Abstract
Colorectal cancer is a prevalent disease in Western countries. While prevention through screening is the best approach to combat the development of colorectal cancer through the removal of precursor adenomas, many patients present with advanced disease that will require surgery and systemic therapy to improve survival. With reference to systemic therapy, the median survival of patients with metastatic colorectal cancer (those with tumor spread to lymph nodes or distant sites) has improved over the past three decades due to the introduction of 5-fluorouracil (5-FU), its subsequent biomodulation, and the addition other chemotherapeutic agents. There is now evidence that the biology of the colorectal tumor, in addition to the stage of colorectal cancer, may predict response to 5-FU-based therapy. More recently, systemic biological therapies that target signaling processes for tumor growth, such as epidermal growth factor receptor, and vascular endothelial growth factor, are also effective in improving patient survival with metastatic colorectal cancer. The use of a combination of systemic therapies that include chemotherapy and biologic therapy should continue to increase patient survival with metastatic colorectal cancer through appropriately designed clinical trials. Treatments based on the biology of the colorectal tumor also need to be examined through clinical trials.
Keywords: colorectal cancer, chemotherapy, biologic therapy, bevacizumab, cetuximab, 5-fluorouracil, epidermal growth factor receptor, tyrosine kinase inhibitor, vascular endothelial growth factor
Introduction
Colorectal cancer is one of the most prevalent cancers in the United States, affecting 1 in 18 Americans during an average lifetime. It has the highest incidence among gastrointestinal cancers in the United States, affecting 148,810 persons in 2008, and is the second most common cause of cancer deaths (behind lung cancer), with 49,960 deaths in 2008 (Jemel et al. 2008). Worldwide, it is estimated that there are more than 940,000 cases of colorectal cancer, and over 500,000 annual deaths (World Health Organization, 2008).
The prognosis of colorectal cancer is principally based on the stage of the disease at presentation and there is a significantly better survival outcome when detected in earlier stages (Table 1). Less than half of colorectal cancer patients are found in stage I and II, with the majority of cases diagnosed with advanced disease. As adenomas and early cancers are often asymptomatic but potentially curable if found and removed as compared to symptomatic colorectal cancer that is more likely to be advanced in stage, screening asymptomatic men and women over the age of 50 years for colorectal neoplasia has shown to detect cancers at an earlier stage compared to those not screened [Hardcastle et al. 1996; Kronborg et al. 1996; Mandel et al. 1993].
Table 1.
Colorectal cancer stages and survival.
| Stage | AJCC TNM stage | Astler-coller-dukes stage | Percent of colorectal cancer patients presenting at stage (%) | Five-year survival (%) |
| I | T1-2, NO, MO | A, B1 | 3-6 | 85-95 |
| II | T3-4, NO, MO | B2, B3 | 35-38 | 60-80 |
| III | Any T, N1-3, MO | C | 38 | 25-60 |
| IV | Any T, any N, M1 | D | 20 | <5 |
Therapy for advanced colorectal cancer can be divided into colon and rectal cancer components, with surgery as the main component of therapy for both subtypes. Rectal cancers are treated surgically by low anterior resection or abdominal perineal resection, often in combination with 5-fluoruracil (5-FU)-based chemoradiation [Boland et al. 2000]. Stage I disease is approached with wide surgical resection generally without chemoradiation. Stage II and III disease is treated by wide surgical resection in combination with adjuvant or neo-adjuvant (with an attempt at anal sphincter preservation) chemoradiation. The approach to stage IV disease is generally palliative with surgical bypass of local bowel obstruction and chemoradiation for local management. For colon cancers, surgery with wide resection margins is the only therapy needed for stage I and II disease, although some stage II patients receive chemotherapy [Boland et al. 2000]. For stage III disease, adjuvant 5-FU-leucovorin or FOLFOX (5-FU, leucovorin, and oxaliplatin) (capecitabine, an oral 5-FU prodrug, can be used in substitute of infused 5-FU) for 6 months is standard and has been shown to improve survival [Goldberg et al. 2004; de Gramont et al. 2000; Moertel et al. 1995; Moertel et al. 1990; Laurie et al. 1989; Petrelli et al. 1989]. For stage IV, surgery may be curative in highly selected patients with resectable bowel disease and resectable isolated hepatic or pulmonary metastases. Unresectable liver lesions may also be managed locally with transarterial chemo-embolization or radiofrequency ablation if symptomatic. In many patients with stage IV disease, surgery is often palliative to prevent bowel obstruction.
Chemotherapy is offered but may not improve overall survival. Palliative chemotherapy regimens for stage IV colon cancer include 5-FU-leucovorin, FOLFOX, and FOLFIRI, and IFL, although IFL is no longer used due to toxicity [Goldberg et al. 2004; de Gramont et al. 2000; Douillard et al. 2000; Saltz et al. 2000; Petrelli et al. 1989]. Specific growth factor inhibitors, such as bevacizumab [antibody to VEGF] and cetuximab [antibody to EGFR] added to the 5-FU-based regimens improve tumor shrinkage and increase months to the survival of stage IV patients [Jonker et al. 2007; Hurwitz et al. 2004].
