Abstract
Background and Aims
Alpine plants are considered one of the groups of species most sensitive to the direct and indirect threats to ecosystems caused by land use and climate change. Collecting and banking seeds of plant species is recognized as an effective tool for providing propagating material to re-establish wild plant populations and for habitat repair. However, seeds from cold wet environments have been shown to be relatively short lived in storage, and therefore successful long-term seed conservation for alpine plants may be difficult. Here, the life spans of 69 seed lots representing 63 related species from alpine and lowland locations from northern Italy are compared.
Methods
Seeds were placed into experimental storage at 45 °C and 60 % relative humidity (RH) and regularly sampled for germination. The time taken in storage for viability to fall to 50 % (p50) was determined using probit analysis and used as a measure of relative seed longevity between seed lots.
Key Results
Across species, p50 at 45 °C and 60 % RH varied from 4·7 to 95·5 d. Seed lots from alpine populations/species had significantly lower p50 values compared with those from lowland populations/species; the lowland seed lots showed a slower rate of loss of germinability, higher initial seed viability, or both. Seeds were progressively longer lived with increased temperature and decreased rainfall at the collecting site.
Conclusions
Seeds of alpine plants are short lived in storage compared with those from lowland populations/related taxa. The lower resistance to ageing in seeds of alpine plants may arise from low selection pressure for seed resistance to ageing and/or damage incurred during seed development due to the cool wet conditions of the alpine climate. Long-term seed conservation of several alpine species using conventional seed banking methods will be problematic.
Keywords: Seed longevity, climate, alpine plants, seed bank storage
INTRODUCTION
Alpine plants are particularly vulnerable to current threats to plant diversity such as land management changes (Rossi et al., 2008), tourism (Ferrarini et al., 2008) and climate change (Thuiller, 2007; Parolo and Rossi, 2008). Ex situ conservation through seed banks will play an important role in safeguarding alpine species and will provide propagating material for in situ conservation and habitat restoration (UNEP, 1992). Seed banking projects, targeting endangered and narrow endemic alpine plants, have already been initiated (e.g. Bonomi et al., 2005) and are recognized as a valid option for the survival of these plants (Rossi et al., 2007). However, so far little is known about seed life span in dry storage of alpine plants.
One of the original tenets of the viability equations of Ellis and Roberts (1980), which are used to predict seed lot longevity in air-dry storage including seed bank conditions, is that different seed lots of the same species stored under identical conditions will follow the same normal distribution of seed deaths over time, differing only in initial viability. In contrast, for seeds of different species stored under identical conditions, there may be considerable variation in both initial viability and the distribution of seed deaths over time, leading to very large differences in the time for viability to fall to, for example, 50 % (p50). As a measure of seed longevity across species, this p50 parameter has been shown to vary in relation to taxonomy/seed structure and macroclimate at the geographic origin of the species (Probert et al., 2009). Walters et al. (2005) similarly reported taxonomic- and origin-related trends, using Avrami kinetics to estimate p50 from seed bank seed survival data.
Probert et al. (2009) went further and proposed a model, developed from seed survival data for 195 species, in which p50 is a function of the presence or absence of endosperm, mean annual temperature and mean total annual rainfall. In this model, seeds of species from cool, wet climates are predicted to be shorter lived in air-dry storage than those of species originating in warm, dry climates. Seeds from plants growing in alpine regions may be inherently short lived in air-dry storage. However, much of the variation in p50 was not explained by the fitted model. In order to provide more accurate predictions of which species' seeds will be short lived, as well as to gain greater understanding of the factors that result in greater inherent seed longevity, it is necessary to explore further within smaller taxonomic groups and/or geographic ranges.
Within species, it is known that the effects of maternal environment during seed development and maturation can affect various seed traits, including desiccation tolerance (Daws et al., 2004) and longevity (Kochanek et al., 2010), and a number of reports have shown that immature seeds are significantly shorter lived than seeds that have attained peak maturity (e.g. Hay and Smith, 2003; Probert et al., 2007). Since alpine regions are characterized by a short growing season as well as cool temperatures, it is possible that one of the reasons why the seeds are short lived is because seed development, particularly the late maturation (or desiccation) phase, is contracted.
The aim of the work presented herein was to assess the goodness of fit of the model of Probert et al. (2009), thereby (a) testing the hypothesis that seeds from alpine plants are short lived in air-dry storage compared with seeds of the same or related species from nearby, lowland sites; and (b) determining how much of the variation in seed longevity between alpine and lowland plants is correlated with differences in climate and seed structure (presence of endosperm). By looking at seed weights, we also considered whether, for species collected from both alpine and lowland populations, differences in longevity could be due to differences in seed maturity at the time of collection.
