Abstract
Background and Aims
Many wetland species form aerenchyma and a barrier to radial O2 loss (ROL) in roots. These features enhance internal O2 diffusion to the root apex. Barrier formation in rice is induced by growth in stagnant solution, but knowledge of the dynamics of barrier induction and early anatomical changes was lacking.
Methods
ROL barrier induction in short and long roots of rice (Oryza sativa L. ‘Nipponbare’) was assessed using cylindrical root-sleeving O2 electrodes and methylene blue indicator dye for O2 leakage. Aerenchyma formation was also monitored in root cross-sections. Microstructure of hypodermal/exodermal layers was observed by transmission electron microscopy (TEM).
Key Results
In stagnant medium, barrier to ROL formation commenced in long adventitious roots within a few hours and the barrier was well formed within 24 h. By contrast, barrier formation took longer than 48 h in short roots. The timing of enhancement of aerenchyma formation was the same in short and long roots. Comparison of ROL data and subsequent methylene blue staining determined the apparent ROL threshold for the dye method, and the dye method confirmed that barrier induction was faster for long roots than for short roots. Barrier formation might be related to deposition of new electron-dense materials in the cell walls at the peripheral side of the exodermis. Histochemical staining indicated suberin depositions were enhanced prior to increases in lignin.
Conclusions
As root length affected formation of the barrier to ROL, but not aerenchyma, these two acclimations are differentially regulated in roots of rice. Moreover, ROL barrier induction occurred before histochemically detectable changes in putative suberin and lignin deposits could be seen, whereas TEM showed deposition of new electron-dense materials in exodermal cell walls, so structural changes required for barrier functioning appear to be more subtle than previously described.
Keywords: Aerenchyma, barrier to radial O2 loss, cylindrical root-sleeving O2 electrode, transmission electron microscopy, hypoxia, methylene blue, root aeration, waterlogging
INTRODUCTION
Root growth into anoxic, waterlogged substrates relies on internal diffusion of O2 (Armstrong, 1979). Internal diffusion of O2 to the root apex is enhanced by formation of aerenchyma and a barrier against radial O2 loss (ROL) (Armstrong, 1979). In roots of rice (Oryza sativa), aerenchyma is formed constitutively, and the amount can be further enhanced by soil waterlogging (Armstrong, 1971; Pradhan et al., 1973). The barrier to ROL is also inducible in rice roots, forming in stagnant or waterlogged conditions, but not (or only weakly) in aerated conditions (Colmer et al., 1998, 2006; Colmer, 2003a). Aerenchyma and the barrier to ROL are regarded as key features contributing to waterlogging tolerance in many wetland species (Armstrong, 1979; Jackson and Drew, 1984; Justin and Armstrong, 1987; Colmer, 2003b; Colmer and Voesenek, 2009), yet barrier induction is poorly understood in comparison with aerenchyma formation (Shiono et al., 2008). The physical resistance that greatly impedes ROL from roots of rice is considered to result from suberin and/or lignin depositions in the exodermis and/or sclerenchyma (Insalud et al., 2006; Kotula et al., 2009), although the microstructure of the barrier is not known.
Ethylene signalling during waterlogging enhances aerenchyma formation (Justin and Armstrong, 1991), but does not induce the barrier to ROL, in adventitious roots of rice (Colmer et al., 2006). Thus, induction of a barrier to ROL and of aerenchyma appear to be differentially regulated (Colmer et al., 2006). Previous studies of the inducible barrier to ROL evaluated roots of rice grown for days, or even weeks, under contrasting treatments (e.g. Colmer et al., 1998), with the exception of Insalud et al. (2006) who showed barrier induction had commenced within 1 d of transfer into stagnant conditions. The present study evaluated the detailed dynamics of ROL barrier induction, as compared with aerenchyma formation, in roots of rice of two contrasting lengths. Experiments were designed to address three main questions: (1) the timing of enhanced aerenchyma formation in adventitious roots of two lengths (i.e. short and long roots) following transfer to deoxygenated stagnant conditions, (2) the timing of induction of the barrier to ROL in these different length roots and (3) what microstructural changes occurred in the roots that formed the barrier. Two root lengths were studied, as earlier work (Colmer et al., 2006) had indicated possible stronger barrier induction in long, compared with short, roots.
ROL was assessed using two methods: (1) cylindrical root-sleeving O2 electrodes and (2) an indicator dye (reduced/oxidized methylene blue). The O2 electrode-based technique enables quantitative measurements of O2 fluxes from intact roots (e.g. Armstrong, 1971). Measurements of ROL at several positions along roots are, however, time consuming, and the O2 electrode-based method requires specialized equipment. Qualitative information on spatial patterns of ROL can be obtained in a shorter time by use of indicator dyes, such as reduced/oxidized methylene blue, to show regions of ROL from roots in an O2-free medium (e.g. Armstrong and Armstrong, 1988; Armstrong et al., 1992; Kotula and Steudle, 2009). The relationship, however, between colour development of methylene blue and rates of ROL from roots in O2-free media had not previously been assessed. Therefore, a fourth (4) question was addressed in the present studies by making direct comparisons of these two approaches, so that the suitability of using the dye method for testing induction of the barrier to ROL in adventitious roots of rice could be established. If reliable, the more rapid dye method could enable screening of large numbers of mutant lines for root aeration traits, because the rice cultivar we used in the present study (‘Nipponbare’) has been sequenced at the whole genome level and is the background of 47 196 Tos17-induced insertion mutants (Miyao et al., 2003).
MATERIALS AND METHODS
Plant material and culture
Lowland rice (Oryza sativa L. ‘Nipponbare’) was grown in a nutrient solution of the same composition as used in earlier studies of rice (Colmer, 2003a). Plants were supported at the stem base in pots (light-shielding pots so roots were in darkness) within a controlled-environment chamber (12-h light/12-h dark cycle; 30 °C/25 °C; relative humidity over 50 %; photosynthetic photon flux density at 214 µmol m−2 s−1 during the light period).
In each experiment, seeds were washed in deionized water, and then soaked in 0·1 % (w/v) benlate solution, 1-[(butylamino) carbonyl-1H-benzimidazol-2-yl] carbamic acid methyl ester (Sumitomo Chemical, Tokyo, Japan), for surface sterilization for 1 d in darkness at 30 °C. Seeds were then washed thoroughly with deionized water, and then incubated in deionized water for 1 d, all still in darkness at 30 °C. Two days after imbibition, seeds were placed on stainless mesh floating on aerated quarter-strength nutrient solution and exposed to light. Five days after imbibition, each seedling was held with a soft sponge floating on aerated full-strength nutrient solution. Eight-day-old seedlings were transplanted into 6-L pots (diameter, 200 mm; height, 220 mm; each pot held four seedlings) containing full-strength nutrient solution. Solutions were renewed every 7 d. In all experiments, pots were arranged in a completely randomized design.
