Abstract
Chronic hepatitis B (CHB) is a major public health problem affecting up to 400 million people globally. Complications of CHB including liver failure and hepatocellular carcinoma result in 1.2 million deaths per year, making CHB the 10th leading cause of mortality worldwide. The natural history of CHB is variable and complex. The past decade witnessed important developments for the therapy of hepatitis B and marked the new era of oral therapy. The ultimate goal of CHB therapy is to arrest the progression of liver injury and to prevent the development of liver failure and hepatocellular carcinoma. Currently, six agents are approved for the treatment of CHB. Each of these agents, given as monotherapy, has been shown to produce virological, biochemical, and histological benefits for both HBeAg positive and negative CHB. There are, however, limitations in spite of their efficacy. The significant side-effect profile of interferon, for example, limits its long-term use. The approved oral agents are tolerable with prolonged use but drug resistance could limit long-term monotherapy. To date, combination therapy with nucleoside analogue and pegylated interferon or two nucleos(t)ide analogues given for one year does not show superiority in durability of response compared to monotherapy. Ongoing research effort is critical to identify the ideal hepatitis B therapy that is safe, effective, and produces durable response with a finite course of therapy. It is equally important to conduct a well designed, prospective natural history study to identify predictors of disease progression. This will accurately guide treatment strategy for this important disease.
Keywords: Hepatitis B virus, pegylated interferon, nucleos(t)ide analogues, drug resistance
Introduction
Hepatitis B virus (HBV) infection is a major public health problem worldwide, responsible for significant morbidity and mortality from chronic liver disease. It is estimated that there are 350 to 400 million HBV carriers globally [Lee, 1997]. In the United States, approximately 1.5 million people are infected and 50,000– 100,000 new cases are reported annually despite the availability of effective vaccines [McQuillan et al. 1999]. This is likely an underestimate since prevalence of chronic hepatitis B (CHB) among immigrants from HBV endemic areas is much higher than that in the general population [Margolis et al. 1991].
HBV is a DNA virus in the family of Hepadnaviridae [Tacke et al. 2004]. There are eight major genotypes of HBV and their prevalence varies amongst geographic regions (Table 1) [Magnius and Norder, 1995]. The compact genome of HBV consists of four partially overlapping open reading frames encoding for the envelope (pre-S/S), core (precore/core), polymerase, and X proteins [Tacke et al. 2004]. Through the process of endocytosis, HBV gains entry into the hepatocyte. However, its surface receptor has not been identified [Doo and Liang, 2001]. After uncoating, the relaxed circular genome is converted in the nucleus to a covalently closed circular (ccc) DNA that is the template for viral replication [Locarnini and Mason, 2006; Doo and Liang, 2001]. The persistence of HBV in the liver, despite antiviral therapy, is due to the maintenance of HBV cccDNA in the nuclei of infected cells. HBV replicates asymmetrically via reverse transcription of an RNA intermediate. Since its polymerase/reverse transcriptase (Pol/Rt) lacks proofreading activity, spontaneous mutations are estimated to occur at a rate of one error per 104–105 nucleotides daily [Locarnini and Mason, 2006]. The resulting random mutations at the polymerase/reverse transcriptase active site may overlap with the antiviral-induced mutations and facilitate drug resistance.
Table 1.
Global distribution of HBV genotypes.
| Genotype | Geographical distribution |
| A | North America, Pandemic |
| B and C | Asia |
| D | Middle East, South Europe (Mediterranean), India |
| E | Africa, especially Sub-Sahara |
| F | Native Americans and Polynesians |
| G | USA, Europe (France, Germany, The Netherlands) |
| H | South and Central America |
CHB is defined by the persistence of serum hepatitis B surface antigen (HBsAg) for six months or longer [Hollinger and Lau, 2006]. The natural history of CHB can be classified into four major clinical phases based on levels of serum alanine aminotransferase (ALT) and HBV DNA, presence of HBeAg, and suspected immune status [Lok et al. 2001]. These phases are: (1) immune tolerance, (2) HBeAg-positive CHB, (3) inactive carrier, and (4) HBeAg-negative CHB. The disease, however, can be variable and the patients may not proceed through all phases of the disease during the course of infection (Table 2) [Lok et al. 2001]. The emergence of pre-core [nucleotide 1896 mutation from guanine (G) to adenine (A)] and basal core promoter (BCP) [adenine (A) to thymine (T) transversion at nucleotide 1762 together with a guanine (G) to adenine (A) transition at nucleotide 1764] mutants lead to HBeAg-negative CHB [Hunt et al. 2000; Okamoto et al. 1994; Okamoto et al. 1990]. These patients continue to have moderate HBV replication and active liver disease. The frequency of these HBV mutants varies worldwide as a result of the different geographic distribution of the HBV genotypes. Patients with HBeAg-negative CHB typically have heterogeneity of disease activities characterized by fluctuating levels of serum aminotransferases and HBV DNA [Hadziyannis and Vassilopoulos, 2001]. These observations underscore the importance of regular assessments of HBsAg positive patients over time in order to confirm the diagnosis of HBeAg-negative CHB versus inactive HBV carrier. HBeAg positive and HBeAg negative-CHB patients with persistent or intermittent elevation of aminotransferases and HBV DNA levels, and histological evidence of active hepatitis should be considered for antiviral therapy.
Table 2.
Phases in the natural history of chronic hepatitis B (CHB).
| Spontaneous recovery | Immune tolerance | HBeAg positive CHB | HBeAg negative CHB | Inactive carrier | |
| HBsAg | Absent | Present | Present | Present | Present |
| HBeAg | Absent | Present | Present | Absent | Absent |
| ALT | Normal | Normal | Elevated | Elevated (fluctuate) | Normal |
| HBV DNA | Undetectable in serum | High 108-1011C/ml | High 106-1010C/ml | Moderate (fluctuate) 103-108C/ml | Low <104C/ml |
C/mL, Copies/mL. It is estimated that 1IU/ml is approximately 5.6 copies/mL.
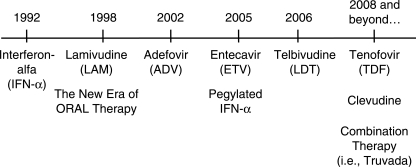
The past decade witnessed important developments for the therapy of hepatitis B. The availability of lamivudine in 1998 not only marked the new era of oral therapy, it also represents a paradigm shift in the management of this important disease (Figure 1). The focus of this review is to discuss both the advances and the unmet needs with the current paradigm.
Figure 1.
Timeline of the FDA-approved therapy for chronic hepatitis B in the United States.
Therapy for chronic hepatitis B
There are six agents approved for the treatment of CHB by the U.S. Food and Drug Administration (FDA) [Hoofnagle et al. 2007; Hollinger and Lau, 2006]. They are peginterferon and standard interferon-alpha (pegIFN-a, IFN-a), nucleoside (lamivudine, entecavir, and telbivudine) and nucleotide (adefovir) analogues. Tenofovir disoproxil fumarate and the combination of tenofovir and emtricitabine (TruvadaTM) both have potent activity against HBV, and are currently approved for use in the treatment of human immunodeficiency virus (HIV). It is anticipated that tenofovir will become FDA approved for CHB in 2008.