MATERIALS AND METHODS
Seeds
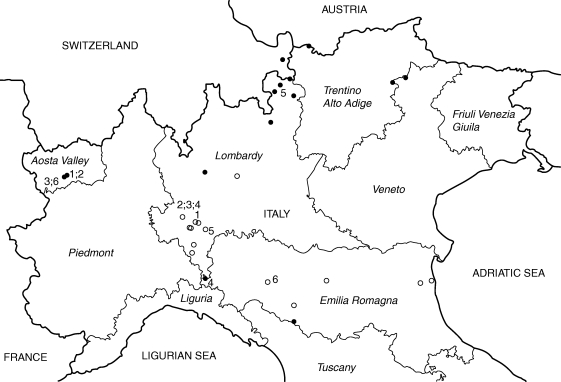
Seeds were collected on the point of natural dispersal from various locations in northern Italy (Fig. 1) between May and September 2008 (one collection per population) and held at the Lombardy Seed Bank (Banca del germoplasma delle piante lombarde/Centro Flora Autoctona della Regione Lombardia) under international seed bank standard conditions of –20 °C after drying at 15 % relative humidity (RH) and 15 °C (FAO/IPGRI, 1994). Based on their occurrence and abundance in the study area, 27 species were selected from lowland sites (approx. 0–400 m a.s.l.), 36 species from alpine sites (approx. 1800–2400 m a.s.l.) and six species from both alpine and lowland sites (Table 1). For this latter group of species, the distance between sampled populations ranged between 69 km (Plantago lanceolata) and 255 km (Verbascum thapsus). Collections represented 20 genera and ten families. The high initial viability (>80 %) of all seed lots was confirmed through germination tests (Table 1). Mean seed weight (seeds equilibrated at 15 °C and 15 % RH) was determined for five replicates of 50 seeds each for each seed lot of the six species collected from both lowland and mountain populations. Finally, for each species, the presence/absence of endosperm was determined with reference to Martin (1945).
Fig. 1.
Map of the collecting sites in lowland (open symbols) and alpine (filled symbols) locations of northern Italy. Numbers represent the species collected from both alpine and lowland populations: 1, Achillea millefolium; 2, Dianthus carthusianorum; 3, D. sylvestris; 4, Plantago lanceolata; 5, Silene vulgaris; 6, Verbascum thapsus.
Table 1.
Details of the species used in the study
| Species name | Family | Provenance | p50 ± s.e. (d) | Germination temperature (°C) |
|---|---|---|---|---|
| Species collected from lowland and alpine populations | ||||
| Achillea millefolium L. | Asteraceae | L | 59·5 ± 1·84 | 30/20* |
| A | 27·4 ± 1·28 | 30/20* | ||
| Dianthus carthusianorum L. | Caryophyllaceae | L | 59·5 ± 2·19 | 15 |
| A | 37·5 ± 0·78 | 15 | ||
| Dianthus sylvestris Wulfen | Caryophyllaceae | L | 41·7 ± 1·80 | 15 |
| A | 34·2 ± 1·38 | 15 | ||
| Plantago lanceolata L. | Plantaginaceae | L | 58·9 ± 1·87 | 25† |
| A | 22·4 ± 1·90 | 20† | ||
| Silene vulgaris (Moench) Garcke | Caryophyllaceae | L | 59·7 ± 1·93 | 25 |
| A | 15·1 ± 0·93 | 25 | ||
| Verbascum thapsus L. | Scrophulariaceae | L | 57·5 ± 2·15 | 25 |
| A | 32·7 ± 1·27 | 25 | ||
| Species collected from either a lowland or an alpine population | ||||
| Achillea clavenae L. | Asteraceae | A | 14·5 ± 0·95 | 30/20* |
| Achillea ligustica All. | Asteraceae | A | 18·2 ± 0·91 | 25 |
| Achillea moscata Wulfen | Asteraceae | A | 19·3 ± 1·97 | 30/20* |
| Armeria plantaginea (All.) Willd. | Plumbaginaceae | L | 34·6 ± 1·30 | 20† |
| Armeria alpina Willd. | Plumbaginaceae | A | 9·6 ± 0·67 | 25/15*,† |
| Artemisia glacialis L. | Asteraceae | A | 26·4 ± 0·92 | 15 |
| Artemisia umbelliformis Lam. | Asteraceae | A | 10·9 ± 0·87 | 15 |
| Artemisia vulgaris L. | Asteraceae | L | 40·0 ± 1·84 | 25 |
| Campanula barbata L. | Campanulaceae | A | 13·2 ± 0·73 | 20† |
| Campanula rapunculus L. | Campanulaceae | L | 39·9 ± 1·60 | 25 |
| Campanula scheuchzeri Vill. | Campanulaceae | A | 13·0 ± 0·85 | 20† |
| Centaurea deusta Ten. | Asteraceae | L | 65·8 ± 3·09 | 25 |
| Centaurea maculosa Lam. | Asteraceae | L | 76·3 ± 2·87 | 25 |
| Centaurea nervosa Willd. | Asteraceae | A | 34·2 ± 1·84 | 30/20*,† |
| Centaurea scabiosa L. | Asteraceae | A | 27·3 ± 2·54 | 30/20* |
| Cerastium arvense L. | Caryophyllaceae | A | 11·6 ± 0·76 | 25/15*,†,‡ |
| Cerastium holosteoides Fries ampl. Hylander | Caryophyllaceae | L | 34·0 ± 0·95 | 20/10*,‡ |
| Cirsium arvense (L.) Scop. | Asteraceae | L | 22·7 ± 1·63 | 30/20* |