Two main types of experiments were conducted, depending upon the objective (explained below). In experiments with long exposure times to treatments, 8- to 9-d-old seedlings were either continued in aerated solution or transplanted into stagnant solution (described below) for an additional 14–21 d. In experiments with short treatments (i.e. up to 120 h of treatment), plants were grown in aerated solution for 3–4 weeks, and then continued in aerated solution or transferred into stagnant solution. Stagnant solution contained 0·1 % (w/v) dissolved agar and was deoxygenated (dissolved O2 < 1·0 mg L−1) prior to use by flushing with N2 gas. The dilute agar prevents convective movements in solution (‘stagnant’ treatment) so this treatment mimics better than other solution culture methods the changes in gas composition found in waterlogged soils (e.g. decreased O2, increased ethylene) (Wiengweera et al., 1997).
Adventitious roots were classified as short (65–90 mm in length) or long (105–130 mm in length), based on length of the main axis at commencement of treatments. Selected short and long adventitious roots were marked near the base using charcoal powder, so these could be recognized over time.
Measurement of radial O2 loss
For experiments that assessed the dynamics of barrier induction and aerenchyma formation, plants were raised for 3–4 weeks so that sufficient adventitious roots had formed prior to imposing two treatments: continuously aerated or stagnant deoxygenated agar nutrient solution.
Cylindrical root-sleeving electrodes were used to measure ROL at selected positions along adventitious roots. Some experiments made continual ROL measurements at the basal part (approx. 15 mm below the root–shoot junction) of short and long adventitious roots during 48 h in an O2-free medium, whereas other experiments measured profiles of ROL along roots after 48 h. Plants were sealed into rubber lids fitted in 2·5-L glass chambers (diameter, 80 mm; height, 460 mm) filled with an O2-free medium containing 0·1 % (w/v) agar, 0·5 mm CaSO4 and 5 mm KCl. The intact root system was immersed in O2-free medium while the shoot remained in air. A cylindrical root-sleeving O2 electrode (internal diameter, 2·25 mm; height, 5·0 mm) (Armstrong and Wright, 1975; Armstrong, 1994), fitted with root-centralizing guides, was placed around a selected adventitious root with few lateral roots. The equilibrium flux of O2 from adventitious roots was measured at selected positions, with appropriate electrode polarization based on polarographs determined for each measurement (see Armstrong 1971; Armstrong, 1994). Root diameters at the positions measured for ROL were determined using a loupe with scale (minimum scale, 0·05 mm). ROL measurements were at 28 °C in a constant temperature room with lights providing photosynthetically active radiation of 67 µmol m−2 s−1, except during measurements spanning 48 h, where 12-h light (67 µmol m−2 s−1)/12-h dark cycles were used.
Histochemical detection of suberin and lignin
Basal parts (12–25 mm below the root–shoot junction) of the short and long adventitious roots were sampled from plants in aerated solution, and after 2, 5 or 14–21 d treatment in stagnant deoxygenated agar nutrient solution. Fresh roots were embedded in 5 % agar and 85-μm sections were made using a vibrating microtome (HM659V; Thermo Fisher Scientific Inc.). Root cross-sections were cleared by incubation in lactic acid saturated with chloral hydrate at 70 °C for 1 h (Lux et al., 2005). Sections were stained with Fluorol Yellow 088 at room temperature for 1 h to visualize suberin lamellae (Brundrett et al., 1991). Suberin lamellae were observed as a yellow–green fluorescence when excited by UV light (UV filter set, UV1-A, Nikon: Band pass = 365 nm, diachronic mirror = 400, band barrier > 400 nm; microscopy: ECLIPSE E600, Nikon; CCD camera: DA-Ri1, Nikon). Other sections were stained for 3 min with saturated phloroglucinol in 20 % hydrochloric acid at room temperature, to visualize lignin with cinnamyl aldehyde groups as these appear orange/red under white light (Jensen, 1962).
Microstructure observation by transmission electron microscopy (TEM)
Basal parts (12–25 mm below the root–shoot junction) of the short and long adventitious roots were sampled from plants in aerated solution or after 48 h of treatment in stagnant deoxygenated agar nutrient solution. Samples were fixed in 2·5 % glutaraldehyde in 0·1 m phosphate buffer (pH 7·4) at 4 °C, and postfixed in 1 % osmium tetraoxide in 0·1 m phosphate buffer (pH 7·4) for 3 h at 4 °C. After stepwise dehydration with an ethanol series (50–100 %), samples were embedded in Spurr's resin and ultrathin sections (80–100 nm) were made using an ultramicrotome. The sections were stained with uranyl acetate and lead citrate, and then observed by TEM (JEM1210, JEOL Ltd).
Observation of ROL pattern using methylene blue
Use of indicator dyes, such as reduced/oxidized methylene blue, in stagnant deoxygenated agar nutrient solutions (e.g. Armstrong and Armstrong, 1988; Kotula and Steudle, 2009) or in waterlogged soil (e.g. Armstrong et al., 1992) provides qualitative information on spatial patterns of ROL. Methylene blue is colourless when reduced and blue when oxidized (Barton and Ollis, 1979).
Methylene blue staining was applied for evaluation of the barrier to ROL during 120 h of aerated or stagnant treatments. In addition, we examined the relationship between colour development along adventitious roots as visualized with methylene blue and rates of ROL from these same adventitious roots measured using root-sleeving O2 electrodes. Rates of ROL from intact roots were measured at 30, 40, 60 and 80 mm behind the apex in an O2-free medium (lacking methylene blue), and the pattern of colour development along these same roots was then assessed following transfer into a solution containing reduced methylene blue. To enable observations on the targeted adventitious roots when in methylene blue, some other roots were trimmed off prior to insertion into this solution.
A solution containing 0·1 % (w/v) agar was prepared, and after cooling, methy1ene blue was added at 13 mg L−1. The blue solution containing oxidized dye was reduced by addition of 130 mg L−1 sodium dithionite (Na2S2O4) so that it was then colourless. The test solution was in glass vessels (length, 350 mm; width, 15 mm; height, 350 mm) and the plant was held with the root–shoot junction at 50 mm below the surface, and the remainder of the shoot in air. The experiment was conducted in a dark room at constant temperature (28 °C). After 30–60 min, colour intensity of ‘blue halos’ forming adjacent to roots was compared against a standard colour chart.