The ultimate goal of therapy for CHB is to arrest the progression of liver injury and to prevent the development of liver failure and hepatocellular carcinoma (HCC). The most important short-and intermediate-term objective of therapy is to maximize HBV DNA suppression. Complete eradication of the HBV is difficult for it has a tendency to integrate into the host genome or remain latent as cccDNA [Laras et al. 2006]. Patients who beco-me HBsAg negative and develop anti-HBs generally have resolution of liver disease. Thus, HBsAg seroconversion should be considered a complete therapeutic response, the most desired endpoint of therapy [Werle-Lapostolle et al. 2004; Lau et al. 1997]. A significant reduction in serum HBsAg titer has been observed with antiviral therapy, which correlated with changes in cccDNA, total intra-cellular HBV DNA and serum HBV DNA [Werle-Lapostolle et al. 2004]. The cccDNA is the major template for transcription and translation of viral antigens, including HBsAg. Changes in serum HBsAg titer might be used as a surrogate for liver cccDNA level, especially the latter requiring a liver biopsy [Zoulim, 2005].
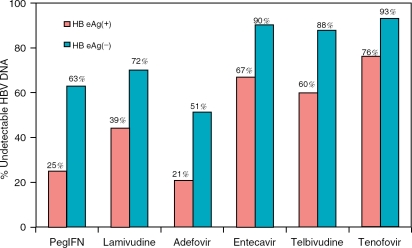
Each of these agents, given as monotherapy, has been shown to produce virological, biochemical, and histological benefit for both HBeAg positive and negative CHB. The biochemical and histological responses usually parallel HBV DNA suppression. Comparison of the potency of these medications for both HBeAg positive and negative CHB during the first year of therapy from representative publications is shown in Figure 2 and Table 3 [Lai et al. 2007; Lok and McMahon 2007; Lai et al. 2006; Marcellin et al. 2004;
Figure 2.
Virological response with undetectable HBV DNA by RT-PCR at week 48–52. In all of these studies, the lower limit for HBV DNA detection was 300 copies/ml with the exception of tenofovir (LLD 400 copies/mL). Tenofovir data for Figures 2 and 3 derived from Marcellin et al. and Heathcote et al., AASLD 2007 Abstract nos. LB2 and LB6.
Table 3.
Biochemical and histological response at week 48-52.
| Normalization of ALT | Improvement in Histology | |||
| Drug | HBeAg+ CHB (%) | HBeAg-CHB (%) | HBeAg+ CHB (%) | HBeAg-CHB (%) |
| Peginterferon | 39 | 38 | 38 | 48 |
| Lamivudine | 66 | 74 | 59 | 63 |
| Adefovir | 48 | 72 | 53 | 64 |
| Entecavir | 68 | 78 | 72 | 70 |
| Telbivudine | 77 | 74 | 65 | 66 |
| Tenofovir | 69 | 77 | 74 | 72 |
| Placebo | 21 | 24 | 25 | 28 |
Tenofovirdata was derived from Marcellin et al. and Heathcote et al. AASLD 2007 Abstract nos. LB2 and LB6, respectively. ALT, alanine aminotransferase, CHB, chronic hepatitis B.
Hadziyannis et al. 2003; Lok et al. 2001]. Patients with HBeAg-negative CHB tend to have lower baseline serum HBV DNA level compared to HBeAg-positive patients. As a result, there was a higher rate of complete viral suppression for HBeAg-negative CHB with every antiviral agent. Nucleos(t)ide analogues are more potent in HBV DNA suppression compared to interferon for both HBeAg positive and negative CHB.
Interferons
Standard IFN-a was the first drug available for treatment of CHB. More recently, long-acting, once weekly pegIFN a-2a (40 kD branched pegylated molecule) was approved by FDA of the United States in 2005. It has similar safety profiles and is more effective compared to standard IFN. The recommended regimen for CHB is pegIFN a-2a 180μg subcutaneously weekly for one year. The therapeutic effects of IFN are secondary to its direct antiviral function, antiproliferative effect (anti-angiogenic and anti-tumor), immunomodulatory properties, and control of apoptosis. The immunomodulatory effects of IFN can be recognized clinically as flares of hepatitis that often precedes a virological response [Perrillo, 2001].
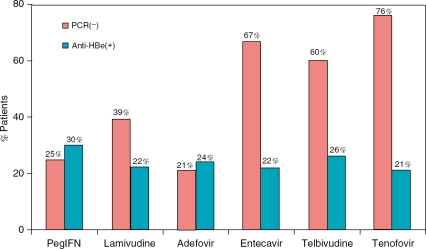
Traditionally, one of the most important treatment endpoints for patients with HBeAg-positive CHB is the loss of HBeAg. PegIFN a-2a has the highest HBeAg seroconversion rate (30% at one year) in spite of its lower antiviral potency compared to the nucleos(t)ide analogues (Figure 3). Long-term follow-up studies of IFN-a therapy from North America and Europe reported that 95-100% of those who cleared HBeAg continued to be HBeAg negative after 5 to 10 years of follow-up and 30-86% of them eventually lost HBsAg [Lau et al. 1997; Niederau et al. 1996]. Liver-related complications and mortality were greater in nonresponders compared to responders, especially among those with pre-existing cirrhosis [Lau et al. 1997]. These studies demonstrated that the loss of HBeAg is a reliable treatment end-point that is associated with long-term disease remission. In contrast, long-term follow-up of patients in Asian studies generally showed a lower rate of durable responses to IFN-a, and inconsistent rates of HBeAg and HBsAg clearance [Yuen et al. 2001; Lin et al. 1999; Lok et al. 1993]. These differences in long-term IFN-α treatment outcomes noted in the Eastern and Western countries could reflect differences in viral factors such as genotypes and in the natural history of the disease in high vs low endemic areas [Alward et al. 1985; McMahon, et al. 1985]. There is evidence that patients with HBV genotype A have the highest rate of IFN-induced HBeAg loss compared to the other genotypes and genotype A is most common in North America and Europe [Janssen et al. 2005; Lau et al. 2005; Cooksley et al. 2003]. In contrast, the HBeAg clearance associated with nucleos(t)ide analogues appears independent of HBV genotype.
Figure 3.
Relationship between antiviral potency and rate of HBeAg seroconversion at week 48-52. There is no positive correlation between potency in HBV DNA suppression and HBeAg seroconversion among the medications. The higher rate of HBeAg seroconversion associated with pegIFN-a is consistent with the evidence that HBeAg and HBsAg clearance is an immune-mediated phenomenon.
The major disadvantage of IFN therapy is its significant side-effect profile that limits its long-term use. It is contraindicated in decompensated cirrhosis and tends to be ineffective in patients with normal aminotransferases. The therapy for HBeAg-negative CHB is particularly challenging due to its high relapse rate and typically requires a prolonged, indefinite course of therapy [Hadziyannis et al. 2003; Manesis and Hadziyannis, 2001]. However, sustained virological response, defined as HBV DNA levels 520,000 copies/mL at 24 weeks after cessation of a 48-week course of therapy, was higher with pegIFN a-2a compared to lamivudine (43% vs 29%, p = 0.007) [Marcellin et al. 2004]. Loss of HBsAg associated with pegIFN a-2a therapy was 4% compared to 0% with lamivudine at one year. Despite its limitations, IFN therapy is associated with the highest rates of both HBeAg and HBsAg seroconversion at one year of therapy, underscoring the importance of immunomodulatory properties on viral clearance.
