often did not remain statistically significant, indicating that species richness effects were indirectly mediated through a high responsiveness of grasses to the presence of legumes which was more likely in experimental communities with higher species richness (Table 3).
Table 3.
Summary of mixed-effects model analyses for functional traits combining all grass species
| Relative height |
Stem mass fraction |
Shoot length |
Specific leaf area |
Leaf length |
Internode length |
Leaf nitrogen |
Biomas s : N ratio |
Foliar δ15N |
Foliar δ13C |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | L ratio | P | |
| (A) Species richness prior to functional group composition | ||||||||||||||||||||
| SR | 5·15 | * ↓ | 4·77 | * ↑ | 5·98 | * ↑ | 6·93 | ** ↑ | 3·55 | 0·91 | 0·62 | 2·99 | . | 29·62 | *** ↓ | 28·39 | *** ↓ | |||
| LEG | 7·77 | ** ↓ | 4·90 | * ↑ | 6·83 | ** ↑ | 26·44 | *** ↑ | 9·89 | ** ↑ | 0·57 | 6·83 | ** ↑ | 6·92 | ** ↓ | 0·17 | 5·19 | * ↓ | ||
| SHERB | 0·13 | 0·05 | 0·02 | 0·08 | 0·10 | 1·95 | 1·02 | 0·05 | 0·79 | 2·13 | ||||||||||
| THERB | 0·31 | 0·99 | 0·25 | 1·39 | 2·56 | 1·23 | 0·13 | <0·01 | 0·34 | 3·59 | ||||||||||
| ID | 80·37 | *** | 209·39 | *** | 252·62 | *** | 165·00 | *** | 204·82 | *** | 134·65 | *** | 37·87 | *** | 29·08 | ** | 27·02 | ** | 155·67 | *** |
| ID × SR | 25·46 | ** | 22·36 | * | 26·72 | * | 20·65 | * | 35·16 | *** | 32·95 | *** | 15·75 | 28·71 | ** | 20·66 | * | 55·66 | *** | |
| ID × LEG | 8·63 | 12·03 | 17·19 | 21·09 | * | 28·86 | *** | 26·43 | ** | 27·39 | ** | 25·11 | ** | 40·92 | *** | 30·51 | ** | |||
| ID × SHERB | 21·87 | * | 13·52 | 14·06 | 13·63 | 15·88 | 32·63 | *** | 15·56 | 21·83 | ** | 7·91 | 11·54 | |||||||
| ID × THERB | 11·86 | 10·84 | 8·72 | 12·83 | 13·98 | 11·98 | 8·79 | 13·62 | 15·53 | 55·27 | *** | |||||||||
| Season | 35·84 | ***M | 409·96 | ***M | 342·48 | ***M | 45·74 | ***A | 73·71 | ***A | – | 44·38 | ***A | 296·46 | ***M | 4·55 | *M | 68·42 | ***A | |
| Season × SR | 1·66 | 0·46 | 1·73 | 0·13 | 3·24 | – | 1·68 | 12·15 | *** | 3·59 | 18·04 | *** | ||||||||
| Season × LEG | 0·57 | 2·09 | 3·48 | 3·00 | 0·02 | – | 9·29 | ** | 0·13 | 1·37 | 11·50 | *** | ||||||||
| Season × SHERB | 6·31 | * | 1·24 | 0·11 | 0·05 | 0·24 | – | <0·01 | 0·71 | 0·23 | <0·01 | |||||||||
| Season × THERB | 0·35 | 0·42 | 0·23 | 6·68 | ** | 0·06 | – | 0·89 | 0·05 | 0·63 | 1·43 | |||||||||
| Season × ID | 30·58 | ** | 213·74 | *** | 117·79 | *** | 54·49 | *** | 172·39 | *** | – | 55·09 | *** | 53·77 | *** | 22·51 | * | 90·21 | *** | |
| (B) Legume presence prior to species richness | ||||||||||||||||||||
| LEG | 12·78 | *** ↓ | 9·49 | ** ↑ | 11·65 | *** ↑ | 32·91 | *** ↑ | 13·46 | *** ↑ | 1·23 | 6·45 | * | 9·89 | ** ↓ | 11·68 | *** ↓ | 21·74 | *** ↓ | |
| SR | 0·14 | 0·18 | 1·16 | 0·46 | 0·01 | 0·25 | 1·01 | 0·02 | 18·11 | *** ↓ | 11·84 | *** ↓ | ||||||||
| ID | 80·50 | *** | 212·25 | *** | 261·99 | *** | 151·13 | *** | 212·06 | *** | 139·39 | *** | 39·03 | *** | 28·30 | ** | 27·03 | ** | 157·39 | *** |
| ID × LEG | 18·12 | 19·69 | * | 33·26 | *** | 24·72 | ** | 53·21 | *** | 27·77 | ** | 13·30 | 16·78 | 49·04 | *** | 66·81 | *** | |||
| ID × SR | 17·95 | 16·26 | 11·65 | 20·78 | * | 12·02 | 33·12 | *** | 32·43 | *** | 38·50 | *** | 12·44 | 20·54 | * | |||||
| Season | 35·94 | *** | 409·79 | *** | 341·79 | *** | 45·79 | *** | 75·80 | *** | – | 44·01 | *** | 303·50 | *** | 4·29 | * | 73·07 | *** | |
| Season × LEG | 1·88 | 2·30 | 5·30 | * | 1·10 | 1·52 | – | 2·06 | 5·86 | * | 0·14 | 28·28 | *** | |||||||
| Season × SR | 0·25 | 0·18 | 0·01 | 2·11 | 1·69 | – | 8·93 | ** | 5·76 | * | 4·96 | * | 1·84 | |||||||
| Season × ID | 31·65 | *** | 214·81 | *** | 131·23 | *** | 54·26 | *** | 182·04 | *** | – | 51·25 | *** | 55·15 | *** | 21·37 | * | 81·44 | *** | |
Models were fitted by stepwise inclusion of the fixed effects. Likelihood ratios (L ratio) were used to assess model improvement and levels of significance are given (* P ≤ 0·050; ** P < 0·010;*** P < 0·001). For models in series (A) the species richness term was fitted prior to contrasts for the presence/absence of a particular plant functional group, while in model series (B) a contrast for the presence/absence of legumes was fitted before species richness.