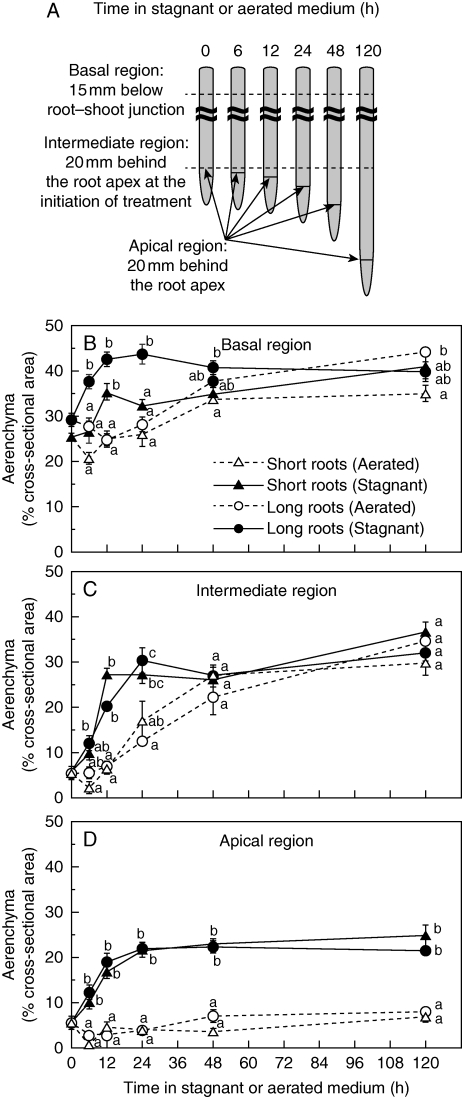
Aerenchyma formation
Formation of aerenchyma during 120 h of aerated or stagnant treatments was measured in cross-sections taken along short and long adventitious roots. Three positions were assessed: 20 mm behind the root apex at each time point (hereafter termed ‘apical region’), 15 mm below the root–shoot junction (‘basal region’) and 20 mm behind the root apex at the initiation of treatment (‘intermediate region’). Root portions were excised and placed in 0·1 m potassium phosphate buffer (pH 7·0). Cross-sections were hand-made using a razor blade, mounted in water and viewed using a microscope (DMIRBE M2FLIII; Leica) fitted with a CCD camera (VB-7000; Keyence) linked to a computer. The proportion of each cross-section occupied by aerenchyma was determined using Image J software (Ver. 1·39u; NIH, Bethesda, MD, USA).
Statistical analysis
Means of aerenchyma (% cross-sectional area), ROL and elongation rate of roots were compared using a two-sample t-test at the 5 % probability level, or one-way ANOVA and then Scheffé's test for multiple comparisons at the 5 % probability level. Frequencies of roots that formed a barrier to ROL as assessed by methylene blue staining were compared using Fisher's exact test at the 5 % probability level. For ROL and aerenchyma data, means ± standard errors (s.e.) were calculated.
RESULTS
Induction of barrier to ROL along short and long adventitious roots
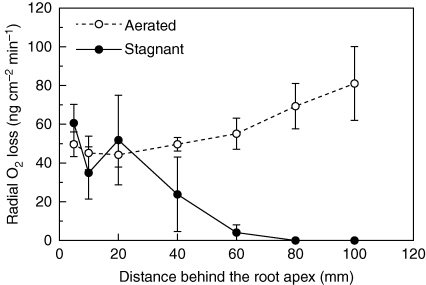
Long adventitious roots of rice (‘Nipponbare’) grown in stagnant deoxygenated agar nutrient solution for 5 d showed ROL profiles consistent with induction of a ‘tight’ barrier to ROL; O2 fluxes from the basal regions were substantially lower than from the more apical positions (Fig. 1). By contrast, when grown in aerated nutrient solution the roots lacked, or expressed only weakly, an ROL barrier; O2 fluxes from basal regions were high relative to rates at 5 mm behind the apex (Fig. 1).
Fig. 1.
Rates of radial O2 loss along intact long adventitious roots of rice in O2-free medium with shoots in air, at 28 °C (mean ± s.e., n = 3). Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 5 d or aerated nutrient solution for 5 d. Lengths of adventitious roots (mean ± s.e., n = 3) were 154 ± 1 mm in aerated and 159 ± 3 mm in stagnant deoxygenated agar nutrient solution.
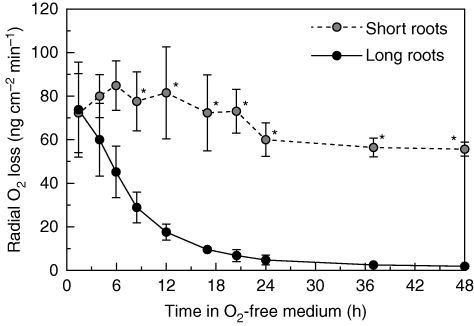
Rates of ROL from a basal position (approx. 15 mm below the root–shoot junction) were monitored at selected times during 48 h following transfer from aerated solution to the O2-free agar solution, both for short and for long adventitious roots. For short adventitious roots, O2 fluxes (measured at 50–91 mm behind the root tip) from the basal region remained high during the first 48 h in the stagnant agar solution; that is, short adventitious roots did not form a barrier to ROL within 48 h (Fig. 2; P < 0·05, two-sample t-test). By contrast, for long adventitious roots, O2 fluxes (measured at 80–106 mm behind the root tip) from the basal region declined soon after transfer into the O2-free agar solution. At 24 h of stagnant treatment, O2 fluxes from the basal region were under 5 ng cm−2 min−1 (Fig. 2); the low O2 fluxes were similar to those observed for basal regions of adventitious roots with a ‘tight’ barrier to ROL (Fig. 1). Subsequently, the O2 fluxes were reduced to 1·9 ng cm−2 min−1 at 48 h of stagnant treatment (Fig. 2), 30-fold lower than that of the basal region of short adventitious roots at 48 h stagnant treatment (P < 0·05, two-sample t-test). This decline in ROL at basal positions in long roots would be caused by ROL barrier formation, rather than changes in internal O2 supply, as internal O2 concentration might even have increased slightly owing to the small increases in aerenchyma (described below). In summary, a barrier to ROL was induced within 24 h for long adventitious roots, whereas for short adventitious roots it was not formed within 48 h of stagnant treatment.
Fig. 2.