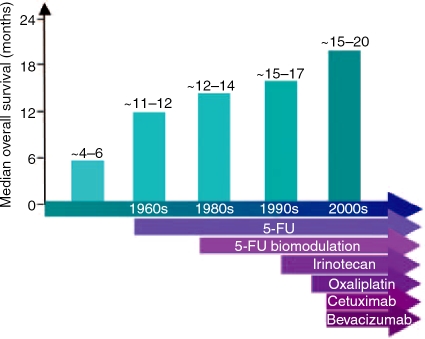
It is important to note the systemic therapeutic progress made in improving survival of patients with metastatic colorectal cancer (Figure 1). Before the 1960s, patients with untreated metastatic colorectal cancer had a median survival of 4-6 months. With the introduction of 5-FU treatment, patient survival was extended to 11–12 months, and 5-FU continues to form the core for colorectal cancer therapy [Boland et al. 2000; Moertel et al. 1995; Moertel et al. 1990; Laurie et al. 1989]. In the 1980s, biomodulators such as leucovorin were added to 5-FU, improving the survival by an additional 2–4 months [Petrelli et al. 1989]. Irinotecan and later oxaliplatin were added to 5-FU as second-line treatments, and later were approved as first-line therapies for metastatic colorectal cancer, further improved overall survival to 15-17 months after diagnosis [Goldberg et al. 2004; de Gramont et al. 2000; Douillard et al. 2000; Saltz et al. 2000]. A metaanalysis of 13 randomized control trials of 1365 patients treated with systemic chemotherapy for metastatic colorectal cancer shows a 35% (95% CI: 24–44%) reduction in the risk of death, translating into an improvement in median survival of 3.7 months [Best et al. 2000]. Biological therapies such as cetuximab and bevacizumab bring average survival lengths close to 20 months after diagnosis, quadrupling the survival times from those observed three decades ago.
Figure 1.
Improved survival of patients with meta-static colorectal cancer with the introduction of new therapies. The addition of 5-FU chemotherapy, followed by its biomodulation and subsequent combining with oxaliplatin and irinotecan, have greatly improved patient survival. Targeted therapies, such as cetuximab and bevacizumab, have also added to the improved survivability from advanced colorectal cancer.
Systemic chemotherapeutic agents for colorectal cancer
5-Fluorouracil
5-FU forms the backbone for almost all chemotherapeutic regimens for patients with advanced colorectal cancer. 5-FU is a fluoropyrimidine that is incorporated into RNA (mRNA, rRNA, and tRNA), and is an inhibitor of thymidylate synthase (which catalyzes the rate limiting conversion of dUMP to dTMP) to inhibit DNA synthesis [Parker and Cheng, 1990]. 5-FU is typically administered with the biomodulator leucovorin, a reduced form of folate that stabilizes the binding between 5-FU and thymidylate synthase [Petrelli et al. 1989]. 5-FU is generally administered as adjuvant therapy for stage III colon cancer patients, and stage II and III rectal cancer patients, with significant improvements in overall survival from 51 to 64% [Gill et al. 2004; Boland et al. 2000]. It can be given as active intravenous or pro-drug oral forms (capecitabine). Side effects can include neutropenia, stomatitis, diarrhea, and palmarplantar erythrodysesthesia (‘hand-foot syndrome’). Deficiency of dihydropyrimidine dehydrogenase (DPD), the exclusive enzymatic step for catabolism and deactivation of fluoropyrimidines, can lead to severe toxicities with 5-FU.
5-FU-based chemotherapy and DNA mismatch repair
Although cancer stage has been the only determinant for evaluation for adjuvant 5-FU-based chemotherapy, there is no current method to determine which patient will have a tumor response to 5-FU. There are likely several reasons why some patients’ colorectal tumors do not respond to treatment with 5-FU. One reason is the loss of DNA mismatch repair (MMR) function within the patient's colorectal cancer. Patients with Lynch syndrome (formerly hereditary nonpolyposis colorectal cancer or HNPCC) as well as 15–20% of sporadic colorectal cancer patients lose DNA MMR, and manifest the phenotype of microsatellite instability along with at least one absent DNA MMR gene expression upon immunohistochemistry of the tumor. Intact DNA MMR repairs DNA polymerase mistakes to maintain the fidelity of replicating DNA. Additionally, the MMR system can recognize certain chemotherapeutic agents that intercalate or get incorporated into DNA, and may be an important trigger to execute cell death [Papouli et al. 2004; Carethers et al. 1999; Carethers et al. 1996]. With MMR-deficiency, repair of polymerase mistakes are lacking and affected cells accumulate mutations that may drive tumorigenesis [Grady and Carethers, 2008]. Importantly, MMR-deficiency may prevent the recognition of DNA-damaging chemotherapy to initiate cell killing by that agent [Meyers et al. 2005; Tajima et al. 2004; Carethers et al. 1999].