| Cirsium spinosissimum (L.) Scop. | Asteraceae | A | 15·3 ± 1·45 | 25/15*,† |
| Dianthus glacialis Haenke | Caryophyllaceae | A | 10·9 ± 0·85 | 25/15*,‡ |
| Epilobium alpestre (Jacq.) Krocker | Onagraceae | A | 13·1 ± 0·84 | 25† |
| Epilobium hirsutum L. | Onagraceae | L | 38·0 ± 1·23 | 25 |
| Festuca alpina Suter | Poaceae | A | 15·2 ± 0·70 | 15 |
| Festuca inops De Not. | Poaceae | L | 24·7 ± 1·94 | 25/15* |
| Festuca violacea Gaudin | Poaceae | A | 5·2 ± 0·60 | 20 |
| Hypericum maculatum Crantz | Guttiferae | A | 30·6 ± 1·05 | 20† |
| Hypericum perforatum L. | Guttiferae | L | 33·3 ± 2·66 | 15‡ |
| Hypericum richeeri Vill. | Guttiferae | A | 9·3 ± 1·07 | 20‡ |
| Plantago alpina L. | Plantaginaceae | A | 10·3 ± 1·24 | 15† |
| Plantago major L. | Plantaginaceae | A | 27·2 ± 1·14 | 25 |
| Plantago maritima L. | Plantaginaceae | L | 86·3 ± 3·23 | 20 |
| Plantago serpentina All. | Plantaginaceae | A | 26·2 ± 0·90 | 25† |
| Rumex alpestris Jacq. | Polygonaceae | A | 26·2 ± 1·31 | 20/10*,† |
| Rumex alpinus L. | Polygonaceae | A | 25·5 ± 0·90 | 25/15 |
| Rumex crispus L. | Polygonaceae | L | 78·2 ± 2·00 | 25/15* |
| Senecio incanus L. ssp. carniolicus (Willd.) Br.-Bl. | Asteraceae | A | 10·3 ± 0·71 | 25/15*,† |
| Senecio vulgaris L. | Asteraceae | L | 13·1 ± 0·70 | 15 |
| Sesleria pichiana Foggi, Gr. Rossi & Pignotti | Poaceae | L | 37·8 ± 1·34 | 15 |
| Sesleria varia (Jacq.) Wettst. | Poaceae | A | 12·1 ± 0·82 | 15‡ |
| Silene acaulis (L.) Jacq. | Caryophyllaceae | A | 13·8 ± 0·85 | 25† |
| Silene alba (Miller) Krause | Caryophyllaceae | L | 51·1 ± 1·90 | 20 |
| Silene armeria L. | Caryophyllaceae | L | 37·0 ± 1·53 | 15 |
| Silene dioica (L.) Clairv. | Caryophyllaceae | A | 15·5 ± 0·70 | 20 |
| Silene nutans L. | Caryophyllaceae | A | 15·4 ± 0·82 | 20 |
| Silene paradoxa L. | Caryophyllaceae | L | 49·2 ± 1·80 | 20 |
| Silene rupestris L. | Caryophyllaceae | A | 11·5 ± 0·73 | 15 |
| Silene saxifraga L. | Caryophyllaceae | A | 21·7 ± 0·97 | 20 |
| Silene suecica (Lodd.) Greuter & Burdet | Caryophyllaceae | A | 18·2 ± 0·94 | 20† |
| Silene vallesia L. | Caryophyllaceae | A | 26·5 ± 0·83 | 20 |
| Silene vulgaris (Moench) Garcke ssp glaerosa (Jordan) Marsd.- J. et Turrill | Caryophyllaceae | A | 11·5 ± 1·02 | 25† |
| Taraxacum alpinum (Hoppe) Hegetschw. | Asteraceae | A | 7·1 ± 0·85 | 15 |
| Taraxacum officinale Weber | Asteraceae | L | 10·7 ± 1·04 | 15 |
| Verbascum alpinum Turra | Scrophulariaceae | A | 25·1 ± 1·23 | 25† |
| Verbascum sinuatum L. | Scrophulariaceae | L | 95·4 ± 4·74 | 25 |
| Veronica alpina L. | Scrophulariaceae | A | 4·7 ± 0·04 | 25/15*,† |
| Veronica hederifolia L. | Scrophulariaceae | L | 12·0 ± 0·11 | 20†,‡ |
| Veronica persica Poiret | Scrophulariaceae | L | 43·4 ± 1·56 | 20† |
Classification to family level (following Pignatti, 1982), provenance [lowland (L) alpine (A)], time to 50 % viability loss (p50 ± s.e.) at 45 °C, 60 % RH and germination conditions used. All non-germinated seeds were cut-test at the end of the test and found to be dead.
*The elevated temperature was applied for 12 h each day, with light provided during the warm phase.
†GA3 at 250 mg L−1 was included in the agar germination medium.
‡Dormancy breaking pre-treatment at 0 °C for 14 d.
Experimental storage
Due to logistical and time constraints with comparing storage life span under seed bank conditions, seed longevity was determined using a standard rapid ageing protocol (Newton et al., 2009; Probert et al., 2009). Ageing experiments commenced in March 2009 and were completed within approx. 5 months. Experiments were carried out in two laboratories: the Millennium Seed Bank (UK) and the Lombardy Seed Bank (Italy), using identical equipment and procedures. To serve as a control, sample of seeds of Myosotis arvensis originating from the same seed lots were included in the ageing experiments performed in these two laboratories and/or started at different times.