Nucleoside and nucleotide analogues
Nucleoside or nucleotide analogues compete with naturally occurring purines and pyrimidines for binding to HBV DNA polymerase. They require intracellular phosphorylation for their activity. Analogues lacking a 3’-OH group on the sugar moiety result in immediate chain termination. Many of these compounds are unnatural L-enantiomers [Leemans et al. 2006]. One of the significant impacts of these oral agents is their beneficial effects on end stage liver disease [Arora and Keeffe, 2007]. Unlike IFN, nucleos(t)ide analogues are well tolerated by patients with decompensated liver disease and significant improvement of hepatic synthetic function has been documented [Arora and Keeffe, 2007]. Among the available nucleos(t)ide analogues, entcavir, telbuvudine and tenofovir are most potent in HBV DNA suppression (Figure 2). At one year, >60% of HBeAg-positive and 485% of HBeAg-negative CHB patients achieved undetectable HBV DNA by RT-PCR assays with these three agents [Lai et al. 2007; Lai et al. 2006]. Adefovir and tenofovir are both structurally related nucleotides. The clinical dosage of tenofovir 300 mg has significantly greater antiviral effect than adefovir dosed at 10mg [Del Poggio et al. 2008; Tan et al. 2008]. Adefovir 10 mg is associated also with a high rate of primary nonresponse in up to 30% of the patients with HBeAg-positive CHB. Although, adefovir at 30 mg has higher antiviral potency, it is not recommended for its potential nephrotoxicity, a Fanconi-like syndrome with phosphaturia and proteinuria [Perazella, 2003]. Of note, nucleos(t)ide analogues are renally excreted so dose adjustment is essential in accordance with creatinine clearance [Izzedine et al. 2005].
An important question is whether the potency of the antiviral agent is associated with an increased rate of HBeAg and HBsAg seroconversion. The one-year HBeAg seroconversion rate is similar across the nucleoside and nucleotide analogues (between 21% and 26%) regardless of their antiviral potency (Figure 3) [Lai et al. 2007; Lai et al. 2006; Marcellin et al. 2004; Hadziyannis et al. 2003]. Similarly, the one-year HBsAg seroconversion rates are 51% for all the nucleos(t)ides. For each agent evaluated, there is a trend toward increased rates of undetectable HBV DNA with prolonged therapy beyond the first year in the absence of drug-associated resistance. Similarly, the rate of HBeAg seroconversion increased to ~30% for lamivudine, adefovir, entecavir, and telbivudine at year two of continuous therapy for patients with HBeAg-positive CHB [Lok and McMahon, 2007; Gish et al. 2007; Hollinger and Lau, 2006; Leung et al. 2001; Liaw et al. 2000]. The durability of HBeAg seroconversion, however, is variable and relapse rates of up to 60% after nucleos(t)ide analogue therapy [Lok et al. 2001]. HBsAg loss also increases with prolonged monotherapy but at a very low rate. Continuous therapy with entecavir for two years is associated with a 5% HBsAg loss and only 2% HBsAg seroconversion [Gish et al. 2007]. Unlike IFN, the nucleos(t)ide analogues are well tolerated even with long-term therapy. The effectiveness and durability of response, unfortunately, could be compromised by the emergence of mutations in the HBV DNA polymerase which confers to the HBV mutants a selective resistance to the drug. To date, IFN-induced resistance has not been reported and HBV resistance to tenofovir has not been well characterized. The primary site(s) of mutations associated with the nucleos(t)ide antiviral agents are showed in Figure 4 [Hollinger et al. 2006; Locarnini et al. 2004; Hoofnagle et al. 1987].
Figure 4.
Anti-viral-induced mutations at HBV DNA polymerase/reverse transcriptase. Only the major primary mutations and clinically relevant compensatory mutations are shown.
Drug resistance and cross-resistance
Antiviral resistance is defined as the selection of HBV mutants conferring reduced susceptibility to a drug that results in primary or secondary treatment failure. While resistance is more likely the cause of secondary treatment failure, it may cause primary treatment failure due to transmission of resistant HBV mutants or due to cross-resistance resulting from previous therapies [Pawlotsky et al. 2008]. The risks of the emergence of drug-resistant mutants in the HBV DNA polymerase/reverse transcriptase increases with duration of therapy, high baseline serum HBV DNA level, incomplete viral suppression during the first six months of therapy, noncompliance to therapy and prior exposure to nucleos(t)ide analogues [Kim et al. 2007; Hollinger et al. 2006]. The first clinical manifestation of antiviral resistance is virologic breakthrough that is defined as a 41 log10 increase in serum HBV DNA from nadir in a patient who had an initial virologic response [Pawlotsky et al. 2008]. Depending on the sensitivity of the genotyping assay, drug-resistant mutations can be detected months prior to the rise of the serum HBV DNA. The subsequent biochemical breakthrough with increased serum aminotransferases tends to occur 3 to 6 months after virologic breakthrough [Lau et al. 2000]. Antiviral resistance can be associated with acute hepatitis flare with decompensation of liver disease especially among those with advanced fibrosis [Liaw et al. 2004]. These observations underscore the importance of regular monitoring for early virologic breakthrough and adjust antiviral therapy accordingly to prevent biochemical breakthrough.
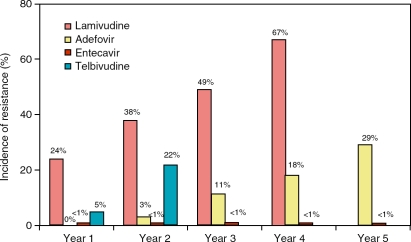
Lamivudine is associated with the highest rate of resistance, reaching near 70% by year four of continuous therapy [Lok et al. 2001]. The primary mutations associated with lamivudine resistance are located in the YMDD catalytic motif of the C domain of the HBV reverse transcriptase (RT) (rtM204V/I) while compensatory mutations (rtV173L, rtL180M) are identified in domain B [Locarnini, 2005]. By phenotypic analysis, the rtM204V and rtL180M combined mutations induce a 1000-fold decrease of susceptibility to lamivudine in vitro by comparison with wild-type (wt) HBV [Locarnini, 2005; Liu et al. 2001] The main effect of the compensatory mutations is to restore replication fitness of the drug-associated HBV mutant. Thus, HBV DNA level usually increases with continuous therapy after the emergence of the primary mutation [Locarnini, 2005; Liu et al. 2001]. Adefovir is generally effective against both wild type HBV and lamivudine resistant mutants [Ono-Nita et al. 1999]. There is evidence to support the ‘addition’ of adefovir to lamivudine in the presence of lamivudine resistance to prevent the subsequent development of adefovir resistance. Fung et al. initially reported a 22% adefovir resistance rate at year-two if lamivudine was ‘switched’ to adefovir monotherapy after the emergence of lamivudine resistance [Fung et al. 2006]. More recently, Lampertico and coworkers compared the efficacy between adefovir monotherapy and adefovir, lamivudine combination on 588 HBeAg-negative patients with lamivudine-resistance CHB. They reported 0% adefovir resistance rate at three years with combination therapy compared to 16% with the switch from lamivudine to adefovir monotherapy [Lampertico et al. 2007].
Despite the initial low resistance rate with adefovir, the cumulative resistance rate increased to 29% by year five [Hadziyannis et al. 2005; Papatheodoridis et al. 2005]. The primary site of adefovir-associated resistance mutation, rtN236T, is located in domain D of the HBV reverse transcriptase. This mutation results in a 3- to 6-fold reduction in susceptibility to ADV in vitro and remains susceptible to nucleoside analogues such as lamivudine, telbivudine, and entecavir. In contrast, the rtA181V/T mutation of adefovir in domain B was found to have reduced responsiveness to lamivudine and telbivudine in phenotypic assays. It remains susceptible to entecavir and tenofovir (Table 4) [Zoulim, 2006; Locarnini, 2005; Ono-Nita et al. 1999].
Table 4.