Note that in series (A) contrasts for each plant functional group were tested in series of alternative models. Arrows indicate a significant increase (↑) or decrease (↓) of trait values in response to increasing species richness or the presence of a particular plant functional group.
(M) for May and (A) for August after the significance level for the term of season indicate that trait values were larger at the respective harvest date.
Abbreviations: SR = species richness (log-linear), LEG = legume presence/absence, THERB = tall herb presence/absence, SHERB = small herb presence/absence, ID = grass species identity.
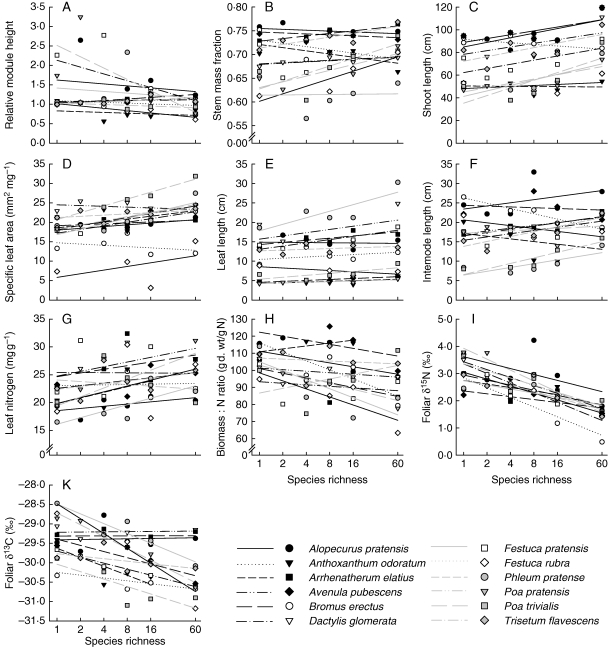
Fig. 1.
Trait values of grasses measured on reproductive modules at estimated peak biomass before first mowing (May 2006) as a function of sown species richness. Regression lines were fitted through mean trait values per species-richness level.
Surrounding canopy height and grass positioning within the canopy
Canopy height of the experimental grasslands increased with increasing species richness (L = 6·95, P = 0·008) and was higher in communities with legumes (L = 6·43, P = 0·011). Relative height of grasses, i.e. species positioning within the canopy, decreased with increasing species richness (Fig. 1A), but this effect was exclusively due to the inclusion of legumes (Fig. 2A and Table 3). Grasses differed largely in their relative height. Early in the season, before first mowing, three grass species were taller than the surrounding vegetation (A. pratensis, A. elatius and D. glomerata; one-sample t-test, P < 0·050), two species were shorter (A. odoratum and F. rubra) and the other species did not differ from the surrounding vegetation in height. Late in the season, before the second mowing, nine grass species reached the average canopy height, whereas three (F. rubra, P. pratensis and P. trivialis) did not.
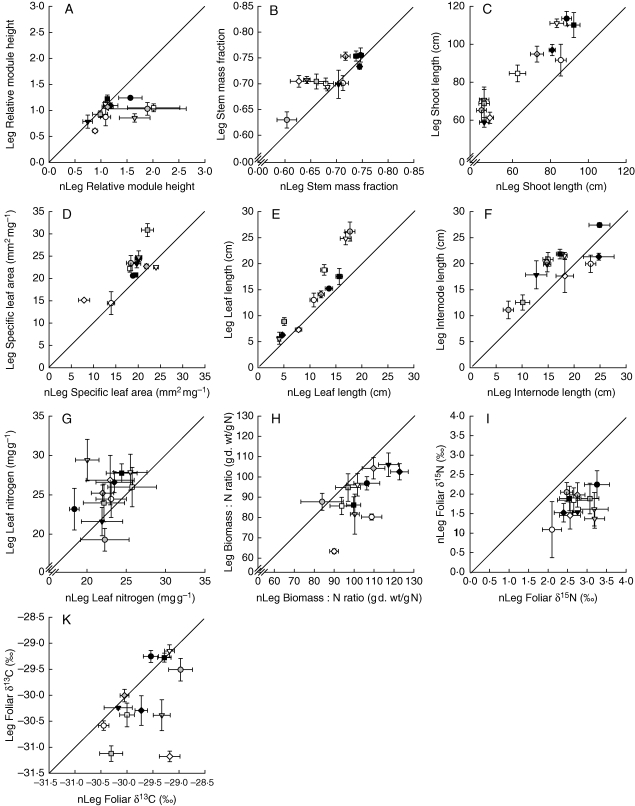
Fig. 2.
Trait values per species averaged across all communities with legumes (Leg; mean ± s.e.) plotted against average trait values across all communities without legumes (nLeg; mean ± s.e.;) measured on reproductive modules at estimated peak biomass before first mowing (May 2006). Positions above the line indicate that species trait values in communities with legumes were larger than in communities without legumes. For species symbols see Fig. 1.