To raise the moisture content of the seeds prior to ageing and to minimize the subsequent adjustment of moisture content when samples were transferred to the ageing conditions, ten samples of 50 seeds each were rehydrated at 47 % RH at 20 °C in open glass vials or Petri dishes; the vials/dishes were placed over a non-saturated solution of LiCl (anhydrous, Laboratory Reagent Grade; Fisher Scientific UK Ltd, Leicester, UK) in distilled water held in a sealed 300 × 300 × 130 mm electrical enclosure box (Ensto UK Ltd, Southampton, UK). At the end of the rehydration period (14 d), seed equilibrium relative humidity (eRH) was checked using a sample of the equilibrating seeds or, if the volume of seeds was too small, a reference sample of Agrostis gigantea seeds that accompanied the test species during rehydration. The eRH was measured using a water activity measuring instrument which comprised a hygrometer sensor housed in an AW-DI0 water activity probe, used in conjunction with a HygroPalm 3 display unit (Rotronic Instruments UK Ltd, Crawley, UK). Once the test species was judged to have reached equilibrium, samples were transferred to a second electrical enclosure box, over a non-saturated solution of LiCl at 60 % RH placed in a compact incubator (LEEC Ltd, Nottingham, UK) without light at 45 ± 2 °C. The RH generated by the LiCl in the box was checked at 4- to 6-week intervals by pipetting a sample of approx. 10 ml of solution and placing it into the sample chamber of the water activity-measuring instrument described above. The bulk solution was adjusted if necessary, usually by adding distilled water, stirring and allowing the solution to equilibrate before rechecking the RH (Hay et al., 2008).
One sample of 50 seeds for each seed lot was removed after 1, 2, 5, 9, 20, 30, 50, 75, 100, 120 and 150 d for germination testing. Seeds were sown on 1 % distilled water agar held in 90 mm diameter Petri dishes and placed in an LMS 250A cooled incubator (LMS Ltd, Sevenoaks, UK) under a temperature regime (constant or alternating) previously found to be optimal for germination of that accession (Table 1). Some of the seed lots required additional treatments including the addition of GA3 (250 mg L−1; Sigma-Aldrich Company Ltd, Dorset, UK) or cold stratification (typically at 0 °C for 14 d) to remove dormancy. Plates were checked weekly for germination and seeds scored as germinated once the radicle had reached approx. 2 mm. At the completion of each germination test, ungerminated seeds were cut-tested to confirm that they were not viable.
Data analysis
Probit analysis was carried out on the data using GenStat Release 11·1 (VSN International Ltd, Oxford, UK) to estimate the time for viability to fall to 50 % (p50) by fitting the viability equation (Ellis and Roberts 1980):
| (1) |
where v is the viability (in normal equivalent deviates, NED) of the seed lot after p days in storage, Ki is the initial viability (NED) of the seed lot, and σ is the time (d) for viability to fall by 1 NED (i.e. the standard deviation of the normal distribution of seed deaths over time). Analysis of residual deviance (variance ratio test following the F-distribution) was used to test for significance when constraining survival curve data for multiple seed lots to common estimates for Ki and/or σ. Analysis of variance (ANOVA) was used for comparing log10 (p50) with seed lot origin (lowland vs. alpine) as a factor, using Minitab version 13 (Minitab Inc., State College, PA, USA).
Monthly mean temperature and total monthly rainfall values, based on data collated between 1950 and 2000, were obtained for the collection location of each seed lot by querying WORLDCLIM data (download version 1·4 http://www.worldclim.org/) at a maximum resolution of 30 arc-seconds (approx. 1 km) using the ‘Extract Values to points’ tool in ERSI ArcMap (Version 9·1). The reproductive period (duration between flowering and seed dispersal) was estimated from phenology information for the Italian flora (Pignatti, 1982). Thus, for each population, the mean temperature and the total rainfall during and outside the reproductive period, as well as the mean annual temperature and total annual rainfall, were calculated. The FITNONLINEAR directive in GenStat Release 11·1 was used to fit the Probert et al. (2009) equation to predict relative seed longevity in the experimental storage conditions used in this study using the equation:
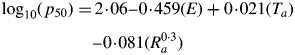 |
(2) |
where Ta is mean annual temperature, Ra is mean total annual rainfall, and E = 1 or E = 0 indicates the presence or absence of endosperm, respectively. Further regression analyses were carried out in GenStat to determine whether other rainfall and temperature parameters better explained the variation in longevity than mean annual temperature and mean total annual rainfall. For these, p50 was log10-transformed to ensure normality.
t-Tests were used to compare seed weights for six species represented by both lowland and alpine populations (paired) or to compare log10 (p50) values for endospermic and non-endospermic seeds (unpaired).