Antiviral resistance, cross resistance and salvage therapy.
| Antiviral resistant mutation | |||||||
| Lamivudine-R | Adefovir-R | Entecavir-R | Telbivudine-R | ||||
| M204V/I ± L180M | A181T | N236T | A181V/T | M204V/I + L180M + T184G or S202I or M250V | 4M204V/I ± L180M | A181T | |
| TDF In Vitro Cross Resistance | ETV, LdT | ADV, LdT | LAM, ETV | LAM, LdT | LAM, LdT | LAM, ETV | LAM, ADV |
| Remain sensitive | ADV, TDF | 3TDV, ETV | LdT | 3TDF, ETV | ADV, TDF | ADV, TDF | 3TDF, ETV |
| 2Salvage therapy | Add ADV or add 1TDF or switch to 1 Truvada | Add 1TDF or switch to 1Truvada | Add LAM or add ETV or add LdT | Add ETV | Add ADV or add 1TDF | Add ADV or add 1TDF | Add 1TDF or switch to 1 Truvada |
ADV = adefovir, TDF = tenofovir, LAM = lamivudine, ETV = entecavir, LdT = telbivudine, Truvada=TDF plus emtricitabine.
TDF and Truvada are not currently FDA-approved for chronic hepatitis B.
The suggested salvage therapy is based on both in vitro cross resistance profiles and clinical findings. They reflect the experience and opinions of the authors.
1-fold decrease in TDF susceptibility for rtA181V/T in vitro (van Bommel et al, poster #960, AASLD 2007).
rtM204V and rtl_180M, in addition to rtM204l, have also been associated with telbivudine use.
A number of recent studies reported that lamivudine monotherapy can promote the emergence of rtA181T mutation in adefovir treatment-naïve patients [Villet et al. 2008; Locarnini, 2005]. This single substitution at position rt181 appears to be sufficient to induce cross-resistance between lamivudine and adefovir. In the specific setting of lamivudine resistance with the presence of both rtM204V/I and rtA181T substitutions, the addition of adefovir will not be effective. The addition of tenofovir to lamivudine or switch to Truvada will be the authors’ therapy of choice in this case based on the available in vitro data and limited clinical presentations (Table 4). These observations with lamivudine and adefovir therapy highlight the important roles of both genotypic and phenotypic assays in identifying the antiviral drug associated mutations and in informing the selection of the subsequent salvage therapy.
The development of entecavir resistance requires pre-existing lamivudine resistance mutations and additional changes in the HBV polymerase/ reverse transcriptase: T184 in domain B, S202 in domain C or M250 in domain E (Figure 4) [Colonno et al. 2006; Tenney et al. 2004]. The relatively low resistance rate of entecavir at 51% in five years among previous treatment-naïve patients can be explained by a combination of its high genetic barrier requiring multiple mutations to reduce its efficacy, and its antiviral potency63 (Tenney et al. APASL 2008) (Figure 5). In contrast, for patients with preexisting lamivudine resistance who were subsequently switched to entecavir, entecavir resistance rate increased to 43% after five years of continuous therapy [Tenney et al. APASL 2008]. This illustrates the important concept of the emergence of drug resistance in the setting of reduced genetic barrier.
Figure 5.
Rate of antiviral resistance with virologic breakthrough on continuous monotherapy.
Even though both entecavir and telbivudine have excellent antiviral potency, telbivudine monotherapy is associated with much higher rate of resistance, up to 22% for HBeAg-positive CHB at two years [Keeffe et al. 2008; Lai et al. 2007]. This could be partially explained by the difference in genetic barrier in the development of resistance between the two drugs. Unlike entecavir, telbivudine only requires the single mutation to confer resistance. Cross-resistance between lamivudine and telbivudine is unavoidable since both drugs induce mutations at HBV reverse transcriptase position 204. Similar to lamivudine, the presence of telbivudine resistance would likely predispose to the emergence of entecavir resistance based on the in vitro data [Zoulim, 2006; Yang et al. 2005]. Similar to lamivudine, there is evidence that telbivudine can promote the emergence of rtA181T in treatment-naïve patients.
Monitoring and management of antiviral resistance
Antiviral resistance is the major limitation of prolonged nucleos(t)ide analogue therapy. Careful consideration is needed to select first-line therapy in order to avoid the emergence of resistance; especially that may limit future treatment choices due to cross resistance with other agents. Lamivudine, in the authors’ opinion, is no longer considered a first-line monotherapy because of its high rate of resistance. Even though the wild-type HBV repopulates and becomes the dominant viral species after the discontinuation of antiviral therapy in the setting of resistance, the drug resistant mutants will persist indefinitely in low level. Upon rechallenged with the same drug or drugs with cross-resistant profiles, the resistant mutants will have growth advantage and replicate in high levels [Lau et al. 2000].
HBV DNA quantification is important for initial patient evaluation, for monitoring treatment response and for early detection of virological breakthrough on therapy. The ideal HBV quantification assay should be sensitive, reproducible and have a broad dynamic range of at least 5 log10. The real-time PCR quantification assays possess these properties and, therefore, are recommended for HBV DNA baseline determination and monitoring during therapy [Lole and Arankalle, 2006; Gordillo et al. 2005]. All patients should have baseline serum HBV DNA, ALT, liver function tests, HBeAg/anti HBe prior to initiating the treatment. Thereafter, serum HBV DNA and ALT should be checked every 3-6 months to ensure adequate response to the treatment and early detection of treatment failure [Pawlotsky et al. 2008].
For nucleos(t)ide analogue, its antiviral effect is defined as >1 log10 decrease in HBV DNA within three months of starting the treatment while its antiviral efficacy is the quantitative log10 reduction in viral load when compared to pretreatment level [Pawlotsky et al. 2008]. Treatment failure can be primary and secondary.
Primary treatment failure is defined as a decrease in serum HBV DNA of ≤1 log10IU/mL from baseline after three months of starting therapy [Pawlotsky et al. 2008]. Secondary treatment failure is a rebound of serum HBV DNA resulting in an increase of ≥1 log10IU/mL in patients with initial antiviral treatment effect [Pawlotsky et al. 2008; Lok and McMahon, 2007]. This should be confirmed by two consecutive determinations at a one-month interval. For patients with primary or secondary treatment failure, medication non-compliance should be excluded and if drug resistance is suspected, resistance testing should be performed [Pawlotsky et al. 2008; Lok and McMahon, 2007].
The sensitivity of the different genotypic assays for the detection of drug resistance varies significantly in their ability to identify minor strains of viruses (Table 5)[Sablon and Shapiro, 2005; Sablon et al. 2003]. Direct sequencing, for example, has relative low sensitivity and can only detect mutant viruses if they exceed 15-50% of the total viral population. The advantage of direct sequencing is its ability to identify new mutations. LiPA and MALDI-TOF assays, in contrast, can identify very low levels (5%) of the mutant viruses. It is, therefore, important to know which resistance assay was applied. Standardization of the genotypic resistance assays is necessary to determine the incidence and prevalence of nucleos(t)ide-induced resistant HBV mutations.
Table 5.
Comparison of genotypic assays for detection of drug resistance.1
| Method | Sensitivity | Information details | Commercial | Complexity of interpretation |
| Direct sequencing | 15-50% | High | Yes | High |
| RFLP | 5-10% | Low | No | Intermediate |
| RT-PCR | 5-10% | Low | No | Intermediate |
| Li PA | 5% | Low | Yes | Low |
| Florescence | Not determined | Intermediate | No | Intermediate |
| 2MALDI-TOF | <5% | Intermediate | No | High |
Modified from Erwin Sablon and Fred Shapiro, [Sablon and Shapiro, 2005].
matrix-assisted laser desorption and ionization time-of-flight mass.
Combination therapy
Mathematical modeling of the HBV kinetics with nucleotide analogue therapy showed a biphasic decline of the HBV levels. The initial, faster phase of viral load decline reflects the clearance of HBV particles from plasma. The second, slower phase of viral load decline closely mirrors the rate-limiting process of infected cell loss [Tsiang et al. 1999]. Since the second phase of viral decline is likely to be induced by an immune-mediated process, the immune clearance of the virus should be up-regulated by immuno-modulators such as the IFNs. This suggests that there may be at least a theoretical advantage to the use a combination of a nucleoside or nucleotide analogue with IFN.