Time courses of rates of radial O2 loss at the basal part (15 mm below the root–shoot junction) of short and long adventitious roots following transfer into an O2-free medium with shoots in air, at 28 °C (short adventitious roots, n = 3; long adventitious roots, n = 4; mean ± s.e.). Plants were raised for 3–4 weeks in aerated nutrient solution, and then transferred for 48 h to O2-free stagnant 0·1 % agar solution that contained 5 mm KCl and 0·5 mm CaSO4. Short (65–90 mm) and long (105–130 mm) adventitious roots were used in experiments (lengths at commencement of treatment); after 48 h in the stagnant agar, root lengths were: short, 66–94 mm (growth rate 1·5 mm d−1); long, 110–131 mm (growth rate 3·4 mm d−1). *Significant difference between short and long adventitious roots (P < 0·05, two-sample t-test).
Microstructure changes and localization of suberin and lignin deposition at exodermis and sclerenchyma
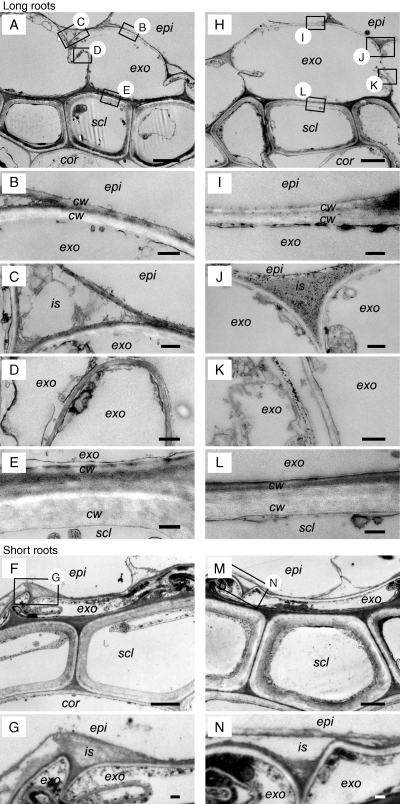
As long roots formed a barrier to ROL rather quickly, microstructural differences at the exodermis and outer-side of the sclerenchyma layers of roots were investigated by TEM for these roots when in aerated solution or after 48 h of stagnant treatment (Fig. 3). Intercellular spaces between exodermis and epidermis cell layers were evident in roots from aerated conditions (Fig. 3C); however, these areas were occupied by a high-density material in roots from stagnant conditions (Fig. 3J). Dark granules were also present in the intercellular spaces between exodermal cells and also between the sclerenchyma cells in roots from stagnant conditions (Fig. 3K), whereas granules were absent in roots from aerated conditions (Fig. 3D). Short adventitious roots from aerated solution and at 2 d of stagnant treatment (i.e. roots without barrier to ROL) showed intercellular spaces between exodermis and epidermis cell layers; in addition, granules were absent (Fig. 3G, N). The other parts of these short roots were also similar to the long roots in aerated solution (Supplementary Data Fig. S1, available online). No obvious changes were seen at the cortex side (i.e. inner side) of the sclerenchyma and their cell-wall structures. Thus, the obvious microstructural changes between roots with or without a barrier to ROL had occurred in the intercellular spaces between exodermis and epidermis cell layers.
Fig. 3.
Comparison of the microstructure of the exodermis and the sclerenchyma in the basal part (15 mm below the root–shoot junction) of short or long adventitious roots grown continuously in aerated nutrient solution (short roots, F, G; long roots, A–E) or following transfer to stagnant deoxygenated agar nutrient solution for 48 h (short roots, M, N; long roots, H–L). Sections stained with uranyl acetate and lead citrate were observed by TEM. We observed the exodermis and sclerenchyma region (A, F, H, M; scale bar = 2 µm) and then specific cells at higher magnification (scale bar = 0·2 µm) for the epidermis side of the exodermis (B, C, G, I, J, N), between the exodermis cells (D, K), and also of the sclerenchyma side of the exodermis (E, L). Abbreviations: cor, cortex; cw, cell wall; epi, epidermis; exo, exodermis; is, intercellular space; scl, sclerenchyma. Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 48 h or aerated nutrient solution for 48 h. At the commencement of treatments the roots studied were short (65–90 mm) and long (105–130 mm) adventitious roots. Small boxes with letters in A and H and F and M indicate areas viewed at higher magnification and shown in the other panels of this figure.
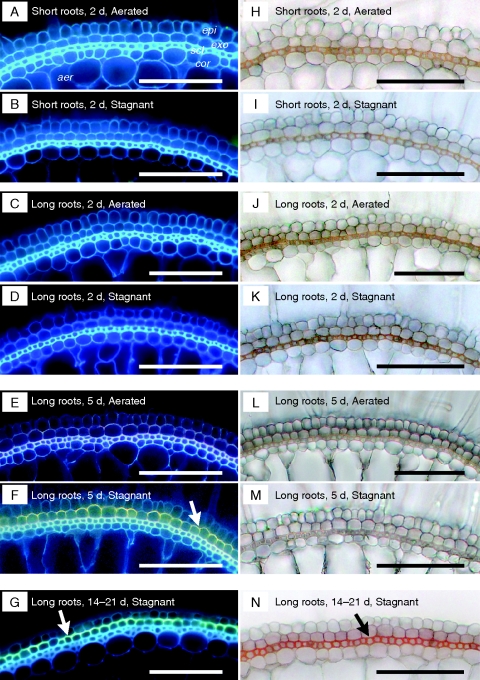
In contrast to the TEM studies, histochemical staining of fresh sections taken after 2 d of the stagnant treatment failed to detect any ‘gross changes’ in suberin lamellae or lignin deposits in outer layers of basal regions of long adventitious roots (Fig. 4D, K) as well as short adventitious roots (Fig. 4B, I), whereas microstructural differences were already observed at that time (Fig. 3) and ROL would already have diminished (Fig. 2). In long roots at 5 d of the stagnant treatment (i.e. at the time the barrier had formed completely), clear yellow–green fluorescence typical of suberin lamellae was observed at exodermis (Fig. 4F), although the orange/red colour of lignin deposits was absent at sclerenchyma (Fig. 4M). At 14–21 d of the stagnant treatment, we observed not only suberin lamellae at exodermis (Fig. 4G) but also lignified sclerenchyma (Fig. 4N) in long roots. Thus, barrier induction occurred (Fig. 2) before these histochemically detectable changes, indicating suberin and lignin deposits could be seen (Fig. 4). Together, these results indicate that structural changes required for barrier function might initially be subtle (e.g. Fig. 3), and suberin lamellae presumably contribute to diminish O2 diffusivity prior to changes in lignin.
Fig. 4.