Traits associated with light acquisition
On average, grass species formed longer shoots, invested a larger proportion of biomass in supporting tissue (higher stem mass fraction) and had leaves with a higher specific leaf area (SLA) in plant communities of increasing species richness (Fig. 1B–D), which was mainly attributable to the inclusion of legumes in the experimental communities (Fig. 2B–D and Table 3). In addition, grasses had generally longer leaves in communities with legumes (Figs 1E and 2E). The formation of longer internodes (Fig. 1F and 2F) as well as the amount of plasticity in the previously mentioned traits in response to increasing species richness and legume presence depended on grass species identity (Table 3).
Traits associated with nitrogen acquisition and use
Leaf nitrogen concentrations were higher and biomas s : N ratios in shoots of grasses were lower in plant communities with legumes, while species richness on average did not affect N concentrations in grasses (Figs 1G, H and 2G, H, and Table 3). However, irrespective of legume presence, increasing species richness explained residual variation in biomas s : N ratios dependent on grass species identity (interaction ‘ID × SR’ significant if fitted after legume presence; Table 3).
Foliar isotopic signatures
Foliar δ15N values decreased with increasing species richness, while effects of legumes on 15N/14N isotopic ratios were not significant when fitted after species richness (Table 3 and Figs 1I and 2I). However, foliar δ15N values and the effects of increasing species richness and legume presence differed largely among grass species (Table 3). On average, foliar δ15N values were not related to leaf N concentrations (L = 1·57, P = 0·211).
Both increasing species richness and legume presence resulted in a decline of foliar δ13C values of grass species, but variation in foliar δ13C values in response to plant diversity depended on grass species identity (Table 3 and Figs 1K and 2K). Neither SLA (L = 2·57, P = 0·109), foliar N concentrations (L = 0·50, P = 0·481) nor species positioning within the canopy (L = 2·73, P = 0·099) explained variation in foliar δ13C values, but the relationships among these variables depended largely on species identity. Variation in foliar δ13C values was best explained by SLA in five grass species (A. odoratum, A. pubescens, F. pratensis, Ph. pratense and T. flavescens), by plant positioning within the canopy in three grass species [A. elatius (late season only), F. rubra and P. trivialis], by leaf N concentrations in two grass species [A. pratensis (early season only), D. glomerata (late season only)] and did not correlate with these variables in B. erectus.
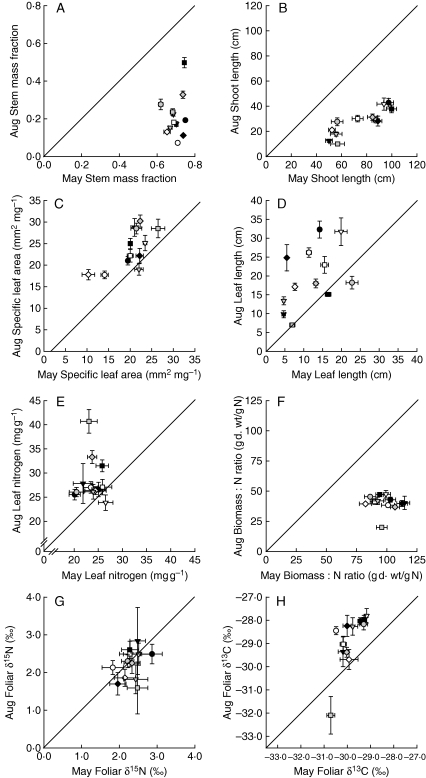
Seasonal differences in trait values
Grasses differed in morphological characteristics between early and late seasons. These differences correlated with differences between reproductive modules (before first mowing) and newly developed vegetative modules (before second mowing). Reproductive modules of grass species before first mowing were generally taller, had a larger stem mass fraction, lower SLA and shorter leaves than vegetative modules before the second mowing (Fig. 3 and Table 3). After regrowth before the second mowing, grasses had larger leaf nitrogen concentrations and their biomas s : N ratio was reduced. Foliar δ15N values were slightly larger in reproductive modules before first mowing, while foliar δ13C values were higher in vegetative modules before the second mowing. Seasonal differences in trait values were largely species-dependent (highly significant interaction ‘ID × Season’ in all traits), indicating different resource acquisition strategies in the reproductive and vegetative stages of different grass species. However, plant diversity effects on trait values of grasses were largely independent of season, except for traits reflecting nitrogen acquisition and use (leaf nitrogen concentrations, shoot biomas s : N ratios) and for foliar δ15N and δ13C values.
Fig. 3.
Trait values per species averaged across all plots in late summer before second mowing in August 2006 (Aug; mean ± s.e.) plotted against trait values averaged across all plots before first mowing in May 2006 (May; mean ± s.e.). Positions above the line indicate that species trait values in August were larger than in May. For species symbols see Fig. 1.
