Abstract
High participation is a key requirement for effective cancer screening. Many strategies to improve participation hold that a person's knowledge and beliefs dictate screening behavior. We compared perception of colon cancer risk in participants and nonparticipants in a population-based study of screening colonoscopy, and also assessed past screening behavior. Surprisingly, while past screening behavior was a predictor of participation, we found that participants perceived their risk of colorectal cancer to be significantly and substantially lower than the real figure and that of nonparticipants. Our data suggest that health promotion strategies aimed at improving health knowledge may not be effective in improving population screening rates.
Keywords: colorectal cancer screening; colonoscopy; health knowledge/attitudes/practice; patient participation/psychology; mass screening, questionnaires
Introduction
Colorectal cancer (CRC) is a common and severe illness [Weitz et al. 2005; World Health Organization, 2003], which has led to programmes aimed at prevention. CRC mortality can be reduced by screening programmes and colon cancer incidence may be prevented by screening and polypectomy [Levin et al. 2008]. Screening for CRC can be performed by faecal occult blood test or flexible sigmoidoscopy followed by colonoscopy (two-stage screening), colonoscopy alone, and potentially other modalities; these methods are predicted to be highly cost-effective [O'Leary et al. 2004; Provenzale, 2002].
The effectiveness of screening in CRC prevention, mortality reduction and cost-effectiveness hinges on participation rates. Other factors, including access to services and service quality, are also important. Suggested strategies to enhance large-scale participation include education about the disease, engagement in the screening process by family physicians and endorsement by public figures [Klabunde et al. 2007; Hoff and Bretthauer, 2006; Podolsky, 2000]. The idea that a person's knowledge and beliefs about disease and screening dictate screening behavior (health belief model) is central to these strategies [Weinstein, 1988; Janz and Becker, 1984].
We explored CRC risk perception in a population-based sample of subjects invited to participate in a colon cancer screening study, using colonoscopy as the primary screening modality [Corbett et al. 2004]. Invitations were sent by mail. Preceived risk of colon cancer and attitudes to cancer screening were then compared between participants and those invitees who did not respond to invitation, to test the role of colon cancer perceived risk in the decision to undergo screening.
Methods
Criteria for selection of subjects
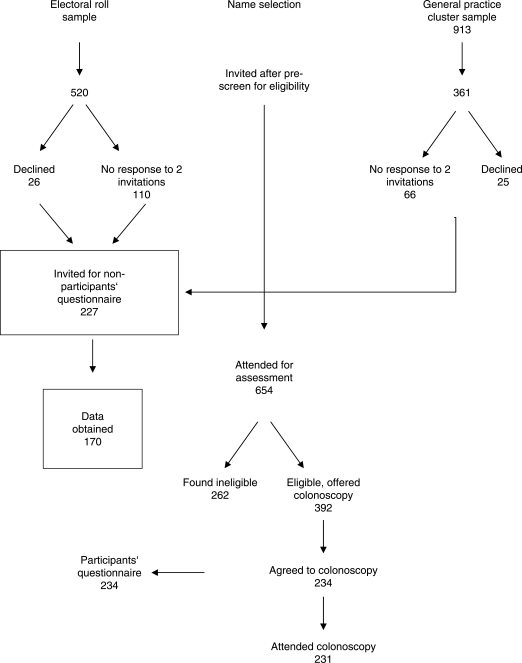
Asymptomatic male and female residents of the Australian Capital Territory (n ¼ 880), aged 55–74 years were randomly selected from the Australian Electoral Roll (n ¼ 520) or from six primary care physicians’ databases (n¼361) and invited by letter to participate in CRC screening by colonoscopy (Figure 1). A single reminder letter was sent if no reply was received after 1 month. Of 880 invitations sent, positive replies were received from 653, of whom 650 (74% response rate) attended for study enrolment. Exclusion criteria included: (1) prior diagnosis of cancer, not including nonmelanomatous skin cancer; (2) colonoscopy, faecal occult blood test, sigmoidoscopy, barium enema or virtual colonoscopy within the previous 10 years; (3) lower gastrointestinal tract symptoms causing GP attendance in the previous 12 months; (4) significant comorbidity; (5) therapeutic anticoagulation; and (6) participation in a clinical trial in the previous 3 months. Of the 650 subjects attending enrolment, 384 (59%) were found to be eligible and 234 agreed to undergo screening colonoscopy with 231 (36%) having colonoscopy. We called the 234 (including the three who did not ultimately attend colonoscopy) ‘the participants’.