in vitro analysis revealed that 5-FU selectively killed colon cancer cells with intact DNA MMR, while cells without intact DNA MMR were 28-fold more resistant [Carethers et al. 1999]. 5-FU-resistant cells in which DNA MMR was restored became sensitive to 5-FU, similar to other MMR-proficient cells [Arnold et al. 2003]. In three retrospective and one prospective study, dichotomizing tumors by the presence or absence of DNA MMR showed that stage II and III colorectal cancer patients (sporadic or Lynch) with DNA MMR-deficient tumors did not derive any survival benefit from 5-FU-based adjuvant chemotherapy [Jover et al. 2006; Carethers et al. 2004; de Vos tot Nedervee Cappel et al. 2004; Arnold et al. 2003; Ribic et al. 2003]. This is in contrast to patients who retained MMR function in their tumors where a significant survival advantage was observed [Jover et al. 2006; Carethers et al. 2004; Arnold et al. 2003; Ribic et al. 2003]. Collectively, these data suggest that 5-FU-based chemotherapy does not prolong survival in patients with MMR-deficient colorectal cancers. At the molecular level, these findings suggest that 5-FU-mediated cytotoxicity may be dependent on intact DNA MMR function.
It had been an accepted concept that incorporation of 5-FU into RNA is the important mechanism of 5-FU toxicity [Carethers et al. 1999; Parker and Cheng 1990; Parker et al. 1987; Lonn and Lonn, 1986; Ingraham et al. 1980]. Under normal conditions, dUTPase prevents the incorporation of dUTP and FdUTP into DNA by dephosphorylating the nucleotides to dUMP and FdUMP, respectively [Parker et al. 1987; Lonn and Lonn, 1986; Ingraham et al. 1980]. However, since 5-FU can inhibit thymidylate synthase, accumulation of dUMP and FdUMP occurs which exhausts the ability of dUTPase to metabolize dUTP and FdUTP. As dUTP and FdUTP levels rise and those of TTP fall, dUTP and FdUTP can replace TTP as a substrate for DNA polymerase, and are incorporated into DNA [Carethers et al. 1999; Parker and Cheng 1990; Parker et al. 1987; Lonn and Lonn, 1986; Ingraham et al. 1980]. It is believed that DNA MMR recognizes 5-FU incorporated into DNA. Two independent laboratories demonstrated biochemically that MMR proteins can bind to 5-FU within DNA [Meyers et al. 2005; Tajima et al. 2004]. How DNA MMR triggers cell death after 5-FU binding is not known. With MMR deficiency, there is no MMR binding or recognition of incorporated 5-FU molecules, and no subsequent triggering for tumor cell demise. Investigation into other chemotherapeutic agents that may circumvent this mechanism for patients with MMR-deficient tumors are being pursued. Some compounds that induce toxicity upon colorectal cancer cells may work independently of DNA MMR, and could prove useful in the treatment of such patients.
Biological information in addition to stage, such as a patient's tumor DNA MMR status, can be considered in the approach to treatment of patients with advanced colorectal cancer.
Irinotecan
Irinotecan, also known as CPT-11, inhibits topoisomerase I, an enzyme involved in the uncoiling of DNA prior to transcription and DNA replication. As a result of the uncoiling, topoisomerase I causes single-strand DNA breaks which are transient and easily repaired. Irinotecan, by blocking topoisomerase I, stabilizes the breaks within DNA, causing DNA fragmentation and ultimately cell death [Klein et al. 2002; Douillard et al. 2000; Saltz et al. 2000]. Irinotecan is metabolized by hepatic carboxylesterases to its active compound, SN-38, and is inactivated through uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) [Klein et al. 2002]. Irinotecan as a single agent and second line to 5-FU-leucovorin improved survival by 2–3 months in patients with advanced colorectal cancer [Douillard et al. 2000; Saltz et al. 2000; Rougier et al. 1998]. Irinotecan used in combination with 5-FU-leucovorin was also shown to increase tumor shrinkage and improve survival by at least 2 months when compared to 5-FU-leucovorin alone [Douillard et al. 2000; Saltz et al. 2000]. Irinotecan is currently used as a first-line therapy for metastatic colon cancer in combination with 5-FU, but has not shown any benefit in the adjuvant setting [Boland et al. 2000]. Side effects can include bone marrow suppression, diarrhea, alopecia, and nausea and vomiting. Neutropenia may be worse in patients homozygous for the UGT1A1∗28 allele which reduces SN-38 detoxification, particularly if administered doses of irinotecan are high [Hoskins et al. 2007].