Nucleoside analogues and pegIFN
There are a number of published multicenter clinical trials using a combination of lamivudine and pegIFN-a. In the study conducted by Janssen et al., 307 HBeAg-positive patients were randomized to receive either a combination of pegIFN a-2b, 100μg/week for 32 weeks then 50 μg/week for 20 weeks in combination with lamivudine 100mg/day, or pegIFN a-2b with placebo [Janssen et al. 2005]. At 26 weeks follow-up, no difference in efficacy endpoints was found between the pegIFN monotherapy and combination therapy which used a relatively low dose of pegIFN. Besides elevated baseline ALT levels, HBV genotype also was identified to be a predictor of response: 60% of the genotype A patients responded compared to 42% for genotype B, 32% for genotype C and 28% for genotype D. Lau et al. and Marcellin et al. reported results of large randomized controlled trials comparing the efficacy and safety of pegIFN a-2a (180 μg weekly), pegIFN a-2a (180 μg weekly) with lamivudine (100 mg daily) and lamivudine (100 mg daily) alone for 48 weeks in HBeAg-positive and negative patients, respectively [Lau et al. 2005; Marcellin et al. 2004]. At 24 weeks of follow-up, the two pegIFN treatment arms (with or without lamivudine) showed the same efficacy in HBV DNA suppression and HBsAg seroconversion, and were superior to that observed with lamivudine alone in both studies. There was a higher rate of lamivudine resistance in the lamivudine monotherapy arm (18%) compared with the pegIFN a-2a plus lamivudine combination arm (<1%) at week 48 (p<0.001). It is important to emphasize that in both studies combination therapy was associated with at least a 1 log10 greater HBV DNA suppression at the end of the 48-week treatment period compared to either monotherapy. This finding raises the possibility that with prolonged therapy, the durability of combination therapy will increase.
Combined nucleoside and nucelotide analogues
To date, there has been limited data on the efficacy of combining nucleoside and nucleotide analogues. Lau et al. evaluated combination therapy with lamivudine and famciclovir in 21 HBeAg-positive Chinese patients [Lau et al. 2000]. They found that patients who received lamivudine 150mg daily and famciclovir 500 mg three times daily had a more rapid fall in HBV DNA levels and a higher rate of HBeAg loss compared to those on lamivudine monotherapy. A recent study compared the efficacy of adefovir with lamivudine vs lamivudine alone in 112 treatment-naïve, predominantly HBeAg-positive patients [Abstract, Sung et al. J Hepatol 38(suppl), A4313. 2003]. The rates of undetectable HBV DNA by PCR (39 and 41%) and HBeAg loss (19 and 20%) were similar in the two arms. However, there was a significantly lower rate of lamivudine resistance in the combination group (2%) compared to lamivudine monotherapy (20%) (p≤ 0.003).
Although, the combination regimens evaluated so far for 48 to 52 weeks did not appear to improve efficacy, they did reduce the rates of resistance to nucleoside or nucleotide monotherapy (see above). Currently, there are no data on prolonged combination therapy beyond a year. An optimal combination regimen should work synergistically in viral suppression, increase rates of HBeAg and HBsAg seroconversion, and prevent the occurrence of viral resistance.
Who should be treated?
Complete eradication of HBV is not achievable with the currently available agents. Most of the patients with CHB require long-term treatment that can be associated with increased risk of developing antiviral resistance and potential side effects. Until ideal therapy becomes available, it is logical to provide therapy for selective patients who are at risk of developing complications from CHB. A number of recently published practice guidelines provided important framework to manage patients with HBeAg-positive and negative CHB [Keeffe et al. 2007; Lok and McMahon, 2007]. The recommendations on therapy are largely based on serum levels of ALT and HBV DNA. There are, however, continuous debates on the optimal ALT and HBV DNA cutoff values to initiate therapy. For patients who do not meet the clear HBV and ALT criteria for therapy, liver biopsy is essential to determine the degree of hepatic inflammation and fibrosis and to treat if there is evidence of disease. The degree of liver injury and its rate of progression vary significantly among patients with CHB. Factors such as serumALT, HBV DNA, HBV genotypes, naturally occurring HBV mutants and hepatic steatosis have been implicated in disease progression but their accuracy is imperfect. A better understanding of the natural history and identification of predictors of disease progression are crucial for the selection of patients for therapy.
Although, serum ALT levels have traditionally been used as an indicator of the severity of hepatic necroinflammatory activity, emerging data suggest that it does not always reflect the degree of underlying disease in CHB. While the REVEAL Study Group noted that patients with higher baseline ALT levels had increased rates of liver disease progression, more than 80% of the cases of cirrhosis and HCC occurred in patients with ALT activity lower than 45 U/L[Chen et al. 2006; Iloeje et al. 2004]. Other studies have shown that 30–40% of patients with normal serum aminotransferases may have significant degree of liver disease on biopsy [Kim et al. 2004]. Taken together, these findings suggest that serum ALT activity within the normal laboratory range may not be a reliable prognostic predictor for CHB. Limitations of these studies are the lack of serial ALT measurements during the follow-up period and the lack of detailed patient characterizations. Since serum aminotransferases fluctuate over time, especially among those with HBeAg-negative CHB, a single, baseline value cannot be expected to reliably predict the course of a chronic disease. In addition, patients with advanced cirrhosis usually have normal or near-normal ALT. Thus, ALT values must be evaluated in the context of other lab results and clinical features.
The serum concentration of HBV DNA is a measure of the level of viral replication in the liver. Chen et al. conducted a long-term observational study on over 3000 HBV carriers in Taiwan for a mean follow-up period of eleven years and found that the risk of cirrhosis and HCC increased significantly proportional to the levels of serum HBV DNA >104 copies/mL [Chen et al. 2006; Iloeje et al. 2003]. The incidence of cirrhosis increased from 4.5% (relative risk, 1.4) for patients with baseline serum HBV DNA concentrations <300 copies/ml to 36.2% (relative risk, 9.8) for patients with serum concentrations of >106 copies/mL. The relationship between serum HBV DNA concentration and cirrhosis remained independent of HBeAg status and ALT level. Likewise, a high baseline and persistently elevated serum HBV DNA concentration increases the risk of HCC. Of the 164 patients in whom HCC developed, the incidence rates of HCC increased in a dose-response relationship beginning with a baseline serum HBV DNA concentration of l04 copies/mL. The findings of this study are important, however; it suffers from a number of limitations similar to those of smaller retrospective studies. The patients in these studies did not have liver biopsies at baseline or during follow-up, so that the subset of patients who developed cirrhosis or HCC within each of the HBV DNA categories could not be assessed as to risk based on histological criteria. For the majority of the cohort, there was no monitoring of serial ALT, HBV DNA levels, or HBeAg serology during follow-up. Other important viral factors such as HBV genotype and HBeAg-negative mutants were not factored into the analysis. In addition, 85% of this study population had HBeAg-negative CHB. These findings require confirmation before they can be generally applied, especially to young subjects in the immune tolerance phase of the disease.