Figure 1.
Sources of subjects for this study.
A further 51 invitees responded to the invitation letter but declined participation, and 176 invitees did not respond. These 227 invitees were then invited by telephone to participate in a two-page questionnaire.
Questionnaire development
The 234 participants (121 male, 113 female) completed a 15-page questionnaire, developed by the investigators, encompassing medical history, family history, health-related quality-of-life short form-36 (SF-36; results have been presented elsewhere [Taupin et al. 2006]) and an eight-domain questionnaire (see below) of colorectal cancer knowledge and risk perception and past screening behaviour.
The 227 nonparticipants were invited by telephone to participate in a two-page questionnaire, developed by the investigators, covering 39 points in 8 domains (nonparticipants questionnaire, see supplementary materials) of CRC knowledge and risk perception and past screening behaviour. Briefly, these eight domains encompassed: (1) perceived discomfort or danger of colonoscopy; (2) demographics (sex, race, marital status, occupation); (3) previous bowel cancer screening, including colonoscopy, barium enema, faecal occult blood test (FOBT), sigmoidoscopy, virtual colonoscopy; (4) previous screening for other cancers [mammography, cervical (Pap) smear, prostate-specific antigen (PSA)]; (5) presence of any serious medical problems including cancer, colon/bowel surgery, or taking warfarin; (6) knowledge of relatives with cancer; (7) bowel cancer knowledge, including discussion of the study with the subject's GP and (8) presence of any bowel symptoms.
No more than two attempts were made to contact these nonparticipants. Subjects were read a list of questions according to a standard script, and were informed, ‘You may decline to answer all or any of the following questions.’ A total of 170 nonparticipants (75%, 75 male, 95 female) provided answers to at least 6 of the 8 domains covered in the nonparticipants questionnaire, with a maximum of 48 responses possible in total. The Australian Capital Territory Human Research Ethics Committee and the Calvary Hospital Medico-Moral Human Research and Ethics Committee approved the study.
Perceived risk assessment
We asked both groups: ‘What is your risk of colon cancer over your whole life?’, and offered the following choices: 1 in 10, 1 in 25, 1 in 75, 1 in 100, 1 in 150, 1 in 250, 1 in 350, 1 in 500, and 1 in 1000 or less. We asked participants only: ‘What is your risk of colon cancer in the next 5 years?’, and offered the same choices. We then asked participants only: ‘If you get colon cancer what is your chance of dying from it?’, and provided the following selection of percentages: 10%, 25%, 33%, 50%, 66%, 75% and 100%. Participants answered these questions by checking a box in a paper questionnaire, with assistance from the interviewer when requested, while nonparticipants gave their answers by telephone.
Endorsement by general practitioners
This study recruited subjects from the electoral roll and from primary care physicians’ databases [Corbett et al. 2004]. In Australia, primary care physicians are referred to as ‘GPs’. Those invited from this latter group received letters of invitation cosigned by the primary care physician and the study investigators. We asked the electoral roll participants (who had not been specifically contacted by their primary care physicians) and electoral roll nonparticipants: ‘Have you discussed this study with your GP?’ We also asked both participants and nonparticipants to assess the anticipated discomfort of colonoscopy according to a 10-point Likert scale.
Statistical analysis
In addition to descriptive statistics, we performed Chi-squared, Fisher exact and t-tests for comparison of groups. Multiple logistic regression was used to explore predictors of participation. We performed sensitivity analysis to examine the significance of comparisons between participants and nonparticipants where there were missing responses; predominantly, missing responses were in the nonparticipants group. Occupational category was recorded according to the Australian Bureau of Statistics (ABS) Australian Standard Classification of Occupations [Austrialian Bureau of Statistics, 1997]. Stata 9.2 was used for statistical analysis.