Comparison of the development of suberin lamellae (A–G) and lignin deposition (H–N) of the exodermis and the sclerenchyma in the basal part (15 mm below the root–shoot junction) of short and long adventitious roots grown continuously in aerated nutrient solution [short roots, A, H; long roots: C, J (2 d), E, L (5 d)] or following transfer to stagnant deoxygenated agar nutrient solution for 2 d (short roots, B, I; long roots: D, K), 5 d (long roots: F, M) and 14–21 d (G, N). Suberin lamella is indicated by yellow–green fluorescence with Fluorol Yellow 088 (white arrow). Autofluorescence was detected as blue in the cell walls. Lignin with cinnamyl aldehyde groups stained orange/red with phloroglucinol-HCl (Black arrows). Abbreviations: aer, aerenchyma; cor, cortex; epi, epidermis; exo, exodermis; scl, sclerenchyma. Scale bars = 100 µm. Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 2 or 5 d, or aerated nutrient solution for 2 or 5 d. At the commencement of treatments the roots studied were short (65–90 mm) and long (105–130 mm) adventitious roots. For plants exposed to 14–21 d of treatments, seedlings were raised for 9 d in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 14–21 d. Long roots in the continuous aerated controls (C, J and E, L) were of similar ages to those roots exposed to stagnant treatment for 14–21 d (G, N), so comparison of G, N can be made with C, J or E, L to see the influence of the 14–21 d stagnant treatment.
Induction of aerenchyma along short and long adventitious roots during 120 h
Aerenchyma formation (% cross-sectional area) was measured for short and long adventitious roots at three different positions (Fig. 5A). In summary, aerenchyma formation in each region was enhanced by stagnant treatment within 12 h, and the trend of induction (i.e. stagnant solution) or constitutive development (i.e. aerated solution) of aerenchyma was essentially the same for short and long roots (Fig. 5). In both root length classes, for the three positions measured, most aerenchyma was present in the basal region, and least at the most apical region.
Fig. 5.
Aerenchyma at three positions along adventitious roots of rice, following transfer into aerated or stagnant deoxygenated agar nutrient solutions (A). Cross-sections at 15 mm below the root–shoot junction – termed ‘basal region’ (B), at 20 mm behind the root apex at the initiation of treatment – termed ‘intermediate region’ (C), and 20 mm behind the root apex at each time point – termed ‘apical region’ (D), were taken by hand with a razor blade and photographed under a microscope and the proportion of aerenchyma was determined. Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 0, 6, 12, 24, 48 or 120 h. Values are means ± s.e. Sample number and length of roots used in these measurements are given in Table 1. Different lower-case letters denote significant differences among root length classes and treatments (P < 0·05, one-way ANOVA and then Scheffé's test for multiple comparison).
At the basal region of both root length classes, aerenchyma was already well developed (i.e. short adventitious roots, 25 %; long adventitious roots, 30 %) for plants raised in aerated solution (Fig. 5B). Upon transfer to stagnant solution, both the short and the long roots formed additional aerenchyma in the basal position, within 12 h (Fig. 5B). In aerated conditions, aerenchyma formation gradually increased with tissue age over 120 h. At the apical region and intermediate region, aerenchyma formations were markedly enhanced by stagnant treatment within 12 h in both the short and the long adventitious roots. Moreover, in these more apical positions, aerenchyma continued to develop, although slowly, until 120 h (Fig. 5C, D). After 120 h in stagnant solution, aerenchyma at the intermediate region of short and long adventitious roots was 37·1 ± 2·0 and 32·1 ± 1·7 %, respectively, but by this time these values were similar to those in roots in aerated solution of 30·0 ± 2·7 and 34·8 ± 2·2 % in short and long adventitious roots, respectively. At 20 mm behind the root apex, however, aerenchyma in roots after 120 h of the stagnant treatment was 24·9 ± 2·3 and 21·5 ± 1·1 % for short and long roots, respectively, values greater than in the aerated treatment (short roots, 7·1 ± 1·4 %; long roots, 8·0 ± 0·6 %).
Short and long adventitious roots continued to grow in stagnant deoxygenated agar nutrient solution, for example during 5 d of treatment at 3·0 ± 0·1 and 5·2 ± 0·4 mm d−1, respectively (Table 1). This root growth was, however, slower than in aerated nutrient solution with short roots at 8·3 ± 1·2 mm d−1 and long roots at 12·4 ± 1·1 mm d−1 (P < 0·05, two-sample t-test). After 120 h in stagnant solution, the length of short adventitious roots had increased to 107 ± 2 mm, which was close to the length of roots classified as long when the treatments were commenced (i.e. the short roots grew and so became long roots at 120 h of stagnant treatment).
Table 1.
Growth in length (mm) of short and long adventitious roots in aerated nutrient solution or stagnant deoxygenated agar nutrient solution
| Time after treatment (h) |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 24 | 48 | 120 | ||
| Short roots | |||||||
| Aerated | Mean | 73 | 74 | 75 | 81 | 110 | 123 |
| s.e. | 1 | 4 | 2 | 3 | 4 | 12 | |
| n | 9 | 5 | 3 | 5 | 11 | 9 | |
| Stagnant | Mean | 73 | 72 | 74 | 80 | 93 | 107 |
| s.e. | 1 | 2 | 1 | 2 | 3 | 2 | |
| n | 9 | 9 | 9 | 8 | 9 | 7 | |
| Long roots | |||||||
| Aerated | Mean | 116 | 129 | 126 | 137 | 151 | 177 |
| s.e. | 5 | 3 | 4 | 4 | 8 | 5 | |
| n | 5 | 6 | 8 | 7 | 5 | 8 | |
| Stagnant | Mean | 116 | 121 | 117 | 123 | 125 | 133 |
| s.e. | 5 | 4 | 3 | 3 | 4 | 6 | |
| n | 5 | 7 | 7 | 7 | 7 | 11 | |
Short (65–90 mm) and long (105–130 mm) adventitious roots were used (lengths at commencement of treatment). Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 0, 6, 12, 24, 48 or 120 h. The samples in this table correspond to the roots in Fig. 5.