Oxaliplatin
Oxaliplatin is a platinum derivative, and unlike it's cousins cisplatin and carboplatin, has synergistic activity with 5-FU in treating colorectal cancer [Goldberg et al. 2004; de Gramont et al. 2000]. Oxaliplatin forms bulky DNA adducts, and ultimately induces cell death, but may also down regulate thymidylate synthase, possibly potentiating the efficacy of 5-FU [Raymond et al. 2002; Boland et al. 2000]. Oxaliplatin as single agent has limited efficacy, and is most efficacious clinically when combined with 5-FU-leucovorinin the FOLFOX regimen for patients with advanced colorectal cancer [Goldberg et al. 2004; de Gramont et al. 2000]. Side effects can include rare kidney dysfunction, and/or ototoxicity, but more commonly neuropathy, which comes in an acute and chronic form [Raymond et al. 2002]. Acute transient dysesthesias, with tingling in the hands, feet, or perioral regions can occur and be exacerbated by low ambient temperatures. This has been theorized to be caused by a prolonged opening of sodium channels resulting in a hyper-excitable state (acute channelopathy). A more chronic cumulative, dose-dependent sensory neuropathy can also occur in which peripheral parasthesias and dysesthesias persist between cycles of oxaliplatin therapy, and which diminish after therapy has been fully completed. The chronic neuropathy may be due to accumulation of oxaliplatin in the dorsal root ganglia, much like which occurs with cisplatin therapy.
Targeted biologic therapies for colorectal cancer
Targeted therapies hold the promise of interrupting key cell pathways that are essential for the growth of the tumor, and involved in the cancer cell survival and metastasis. Some of these targeted therapies are antibodies that can inhibit a receptor, and some are small molecules – termed biologic therapy. Given their specific targeted nature, side effects might be less than general systemic chemotherapies.
Vascular endothelial growth factor inhibitor
Bevacizumab Bevacizumab is a humanized monoclonal antibody directed against VEGF, a soluble protein instrumental in angiogenesis [Ferrara et al. 2003]. Bevacizumab alone as mono-therapy is ineffective; it has been shown to increase survival in patients with metastatic colorectal cancer (20.3 vs 15.6 months) when used with the IFL regimen [Hurwitz et al. 2004]. In the United States, bevacizumab was approved by the Food and Drug Administration for use in combination with any 5-FU-containing regimen. Side effects include reversible hypertension and proteinuria (bevacizumab toxicity syndrome, or BETS), but can also include hemorrhage, gastrointestinal perforation, and arterial thromboembolism. Any surgery after bevacizumab therapy should be delayed by 6 weeks due to the reduced ability to heal wounds. Although the mechanism of bevacizumab is to inhibit VEGF, it is not clear whether its activity is solely by blocking VEGF activity or if it affects tumor vasculature to enhance the intra-cellular access for chemotherapy.
Epidermal growth factor receptor inhibitors
Cetuximab Cetuximab is a monoclonal chimeric human/mouse IgG1 antibody directed against the extracellular domain of the EGFR, part of the ErbB family of receptors (EGFR is also known as ErbB1). EGFR is normally present on epithelium, but is overexpressed in some colorectal cancers and associated with a poorer prognosis. EGFR normally enhances pathways involved in cell growth and proliferation; thus inhibition of overexpressed EGFR should slow proliferation of cancer cells. Clinically, cetuximab has activity in irenotecan-resistant tumors, as well as independent activity that favor slowing of disease progression and improved overall survival [Jonker et al. 2007; Cunningham et al. 2004]. The main side effect of cetuximab is an acneiform rash for which its appearance predicts improved survival [Jonker et al. 2007]. Hypomagnesemia can also occur, as can hyper-sensitivity and anaphylaxis when infused in some patients due to the pre-existing presence of IgE antibodies directed against galactose-a-1,3-galac-tose present on the murine portion of the Fab portion of the antibody [Chung et al. 2008]. It should be noted that clinical trials assessed over-expression of EGFR before patient entry; however, it is unclear if the degree of expression is related to the likelihood of response to cetuximab. Thus, cetuximab might be interacting with other molecular targets in addition to EGFR for its effect on cell growth, or as an IgG1 antibody triggering antibody-dependent cytotoxicity, or other mechanism [Ponz-Sarvise et al. 2007; Chung et al. 2005]. Activating mutations in KRAS, downstream of EGFR, has been associated with resistance to cetuximab therapy, and portends a poorer survival [Lièvre et al. 2008] (Table 2).
Table 2.
Agents approved in the United States for use in advanced colorectal cancer therapy.
| Agent | Trade name | Mechanism of action | Colorectal cancer stage when used | Side effects |
| 5-Fluorouracil, Capecitabine | Generic, Xeloda | Blocks thymidylate synthase, RNA, gets inserted into DNA | II (rarely), III, IV (5-FU-leucovorin, FOLFOX, FOLFIRI) | Neutropenia, stomatitis, diarrhea, hand-foot syndrome |
| Irinotecan | Camptosar | Topoisomerase 1 inhibitor | III, IV (FOLFIRI) | Bone marrow suppression, diarrhea, alopecia, nausea, vomiting |
| Oxaliplatin | Eloxatin | Platination of DNA | III, IV (FOLFOX) | Kidney dysfunction, ototoxicity, neuropathy |
| Bevacizumab | Avastin | Anti-VEGF | IV, first line (with chemotherapy) | Hypertension, proteinuria, delayed wound healing |
| Cetuximab | Erbitux | Anti-EGFR | IV, second line (with chemotherapy) | Acneiform rash, hypomagnesemia |
| Panitumumab | Vectibix | Anti-EGFR | IV, second line (with chemotherapy) | skin toxicity, nausea, diarrhea, anorexia, hypomagnesemia |
VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; first line, initial therapy; second line, therapy for those refractory to prior therapy.