Results
Levels of knowledge
To assess whether participants were able to make a ‘competent’ assessment of risk, we examined those responses where the subject's perceived risk of developing CRC in the next five years was greater than the perceived lifetime risk. Of 234 participants, 62 reported a higher 5-year risk than lifetime risk, 115 reported equal risk assessments, and 57 correctly reported a lower 5-year risk than lifetime risk. Using this criterion, 172 of 234 participants (74%) answered these questions ‘competently’. There was no relationship between this criterion and occupational category (9 × 9 contingency table: χ2= 12.9 with 16 degrees of freedom, p = 0.68), or according to professional versus nonprofessional occupation (2 × 3 contingency table: χ2 = 3.5 with two degrees of freedom, p = 0.17). Furthermore, we observed no differences in the absolute levels of perceived risk according to occupational category, whether professional vs nonprofessional or manual vs clerical, albeit that we were only able to perform these comparisons in the participant group.
Perceived risk of CRC incidence and mortality
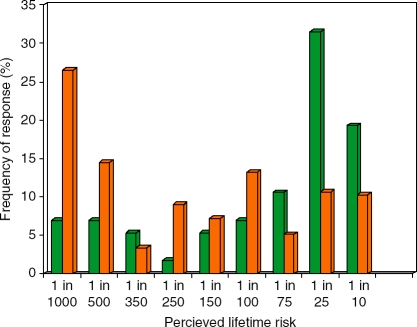
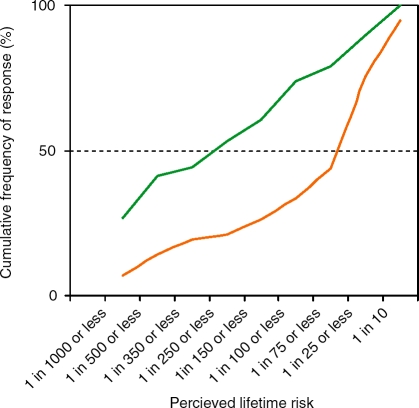
We next determined the perceived risk of CRC in participants and nonparticipants. We asked both groups to choose from an offered range. The response rate to this question was 234/234 for participants and 161/170 (95%) for nonparticipants. In both groups, the majority of respondents perceived their lifetime risk of CRC to be less than the true figure (approximately 1 in 20). As shown in Figure 2, nonparticipants most frequently assessed lifetime risk of CRC to be 1 in 25. Participants most frequently perceived lifetime risk to be 1 in 1000. We then represented these frequencies of response cumulatively. Nonparticipants perceived higher lifetime risk at all ranges of risk, indicating that the high rates of risk perception in some nonparticipants were not offset by lower rates of risk perception in others (Figure 3). This ‘optimistic bias’ in participants compared to nonparticipants was statistically significant (χ2 = 30.02, p<.001).
Figure 2.
Perceived lifetime risk of CRC. For nonparticipants (green bars), the most frequent perceived lifetime risk was 1 in 25, while for participants (orange bars), the most frequent lifetime risk was 1 in 1000.
Figure 3.
Cumulative risk of CRC. Nonparticipants (green line) perceived higher lifetime risk than participants (orange line) at all ranges of risk, indicating that the high rates of risk perception in some nonparticipants were not offset by lower rates of risk perception in others. This difference was statistically significant (χ2 = 30.02, p <.001).
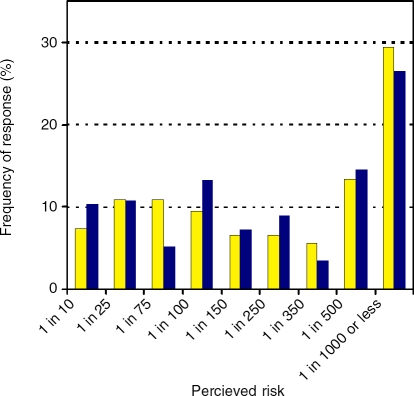
We then asked participants to rate their risk of developing CRC in the next 5 years. This question was immediately below the lifetime risk question on the paper questionnaire, and provided the same ranges represented in parallel. While again the most frequent response was the lowest risk (1 in 1000, 29.4%, Figure 4), most participants did not rate the risk in the next five years to be appreciably lower than lifetime risk. Indeed, as described above, 26% of participants believed their lifetime risk was lower than their risk in the next five years.
Figure 4.
Comparative perceived risk of developing colorectal cancer in the next 5 years (yellow bars) vs perceived lifetime risk overall of developing colorectal cancer (blue bars) (participants only).
Participants were then asked what the mortality rate of CRC was (‘If you develop bowel cancer, what are your chances of dying from it?’).