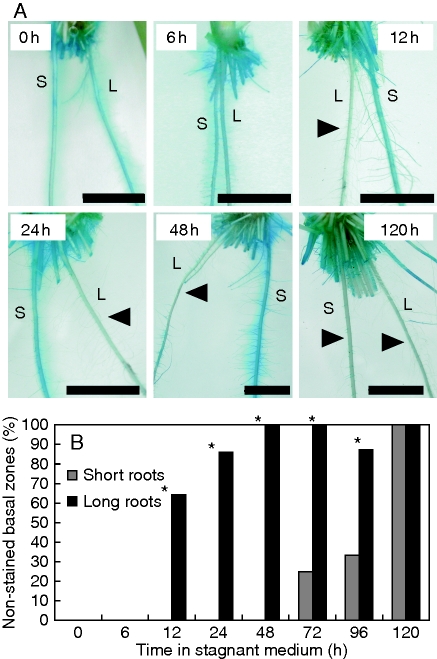
Induction of barrier to ROL along short and long adventitious roots over 120 h, as assessed using methylene blue staining
Induction of the barrier to ROL in adventitious roots was also assessed by methylene blue staining, a method that can quickly provide qualitative patterns of ROL (Armstrong and Armstrong, 1988; Armstrong et al., 1992; Colmer, 2003b). Plants grown in aerated solution, or those exposed to stagnant deoxygenated agar nutrient solution for different lengths of time, were transferred to an O2-free agar solution with methylene blue added (Fig. 6). Methylene blue is colourless when reduced, and blue when oxidized. Basal regions of short and long adventitious roots of plants from aerated solutions formed ‘blue halos’ in the methylene blue solution (Fig. 6A), indicating lack of a barrier to ROL. For plants transferred into the methylene blue assay after 12 h of stagnant treatment in the nutrient solution, many (i.e. 64 %) of the long adventitious roots did not form ‘blue halos’ (i.e. roots had a barrier induced) and after 48 h of treatment prior to the assay all of the long adventitious roots did not form ‘blue halos’ in basal zones at 48 h after treatment (i.e. all long roots had a barrier induced) (Fig. 6B; P < 0·05, Fisher's exact test). By comparison, the short adventitious roots still formed ‘blue halos’ in basal zones even after 48 h of treatment, and even after 72 h of stagnant treatment only 25 % of the short adventitious roots no longer formed ‘blue halos’, and this increased to only 33 % at 96 h. Finally, at 120 h, no adventitious roots formed ‘blue halos’ (i.e. at 120 h the barrier had been induced even in initially short roots) (Fig. 6B). However, the length of short adventitious roots increased to 119 ± 5 mm after 120 h in stagnant solution, close to the length of roots initially classified as being long. All short and long adventitious roots growing in aerated nutrient solution for the 120 h treatment period still formed ‘blue halos’ in basal zones when transferred to the methylene blue assay (i.e. all aerated roots had substantial ROL from basal zones).
Fig. 6.
Short (S) and long (L) adventitious roots of rice plants transferred into an O2-free agar solution with methylene blue. Methylene blue is colourless when reduced and blue when oxidized. Arrow denotes the ‘blue halos’ adjacent to adventitious roots. Plants were raised for 3–4 weeks in aerated nutrient solution, prior to transfer to stagnant deoxygenated agar nutrient solution for 0, 6, 12, 24, 48 or 120 h (A). Scale bar = 10 mm. Percentage of roots with ‘blue halos’ along short and long adventitious roots (B). Lengths at the commencement of treatment were: short (65–90 mm) and long (105–130 mm) adventitious roots. Sample numbers and length of roots used in these measurements are given in Supplementary Data Table S1, available online. *Significant difference between short and long adventitious roots (P < 0·05, Fisher's exact test).
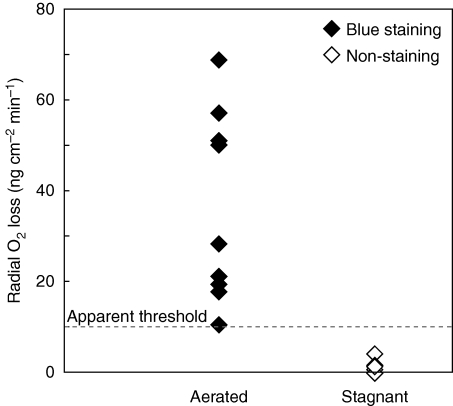
Threshold of methylene blue staining to test for formation of a barrier to ROL
The methylene blue method has been used to visualize sites of O2 loss from roots in O2-deficient media (Armstrong and Armstrong, 1988; Armstrong et al., 1992; Kotula and Steudle, 2009), but the relationship between ‘blue halo’ formation with methylene blue treatment and the rate of ROL from roots had not previously been tested. We measured rates of ROL using cylindrical root-sleeving O2 electrodes, and then used the same plants to examine the same roots in the methylene blue assay for ROL. Basal parts of roots grown in aerated nutrient solution (i.e. no ROL barrier formed) showed high rates of ROL and also had ‘blue halos’ with methylene blue treatment (Fig. 7). Basal parts of roots grown in stagnant deoxygenated agar nutrient solution (i.e. ROL barrier formed) showed very low ROL and also did not form ‘blue halos’ with methylene blue treatment (Fig. 7). For the methylene blue assay used, a number of roots formed ‘blue halos’ when the rates of ROL were over 10 ng cm−2 min−1, whereas the ‘blue halos’ were not formed when ROL was less than 10 ng cm−2 min−1. Thus, the apparent ROL threshold, to obtain methylene blue staining over exposures of 30–60 min, was approx. 10 ng cm−2 min−1. These results supported that methylene blue staining (e.g. Fig. 6) could be used to distinguish whether rice roots have formed a barrier to ROL. Methylene blue staining should therefore be useful to test large numbers of lines quickly to determine whether roots form a barrier to ROL (e.g. rice mutant screening), although it is not a quantitative method.
Fig. 7.
Rates of radial O2 loss, and assessment of ‘blue halos’ formed with methylene blue treatment, for adventitious roots of rice grown in aerated or stagnant deoxygenated agar nutrient solution. The basal region of root, 11–50 mm below the root–shoot junction (i.e. 30–60 mm behind the root apex), was measured for ROL and then observed for staining with methylene blue. Before measurements, plants were raised for 8 d in aerated nutrient solution and then grown in either aerated or stagnant deoxygenated nutrient solution for 14–21 d. ROL was measured using cylindrical root-sleeving O2 electrodes in an O2-free agar solution with shoots in air, and subsequently the measured adventitious roots were transferred into O2-free agar solution containing methylene blue. Methylene blue is colourless when reduced and blue when oxidized. Measurements and observations were taken at 28 °C using intact adventitious roots 57–107 mm in length (three plants, n = 6) grown in aerated nutrient solution and 60–81 mm in length (four plants, n = 9) grown in stagnant deoxygenated agar nutrient solution. Dashed line: apparent threshold of methylene blue staining for 30–60 min.