Panitumumab Panitumumab, like cetuximab, is a monoclonal antibody directed against the extracellular domain of EGFR. However, panitumumab is a fully humanized IgG2 antibody, which may confer a smaller risk for infusion reactions over cetuximab. Colorectal cancer patients refractory to therapy had decreased tumor progression with panitumumab, and there was improved progression free survival when panitumumab was combined with FOLFIRI [Berlin et al. 2007; Van cutsem et al. 2007]. Similar to cetuximab, efficacy is best with wild type KRAS, as those with mutant KRAS are resistant to therapy and have a poorer survival [Amado et al. 2008]. Also like cetuximab, side effects can include skin toxicity, but also nausea, diarrhea, anorexia, and hypomagnesemia.
Tyrosine kinase inhibitors
Gefitinib Gefitinib is a small molecule that targets and inhibits intracellular EGFR tyrosine kinase activity. Unlike the extracellular portion of EGFR where monoclonal antibodies are used to block EGFR activation, gefitinib inhibits the intracellular tyrosine kinase catalytic domain of EGFR that activates downstream signaling events and effector pathways. There is evidence that the tyrosine kinase domain may still be a useful target in cetuximab-resistant cells, suggesting that tyrosine kinase inhibitors may further modulate signaling that is not fully blocked with EGFR antibodies [Huang et al. 2004]. Gefitinib in combination with irinotecan-based therapies such as FOLFIRI enhanced irinotecan's toxicities of diarrhea and neutropenia [Verones et al. 2005]. Gefitinib combined with FOLFOX achieved a median overall survival of twelve months in those patients, who previously failed chemotherapy [Kuo et al. 2005]. Side effects can include skin rashes and diarrhea (Table 3).
Table 3.
Newer, not yet approved, therapies for advanced colorectal cancer.
| Agent | Target | Comments |
| Matuzumab (EMD72000) | EGFR extracellular domain | Humanized monoclonal antibody |
| AEE788 | EGFR, ErbB2, VEGFR-2 extracellular domains | Oral; poor clinical activity for patients with metastatic colorectal cancer to the liver |
| HKI-272 | Pan-ErbB extracellular domains | Irreversible agent |
| hR3 | EGFR extracellular domain | Monoclonal antibody |
| ICR62 | EGFR extracellular domain | Monoclonal antibody |
| Gefitinib (Iressa) | EGFR tyrosine kinase domain | Used only in locally advanced or metastatic nonsmall cell lung cancer |
| Erlotinib (Tarceva) | EGFR tyrosine kinase domain | Approved for locally advanced or metastatic nonsmall cell lung cancer, and advanced, unresectable, or metastatic pancreatic cancer |
| Lapatinib (Tyverb) | ErbB1 and ErbB2 tyrosine kinase domain | Approved for advanced metastatic breast cancer in conjunction with capecitabine |
| EKB-569 | Pan-ErbB tyrosine kinase domain | |
| Sorafenib (Nexavar) | Multi-kinase inhibitor (Raf, tyrosine kinases, VEGFR, PDGFR, c-kit, c-Ret) | Approved for advanced hepatocellular cancer and advanced renal cell cancer |
| AMT2003 (Auron Misheil Therapy) | Unknown |
Erlotinib Erlotinib is a small molecule that competes with ATP for EGFR's tyrosine kinase domain. This inhibits receptor autophosphorylation and blocks downstream signaling from the intracellular portion of EGFR. Erlotinib has some activity as a single agent, and trials are underway as phase III studies and in combination with traditional chemotherapy regimens [Townsley et al. 2006]. Side effects can include skin rash, diarrhea, nausea, renal dysfunction, hyperbilirubinemia, and neutropenia.
Lapatinib Lapatinib is an ATP-competitive small molecule that is a reversible inhibitor of both the ErbB1 and ErbB2 (Her2/Neu) tyrosine kinases [Ponz-Sarvise et al. 2007]. Lapatinib as a single agent showed some benefit in preventing progression of colorectal cancer metastasis in patients who progressed with prior therapy. Side effects include diarrhea, rash, fatigue, nausea, anorexia, and vomiting.
Multi-kinase inhibitor
Sorafenib Sorafenib is an oral multi-kinase inhibitor that has activity against Raf kinase (a serine-threonine kinase) and tyrosine kinases, including VEGF receptor, platelet-derived growth factor receptor (PDGFR), c-kit, and c-Ret [Wilhelm et al. 2006]. It inhibits tumor cell proliferation by targeting the Ras/Raf/ extracellular signal regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signaling pathway at the level of Raf kinase, a pathway that is often activated in colorectal cancer, and exerts its anti-angiogenic effect by targeting the receptor tyrosine kinases VEGF receptor 2, VEGF receptor 3, and PDGFR, and their associated signaling cascades [Grady and Carethers, 2008; Wilhelm et al. 2006]. It is also found to induce apoptosis in some human cancer cell lines by downregulating the levels of antiapoptotic protein myeloid cell leukemia sequence 1 [Yu et al. 2005]. Single agent therapy showed some clinical benefit in patients with advanced colorectal cancer [Strumberg et al. 2005]. Side effects mainly include diarrhea and skin toxicity.