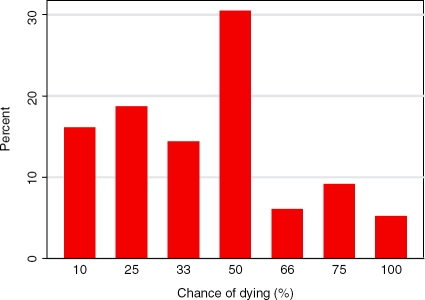
Most frequently, participants felt the mortality of CRC was 50% (30.4% of participants, Figure 5). Cumulatively, 49.1% participants felt the mortality was less than 50%, while only 20.4% felt the mortality was more than 50%.
Figure 5.
Perceived risk of dying from colorectal cancer, once diagnosed (participants only).
Other predictors of participation
Women who had previously had a mammogram or Pap smear were significantly more likely to participate, as were men who had had a PSA test (Table 1). Previous faecal occult blood test (FOBT) was negatively associated with participation. However, as shown in Table 1, only 20 subjects reported previous FOBT, of whom 5 participated and 15 did not participate. Occupational category (professional vs nonprofessional) was not associated with participation.
Table 1.
Proportions participating, according to past screening behaviour and occupational category.
| Yes | No | Unknown | OR for participation∗ | FET∗p-value | |
| Previous FOBT | 5/15 participated (33%) | 229/363 participated (59%) | 0/26 (0%) | 0.29 | 0.028 |
| Sigmoidoscopy | 4/6 (67%) | 230/372 (62%) | 0/26 (0%) | 1.23 | 1.0 |
| Mammography | 109/167 (65%) | 4/18 (22%) | 0/24 (0%) | 6.58 | 0.001 |
| Pap smear | 110/168 (65%) | 3/13 (23%) | 0/28 (3%) | 6.32 | 0.005 |
| PSA | 69/98 (70%) | 49/78 (63%) | 3/21 (14%) | 1.41 | 0.33 |
| Virtual CT | 0/1 (0%) | 234/376 (62%) | 0/27 (0%) | 0 | 0.38 |
| 1st degree relative with cancer | 120/167 (72%) | 114/237 (48%) | 0/0 | 2.75 | 0.000 |
| Higher occupation | 141/199 (71%) | 87/127 (69%) | 7/79 (8%) | 1.09 | 0.80 |
Comparing ‘yes' vs ‘no', omitting ‘unknown'. FET, Fisher's exact test; FOBT, faecal occult blood test; OR, odds ratio.
We asked participants and nonparticipants if they had knowledge of a first-degree relative with any cancer, the relationship with the subject, and cancer type if known. Participants were more likely to report a first-degree relative with any cancer, and more likely to report a first-degree relative with CRC, breast cancer (Table 2) and lung cancer (data not shown).
Table 2.
Association between anticipated discomfort, discussion with GP and knowledge of a relative with cancer, and participation in colonoscopic screening.
| 1. Anticipated discomfort score | 5-10 | Less than 5 | Missing | FET p value (missing omitted) |
| Participants | 31 | 202 | 1 | |
| Nonparticipants | 27 | 13 | 130 | 0.000 |
| 2. Discussed with GP (electoral roll arm) | Yes | No | Missing | |
| Participants | 47 | 72 | 1 | |
| Nonparticipants | 18 | 104 | 10 | 0.000 |
| 3. GP recommended | Yes | No | Missing | |
| Participants | 44 | 3 | 0 | |
| Nonparticipants | 8 | 10 | 0 | 0.000 |
| 4. First degree relative with any cancer | Yes | No | Missing | |
| Participants | 116120 | 128114 | 0 | |
| Nonparticipants | 4847 | 122123 | 0 | 0.000 |
| 5. First degree relative with colorectal cancer | Yes | No | Missing | |
| Participants | 40 | 194 | 0 | |
| Nonparticipants | 11 | 159 | 0 | 0.001 |
| 6. First degree relative with breast cancer | Yes | No | Missing | |
| Participants | 48 | 186 | 0 | |
| Nonparticipants | 9 | 161 | 0 | 0.000 |
FET, Fisher's exact test.