DISCUSSION
Aerenchyma and a barrier to ROL enhance internal diffusion of O2 towards the root tip, and thus root elongation into anoxic substrates (Armstrong, 1979). Several cultivars of rice form a ‘tight’ barrier to ROL, induced under waterlogged or deoxygenated stagnant conditions (Colmer et al., 1998, 2006; Colmer, 2003a). Rice ‘Nipponbare’ was shown in the present study to also form an inducible ‘tight’ barrier to ROL (Fig. 1); this is of significance for future work, as the whole genome of ‘Nipponbare’ has been sequenced (International Rice Genome Sequencing Project, 2005) and many tools are available for molecular and genetic studies (e.g. oligo microarray, mutant collection and database). The barrier to ROL was induced within 24 h in long adventitious roots, but not until after 48 h of stagnant treatment in short adventitious roots (Figs 2 and 5). Data for ROL after 1 d of stagnant treatment were available for long adventitious roots of ‘Amaroo’ (Insalud et al., 2006) for which ROL had decreased by 90 % in basal regions, and the reduction after 24 h in ‘Nipponbare’ was similar. The present study of ‘Nipponbare’ provides detailed time-series data for ROL barrier induction, revealing that the process commences soon after transfer to stagnant medium.
In Amazonian tree species (De Simone et al., 2003), Glyceria maxima and Phragmites australis (Soukup et al., 2007) and Hordeum marinum (Garthwaite et al., 2008), the barrier to ROL has been linked to suberin deposits in the exodermis/hypodermis. In rice, the barrier has been suggested to result from suberin and/or lignin deposition in the exodermis and sclerenchyma, forming a physical resistance to diminish ROL (Insalud et al., 2006; Kotula et al., 2009). Thus, the relative contributions of suberin and lignin were uncertain and microstructural changes associated with barrier formation had not previously been examined. The present study showed microstructural changes at the exodermis in the long adventitious roots within 48 h, when barrier formation is already complete in the roots of rice. In particular, intercellular spaces between exodermis and epidermis in the long roots from stagnant conditions were occupied by a high-density material that was absent in aerated roots (Fig. 3). This apparent deposition of additional material might have blocked these spaces and thus diminished O2 diffusivity. The present observations add to the larger-scale changes of suberization and lignification that have previously been visualized using histochemical staining and light microscopy in roots after longer treatment periods (Kotula et al., 2009). Histochemical staining of root sections in the present study (Fig. 4G, N) found similar changes to those described earlier for rice after 2–3 weeks in stagnant agar (Kotula et al., 2009), but these suberin deposits were not evident in roots within the first 2 d in the present study, during which time barrier induction was already complete (Fig. 4D, K). By 5 d of stagnant treatment, suberin deposits were evident in roots (Fig. 4F) although changes in lignin were not yet evident (Fig. 4M). Thus, the microstructural changes described here (Fig. 3) might, in addition to suberin depositions that can be visualized using histochemical stains at later stages, contribute to diminishing ROL. At 2 d of stagnant treatment, any suberin and lignin deposits might be lower than the detection limits of these histochemical methods. Thus, elucidation of the dynamics in ROL barrier induction was far superior using physiologically based assays of barrier functioning (i.e. ROL measurements), as compared with anatomical studies based on histochemical staining. Moreover, suberin deposits were accumulated before lignin, supporting the notion that suberin is of greater importance than lignin for the barrier to ROL in roots of rice.
The reason why long adventitious roots might form a barrier to ROL more quickly than short adventitious roots, as described in the present study (e.g. Fig. 2), could relate to tissue age. Basal tissues of long roots are older than those of short roots, and both suberin and lignin accumulation can be influenced by developmental stage (Schreiber et al., 1999; Kotula et al., 2009). Therefore, short adventitious roots might not yet be able to form a barrier to ROL owing to the young developmental stage of the tissues.
Rice forms lysigenous aerenchyma constitutively in well-drained soils or under aerated conditions (Webb and Jackson, 1986; Justin and Armstrong, 1991; Colmer et al., 2006). Aerenchyma formation in rice is further enhanced when in waterlogged soil (Armstrong, 1971) or in stagnant deoxygenated agar nutrient solution (Colmer, 2003a; Colmer et al., 2006; present study). Webb and Jackson (1986) showed degeneration in cortex of rice roots starting within 12-h-old tissue. The present study showed enhanced formation of aerenchyma within 12 h by stagnant treatment. Rice is able to form aerenchyma relatively quickly (12 h) as compared with some non-wetland plants (e.g. wheat took 24–48 h to start to form aerenchyma under deoxygenated stagnant conditions; Malik et al., 2003). The constitutive aerenchyma already present, and ability for rapid additional aerenchyma formation, would assist rice in minimizing or even escaping transient conditions of hypoxia when waterlogging first occurs, unless roots are longer than the maximal length (cf. Armstrong, 1979) supported by internal O2 diffusion.
Ethylene signalling has been implicated in enhanced aerenchyma formation in rice (Justin and Armstrong, 1991; Colmer et al., 2006) and other species [e.g. Zea mays (Drew et al., 1979; Konings, 1982)]. Aerenchyma formation in the present study of rice was especially enhanced in the apical region, as compared with basal regions (Fig. 5). Ethylene accumulates in stagnant deoxygenated agar nutrient solution (Wiengweera et al., 1997) and exogenous ethylene applied to rice also induced formation of aerenchyma more strongly in apical than in basal regions (Justin and Armstrong, 1991; Colmer et al., 2006).
Exogenous ethylene does not induce the barrier to ROL in roots of rice (Colmer et al., 2006), even though it enhances aerenchyma formation (Justin and Armstrong, 1991; Colmer et al., 2006). This implies that development of a barrier to ROL and development of aerenchyma are differentially regulated (Colmer et al., 2006; Shiono et al., 2008). Root length affected induction rate of the barrier to ROL (Figs 2 and 6), but root length did not influence aerenchyma formation (Fig. 5), which further supports the hypothesis that these two acclimations are differentially regulated. The factor that induces the barrier to ROL remains to be discovered, but based on barrier induction in response to phytotoxins in roots of rice (Armstrong and Armstrong, 2001, 2005), it has been suggested by Garthwaite et al. (2008) that accumulation of root exudates or of cellular degradation products, in stagnant media, might be involved in barrier induction.