RESULTS
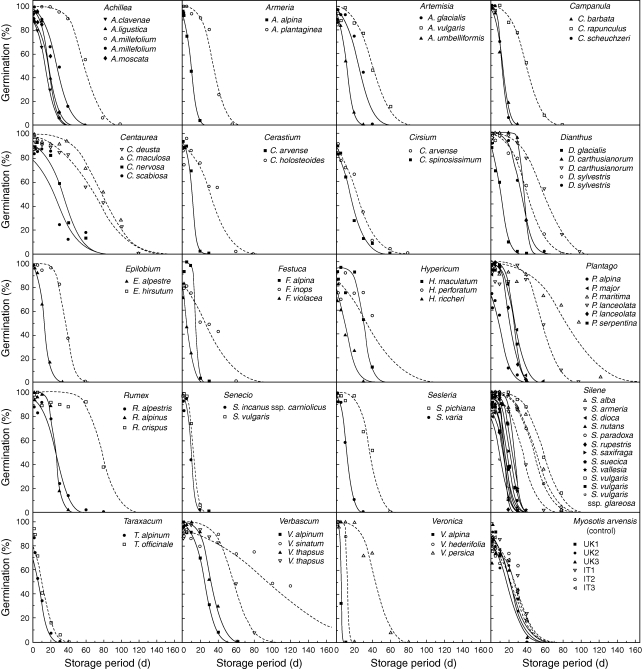
Seed viability declined as the period of experimental storage increased, for all seed lots (Fig. 2), but with wide variation (4·7–95·4 d) in the time taken for viability to fall to 50 % (p50; Table 1). Variation in p50 between seed lots was explained by differences in the rate at which seeds lost the ability to germinate (1/σ) and the initial seed viability (Ki) (not shown, but compare slopes and intercepts, respectively, for the survival curves shown in Fig. 2). This was also the case when comparing seed survival data for congeneric species or for seed lots of the same species collected from both alpine and lowland populations. Comparing seed survival data across genera, Taraxacum, Senecio and Veronica were the shortest lived, with p50 values less than approx. 15 d, while Verbascum, followed by Plantago had the highest p50 values of 95 and 86 d, respectively (Table 1 and Fig. 2).
Fig. 2.
Survival curves fitted by probit analysis for all lowland (open symbols, broken lines) and alpine (filled symbols, continuous lines) seed lots. The final graph at bottom-right shows the survival curves of the control species (Myosotis arvensis) in the UK (filled symbols, continuous lines) and Italian (open symbols, broken lines) laboratories. Seeds were placed into experimental storage conditions of 45 °C and 60 % RH. All non-germinated seeds were cut-test at the end of the test and found to be dead.
The lowland seed lots consistently showed a slower rate of loss of germinability, higher initial seed viability, or both (Fig. 2). In some cases, although there were significant differences in the seed survival curves for species within genera collected from similar environments (i.e. alpine or lowland), these differences were small compared with the differences between survival curves of congeneric species collected from contrasting environments (e.g. see Fig. 2, Plantago and Silene species). For example, the mean p50 value for seed lots of alpine Silene sp. is 16 ± 2·2 d (± s.e.), while that of seed lots from the lowland Silene sp. was >3 times larger (50 d). ANOVA confirmed that, across all seed lots tested, alpine seed lots had significantly lower p50 values compared with those from the lowland seed lots (F = 24·81; P < 0·001).
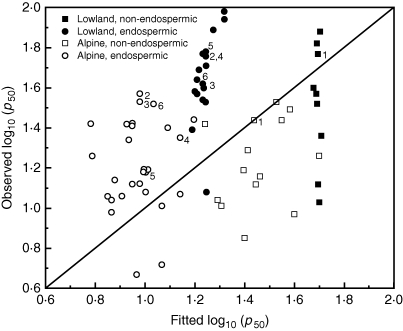
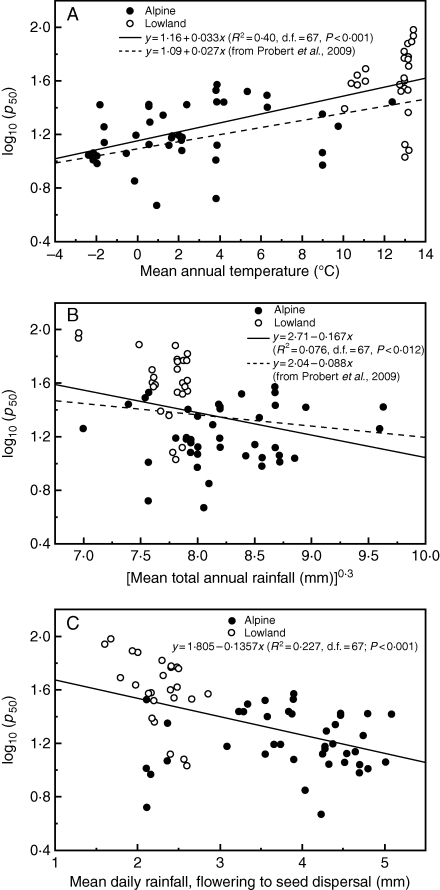
Using the Probert et al. (2009) model to predict log10 (p50) for these seed lots under the standard experimental storage conditions used here, the residual variance was greater than the observed variance in log10 (p50). In general, the model overestimated the longevity of non-endospermic seeds and underestimated the longevity of endospermic seeds (Fig. 3). Furthermore, the model explained very little of the variation in p50 for lowland seed lots. It was not possible to carry out further multiple regression analysis since, for these seed lots, these climate variables were correlated (alpine habitats tend to experience both cooler temperatures and greater rainfall than lowland habitats). However, simple linear regression analysis indicated a similar relationship between log10 (p50) and mean annual temperature to that shown in Probert et al. (2009), accounting for 40·2 % of the variance (Fig. 4A). There were also significant relationships between log10 (p50) and other temperature variables (mean temperature during or outside the reproductive period); however, none accounted for more of the total variation in p50 (not shown). The relationship between log10 (p50) and mean total annual rainfall was weaker, accounting for <10 % of the variation (Fig. 4B). A better fit was observed if only the mean daily rainfall between flowering and seed dispersal was considered, rather than total annual rainfall, accounting for >20 % of the variation (Fig. 4C). Thus, seed longevity increased with an increase in the mean annual temperature and a decrease in the mean daily rainfall during seed development and, accordingly, seeds from alpine species/populations were shorter lived than seeds from lowland species/populations (Fig. 4). There was no significant difference in the mean log10 (p50) values for endospermic and non-endospermic seeds (t = –0·56, d.f. = 0·67, P = 0·58).