Following univariate analysis, we performed multiple regression to adjust for confounding between these various predictors. Up to 15% of responses in these categories were missing (or unknown) from nonparticipants (Table 1). We therefore performed this analysis separately, according to treatment of unknown responses as ‘no’ (Table 3) or as missing data (Table 4). The modelling was done separately for each sex. The results seen in single-variable analysis were also present in multivariable analysis under the condition that unknowns were ‘no’, except that the negative association between past FOBT and participation was significant only in males. Under the condition that unknowns were omitted, past mammogram but not Pap smear was associated with participation, and the association between past FOBT and participation was again significant only in males.
Table 3.
Multiple logistic regression of past screening behaviour and participation, unknowns treated as ‘No'.
| Females (n = 207) | Males (n=195) | |||
| OR for participation (CI) | p value | OR (CI) | p value | |
| FOBT | 0.80 (0.17, 3.79) | 0.78 | 0.067 (0.007, 0.655) | 0.020 |
| Sigmoidoscopy | 1.08 (0.09, 12.7) | 0.95 | 1.04 (0.08, 13.5) | 0.98 |
| 1st degree relative with cancer | 2.42 (1.26,4.64) | 0.008 | 2.49 (1.28,4.85) | 0.007 |
| Mammography | 5.88 (1.72, 20.1) | 0.005 | – | – |
| Pap smear | 7.80 (2.07, 30.6) | 0.002 | – | – |
| PSA | – | – | 2.41 (1.29,4.50) | 0.006 |
FOBT, faecal occult blood test; OR, odds ratio; PSA, rostate specific antigen.
Table 4.
Multiple logistic regression of past screening behaviour and participation, unknowns omitted.
| Females (n=179) | Males (n=169) | |||
| OR (CI) | p value | OR (CD | p value | |
| FOBT | 0.74 (0.16, 3.41) | 0.70 | 0.063 (0.007, 0.59) | 0.026 |
| Sigmoidoscopy | 1.07 (0.09, 12.6) | 0.96 | – | – |
| 1st degree relative with cancer | 2.23 (1.17,4.27) | 0.015 | 2.08 (1.01,4.28) | 0.047 |
| Mammography | 4.35 (1.23, 15.4) | 0.023 | – | – |
| Pap smear | 3.50 (0.80, 15.2) | 0.095 | – | – |
| PSA | – | – | 1.68 (0.85, 3.31) | 0.14 |
FOBT, faecal occult blood test; OR, odds ratio; PSA, prostate specific antigen.
Endorsement by general practitioners
We asked the electoral roll participants (who had not been specifically contacted by their primary care physicians), and electoral roll nonparticipants: ‘Have you discussed this study with your GP?’. Of 234 participants, 47 had discussed the study with their primary care physician (Table 2), while only 18 of 170 nonparticipants volunteered this information (χ2 = 18,7, p = 0.00). The primary care physician had recommended participation in 44 of 47 participants, and 8 of 18 nonparticipants. This also was a statistically different proportion (χ2 =19, 7, p = 0.00), although numbers in these groups were small.
We asked both participants and nonparticipants to assess the anticipated discomfort of colonoscopy according to a 10-point Likert scale.
Participants were less likely to assess the anticipated discomfort as more than five on a 10-point scale (Table 2) than nonparticipants (χ2 = 60.6, p = 0.00). However, many nonparticipants chose not to respond to this question.
Discussion
The effectiveness of cancer screening in populations hinges on achieving high participation rates in diverse communities [Smith et al. 2006]. Education of the public and screening providers is viewed as an essential element in effective cancer screening. We performed this cross-sectional analysis to determine if risk perception was associated with participation in colon cancer screening with colonoscopy. We confirmed that participation was associated with participation in mammography and cervical cancer screening, in line with the literature. We were therefore surprised that perception of higher colon cancer risk was associated with nonparticipation with colon cancer screening.
Reasons for participation in CRC screening may be random or chaotic and therefore unknowable. Illustrating this possibility, an Australian study of CRC screening by flexible sigmoidoscopy, using mailout of invitations to a population aged 55–59 in the Fremantle area of Western Australia, resulted in only 12% participation in the screening procedure [Olynyk et al. 1996]. A similar mailout to Boston city employees age 50 and older resulted in approximately 3% participation (not corrected for eligibility) in screening flexible sigmoidoscopy [Schroy et al. 1996]. Most of the difference between these results and our participation rate of 59% can be attributed to differences in crude response rate. It is unlikely, on face value, that any characteristics of our invitation letter could have been responsible. Could attributes of the different study populations, the timing of the studies (a decade between the Boston and Fremantle studies and our study), or of the procedure on offer (flexible sigmoidoscopy vs colonoscopy) really explain these large differences?