Summary
Patients with advanced colorectal cancer have improved options for systemic therapy that has quadrupled survival from metastatic disease over the past three decades. Chemotherapy with 5-FU-leucovorin is the mainstay, but first-line therapies also include oxaliplatin or irinotecan which enhance survival. For patients with meta-static colorectal cancer, there is evidence that sequential therapy (i.e., 5-FU until failure, followed by either single or combination therapy consisting of irinotecan or oxaliplatin) is an alternative option as compared to maximum tolerated combination therapy at the onset, with similar patient survival [Koopman et al. 2007; Seymour et al. 2007]. Biologic therapies that include blockage of EGFR activation with antibodies and inhibition of EGFR tyrosine kinase activity utilizing small molecules also are beneficial to survival. Inhibition of angiogenesis with antibodies to VEGF also significantly improves survival. Newer targeted therapies are under investigation for efficacy, and more are sure to be developed as the molecular pathways that drive the pathogenesis and metastasis of colorectal cancer are more completely understood. Combination therapy might eventually prove beneficial, targeting multiple proliferative pathways to arrest tumor growth and spread.
In addition to targeted therapies, a patient's individual tumor biological characteristics may need to be understood as there is evidence that 5-FU-based chemotherapy is more effectual in patients who retain MMR, and not effectual for patients who lose MMR in the tumor. Individualizing treatment based on the patient's tumor biology may further increase survival in those with meta-static colorectal cancer.
Acknowledgements
Supported by the U.S. Public Health Service (DK067287) and the VA Research Service
Conflict of interest statement
None declared.
Glossary
Abbreviations
- 5-FU
5-fluorouracil
- ATP
adenosine triphosphate
- BETS
bevacizumab toxicity syndrome
- dUTPase
deoxyuracil triphosphatase
- dUMP
deoxyuracil monophosphate
- dUTP
deoxyuracil triphosphate
- DPD
dihydropyrimidine dehydrogenase
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal regulated kinase
- FOLFOX
5-FU/leucovorin/oxaliplatin
- FOLFIRI
folic acid/5-FU/irinotecan
- FdUMP
5-fluoro-deoxyuracil monophosphate
- FdUTP
5-fluoro-deoxyuracil triphosphate
- HNPCC
hereditary nonpolyposis colorectal cancer
- IFL
irinotecan/5-FU/leucovorin
- MMR
mismatch repair
- MAPK
mitogen activated protein kinase
- PDGFR
platelet-derived growth factor receptor
- TTP
thymidine triphosphate
- TMP
thymidine monophosphate
- UGT1A1
uridine diphosphate glucuronosyltransferase 1A1
- VEGF
vascular endothelial growth factor
References
- Amado R.G., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D.J.et al. (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer, J Clin Oncol 26:1626–34 [DOI] [PubMed] [Google Scholar]
- Arnold C.N., Goel A., Boland C.R. (2003) Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluoruracil in colorectal cancer cell lines, Int J Cancer 106:66–73 [DOI] [PubMed] [Google Scholar]
- Berlin J., Posey J., Tchekmedyian S., Hu E., Chan D., Malik I.et al. (2007) Panitumumab with irinotecan/leucovorin/5-fluoruracil for first-line treatment of metastatic colorectal cacner, Clin Colorectal Cancer 6:427–432 [DOI] [PubMed] [Google Scholar]
- Best L., Simmonds P., Baughan C., Buchanan R., Davis C., Fentiman I.et al. (2000) Colorectal meta-analysis Collaboration. Palliative chemotherapy for advanced or metastatic colorectal cancer, Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD001545. 10.1002/14651858.CD001545 Accessed April 8, 2008 [DOI] [PMC free article] [PubMed]
- Boland C.R., Sinicrope F.A., Brenner D.E., Carethers J.M. (2000) Colorectal cancer prevention and treatment, Gastroenterology 118:S115–S128 [DOI] [PubMed] [Google Scholar]
- Carethers J.M., Chauhan D.P., Fink D., Nebel S., Bresalier R.S., Howell S.B.et al. (1999) Mismatch repair proficiency and in vitro response to 5-fluorouracil, Gastroenterology 117:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carethers J.M., Hawn M.T., Chauhan D.P., Luce M.C., Marra G., Koi M.et al. (1996) Competency in mismatch repair prohibits clonal expansion of cancer cells treated with N-methyl-N'-nitro-N-nitrosoguanidine, J Clin Invest 98:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carethers J.M., Smith E.J., Behling C.A., Nguyen L., Tajima A., Doctolero R.T.et al. (2004) Use of 5-fluorouracil and survival in patients with microsatellite unstable colorectal cancer, Gastroenterology 126:394–401 [DOI] [PubMed] [Google Scholar]