Available data suggest that HBV genotype may be related to disease outcome. Due to the variability of the HBV genotypes in different regions of the world, a comprehensive comparison of disease outcome based on genotypes using standardized study criteria is unavailable. In Asia where genotype B and C predominant, genotype C is found to be associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B [Chan et al. 2004; Yuen et al. 2004]. In Europe, genotype D is associated with more active disease than genotype A [Kidd-Ljunggren et al. 2002]. Genotype F was recently implicated in conferring increased risk of HCC in Alaskan natives [Livingston et al. 2007] To date, there has been little information regarding disease outcomes associated with genotype E, G, and H. Besides HBV genotypes, naturally occurring HBV mutants have been implicated in disease progression. A number of Asian studies have provided evidence that BCP mutants increase the risk of liver disease progression and HCC development. Since genotype C has a higher prevalence of BCP mutation than genotype B (odds ratio, 5.18), it is uncertain whether BCP mutant alone is an independent risk. Deletions in the pre-S gene of HBV genome have also been implicated in progressive liver injury and hepatocarcinogenesis [Sugauchi et al. 2003; Fan et al. 2001].
These naturally history studies collectively provide evidence that high HBV DNA, genotype C, BCP mutation and pre-S deletion are associated with liver disease progression and HCC development in patients with CHB. It is possible that a combination of these viral factors synergistically increases risk for disease complications. Large-scale prospective studies are needed to confirm the causal relationship between these viral factors and clinical outcomes of chronic HBV infection.
There is strong evidence that nonalcoholic fatty liver disease (NAFLD) and insulin resistance are associated with increased fibrogenesis in chronic hepatitis C [Hu et al. 2007; Leandro et al. 2006; Patton et al. 2004]. The impact of NAFLD and metabolic syndrome on CHB is not well understood. In a retrospective study, Bondini and colleagues found that among 64 HBV patients with available liver biopsies, 8 had nonalcoholic steatohepatitis (NASH) and 4 had simple steatosis [Bondini et al. 2007]. They reported that patients with hepatitis B superimposed with NASH were older, more likely to have hypertension, dyslipidemia, and increased waist circumference. Interestingly, Chu et al. found that increased body mass index (BMI) and hepatic steatosis are associated with HBsAg clearance [Chu et al. 2007]. The authors compared BMI and ultrasound grading of hepatic steatosis between 54 patients with documented HBsAg clearance and 108 age- and gender-matched HBsAg carriers. High BMI (426) and moderate to severe hepatic steatosis were significantly more prevalent among those with HBsAg clearance. The major limitation of the study is the lack of histological evaluation of steatosis, inflammation and fibrosis. In spite of these limitations, the impact of metabolic syndrome and hepatic steatosis on hepatitis B merits careful evaluation in a prospective study since the prevalence of obesity and NAFLD is increasing among Asian Americans.
Conclusions
HBV continues to be a major cause of significant morbidity and mortality despite the availability of effective vaccines and improved therapeutic options. The natural history of hepatitis B is not well understood. A number of viral and host factors have been implicated in disease progression and development of HCC. However, histological data is lacking in most published studies. Furthermore, viral and host factors may work additively or even synergistically in modifying disease status. A large, prospective clinical study with uniform histological assessment and well-represented populations of CHB will be instrumental to accurately establish predictors and confounders of disease outcomes. This will not only extend our understanding of the natural history of hepatitis B but will also accurately guide treatment strategy for this important disease. The ultimate goal of therapy for CHB is to arrest the progression of liver injury and to prevent the development of hepatic complications such as liver failure and HCC. Sustained inhibition of HBV replication has been shown to be associated with normalization of aminotransferases and histological improvement, while HBsAg seroconversion is the best surrogate marker for viral clearance. Choice of first-line therapy taking into account antiviral potency, safety, and low risk of antiviral resistance is critical. The ideal hepatitis B therapy should be safe, effective with a finite course of therapy and associated with a sustained and durable response. Ongoing research is essential to evaluate the new and currently available agents, not only as monotherapy, but as combination therapy to identify the synergies necessary to reach the ultimate goal of therapy.
Conflict of interest statement
Daryl Lau: Bristol-Myers Squibb (research support), Gilead Sciences (consultant), Idenix Pharmaceuticals (consultant), Roche Pharmaceuticals (consultant and research support).
Contributor Information
Daryl T.-Y. Lau, Associate Professor of Medicine, Harvard Medical School (HMS), Director of Translational Liver Research, Beth Israel Deaconess Medical Center, HMS Liver Center, Division of Gastroenterology, Department of Medicine, 110 Francis Street, Suite 4A, Boston, MA 02215. dlau@bidmc.harvard.edu
Wissam Bleibel, Clinical fellow from the Liver Center, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
References
- Alward W.L., McMahon B.J., Hall D.B., Heyward W.L., Francis D.P., Bender T.R. (1985) The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J Infect Dis 151(4):604–609 [DOI] [PubMed] [Google Scholar]
- Arora G., Keeffe E.B. (2007) Chronic hepatitis B with advanced fibrosis or cirrhosis: impact of antiviral therapy. Rev Gastroenterol Disord 7(2):63–73 [PubMed] [Google Scholar]
- Bondini S., Kallman J., Wheeler A., Prakash S., Gramlich T., Jondle D.M.et al. (2007) Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int 27(5):607–611 [DOI] [PubMed] [Google Scholar]
- Chan H.L., Hui A.Y., Wong M.L., Tse A.M., Hung L.C., Wong V.W.et al. (2004) Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 53(10):1494–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.J., Yang H.I., Su J., Jen C.L., You S.L., Lu S.N.et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama 295(1):65–73 [DOI] [PubMed] [Google Scholar]
- Chu C.M., Lin D.Y., Liaw Y.F. (2007) Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond) 31(5):871–875 [DOI] [PubMed] [Google Scholar]
- Colonno R.J., Genovesi E.V., Medina I., Lamb L., Durham S.K., Huang M.L.et al. (2001) Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J Infect Dis 184(10):1236–1245 [DOI] [PubMed] [Google Scholar]
- Colonno R.J., Rose R., Baldick C.J., Levine S., Pokornowski K., Yu C.F.et al. (2006) Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 44(6):1656–1665 [DOI] [PubMed] [Google Scholar]
- Cooksley W.G., Piratvisuth T., Lee S.D., Mahachai V., Chao Y.C., Tanwandee T.et al. (2003) Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 10(4):298–305 [DOI] [PubMed] [Google Scholar]