In this study, we reasoned that if perceived risk played a dominant role in the decision to screen, there would be a significant difference between degree of perceived risk in participants and nonparticipants, with the highest risk perception in participants. In fact, the reverse was found, with a significantly higher risk perception in nonparticipants (Figure 2). This significant difference was apparent at all levels of perceived risk (Figure 3). This finding suggests that perceived risk does not play a significant role in the choice to undergo screening.
Our study was also designed to test the role of the primary care physician in participation in colon cancer screening. We have reported that recruiting subjects from primary care physician practices rather than from the broad population does increase participation modestly; this difference can be ascribed to superior eligibility and is offset by the effort this requires. Crude response rates did not differ according to source of invitation [Corbett, 2004]. In this study, we further assessed the role of the primary care physician in the decision to screen, by asking invitees whether they had discussed the study with their primary care physician and whether the primary care physician had recommended the study (these questions were asked of invitees in the electoral roll arm). Both discussion with primary care physicians and recommendation from primary care physicians was significantly associated with participation. The strength of this association tempts us to speculate that the most effective role for primary care physicians in cancer screening is in consultation rather than promotion; however, our study was not designed to specifically compare the two activities.
We found that participants were more likely to report a first-degree relative with any cancer, and a first-degree relative with CRC. This suggests that knowledge of a close relative with cancer may influence the decision to screen, a proposition that has face value. We did not ask subjects if this knowledge affected their decision on colonoscopic screening. We also found that participants were significantly more likely to report a first-degree relative with breast cancer and lung cancer. We caution therefore that these responses may have been subject to bias; for example, because participants had longer time to answer this question thoughtfully.
One of the strengths of this study is that the population sample was geographically representative of the entire community; furthermore, the community is rather homogeneous [Corbett, 2004]. We were able to associate screening behaviour and beliefs and knowledge. This allowed the health belief model, which underpins some screening philosophies [Weinstein, 1988; Janz and Becker, 1984], to be directly tested.
We obtained useful data from nonparticipants by questionnaire. This can be difficult data to obtain; however, we obtained useful data in 170 of 227 (75%) nonparticipants. This however came at the expense of many incomplete answers to the nonparticipants questionnaire, and many of these comparisons therefore did not survive sensitivity analysis and were not presented in this report.
There is a possibility that many individuals were not capable of making a competent assessment of risk. We made no direct assessment of health literacy, overall literacy or numeracy. Numeracy has been shown in one screening population to predict accuracy of risk perception [Schwartz et al. 1997]. However, we assumed responses were ‘competent’ if the rating for ‘what is the chance of developing bowel cancer in the next 5 years?’ was not greater than the rating for ‘What is the chance of developing bowel cancer in your lifetime?’ Using this criterion, 172 of 234 participants (74%) answered these questions ‘competently’. There was no relationship between this criterion and occupational category, or according to professional vs nonprofessional occupation. Furthermore, we observed no differences in perceived risk according to occupational category, whether professional vs nonprofessional or manual vs clerical, albeit that we were only able to perform these comparisons in the participant group. This argues against the possibility that the reverse relationship between participation and risk perception was due to heterogeneity between the two groups with respect to vocation, which we did not assess in nonparticipants. Our assessment of ‘competence’ is in line with previous data that show that many people do not adjust risk perception according to time span [Zikmund-Fisher et al. 2005], although this was shown for assessments of hypothetical rather than actual risk. Perception of risk may be inaccurate if the form in which it is conveyed is not understood. It is possible that this may explain our unexpected results. However, we observed that the significant difference in risk perception between the participants and nonparticipants was concordant at all levels of perceived risk (Figure 3). We chose to convey risk as a frequency rather than a percentage, because of existing data that demonstrate better perception of frequencies for risk perception [Fagerlin et al. 2007]. Possibly the best method for risk perception may be graphical; however, this would not have been possible for our telephone sample.