Fig. 3.
Co-plot of log10 (p50) values determined in this study and those predicted, based on climate data and presence/absence of endosperm, using the Probert et al. (2009) model (eqn 2). The solid line shows the exact correlation between the two (y = x). Numbers represent the species collected from both alpine and lowland populations: 1, Achillea millefolium; 2, Dianthus carthusianorum; 3, D. sylvestris; 4, Plantago lanceolata; 5, Silene vulgaris; 6, Verbascum thapsus.
Fig. 4.
Relationship between p50 and (A) mean annual temperature at the collecting site, (B) mean total annual rainfall and (C) mean daily rainfall during the reproductive phase (flowering to seed dispersal) for alpine and lowland seed lots collected in northern Italy from 63 species. Solid lines show the results of linear regression analysis; dashed lines show the results of linear regression analysis for 195 species as described in Probert et al. (2009).
Seeds from alpine species/populations were either significantly heavier or not significantly different in weight compared with seeds from lowland species/populations (Table 2).
Table 2.
Mean seed weight and results of the t-test analysis used to investigate the variation between lowland and mountain populations of the same species
| Weight (mg) mean ± s.e. |
||||
|---|---|---|---|---|
| Species | Alpine | Lowland | t-value | P-value |
| Achillea millefolium | 0·007 ± 0·0001 | 0·003 ± 0·0001 | –12·58 | <0·001 |
| Dianthus carthusianorum | 0·027 ± 0·0005 | 0·023 ± 0·0003 | 5·63 | 0·005 |
| Dianthus sylvestris | 0·044 ± 0·0005 | 0·046 ± 0·0017 | –1·08 | 0·340 |
| Plantago lanceolata | 0·142 ± 0·0038 | 0·052 ± 0·0028 | –19·08 | <0·001 |
| Silene vulgaris | 0·039 ± 0·0012 | 0·026 ± 0·0002 | –9·18 | <0·001 |
| Verbascum thapsus | 0·006 ± 0·0001 | 0·006 ± 0·0001 | –1·83 | 0·142 |
Viability loss among seed lots of the control species M. arvensis was not significantly different (P > 0·05), justifying the consistency of the ageing environments between experiments carried out in the different laboratories and/or started at different times.
DISCUSSION
Understanding species difference in seed longevity is crucial to the effective management of seed conservation collections because it underpins the selection of viability re-test intervals and hence regeneration or re-collection strategies (Probert et al., 2009). The results presented here confirm that, for seeds stored under identical conditions (60 % RH, 45 °C), there can be significant variation in seed longevity both between and within species. The estimates for p50 ranged between 4·7 d (Veronica alpina) and 95·4 d (Verbascum sinuatum). For seed lots of 195 taxonomically diverse species collected worldwide and placed into the same conditions of experimental storage, Probert et al. (2009) reported estimates for p50 ranging between 0·1 and 771 d. Thus, compared with this broader data set, all the seed lots in the present study can be described as having short to medium longevity in air-dry storage (based on a logartihmic scale to categorize species according to their relative longevity: p50 ≤ 1 d indicates ‘very short’ seed longevity; p50 > 1 to ≤ 10, ‘short’; p50 > 10 to ≤ 100, ‘medium’; p50 > 100 to ≤ 1000, ‘long’; p50 > 1000, ‘very long’).
Using climate data for the collection locations, p50 values were predicted for these lowland and alpine seed lots, using eqn (2) (Probert et al., 2009). There was much more variation in the mean annual temperature and mean annual rainfall between the alpine seed lots than the lowland seed lots (Fig. 4A, B), resulting in a wider range of predicted values (Fig. 3). However, for both the whole data set and for the data for alpine seed lots only, eqn (2) did not explain a significant amount of the observed variation in p50. Further, the model tended to overestimate the longevity of non-endospermic seeds and underestimate the longevity of endospermic seeds (Fig. 3). t-Test analysis showed that there was not a significant difference in the mean log10 (p50) of endospermic and non-endospermic seeds; thus seed structure was not a significant factor in accounting for variation in seed longevity for this set of species.