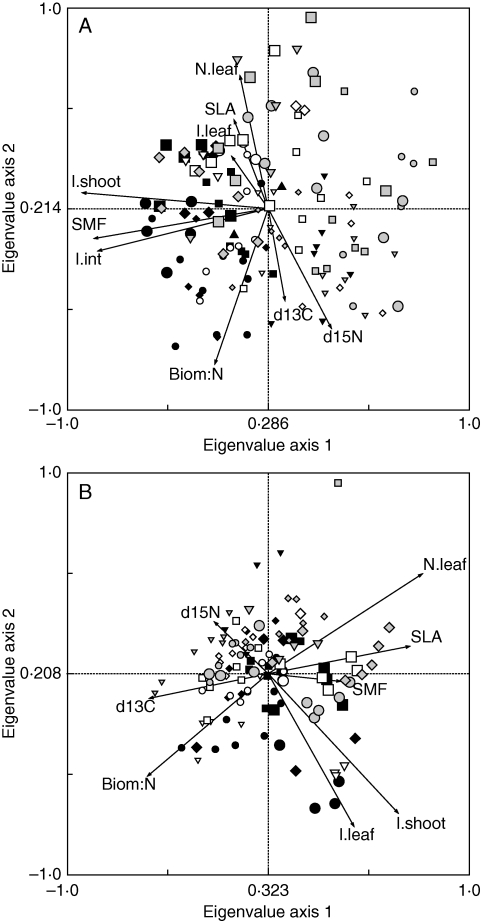
Trait combinations reflecting grass species strategies
The first and second axes of standardized PCA of trait values of grasses explained >50 % of the trait variation of reproductive shoots (before first mowing in May) and of vegetative shoots (before second mowing in August). The first axis in the analysis of reproductive shoots (Fig. 4A) correlated with traits characterizing height growth, i.e. shoot length, internode length and stem mass fraction, and separated taller grass species (A. pratensis, A. elatius, A. pubescens, D. glomerata and T. flavescens) from grass species with smaller reproductive shoots (A. odoratum, F. rubra, Ph. pratense, P. pratensis and P. trivialis). The second axis had high positive loadings for leaf nitrogen concentration and SLA and negative loadings for shoot biomas s : N ratio and foliar δ13C and δ15N values.
Fig. 4.
Standardized principal components analysis (PCA) for species trait values of reproductive modules at estimated peak biomass before first mowing (A), and species trait values of vegetative modules before second mowing (B) across all plots. For species symbols see Fig. 1; small symbols indicate grasses in communities without legumes, and large symbols indicate grasses in communities with legumes. Abbreviations of traits are explained in Table 2.
The first axis in the analysis of vegetative shoots (Fig. 4B) also suggested a differentiation of grass species with respect to light as well as to nitrogen acquisition and use. The axis correlated positively with leaf nitrogen concentrations, shoot length and SLA, and negatively with shoot biomas s : N ratios and foliar δ13C values. The second axis had high negative loadings for shoot and leaf length and positive loadings for stem mass fraction, leaf nitrogen concentration and foliar δ15N values.
For both analyses of reproductive and vegetative modules legume presence was the main experimental factor that explained species positioning on the first ordination axis (Table 4). Both species richness and legume presence explained species positioning at the second principal component in analysis of reproductive modules, while species positioning at the second ordination axis in analysis of vegetative modules was attributable to species-richness effects. Ordination axes indicated highly significant differences among species in their trait ‘syndroms’ and differential effects of increasing species richness or legume presence on trait values. For instance, some species such as A. pratensis, A. pubescens and D. glomerata increased their investment in height growth (longer shoots and leaves) in communities with legumes. Other species such as F. pratensis, F. rubra, Ph. pratense and P. trivialis increased their leaf nitrogen concentrations and SLA or the trait plasticity in response to legume presence differed between reproductive and vegetative shoots (e.g. T. flavescens).
Table 4.
Summary of mixed-effects model analyses of the principle component scores of the two leading PCA axes of analyses of multiple traits of reproductive modules (May) and vegetative modules (August) combining all grass species (see Fig. 4)
| May |
August |
|||||||
|---|---|---|---|---|---|---|---|---|
| Axis 1 |
Axis 2 |
Axis 1 |
Axis 2 |
|||||
| L ratio | P | L ratio | P | L ratio | P | L ratio | P | |
| Species richness prior to functional group composition | ||||||||
| SR | 5·06 | * | 27·86 | *** | 6·60 | * | 9·65 | ** |
| LEG | 4·39 | * | 7·58 | ** | 18·37 | *** | 0·08 | |
| SHERB | 0·65 | 0·55 | 0·10 | 0·06 | ||||
| THERB | 1·11 | 0·33 | 0·20 | 2·64 | ||||
| ID | 170·02 | *** | 34·91 | *** | 49·98 | *** | 93·38 | *** |
| ID × SR | 48·90 | *** | 19·56 | 11·87 | 20·40 | * | ||
| ID × LEG | 20·16 | * | 22·20 | * | 31·61 | *** | 14·33 | |
| ID × SHERB | 12·44 | 16·40 | 16·02 | 9·37 | ||||
| ID × THERB | 12·92 | 9·73 | 5·69 | 6·60 | ||||
| Legume presence prior to species richness | ||||||||
| LEG | 7·77 | ** | 25·72 | *** | 24·89 | *** | 3·93 | * |
| SR | 1·68 | 9·72 | ** | 0·08 | 5·79 | * | ||
| ID | 175·57 | *** | 32·57 | *** | 49·68 | *** | 93·65 | *** |
| ID × LEG | 37·16 | *** | 19·94 | * | 22·96 | *** | 19·64 | * |
| ID × SR | 31·68 | *** | 22·64 | * | 23·98 | ** | 14·81 | |
Likelihood ratios (L ratio) were used to assess model improvement and levels of significance are given (* P ≤ 0·050; ** P < 0·010; *** P < 0·001).
For an explanation of statistical analyses and abbreviations, see Table 3.