Our study had a number of limitations. The nonparticipants questionnaire was considerably briefer that that administered to participants. For example, while perceived overall risk of colorectal cancer was assessed in both groups, perceived risk in the next 5 years and perceived mortality was asked only in the participants group. We did not obtain useful information on occupation from the nonparticipants. Although we obtained useful responses from 75% of nonparticipants, the responses may still have reflected selection bias (e.g. the disadvantaged or itinerant were likely not adequately represented). By allowing interviewees to answer the nonparticipants questionnaire selectively, we created a question response bias, whereby a nonparticipant may have chosen to respond only if he/she had strong beliefs regarding the question. In addition, both questionnaires were developed by the investigators and are still undergoing validation in different screening groups in our population.
Our sample of participants was also depleted in subjects who had a previous colonoscopy, as this was an exclusion criterion of the study. Of those who responded to our invitation letter, then were excluded, 53% were for reason of colonoscopy in the last 10 years, and 19% of the electoral roll sample were so excluded [Corbett, 2004]. It is possible that this depleted our sample of subjects that may have apprehended a higher risk of CRC.
In addition, the participant questionnaires were answered in writing with the participants on-site, with assistance from an investigator where requested, as part of a number of questionnaires comprising 15 pages and taking 30–40 min, while nonparticipants answered orally, at home, in a process taking approximately 15min, and with no other assessments taking place. Therefore, it is probable that there were differences in attention between the two groups. Furthermore, the nonparticipants questionnaire was administered between 6 and 12 months after the invitation letter was sent, and therefore at least 6 months since the decision not to screen in the case of nonparticipants, whereas participants were administered their questionnaires 1–12 months after the invitation letter. Inter-group comparisons must therefore be interpreted in this light, although the finding that nonparticipants rated the risk of CRC higher than participants argues against this being a weakness of the study.
A recognised phenomenon of questionnaires where a range of possible responses is offered is that respondents have a tendency to select the middle of the range. However, in our study this was not apparent, except for the perceived mortality of colorectal cancer (Figure 4). Indeed the distribution of responses to risk assessment was markedly asymmetrical. Furthermore, the distribution was positively skewed in nonrespondents and negatively skewed in participants (Figure 2).
In our study, nonparticipants anticipated significantly greater discomfort from colonoscopy than attendees. This analysis was significantly weakened by the large proportion of nonparticipants who chose not to answer this question, and did not survive sensitivity analysis. There is some face validity to the idea that nonparticipants who answered would perceive more discomfort, and would volunteer this, than those who chose not to answer this question. This is a difficult idea to verify scientifically.
Characteristics of the study population that have been correlated with higher participation in colorectal cancer screening by FOBT and flexible sigmoidoscopy include female gender, income and completion of higher education, but not ethnicity [Shapiro et al. 2001; Vernon, 1997]. Our study of screening colonoscopy showed no influence of gender on risk perception or overall participation. We used occupation as a marker of education and income, and, with this caveat, saw no association with risk perception. Our study was not powered to analyze the effect of ethnicity.
We observed a clear correlation between participation in colonoscopic screening and some previous screening behaviour. Female nonparticipants were less likely to have had screening mammograms or Pap smears. This has also been a clear finding in other forms of CRC screening [Shapiro, 2001]. However, male nonparticipants were no less likely to have had previous PSA test for prostate cancer (assuming ‘unknowns’ were treated as a ‘no’ response). It must be emphasised that population screening by PSA is not endorsed in Australia and testing where performed is usually initiated by general practitioners [Pinnock, 2004]. We draw no conclusions from the significant negative association between past FOBT and participation in CRC screening through colonoscopy, due to the small numbers of subjects who had undergone previous FOBT.
Health behavior change theories hold that individuals who perceive greater CRC risk are more likely to screen for CRC than those with lower perceived risk [Vernon, 1999; Weinstein, 1988; Janz and Becker, 1984]. The data supporting this concept are variable [Vernon, 1999]. The theory also suggests that strategies aimed at increasing perceived risk will increase screening behavior; on the other hand ‘optimistic bias’, the tendency to believe one is at lower risk than others, mitigates against these strategies [Kreuter and Strecher, 1995; Weinstein, 1980]. Indeed, individuals given specific information on risk and severity before an offer of screening were no more likely to participate [Lipkus, 2005, 2003].