Considering climate variables independently (necessary since, for these seed lots, rainfall and temperature were correlated), there was still a highly significant relationship between log10 (p50) and mean annual temperature (Fig. 4A). Seed longevity increased as mean annual temperature increased; temperature conditions during the reproductive period did not account for more of the variation in seed longevity than the mean annual temperature (result not shown). There was also a significant relationship between log10 (p50) and mean total annual rainfall (P < 0·012); however a stronger relationship was apparent when rainfall during the reproductive period was considered (P < 0·001; Fig. 4B, C). The seed lots collected from alpine areas that tended to have cooler and wetter climates were shorter lived than the seed lots collected from lowland sites, even when comparing seed lots from congeneric species, the only exceptions being Taraxacum and Senecio (Fig. 2). Most notably, this also held for seed lots of the same species collected from both alpine and lowland populations: Achillea millefolium, Dianthus carthusianorum, D. sylvestris, Plantago lanceolata, Silene vulgaris and Verbascum thapsus. For example, the p50 for the lowland Silene vulgaris seed lot was 59 d compared with 15 d for the alpine seed lot, with the latter having both lower initial seed viability and a faster rate of loss of ability to germinate (P < 0·001). The Taraxacum officinalis and Senecio vulgaris seed lots were relatively short lived despite originating from lowland sites; however, both are early spring flowering species (Pignatti, 1982), and therefore normally experience relatively low temperatures and high rainfall during the flowering and seed development period.
In a recent study of the effect of maternal environment on seed longevity in Wahelenbergia tumidifructa (Kochanek et al., 2010), the effect of temperature on seed longevity depended on the growth phase of the plants. While low temperatures prior to seed set had either no effect or enhanced seed longevity compared with high temperatures, low temperatures during the seed development and ripening phase significantly reduced seed longevity. This is consistent with our finding that rainfall during the reproductive phase was more critical than mean annual rainfall or rainfall outside of the reproductive phase. Kochanek et al. (2010) also found that dry conditions combined with warmth during seed maturation led to increased seed longevity, and Sinniah et al. (1998) found that the longevity of Brassica campestris seeds was greater if maternal plants experienced drought during seed development.
Reduced viability and vigour of seeds ripened under cool, wet conditions may be due to higher rates of oxidative stress during maturation; Waterworth et al. (2010) have shown that prolonged imbibition of Arabidopsis thaliana seeds at low temperature results in additional DNA damage due to increased oxidative stress under these conditions. Another explanation for the observed differences in longevity is that the climate conditions that alpine plants normally experience during the reproductive period may affect seed development and maturation, reducing seed quality at dispersal. In general, the more immature the seed lot is, the lower its initial viability and/or the faster its loss of germinability (Ellis et al., 1993; Ellis and Hong, 1994; Hay and Probert, 1995; Sanhewe and Ellis, 1996). For example, the survival curves of immature seeds of Digitalis purpurea showed both lower initial seed viability and faster loss of germinability, compared with those at an advanced developmental stage (Hay and Probert, 1995). Furthermore, across five growing sites of Aesculus hippocastanus spanning 19° of latitude in Europe, Daws et al. (2004) found that desiccation tolerance increased when seeds developed under warmer conditions because development progressed further before seed shed. In that study, seed weight was found to be positively correlated with the degree of desiccation tolerance. Although our seed lots were neither desiccation sensitive (as are seeds of Aesculus hippocastanus) nor collected across a range of latitudes, and despite being collected at the point of natural dispersal, the cooler, wetter conditions experienced by the alpine plants could have had a negative affect on seed ripening and thus longevity. However, if this were the case we would have expected that seeds collected from plants growing at the alpine locations would have been smaller than their lowland counterparts which would have developed under more favourable conditions. In fact, for the six species for which we had both lowland and alpine populations, there was either no significant difference in seed weight or the seeds from the alpine sites were significantly heavier (Table 2). Seed size was therefore not positively correlated with seed quality in terms of longevity in air-dry storage. Consistent with this, Lehtilä and Ehrlén (2005) concluded that factors that may influence seed size do not necessarily also influence seed quality. The weaker resistance to ageing in seeds from alpine plants does not seem to be simply related to maturity.
Whilst there is compelling evidence that environmental conditions strongly influence seed longevity, the possibility that differences between alpine and lowland plants may also be explained by adaptive, genetic differences linked to climate cannot be ruled out. Supporting this view, preliminary experiments carried out at the Millennium Seed Bank laboratories (RBG, Kew, UK) have shown that seed longevity differences in the offspring of plants of Silene vulgaris originating from Morocco and England grown under identical glasshouse conditions were identical to the differences observed in the seeds from which they were grown. It is possible that populations and species adapted to alpine habitats have not experienced the same selection pressure for seed resistance to ageing as populations and species growing in warmer, drier, lowland habitats. Seeds buried in the substrate at alpine sites are certainly likely to deteriorate at a slower rate than seeds buried in lowland soils following dispersal simply because the average temperature experienced will be lower. This alone means that there will be less selective pressure on resistance to ageing. Supporting the idea that seeds of alpine plants deteriorate slowly under natural conditions, the seeds of several alpine plants have been shown to survive for many years in their natural habitat (Schwienbacher et al., 2008). This possibility that measured differences between lowland and alpine collections could be explained by genetic differences will be explored by reciprocal transplant experiments.
This study has highlighted a significant concern for the successful ex situ conservation of alpine plants, which represent one of the groups most sensitive to the direct and indirect human impacts on plant diversity (Korner, 1999; Thuiller, 2007; Parolo and Rossi, 2008). We have shown that seeds of alpine populations and species are significantly shorter lived than lowland counterparts. Conservation collections of alpine species held in seed banks will need to be re-tested more frequently to monitor their potential decline in viability during storage, and alternative storage techniques such as cryopreservation should be considered for the shortest lived cases (Li and Pritchard, 2009). Further research is underway to resolve the extent to which seed longevity is determined by environmental or genetic factors.