- Del Poggio P., Zaccanelli M., Oggionni M., Colombo S., Jamoletti C., Puhalo V. (2007) Low-dose tenofovir is more potent than adefovir and is effective in controlling HBV viremia in chronic HBeAg-negative hepatitis B. W o r ld J Gastroenterol 13(30):4096–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doo E., Liang T.J. (2001) Molecular anatomy and pathophysiologic implications of drug resistance in hepatitis B virus infection. Gastroenterology 120(4):1000–1008 [DOI] [PubMed] [Google Scholar]
- Fan Y.F., Lu C.C., Chen W.C., Yao W.J., Wang H.C., Chang T.T.et al. (2001) Prevalence and significance of hepatitis B virus (HBV) pre-S mutants in serum and liver at different replicative stages of chronic HBV infection. Hepatology 33(1):277–286 [DOI] [PubMed] [Google Scholar]
- Fung S.K., Chae H.B., Fontana R.J., Conjeevaram H., Marrero J., Oberhelman K.et al. (2006) Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol 44(2):283–290 [DOI] [PubMed] [Google Scholar]
- Gish R.G., Lau D.T., Schmid P., Perrillo R. (2007) A pilot study of extended duration peginterferon alfa-2a for patients with hepatitis B e antigen-negative chronic hepatitis B. Am J Gastroenterol 102(12):2718–2723 [DOI] [PubMed] [Google Scholar]
- Gish R.G., Lok A.S., Chang T.T., de Man R.A., Gadano A., Sollano J.et al. (2007) Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology 133(5):1437–1444 [DOI] [PubMed] [Google Scholar]
- Gordillo R.M., Gutierrez J., Casal M. (2005) Evaluation of the COBAS TaqMan 48 real-time PCR system for quantitation of hepatitis B virus DNA. J Clin Microbiol 43(7):3504–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziyannis S.J., Vassilopoulos D. (2001) Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 34(4 Pt 1):617–624 [DOI] [PubMed] [Google Scholar]
- Hadziyannis S.J., Papatheodoridis G.V., Vassilopoulos D. (2003) Treatment of HBeAg-negative chronic hepatitis B. Semin Liver Dis 23(1):81–88 [DOI] [PubMed] [Google Scholar]
- Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.T., Kitis G., Rizzetto M.et al. (2003) Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 348(9):800–807 [DOI] [PubMed] [Google Scholar]
- Hadziyannis S.J., Tassopoulos N.C., Heathcote E.J., Chang T.T., Kitis G., Rizzetto M.et al. (2005) Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 352(26):2673–2681 [DOI] [PubMed] [Google Scholar]
- Hollinger F.B., Lau D.T. (2006) Hepatitis B: the pathway to recovery through treatment. Gastroenterol Clin North Am 35(4):895–931 [DOI] [PubMed] [Google Scholar]
- Hoofnagle J.H., Doo E., Liang T.J., Fleischer R., Lok A.S. (2007) Management of hepatitis B: summary of a clinical research workshop. Hepatology 45(4):1056–1075 [DOI] [PubMed] [Google Scholar]
- Hoofnagle J.H., Shafritz D.A., Popper H. (1987) Chronic type B hepatitis and the “healthy” HBsAg carrier state. Hepatology 7(4):758–763 [DOI] [PubMed] [Google Scholar]
- Hu K.Q., Currie S.L., Shen H., Cheung R.C., Ho S.B., Bini E.J.et al. (2007) Clinical implications of hepatic steatosis in patients with chronic hepatitis C: a multicenter study of U. S. veterans. Dig Dis Sci 52(2):570–578 [DOI] [PubMed] [Google Scholar]
- Hunt C.M., McGill J.M., Allen M.I., Condreay L.D. (2000) Clinical relevance of hepatitis B viral mutations. Hepatology 31(5):1037–1044 [DOI] [PubMed] [Google Scholar]
- Iloeje U.H., Yang H.I., Su J., Jen C.L., You S.L., Chen C.J. (2006) Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130(3):678–686 [DOI] [PubMed] [Google Scholar]
- Izzedine H., Launay-Vacher V., Deray G. (2005) Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 45(5):804–817 [DOI] [PubMed] [Google Scholar]
- Janssen H.L., van Zonneveld M., Senturk H., Zeuzem S., Akarca U.S., Cakaloglu Y.et al. (2005) Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365(9454):123–129 [DOI] [PubMed] [Google Scholar]
- Keeffe E.B., Dieterich D.T., Pawlotsky J.M., Benhamou Y. (2008) Chronic hepatitis B: preventing, detecting, and managing viral resistance. Clin Gastroenterol Hepatol 6(3):268–274 [DOI] [PubMed] [Google Scholar]
- Keeffe E.B., Zeuzem S., Koff R.S., Dieterich D.T., Esteban-Mur R., Gane E.J.et al. (2007) Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol 5(8):890–897 [DOI] [PubMed] [Google Scholar]
- Kidd-Ljunggren K., Miyakawa Y., Kidd A.H. (2002) Genetic variability in hepatitis B viruses. J Gen Virol 83(Pt 6):1267–1280 [DOI] [PubMed] [Google Scholar]
- Kim H.C., Nam C.M., Jee S.H., Han K.H., Oh D.K., Suh I. (2004) Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ 328(7446):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Yu S.K., Seo Y.S., Yim H.J., Yeon J.E., Park J.J.et al. (2007) Clinical outcomes of chronic hepatitis B patients with persistently detectable serum hepatitis B virus DNA during lamivudine therapy. J Gastroenterol Hepatol 22(8):1220–1225 [DOI] [PubMed] [Google Scholar]
- Lai C.L., Gane E., Liaw Y.F., Hsu C.W., Thongsawat S., Wang Y.et al. (2007) Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 357(25):2576–2588 [DOI] [PubMed] [Google Scholar]
- Lai C.L., Shouval D., Lok A.S., Chang T.T., Cheinquer H., Goodman Z.et al. (2006) Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 354(10):1011–1020 [DOI] [PubMed] [Google Scholar]
- Lampertico P., Vigano M., Manenti E., Iavarone M., Sablon E., Colombo M. (2007) Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 133(5):1445–1451 [DOI] [PubMed] [Google Scholar]
- Laras A., Koskinas J., Dimou E., Kostamena A., Hadziyannis S.J. (2006) Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology 44(3):694–702 [DOI] [PubMed] [Google Scholar]
- Lau D.T., Everhart J., Kleiner D.E., Park Y., Vergalla J., Schmid P.et al. (1997) Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology 113(5):1660–1667 [DOI] [PubMed] [Google Scholar]
- Lau D.T., Khokhar M.F., Doo E., Ghany M.G., Herion D., Park Y.et al. (2000) Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 32(4 Pt 1):828–834 [DOI] [PubMed] [Google Scholar]
- Lau G.K., Piratvisuth T., Luo K.X., Marcellin P., Thongsawat S., Cooksley G.et al. (2005) Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 352(26):2682–2695 [DOI] [PubMed] [Google Scholar]
- Lau G.K., Tsiang M., Hou J., Yuen S., Carman W.F., Zhang L.et al. (2000) Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology 32(2):394–399 [DOI] [PubMed] [Google Scholar]
- Leandro G., Mangia A., Hui J., Fabris P., Rubbia-Brandt L., Colloredo G.et al. (2006) Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology 130(6):1636–1642 [DOI] [PubMed] [Google Scholar]
- Lee W.M. (1997) Hepatitis B virus infection. N Engl J Med 337(24):1733–1745 [DOI] [PubMed] [Google Scholar]
- Leemans W.F., Ter Borg M.J., de Man R.A. (2007) Review article: Success and failure of nucleo-side and nucleotide analogues in chronic hepatitis B. Aliment Pharmacol Ther 26 Suppl 2:171–182 [DOI] [PubMed] [Google Scholar]
- Leung N.W., Lai C.L., Chang T.T., Guan R., Lee C.M., Ng K.Y.et al. (2001) Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 33(6):1527–1532 [DOI] [PubMed] [Google Scholar]
- Liaw Y.F., Leung N.W., Chang T.T., Guan R., Tai D.I., Ng K.Y.et al. (2000) Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology 119(1):172–180 [DOI] [PubMed] [Google Scholar]
- Liaw Y.F., Sung J.J., Chow W.C., Farrell G., Lee C.Z., Yuen H.et al. (2004) Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 351(15):1521–1531 [DOI] [PubMed] [Google Scholar]
- Lin S.M., Sheen I.S., Chien R.N., Chu C.M., Liaw Y.F. (1999) Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology 29(3):971–975 [DOI] [PubMed] [Google Scholar]
- Liu C.J., Chen P.J., Lai M.Y., Kao J.H., Chen D.S. (2001) Hepatitis B virus variants in patients receiving lamivudine treatment with breakthrough hepatitis evaluated by serial viral loads and full-length viral sequences. Hepatology 34(3):583–589 [DOI] [PubMed] [Google Scholar]