- Chung C.H., Mirakhur B., Chan E., Le Q.T., Berlin J., Morse M.et al. (2008) Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose, N Engl J Med 358:1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.Y., Shia J., Kemeny N.E., Shah M., Schwartz G.K., Tse A.et al. (2005) Cetuximab shows activity in colorectal cancer patients with turmos that do not express the epidermal growth factor receptor by immunohistochemistry, J Clin Oncol 23:1803–1810 [DOI] [PubMed] [Google Scholar]
- Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C., Chau I., Van Cutsem E. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer, New Engl J Med 351:337–45 [DOI] [PubMed] [Google Scholar]
- de Gramont A., Figer A., Seymour M., Homerin M., Hmissi A., Cassidy J.et al. (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer, J Clin Oncol 18(16):2938–2947 [DOI] [PubMed] [Google Scholar]
- de Vos tot Nedervee Cappel W.H., Neulenbeld H.J., Keibeuker J.H., Nagengast F.M., Menko F.H., Griffioen G.et al. (2004) Survival after adjuvant 5-FU treatment for stage III coon cancer in hereditary nonpolyposis colorectal cancer, In J Cancer 109:468–471 [DOI] [PubMed] [Google Scholar]
- Douillard J.Y., Cunningham D., Roth A.D., Navarro M., James R.D., Karasek P.et al. (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial, Lancet 355(9209):1041–1047 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H.P., LeCounter J. (2003) The biology of VEGF and its receptors, Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- Gill S., Loprinzi C.L., Sargent D.J., Thomé S.D., Alberts S.R., Haller D.G.et al. (2004) Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22:1797–806 [DOI] [PubMed] [Google Scholar]
- Goldberg R.M., Sargent D.J., Morton R.F., Fuchs C.S., Ramanathan R.K., Williamson S.K.et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer, J Clin Oncol 22(1):23–30 [DOI] [PubMed] [Google Scholar]
- Grady W.M., Carethers J.M. (2008) Genomic instability and molecular pathogenesis of colorectal cancer, Gastroenterology (in press)
- Hardcastle J.D., Chamberlain J.O., Robinson M.HE., Moss S.M., Amar S.S., Balfour T.W.et al. (1996) Randomised controlled trial of faecal-occult-blood screening for colorectal cancer, Lancet 348:1472. [DOI] [PubMed] [Google Scholar]
- Hoskins J.M., Goldberg R.M., Qu P., Ibrahim J.G., McLeod H.L. (2007) UGT1A1∗28 genotype and irinotecan-induced neutropenia: dose matters, J Natl Cancer Inst 99:1290–1295 [DOI] [PubMed] [Google Scholar]
- Huang S., Armstrong E.A., Benavente S., Chinnaiyan P., Harari P.M. (2004) Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR); combining anti-EGFR antibody with tyrosine kinase inhibitor, Cancer Res 64:5355–5362 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W.et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer, N Engl J Med 350(23):2335–42 [DOI] [PubMed] [Google Scholar]
- Ingraham H.A., Tseng B.Y., Goulian M. (1980) Mechanism for exclusion of 5-fluorouracil from DNA, Cancer Res 40:998–1001 [PubMed] [Google Scholar]
- Jemel A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M.J. (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Jonker D.J., O'Callaghan C.J., Karapetis C.S., Zalcberg J.R., Tu D., Au H.J.et al. (2007) Cetuximab for the treatment of colorectal cancer, N Engl J Med 357(20):2040–2048 [DOI] [PubMed] [Google Scholar]
- Jover R., Zapater P., Castells A., Llor X., Andreu M., Cubiella J.et al. (2006) Gastrointestinal Oncology Group of the Spanish Gastroenterological Association. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer, Gut 55:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C.E., Gupta E., Reid J.M., Atherton P.J., Sloan J.A., Pitot H.C.et al. (2002) Population pharmacokinetic model for irinotecan and two of its metabolites, SN-38 and SN-38 glucoronide Clin Pharmacol Ther 72:638–47 [DOI] [PubMed] [Google Scholar]
- Koopman M., Antonini N.F., Douma J., Wals J., Honkoop A.H., Erdkamp F.L.et al. (2007) Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial, Lancet 370:135–142 [DOI] [PubMed] [Google Scholar]
- Kronborg O., Fenger C., Olsen J., Jørgensen O.D, Søndergaard O. (1996) Randomised study of screening for colorectal cancer with faecal-occult-blood test, Lancet 348:1467–71 [DOI] [PubMed] [Google Scholar]
- Kuo T., Cho C.D., Hasley J., Wakelee H.A., Advani R.H., Ford J.M.et al. (2005) Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cacner, J Clin Oncol 23:5613–5619 [DOI] [PubMed] [Google Scholar]
- Laurie J.A., Moertel C.G., Fleming T.R., Wieand H.S., Leigh J.E., Rubin J.et al. (1989) Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil, J Clin Oncology 7:1447–1456 [DOI] [PubMed] [Google Scholar]
- Lièvre A., Bachet J.B., Boige V., Cayre A., Le Corre D., Buc E.et al. (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab, J Clin Oncol 26:374–379 [DOI] [PubMed] [Google Scholar]
- Lonn U., Lonn S. (1986) DNA lesions in human neoplastic cells and cytotoxicity of 5-fluoropyrimidines, Cancer Res 46:3866–3870 [PubMed] [Google Scholar]