ACKNOWLEDGEMENTS
This work was supported by the University of Pavia, the Centro Flora Autoctona of the Lombardy Region, the Italian Ministry for Education, University and Research (MIUR, project no. 2007JNJ7MX_004) and the National Research Council (CNR) project SHARE. Financial support was also provided by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew is supported by a grant-in-aid from Defra. The authors would like to express their thanks to Kenwin Liu, Ian Wood and Simone Pedrini.
LITERATURE CITED
- Bonomi C, Rossi G, Mondoni A, et al. XVII International Botanical Congress. Vienna, Austria: 2005. Seed conservation activities for narrow endemic species in the italian Alps. Abstract p.609. [Google Scholar]
- Daws MI, Lydall E, Chmielarz P, et al. Developmental heat sum influences recalcitrant seed traits in Aesculus hippocastanum across Europe. New Phytologist. 2004;162:157–166. [Google Scholar]
- Ellis RH, Hong TD. Desiccation tolerance and potential longevity of developing seeds of rice (Oryza sativa L.) Annals of Botany. 1994;73:501–506. [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- Ellis RH, Hong TD, Jackson MT. Seed production environment, time of harvest, and the potential longevity of seeds of three cultivars of rice (Oryza sativa L.) Annals of Botany. 1993;72:583–590. [Google Scholar]
- FAO/IPGRI. Genebank standards. Rome: Food and Agriculture Organisation of the United Nations/International Plant Genetic Resource Institute; 1994. [Google Scholar]
- Ferrarini A, Rossi G, Parolo G, Ferloni M. Planning low-impact tourist paths within a Site of Community Importance through the optimisation of biological and logistic criteria. Biological Conservation. 2008;141:1067–1077. [Google Scholar]
- Hay FR, Probert RJ. The effect of different drying conditions and maturity on desiccation tolerance and seed longevity in Digitalis purpurea L. Annals of Botany. 1995;76:639–647. [Google Scholar]
- Hay FR, Smith RD. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linnington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. pp. 97–133. [Google Scholar]
- Hay FR, Adams J, Manger K, Probert RJ. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology. 2008;36:737–746. [Google Scholar]
- Kochanek J, Buckley JM, Probert RJ, Adkins SW, Steadman KJ. Pre-zygotic parental environment modulates seed longevity. Austral Ecology. 2010;35:837–848.. [Google Scholar]
- Korner C. Alpine plant life. Functional plant ecology of high mountain ecosystems. 2nd edn. Berlin: Springer; 1999. [Google Scholar]
- Lehtilä K, Ehrlén J. Seed size as an indicator of seed quality: a case study of Primula veris. Acta Oecologica. 2005;28:207–212. [Google Scholar]
- Li DZ, Pritchard HW. The science and economics of ex situ plant conservation. Trends in Plant Science. 2009;14:614–621. doi: 10.1016/j.tplants.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. The American Midland Naturalist. 1945;36:513–660. [Google Scholar]
- Newton R, Hay F, Probert R. Protocol for comparative seed longevity testing. 2009 Technical Information Sheet_01, Royal Botanic Gardens Kew, UK. www.kew.org/msbp/scitech/publications/01-Comparative%20longevity.pdf . [Google Scholar]
- Parolo G, Rossi G. Upward migration of vascular plants following a climate warming trend in the Alps. Basic and Applied Ecology. 2008;9:100–107. [Google Scholar]
- Pignatti S. Flora d'Italia. Bologna: Edagricole; 1982. [Google Scholar]
- Probert RJ, Adams J, Coneybeer J, Crawford A, Hay FR. Seed quality for conservation is critically affected by pre-storage factors. Australian Journal of Botany. 2007;55:326–335. [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Parolo G, Aplin D. Ex situ conservation reaches out to save upwardly-mobile plants from extinction. European Native Seed Conservation Newsletter. 2007;3:3–4. [Google Scholar]
- Rossi G, Parolo G, Ferrarini A. A rapid and cost-effective tool for managing habitats of the European Natura 2000 network: a case study in the Italian Alps. Biodiversity and Conservation. 2008;18:1375–1388. [Google Scholar]
- Sanhewe AJ, Ellis RH. Seed development and maturation in Phaseolus vulgaris. I. Ability to germinate and to tolerate desiccation. Journal of Experimental Botany. 1996;47:949–958. [Google Scholar]
- Schwienbacher E, Marcante S, Erschbamer B. Alpine species seed longevity in the soil in relation to seed size and shape – A 5-year burial experiment in the Central Alps. Flora. 2010;205:19–25. [Google Scholar]
- Sinniah UR, Ellis RH, John P. Irrigation and seed quality development in rapid-cycling Brassica: seed germination and longevity. Annals of Botany. 1998;82:309–314. [Google Scholar]
- Thuiller W. Climate change and the ecologist. Nature. 2007;448:550–552. doi: 10.1038/448550a. [DOI] [PubMed] [Google Scholar]
- UNEP. Convention on biological diversity. United Nations Environmental Programme; 1992. [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research. 2005;15:1–20. [Google Scholar]
- Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE. A plant DNA ligase is an important determinant of seed longevity. The Plant Journal. 2010;63:848–860. doi: 10.1111/j.1365-313X.2010.04285.x. [DOI] [PubMed] [Google Scholar]