- Livingston S.E., Simonetti J.P., McMahon B.J., Bulkow L.R., Hurlburt K.J., Homan C.E.et al. (2007) Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis 195(1):5–11 [DOI] [PubMed] [Google Scholar]
- Locarnini S. (2005) Molecular virology and the development of resistant mutants: implications for therapy. Semin Liver Dis 25(Suppl 1):9–19 [DOI] [PubMed] [Google Scholar]
- Locarnini S., Mason W.S. (2006) Cellular and virological mechanisms of HBV drug resistance. J Hepatol 44(2):422–431 [DOI] [PubMed] [Google Scholar]
- Locarnini S., Hatzakis A., Heathcote J., Keeffe E.B., Liang T.J., Mutimer D.et al. (2004) Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther 9(5):679–693 [PubMed] [Google Scholar]
- Lok A.S., McMahon B.J. (2007) Chronic hepatitis B. Hepatology 45(2):507–539 [DOI] [PubMed] [Google Scholar]
- Lok A.S., Chung H.T., Liu V.W., Ma O.C. (1993) Long-term follow-up of chronic hepatitis B patients treated with interferon alfa. Gastroenterology 105(6):1833–1838 [DOI] [PubMed] [Google Scholar]
- Lok A.S., Heathcote E.J., Hoofnagle J.H. (2001) Management of hepatitis B: 2000-summary of a workshop. Gastroenterology 120(7):1828–1853 [DOI] [PubMed] [Google Scholar]
- Lole K.S., Arankalle V.A. (2006) Quantitation of hepatitis B virus DNA by real-time PCR using internal amplification control and dual TaqMan MGB probes. J Virol Methods 135(1):83–90 [DOI] [PubMed] [Google Scholar]
- Magnius L.O., Norder H. (1995) Subtypes, genotypes and molecular epidemiology of the hepatitis B virus as reflected by sequence variability of the S-gene. Intervirology 1995; 38(1-2):24–34 [DOI] [PubMed] [Google Scholar]
- Manesis E.K., Hadziyannis S.J. (2001) Interferon alpha treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastroenterology 121(1):101–109 [DOI] [PubMed] [Google Scholar]
- Marcellin P., Lau G.K., Bonino F., Farci P., Hadziyannis S., Jin R.et al. (2004) Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 351(12):1206–1217 [DOI] [PubMed] [Google Scholar]
- Margolis H.S., Alter M.J., Hadler S.C. (1991) Hepatitis B: evolving epidemiology and implications for control. Semin Liver Dis 11(2):84–92 [DOI] [PubMed] [Google Scholar]
- McMahon B.J., Alward W.L., Hall D.B., Heyward W.L., Bender T.R., Francis D.P.et al. (1985) Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 151(4):599–603 [DOI] [PubMed] [Google Scholar]
- McQuillan G.M., Coleman P.J., Kruszon-Moran D., Moyer L.A., Lambert S.B., Margolis H.S. (1999) Prevalence of hepatitis B virus infection in the United States: the national health and nutrition examination surveys, 1976 through 1994. Am J Public Health 89(1):14–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Heintges T., Lange S., Goldmann G., Niederau C.M., Mohr L.et al. (1996) Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N Engl J Med 334(22):1422–1427 [DOI] [PubMed] [Google Scholar]
- Okamoto H., Tsuda F., Akahane Y., Sugai Y., Yoshiba M., Moriyama K.et al. (1994) Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J Virol 68(12):8102–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Yotsumoto S., Akahane Y., Yamanaka T., Miyazaki Y., Sugai Y.et al. (1990) Hepatitis B viruses with precore region defects prevail in persistently infected hosts along with seroconversion to the antibody against e antigen. J Virol 64(3):1298–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono-Nita S.K., Kato N., Shiratori Y., Lan K.H., Yoshida H., Carrilho F.J.et al. (1999) Susceptibility of lamivudine-resistant hepatitis B virus to other reverse transcriptase inhibitors. J Clin Invest 103(12):1635–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoridis G.V., Dimou E., Dimakopoulos K., Manolakopoulos S., Rapti I., Kitis G.et al. (2005) Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology 42(1):121–129 [DOI] [PubMed] [Google Scholar]
- Patton H.M., Patel K., Behling C., Bylund D., Blatt L.M., Vallee M.et al. (2004) The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol 40(3):484–490 [DOI] [PubMed] [Google Scholar]
- Pawlotsky J.M., Dusheiko G., Hatzakis A., Lau D., Lau G., Liang T.J.et al. (2008) Virologic monitoring of hepatitis B virus therapy in clin trials and practice: recommendations for a standardized approach. Gastroenterology 134(2):405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazella M.A. (2003) Drug-induced renal failure: update on new medications and unique mechanisms of nephrotoxicity. Am J Med Sci 325(6):349–362 [DOI] [PubMed] [Google Scholar]
- Perrillo R.P. (2001) Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 120(4):1009–1022 [DOI] [PubMed] [Google Scholar]
- Sablon E., Shapiro F. (2005) Advances in molecular diagnosis of HBV infection and drug resistance. Int J Med Sci 2(1):8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablon E., Shapiro F., Zoulim F. (2003) Early detection of hepatitis B drug resistance: implications for patient management. Expert Rev Mol Diagn 3(5):535–547 [DOI] [PubMed] [Google Scholar]
- Sugauchi F., Ohno T., Orito E., Sakugawa H., Ichida T., Komatsu M.et al. (2003) Influence of hepatitis B virus genotypes on the development of preS deletions and advanced liver disease. J Med Virol 70(4):537–544 [DOI] [PubMed] [Google Scholar]
- Tacke F., Manns M.P., Trautwein C. (2004) Influence of mutations in the hepatitis B virus genome on virus replication and drug resistance- implications for novel antiviral strategies. Curr Med Chem 11(20):2667–2677 [DOI] [PubMed] [Google Scholar]
- Tan J., Degertekin B., Wong S.N., Husain M., Oberhelman K., Lok A.S. (2008) Tenofovir monotherapy is effective in hepatitis B patients with antiviral treatment failure to adefovir in the absence of adefovir-resistant mutations. J Hepatol 48(3):391–398 [DOI] [PubMed] [Google Scholar]
- Tenney D.J., Levine S.M., Rose R.E., Walsh A.W., Weinheimer S.P., Discotto L.et al. (2004) Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother 48(9):3498–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang M., Rooney J.F., Toole J.J., Gibbs C.S. (1999) Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29(6):1863–1869 [DOI] [PubMed] [Google Scholar]
- Villet S., Pichoud C., Billioud G., Barraud L., Durantel S., Trepo C.et al. (2008) Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J Hepatol 48(5):747–755 [DOI] [PubMed] [Google Scholar]
- Werle-Lapostolle B., Bowden S., Locarnini S., Wursthorn K., Petersen J., Lau G.et al. (2004) Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126(7):1750–1758 [DOI] [PubMed] [Google Scholar]
- Yang H., Qi X., Sabogal A., Miller M., Xiong S., Delaney W.E., 4th. (2005) Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir Ther 10(5):625–633 [PubMed] [Google Scholar]
- Yuen M.F., Hui C.K., Cheng C.C., Wu C.H., Lai Y.P., Lai C.L. (2001) Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 34(1):139–145 [DOI] [PubMed] [Google Scholar]
- Yuen M.F., Tanaka Y., Mizokami M., Yuen J.C., Wong D.K., Yuan H.J.et al. (2004) Role of hepatitis B virus genotypes Ba and C, core promoter and precore mutations on hepatocellular carcinoma: a case control study. Carcinogenesis 25(9):1593–1598 [DOI] [PubMed] [Google Scholar]
- Zoulim F. (2005) Combination of nucleoside analogues in the treatment of chronic hepatitis B virus infection: lesson from experimental models. J Antimicrob Chemother 55(5):608–611 [DOI] [PubMed] [Google Scholar]
- Zoulim F. (2006) In vitro models for studying hepatitis B virus drug resistance. Semin Liver Dis 26(2):171–180 [DOI] [PubMed] [Google Scholar]