Niche segregation among grass species in monocultures and 60-species-mixtures
Between-species-variance components in shoot length, leaf length and foliar δ13C values were larger in 60-species mixtures than in monocultures, suggesting a larger niche separation among grass species (Table 5). Differences in between-species variance components between both species-richness levels in other traits varied between reproductive and vegetative modules. Between-species variance components in stem mass fraction and internode length of reproductive modules were smaller in 60-species mixtures than in monocultures indicating niche contraction. In contrast, between-species variance components in leaf nitrogen concentrations, foliar δ15N values and biomas s : N ratios of reproductive modules were larger in 60-species mixtures than in monocultures, while the opposite was observed for vegetative modules. Within-species variance components were on average larger in 60-species-mixtures than in monocultures (except for internode length and foliar δ15N values of reproductive modules) indicating an average larger niche width of grass species in the more diverse communities.
Table 5.
Niche separation among grass species and average niche width of grass species in monoculture and the 60-species mixture for traits of reproductive modules (May) and vegetative modules (August)
| Reproductive modules |
Vegetative modules |
|||
|---|---|---|---|---|
| Monoculture | 60-species mixture | Monoculture | 60-species mixture | |
| Niche separation (derived from between-species variance components) | ||||
| Stem mass fraction | 0·041 | 0·035 | 0·088 | 0·139 |
| Shoot length (cm) | 18·5 | 21·0 | 7·1 | 15·4 |
| Specific leaf area (mm2 mg−1) | 4·03 | 4·89 | 4·57 | 4·01 |
| Leaf length (cm) | 5·2 | 7·8 | 5·5 | 12·2 |
| Internode length (cm) | 4·5 | 3·1 | – | – |
| Leaf nitrogen concentration (mg g−1) | 1·59 | 2·92 | 6·90 | 2·96 |
| Biomas s : N ratio [gdw (g N−1)] | 0·0 | 10·9 | 8·4 | 4·5 |
| Foliar δ15N (‰) | 0·26 | 0·28 | 0·72 | 0·53 |
| Foliar δ13C (‰) | 0·50 | 0·67 | 0·46 | 1·60 |
| Niche width (derived from within-species variance components) | ||||
| Stem mass fraction | 0·026 | 0·028 | 0·031 | 0·056 |
| Shoot length (cm) | 5·3 | 6·5 | 3·0 | 4·2 |
| Specific leaf area (mm2 mg−1) | 0·97 | 2·53 | 2·66 | 3·41 |
| Leaf length (cm) | 1·2 | 2·0 | 4·1 | 4·9 |
| Internode length (cm) | 4·1 | 3·7 | – | – |
| Leaf nitrogen concentration (mg g−1) | 2·04 | 3·52 | 1·23 | 3·03 |
| Biomas s : N ratio [gdw (g N−1)] | 10·5 | 12·6 | 2·6 | 3·7 |
| Foliar δ15N (‰) | 0·69 | 0·26 | 0·55 | 0·63 |
| Foliar δ13C (‰) | 0·27 | 0·36 | 0·73 | 0·78 |
Mixed-effects models with species identity as random term were fitted separately for trait values of reproductive modules and vegetative modules in monocultures and 60-species mixtures. The restricted maximum likelihood method was applied to estimate between- and within-species variance components.
The occupied niche space of grass species, estimated as convex hull volume of trait space based on all possible nine trait axes, was higher in 60-species mxtures than in monocultures (reproductive modules in monocultures, 0·009; in 60-species mixtures, 0·064; vegetative modules in monocultures, 0·029; in 60-species mixtures, 0·048).
DISCUSSION
Plant traits have been the focus of many studies during the last decade to understand functional trade-offs, their role in resource acquisition, processing and use and their effects on community composition and ecosystem processes (e.g. Lavorel and Garnier, 2002; McGill et al., 2006). Functional traits of grass species and their relationships to environmental gradients such as climate (e.g. Oyarzabal et al., 2008; Albert et al., 2010), nutrient availability and management (e.g. Craine et al., 2001; Al Haj Khaled et al., 2005; Díaz et al., 2007; Maire et al., 2009; Pontes et al., 2010) have been investigated in several studies. Grass species are known to exhibit different strategies in response to nutrient supply or disturbance, being either fast-growing and maximizing resource exploitation (associated with high SLA, high leaf nitrogen concentrations and a short leaf lifespan) or slow-growing with a maximization of resource conservation (associated with low SLA, low leaf nitrogen concentrations and a long leaf lifespan) (van der Werf et al., 1993; Ryser and Urbas, 2000). Nitrogen and light are the most limiting factors for plant growth in temperate grasslands. The more complete use of these resources in plant communities of increasing diversity suggests an intensified competition among species. Thus, a niche separation through functional differences among plant species is pivotal to maintain large species richness in a small area. The present study focused on functional traits of above-ground plant organs known to be associated with light and nutrient acquisition and use in 12 grass species. It was shown that all investigated traits differed significantly among grass species, contrasting the widespread assumption of high redundancy within a seemingly homogenous plant functional group (Table 3). Large differences in the expression of different trait combinations (Fig. 4) suggested species-specific strategies of grass species to achieve equilibrium in carbon and nitrogen acquisition and use for growth. This implies that ecological differences among grass species allow for their coexistence and complementarity in temperate grasslands (Gross et al., 2007) and that usual classifications into functional groups are critical when examining the assembly of plant communities and ecosystem processes (Wright et al., 2005).