Conclusions
The present study has demonstrated the dynamics in the formation of aerenchyma and induction of the barrier to ROL in roots of rice (Oryza sativa ‘Nipponbare’). Root length affected induction rate of the barrier to ROL, but, did not influence aerenchyma formation, which further supports the hypothesis, based on differential responses to ethylene, that these two acclimations are differentially regulated (Colmer et al., 2006). Moreover, we have shown the detailed dynamics of the formation a barrier to ROL in rice. Barrier induction, as measured in functional assays for patterns of ROL, occurred prior to histochemically detectable changes in suberin and lignin as viewed by light microscopy. So, structural changes required for barrier functioning are more subtle than previously recognized. Microstructural packing of intercellular spaces at the peripheral side of the exodermis may be involved. Suberin deposits may also be of more importance than lignin, as suberin increased prior to changes in lignin. Finally, we also estimated the threshold of ROL of 10 ng cm−2 min−1 for methylene blue staining during a 30–60 min incubation that would be useful to screen large numbers of rice lines (e.g. mutant screening) for changes in expression of a barrier to ROL in adventitious roots.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof. Yukuo Abe, Dr Masaru Ohta, Prof. Nobuhiro Tsutsumi, Prof. William Armstrong, Dr Jean Armstrong, Dr Al Imran Malik and Mr Hirokazu Takahashi for discussion and comments on this study. Prof. Hank Greenway, Prof. Lukas Schreiber, Dr Kosala Ranathunge and Dr Lukasz Kotula provided helpful comments on a final draft of this manuscript. Mr Ray Scott built the root-sleeving Pt electrodes and Ag/AgCl half cells. Prof. Keo Intabon and Mr Seiji Yokota are thanked for their help in setting up the cylindrical root-sleeving O2 electrodes in Japan. Ms Risa Ogawa is thanked for her support with the experiment of aerenchyma formation. Mr Tadashi Sakai, Dr Mayuko Sato, Ms Takako Kawai, Mr Hideo Tsunakawa and Mr Toshio Ito are thanked for their support, comments and suggestions regarding TEM. K.S. was supported by a JSPS Research Fellowship. M.N. received grants-in-aid from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation, IPG-0012), a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the Bio-oriented Technology Research Advancement Institution (Promotion of Basic Research Activities for Innovative Biosciences). H.I. received grants-in-aid from the Global Environment Research Fund of Ministry of Environment and CREST project of the Japan Science and Technology Agency.
LITERATURE CITED
- Armstrong J, Armstrong W. Phragmites australis – A preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytologist. 1988;108:373–382. [Google Scholar]
- Armstrong J, Armstrong W. Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany. 2001;88:1359–1370. [PubMed] [Google Scholar]
- Armstrong J, Armstrong W. Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany. 2005;96:625–638. doi: 10.1093/aob/mci215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W, Beckett PM. Phragmites australis – Venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytologist. 1992;120:197–207. [Google Scholar]
- Armstrong W. Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiologia Plantarum. 1971;25:192–197. [Google Scholar]
- Armstrong W. Aeration in higher plants. Advances in Botanical Research. 1979;7:225–332. [Google Scholar]
- Armstrong W. Polarographic oxygen electrodes and their use in plant aeration studies. Proceedings of the Royal Society of Edinburgh. 1994;102B:511–527. [Google Scholar]
- Armstrong W, Wright EJ. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum. 1975;35:21–26. [Google Scholar]
- Barton DS, Ollis WD. Heterocyclic compounds. In: Sammes PG, editor. Comprehensive organic chemistry. Oxford: Pergamon Press; 1979. pp. 1105–1106. [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA. Efficient lipid staining in plant material with sudan red 7B or fluoral yellow 088 in polyethylene glycol-glycerol. Biotechnic and Histochemistry. 1991;66:111–116. doi: 10.3109/10520299109110562. [DOI] [PubMed] [Google Scholar]
- Colmer TD. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.) Annals of Botany. 2003a;91:301–309. doi: 10.1093/aob/mcf114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell and Environment. 2003b;26:17–36. [Google Scholar]
- Colmer TD, Voesenek LACJ. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology. 2009;36:665–681. doi: 10.1071/FP09144. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany. 1998;49:1431–1436. [Google Scholar]
- Colmer TD, Cox MCH, Voesenek LACJ. Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist. 2006;170:767–778. doi: 10.1111/j.1469-8137.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- De Simone O, Haase K, Müller E, et al. Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four amazonian tree species. Plant Physiology. 2003;132:206–217. doi: 10.1104/pp.102.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, Jackson MB, Giffard S. Ethylene-promoted adventitious rooting and development of cortical air spaces (aerenchyma) in roots may be adaptive responses to flooding in Zea mays L. Planta. 1979;147:83–88. doi: 10.1007/BF00384595. [DOI] [PubMed] [Google Scholar]
- Garthwaite AJ, Armstrong W, Colmer TD. Assessment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist. 2008;179:405–416. doi: 10.1111/j.1469-8137.2008.02467.x. [DOI] [PubMed] [Google Scholar]
- Insalud N, Bell RW, Colmer TD, Rerkasem B. Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Annals of Botany. 2006;98:995–1004. doi: 10.1093/aob/mcl194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature. 2005;436:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Drew MC. Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT, editor. Flooding and plant growth. New York: Academic Press; 1984. pp. 47–128. [Google Scholar]
- Jensen WA. Botanical histochemistry – Principles and practice. San Francisco: WH Freeman; 1962. [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;106:465–495. [Google Scholar]
- Justin SHFW, Armstrong W. Evidence for the involvement of ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.) New Phytologist. 1991;118:49–62. [Google Scholar]
- Konings H. Ethylene-promoted formation of aerenchyma in seedling roots of Zea mays L. under aerated and non-aerated conditions. Physiologia Plantarum. 1982;54:119–124. [Google Scholar]
- Kotula L, Steudle E. Measurements of oxygen permeability coefficients of rice (Oryza sativa L.) roots using a new perfusion technique. Journal of Experimental Botany. 2009;60:567–580. doi: 10.1093/jxb/ern300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula L, Ranathunge K, Schreiber L, Steudle E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany. 2009;60:2155–2167. doi: 10.1093/jxb/erp089. [DOI] [PubMed] [Google Scholar]
- Lux A, Morita S, Abe J, Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AI, Colmer TD, Lambers H, Schortemeyer M. Aerenchyma formation and radial O2 loss along adventitious roots of wheat with only the apical root portion exposed to O2 deficiency. Plant Cell and Environment. 2003;26:1713–1722. [Google Scholar]
- Miyao A, Tanaka K, Murata K, et al. Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. The Plant Cell. 2003;15:1771–1780. doi: 10.1105/tpc.012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan SK, Varade SB, Kar S. Influence of soil water conditions on growth and root porosity of rice. Plant and Soil. 1973;38:501–507. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany. 1999;50:1267–1280. [Google Scholar]
- Shiono K, Takahashi H, Colmer TD, Nakazono M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Science. 2008;175:52–58. [Google Scholar]
- Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- Webb J, Jackson MB. A transmission and cryo-scanning electron microscopy study of the formation of aerenchyma (cortical gas-filled space) in adventitious roots of rice (Oryza sativa) Journal of Experimental Botany. 1986;37:832–841. [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany. 1997;80:115–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.