- Mandel J.S., Bond J.H., Church T.R., Snover D.C., Bradley G.M., Schuman L.M., Ederer F. (1993) Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 328:1365–71 [DOI] [PubMed] [Google Scholar]
- Meyers M., Wagner M.W., Mazurek A., Schmutte C., Fishel R., Boothman D.A. (2005) DNA mismatch repair-dependent response to fluoropyrimidine-generated damage, J Biol Chem 280:5516–5526 [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Fleming T.R., Macdonald J.S., Haller D.G., Laurie J.A., Goodman P.J.et al. (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma, N Eng J Med 322:352–358 [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Fleming T.R., Macdonald J.S., Haller D.G., Laurie J.A., Tangen C.M.et al. (1995) Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report, Ann Intern Med 122:321–326 [DOI] [PubMed] [Google Scholar]
- Papouli E., Cejka P., Jiricny J. (2004) Dependence of the cytotoxicity of DNA-damaging agents on the mismatch repair status of human cells, Cancer Res 64:3391–3394 [DOI] [PubMed] [Google Scholar]
- Parker W.B., Cheng Y.C. (1990) Metabolism and mechanism of action of 5-fluorouracil, Pharmac Ther 48:381–395 [DOI] [PubMed] [Google Scholar]
- Parker W.B., Kennedy K.A., Klubes P. (1987) Dissociation of 5-fluorouracil-induced DNA fragmentation from either its incorporation into DNA or its cytotoxicity in murine T-lymphoma (S-49) cells, Cancer Res 47:979–982 [PubMed] [Google Scholar]
- Petrelli N., Douglass H.O., Jr, Herrera L., Russell D., Stablein D.M., Bruckner H.W.et al. (1989) The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group, J Clin Oncol 7(10):1419–1426 [DOI] [PubMed] [Google Scholar]
- Ponz-Sarvise M., Rodriguez J., Viudez A., Chopitea A., Calvo A., Garcia-Foncillas J.et al. (2007) Epidermal growth factor receptor inhibitors in colorectal cacner treatment: what's new, W o r ld J Gastroenterol 13:5877–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E., Faivre S., Chaney S., Woynarowski J., Cvitkovic E. (2002) Cellular and molecular pharmacology of oxaliplatin, Mol Cancer Ther 1:227–235 [PubMed] [Google Scholar]
- Ribic C.M., Sargent D.J., Moore M.J., Thibodeau S.N., French A.J., Goldberg R.M.et al. (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer, N Engl J Med 349:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier P., Van Cutsem E., Bajetta E., Niederle N., Possinger K., Labianca R.et al. (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cacner, Lancert 352:1407–1412 [DOI] [PubMed] [Google Scholar]
- Saltz L.B., Cox J.V., Blanke C., Rosen L.S., Fehrenbacher L., Moore M.J.et al. (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group, N Engl J Med 343(13):905–914 [DOI] [PubMed] [Google Scholar]
- Seymour M.T., Maughan T.S., Ledermann J.A., Topham C., James R., Gwyther S.J.et al. (2007) FOCUS Trial Investigators; National Cancer Research Institute Colorectal Clinical Studies Group. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial, Lancet 370:143–152 [DOI] [PubMed] [Google Scholar]
- Strumberg D., Richly H., Hilger R.A., Schleucher N., Korfee S., Tewes M.et al. (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors, J Clin Oncol 23:965–972 [DOI] [PubMed] [Google Scholar]
- Tajima A., Hess M.T., Cabrera B.L., Kolodner R.D., Carethers J.M. (2004) The mismatch repair complex hMutS alpha recognizes 5-fluorouracil-modified DNA: implications for chemosensitivity and resistance, Gastroenterology 127:1678–1684 [DOI] [PubMed] [Google Scholar]
- Townsley C.A., Major P., Siu L.L., Dancey J., Chen E., Pond G.R.et al. (2006) Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer, Br J Cancer 94:1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B.et al. (2007) Open-label phase III trial of panitumumab plus best supportive care compated with best supportive care alon in patients with chemotherapy-refractory metastatic colorectal cacner, J Clin Oncol 25:1658–1664 [DOI] [PubMed] [Google Scholar]
- Verones M.L., Sun W., Giantonio B., Berlin J., Shults J., Davis L.et al. (2005) A phase II trial of gefitinib with 5-fluorouracil, leucovorin, and irinotecan in patients with colorectal cancer, Br J Cancer 92:1846–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R.A., Schwartz B., Simantov R., Kelley S. (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer, Nat Rev Drug Discov 5:835–844 [DOI] [PubMed] [Google Scholar]
- World Health Organization. Available at: http://www.who.int/mediacentre/news/releases/2003/pr27/en/ (accessed April 8, 2008)
- Yu C., Bruzek L.M., Meng X.W., Gores G.J., Carter C.A., Kaufmann S.H.et al. (2005) The role of Mcl-1 down-regulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006, Oncogene 24:6861–6869 [DOI] [PubMed] [Google Scholar]