Plant diversity effects on traits associated with light acquisition
Increasing species richness is associated with a decrease in light supply through the development of a taller and denser canopy associated with a higher leaf area index (Spehn et al., 2005; Lorentzen et al., 2008). Closed canopies are characterized by pronounced gradients in spectral light quality and quantity (Jones, 1992). Grasses in the present study exhibited two strategies in adjustment to increasing competition for light: (1) shade avoidance reflected by shoot, internode and leaf elongations associated with a larger investment into supporting tissues; and (2) shade tolerance by increasing SLA. Phytochrome-mediated stem elongation is a typical response to shade triggered by changes in light quality with lower red : far red ratios (Smith, 1982). In the present study, plant diversity effects on morphological traits associated with light acquisition were mainly attributable to the presence of legumes, because species richness effects on variation in these traits mostly did not remain statistically significant when fitted after legume presence (Table 3). In the Jena Experiment the presence of legumes is associated with a higher community leaf area index and canopy density (Roscher et al., 2010), thus reducing light availability for co-occurring grass species, while canopy density within species richness-levels is more variable. Although the investigated grass mostly displayed an intermediate combination of shade tolerance and shade avoidance strategies (Henry and Aarssen, 1997), significant interaction terms of plant diversity with species identity and species separation along the leading axes of a PCA (Fig. 4) indicated species-specific responses. For instance, grass species with taller modules, e.g. A. pratensis, A. elatius, D. glomerata or A. pubescens, showed little variation in SLA in response to legume presence in contrast to smaller grass species which adjusted their shoot length or exhibited a larger plasticity in SLA (e.g. F. rubra, Ph. pratense and P. trivialis) (Figs 2 and 4).
In contrast, declining foliar δ13C values were associated with increasing species richness and legume presence (Figs 1 and 2). Although increasing foliar δ13C values were opposed to increasing SLA and leaf nitrogen concentrations in multiple-trait analyses (Fig. 4), a decreasing relative height and increasing SLA could best explain declining foliar δ13C values in separate analyses of most grass species. In contrast, no indication for positive relationships between leaf nitrogen concentrations and foliar δ13C values was found as expected if an improved nitrogen supply would increase the photosynthetic capacity of grass species (Evans, 1989). Thus, it is more likely that canopy characteristics determined variation in foliar δ13C values. Increasing air humidity and decreasing leaf temperatures in lower canopy layers could affect the ratio of intercellular to ambient CO2 concentrations (Ci : Ca) during assimilation via stomata aperture, thus leading to changes in the 13C : 12C ratio in bulk leaf material (Farquhar et al., 1989). In accordance to Jumpponen et al. (2005), grass species which mostly overtopped the surrounding vegetation, i.e. A. pratensis, A. elatius, D. glomerata, had near constant foliar δ13C values across the species-richness gradient, irrespective of legume presence/absence (Figs 1K and 2K). However, foliar δ13C values are also influenced by variation in δ13C of source CO2 within the canopy (Buchmann et al., 2002). Particularly in the lower layers of dense canopies, CO2 concentrations increase due to low turbulences and soil respiration and thus 13C : 12C ratios decrease (Buchmann and Ehleringer, 1998). In fact, CO2 enrichment in the lower layers of a 60-species mixture has been measured in the present experiment (unpubl. res.). Buchmann et al. (2002) showed that about 30 % of the vertical variations in foliar δ13C values within a canopy are typically due to source air effects, while 70 % of these foliar variations are due to plant ecophysiology. Therefore, the observed decrease in foliar δ13C values of grass species might be partly due to changes in the δ13C of source CO2 with increasing plant diversity, as a consequence of a higher canopy density (Lorentzen et al., 2008) and increased microbial activities (Eisenhauer et al., 2010).
Plant diversity effects on traits associated with nitrogen acquisition and use
Traits related to N acquisition and use, i.e. leaf nitrogen concentrations and shoot biomas s : N ratios, did not change with plant species richness but were strongly influenced by legume presence, suggesting positive effects of legumes on nitrogen nutrition of grass species (Table 3). In contrast, variation in foliar δ15N values was related to increasing species richness, while effects of legume presence on foliar δ15N values depended on grass species identity (Table 3 and Fig. 2I). Facilitation of non-legume species through N2-fixing legumes has been demonstrated in several biodiversity experiments (e.g. Tilman et al., 1997; Hille Ris Lambers et al., 2004; Temperton et al., 2007). Even though the variation in foliar δ15N was partly due to legume presence, the present results support the hypothesis that legumes also indirectly facilitated co-occurring grasses. Due to the additional atmospheric N source, more soil nitrogen might have been spared and thus been available for non-legume species in communities with legumes (Temperton et al., 2007). Further mechanisms might have also contributed to the observed decline in foliar δ15N with increasing species richness. Kahmen et al. (2008) showed that differences in foliar δ15N values among non-legumes can be due to the uptake of different N forms (NO3− and NH4+). Recent results by von Felten et al. (2009) suggested that niche breadth of single species with respect to chemical N forms decreased with increasing species richness. Thus, a shift in the uptake of different N forms was probably responsible for the variation of foliar δ15N values at increasing species richness, supported by reduced levels of soil-extractable nitrate and increasing competition for soil N in the Jena Experiment (Oelmann et al., 2007).
Plant diversity effects on niche segregation among grass species
The larger convex hull volumens of multiple-trait space occupied by the investigated grass species in 60-species mixtures compared with monocultures suggested that trait adjustment of grass species increased niche segregation in more diverse plant communities. The separate inspection of traits showed that niche separation among grass species was dependent on the considered trait (Table 5). For instance, higher between-species-variance components in shoot length, leaf length, foliar δ13C values and SLA (reproductive modules only) in 60-species-mixtures suggested niche separation among grass species in light acquisition. While niche separation among grass species in traits associated with nitrogen acquisition and use were larger in 60-species-mixtures than in monocultures in early summer, when grasses reach maximum biomass production, the opoosite was observed in late summer. These seasonal differences suggest that niche separation in nitrogen acquisition traits might be larger in periods of larger resource demands. The unexpectedly larger average niche width of grass species in 60-species mixtures co-occurring with a larger total niche space (larger convex hull volumes) indicates that trait adjustment may increase complementarity among grass species by niche extension. In contrast, complementarity through niche separation may differ between light and nutrient acquisition.
Conclusions
Although general effects of increasing species richness and, particularly, the presence of legumes on variation in functional traits of grass species were found, trait expression differed strongly among grass species. The present results are supported by studies reporting substantial variation among traits of taxonomically and phylogenetically closely related grass species in response to nutrient availability or disturbance (e.g. Craine et al., 2001; Díaz et al., 2007; Pontes et al., 2010). However, previous studies have shown that ranking of species based on trait values measured in different environments, e.g. under different nutrient availability, remains conserved (Garnier et al., 2001; Al Haj Khaled et al., 2005). The present study provided clear evidence that increasing plant diversity results in species-dependent effects on the expression of traits associated with light and nitrogen acquisition of grass species, which consequently also affects the ‘ranking’ of trait values among grass species. Thus, differences in both, the set of their trait values and in their species-specific strategies to respond to increasing plant diversity are the base for niche partitioning among different grass species and their coexistence in species-rich plant communities.
ACKNOWLEDGEMENTS
We thank K. Sörgel and R. A. Werner for stable isotope measurements and M. Scherer-Lorenzen for helpful discussions. We are grateful to A. Weigelt for co-ordination in the field, the gardeners and many student helpers for maintenance of the experimental plots, and M. Awgustow, J. Bauer, I. Kertscher, J. Reinsperger, B. Schulze and J. Seyfferth for their help with field and laboratory work. This work (as part of The Jena Experiment) was supported by the German Science Foundation (FOR 456) and Friedrich Schiller University of Jena and the Max Planck Society.
LITERATURE CITED
- Albert CH, Thuiller W, Yoccoz NG, et al. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology. 2010;98:604–613. [Google Scholar]
- Al Haj Khaled R, Duru M, Theau JP, Plantureux S, Cruz P. Variation in leaf traits through seasons and N-availability levels and its consequences for ranking grassland species. Journal of Vegetation Science. 2005;16:391–398. [Google Scholar]
- Anten NPR, Hirose T. Shoot structure, leaf physiology, and daily carbon gain of plant species in a tallgrass meadow. Ecology. 2003;84:955–968. [Google Scholar]
- Balvanera P, Pfisterer AB, Buchmann N, et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- Barber CB, Dobkin DP, Huhdanpaa H. The quickhull algorithm for convex hulls. ACM Transactions on Mathematical Software. 1996;22:469–483. [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. Resource limitation in plants – an economic analogy. Annual Review of Ecology and Systematics. 1985;16:363–392. [Google Scholar]
- ter Braak CJF, Šmilauer P. CANOCO reference manual and CanoDraw for Windows user's guide: software for canonical community ordination (version 4.5). Ithaca, NY: 2002. [Google Scholar]
- Buchmann N, Ehleringer JR. CO2 concentration profiles, and carbon and oxygen isotopes in C3 and C4 crop canopies. Agricultural and Forest Meteorology. 1998;89:45–58. [Google Scholar]
- Buchmann N, Brooks JR, Ehleringer JR. Predicting daytime carbon isotope ratios of atmospheric CO2 within forest canopies. Functional Ecology. 2002;16:49–57. [Google Scholar]
- Cornwell WK, Schwilk DW, Ackerly DD. A trait-based test for habitat filtering: convex hull volume. Ecology. 2006;87:1465–1471. doi: 10.1890/0012-9658(2006)87[1465:attfhf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos. 2001;93:274–285. [Google Scholar]
- Craine JM, Elmore AJ, Aidar MPM, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics. 2002;33:507–559. [Google Scholar]
- Díaz S, Cabido M. Plant functional types and ecosystem function in relation to global change. Journal of Vegetation Science. 1997;8:463–474. [Google Scholar]
- Díaz S, Lavorel S, McIntyre S, et al. Plant trait response to grazing – a global synthesis. Global Change Biology. 2007;13:313–341. [Google Scholar]
- Dimitrakopoulos PG, Schmid B. Biodiversity effects increase linearly with biotope space. Ecology Letters. 2004;7:574–583. [Google Scholar]
- Eisenhauer N, Beßler H, Engels C, et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology. 2010;91:485–496. doi: 10.1890/08-2338.1. [DOI] [PubMed] [Google Scholar]
- Ellenberg H. Vegetation ecology of central Europe. Cambridge: Cambridge University Press; 1988. [Google Scholar]
- Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:503–537. [Google Scholar]
- von Felten S, Hector A, Buchmann N, Niklaus PA, Schmid B, Scherer-Lorenzen M. Belowground nitrogen partitioning in experimental grassland plant communities of varying species richness. Ecology. 2009;90:1389–1399. doi: 10.1890/08-0802.1. [DOI] [PubMed] [Google Scholar]
- Garnier E, Laurent G, Bellmann A, et al. Consistency of species ranking based on functional leaf traits. New Phytologist. 2001;152:69–83. doi: 10.1046/j.0028-646x.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Gross N, Suding KN, Lavorel S, Roumet C. Complementarity as a mechanism of coexistence between functional groups of grasses. Journal of Ecology. 2007;95:1296–1305. [Google Scholar]
- Harper JL, White J. The demography of plants. Annual Review of Ecology and Systematics. 1974;5:419–463. [Google Scholar]
- Henry HAL, Aarssen LW. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos. 1997;80:575–582. [Google Scholar]
- Hille Ris Lambers J, Harpole WS, Tilman D, Knops J, Reich P. Mechanisms responsible for the positive diversity–productivity relationship in Minnesota grasslands. Ecology Letters. 2004;7:661–668. [Google Scholar]
- Hirose T. A vegetative plant growth model: adaptive significance of phenotypic plasticity in matter partitioning. Functional Ecology. 1987;1:195–202. [Google Scholar]
- Högberg P. 15N natural abundance in soil–plant systems. New Phytologist. 1997;137:179–203. doi: 10.1046/j.1469-8137.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- Hubbell SP. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press; 2001. [Google Scholar]
- Jones HG. Plants and microclimate: a quantitative approach to environmental plant physiology. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Jumpponen A, Mulder CPH, Huss-Danell K, Högberg P. Winners and losers in herbaceous plant communities: insights from foliar carbon isotope composition in monocultures and mixtures. Journal of Ecology. 2005;93:1136–1147. [Google Scholar]
- Kahmen A, Wanek W, Buchmann N. Foliar δ15N values characterize soil N cycling and reflect nitrate or ammonium preference of plants along a temperate grassland gradient. Oecologia. 2008;156:861–870. doi: 10.1007/s00442-008-1028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge G, Müller-Westermeier G. Das Klima ausgewählter Orte der Bundesrepublik Deutschland: Jena. Berichtes des Deutschen Wetterdienstes. 2000;213:1–290. [Google Scholar]
- Laliberté E, Shipley B. Measuring functional diversity from multiple traits, and other tools for functional ecology. 2010 doi: 10.1890/08-2244.1. R package version 1.0-9. The Comprehensive R Archive Network (CRAN), Vienna, Austria. URL: http://cran.r-project.org/package=FD . [DOI] [PubMed] [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Lorentzen S, Roscher C, Schumacher J, Schulze E-D, Schmid B. Species richness and identity affect the use of aboveground space in experimental grasslands. Perspectives in Plant Ecology, Evolution and Systematics. 2008;10:73–87. [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology and Evolution. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Maire V, Gross N, Pontes L da S, Picon-Cochard C, Soussana JF. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Functional Ecology. 2009;23:668–679. [Google Scholar]
- Oelmann Y, Wilcke W, Temperton VM, et al. Soil and plant nitrogen pools as related to plant diversity in an experimental grassland. Soil Science Society of America Journal. 2007;71:720–729. [Google Scholar]
- Oyarzabal M, Paruelo JM, del Pino F, Oesterheld M, Lauenroth WK. Trait differences between grass species along a climatic gradient in South and North America. Journal of Vegetation Science. 2008;19:183–192. [Google Scholar]
- Pontes L da S, Louault F, Carrère P, Maire V, Andueza D, Soussana J-F. The role of plant traits and their plasticity in the response of pasture grasses to nutrients and cutting frequency. Annals of Botany. 2010;105:957–965. doi: 10.1093/aob/mcq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscher C, Schumacher J, Baade J, et al. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic and Applied Ecology. 2004;5:107–121. [Google Scholar]
- Roscher C, Kutsch WL, Schulze E-D. Light and nitrogen competition limit Lolium perenne in experimental grasslands of increasing plant diversity. Plant Biology. 2010 doi: 10.1111/j.1438-8677.2010.00338.x. doi:10.1111/j.1438-8677.2010.00338.x. [DOI] [PubMed] [Google Scholar]
- Ryser P, Urbas P. Ecological significance of leaf lifespan among Central European grass species. Oikos. 2000;91:41–50. [Google Scholar]
- Schlichting CD. Phenotypic integration and environmental change. Bioscience. 1989;39:460–464. [Google Scholar]
- Silvertown J. Plant coexistence and the niche. Trends in Ecology and Evolution. 2004;19:605–611. [Google Scholar]
- Silvertown J, Dodd M, Gowing D. Phylogeny and the niche structure of meadow plant communities. Journal of Ecology. 2001;89:428–435. [Google Scholar]
- Smith H. Light quality, photoperception and plant strategy. Annual Review of Plant Physiology. 1982;33:481–518. [Google Scholar]
- Spehn EM, Hector A, Joshi J, et al. Ecosystems effects of biodiversity manipulations in European grasslands. Ecological Monographs. 2005;75:37–63. [Google Scholar]
- Suding KN, Goldberg DE, Hartman KM. Relationships among species traits: separating levels of response and identifying linkages to abundance. Ecology. 2003;84:1–16. [Google Scholar]
- Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia. 2007;151:190–205. doi: 10.1007/s00442-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- Valladares F, Niinemets Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution and Systematics. 2008;39:237–257. [Google Scholar]
- Violle C, Navas ML, Vile D, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- van der Werf A, van Nuenen M, Visser AJ, Lambers H. Effects of N-supply on the rates of photosynthesis and shoot and root respiration of inherently fast- and slow-growing monocotyledonous species. Physiologia Plantarum. 1993;89:563–569. [Google Scholar]
- Wright IJ, Naeem S, Hector A, et al. Conventional functional classification schemes underestimate the relationships with ecosystem functioning. Ecology Letters. 2005;9:111–120. doi: 10.1111/j.1461-0248.2005.00850.x. [DOI] [PubMed] [Google Scholar]