Overall, our results challenge the importance of communicating risk, in order to enhance risk perception and thereby achieve high participation rates in colorectal (and potentially other) cancer screening. If reproduced in other populations and other forms of cancer screening, this finding may have implications for how screening resources should be deployed, with efforts at improving access to screening potentially more effective than risk communication strategies.
Acknowledgements
Funding for this study was provided by the Canberra Hospital Private Practice Trust Fund and Medical Specialists Associated. The Canberra Hospital and Medical Specialists Associated did not influence the design, conduct, management, analysis or interpretation of the data, or the preparation of the manuscript.
Conflict of interest statement
None declared.
Contributor Information
Keith Dear, National Centre for Epidemiology and Population Health, The Australian National University, Canberra, Australia. keith.dear@anu.edu.au.
Leitha Scott, Gastroenterology and Hepatology Unit, The Canberra Hospital, Garran, Australia.
Sharon Chambers, Gastroenterology and Hepatology Unit, The Canberra Hospital, Garran, Australia.
Mike C. Corbett, Gastroenterology and Hepatology Unit, The Canberra Hospital, Garran, Australia
Doug Taupin, Gastroenterology and Hepatology Unit, The Canberra Hospital, Garran, Australia.
References
- Australian Bureau of Statistics (ABS) (1997) Australian Standard Classification of Occupations (ASCO) 2nd edn.Australian Bureau of Statistics; Catalogue number 1220.0 [Google Scholar]
- Corbett M., Chambers S.L., Shadbolt B., Hillman L.C., Taupin D. (2004) Colonoscopy screening for colorectal cancer: the outcomes of two recruitment methods, Med J Aust 181(8):423–427 [DOI] [PubMed] [Google Scholar]
- Fagerlin A., Ubel P.A., Smith D.M., Zikmund-Fisher B.J. (2007) Making numbers matter: present and future research in risk communication, Am J Health Behav 31(Suppl. 1):S47–56 [DOI] [PubMed] [Google Scholar]
- Hoff G., Bretthauer M. (2006) The science and politics of colorectal cancer screening, PLoS Med 3(1):e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz N.K., Becker M.H. (1984) The health belief model: a decade later, Health Educ Q 11(1):1–47 [DOI] [PubMed] [Google Scholar]
- Klabunde C.N., Lanier D., Breslau E.S., Zapka J.G., Fletcher R.H., Ransohoff D.F.et al. (2007) Improving colorectal cancer screening in primary care practice: innovative strategies and future directions, J Gen Intern Med 22(8):1195–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter M.W., Strecher V.J. (1995) Changing inaccurate perceptions of health risk: results from a randomized trial, Health Psychol 14(1):56–63 [DOI] [PubMed] [Google Scholar]
- Levin B., Lieberman D.A., McFarland B., Andrews K.S., Brooks D., Bond J.et al. (2008) Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology, Gastroenterology 134(5):1570–1595 [DOI] [PubMed] [Google Scholar]
- Lipkus I.M., Green L.G., Marcus A. (2003) Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors, J Health Commun 8(3):213–228 [DOI] [PubMed] [Google Scholar]
- Lipkus I.M., Skinner C.S., Dement J., Pompeii L., Moser B., Samsa G.P.et al. (2005) Increasing colorectal cancer screening among individuals in the carpentry trade: test of risk communication interventions, Prev Med 40(5):489–501 [DOI] [PubMed] [Google Scholar]
- O'Leary B.A., Olynyk J.K., Neville A.M., Platell C.F. (2004) Cost-effectiveness of colorectal cancer screening: comparison of community-based flexible sigmoidoscopy with fecal occult blood testing and colonoscopy, J Gastroenterol Hepatol 19(1):38–47 [DOI] [PubMed] [Google Scholar]
- Olynyk J.K., Aquilia S., Fletcher D.R., Dickinson J.A. (1996) Flexible sigmoidoscopy screening for colorectal cancer in average-risk subjects: a community-based pilot project, Med J Aust 165(2):74–76 [DOI] [PubMed] [Google Scholar]
- Pinnock C.B. (2004) PSA testing in general practice: can we do more now? Med J Aust 180(8):379–381 [DOI] [PubMed] [Google Scholar]
- Podolsky D.K. (2000) Going the distance - the case for true colorectal-cancer screening, N Engl J Med 343(3):207–208 [DOI] [PubMed] [Google Scholar]
- Provenzale D. (2002) Cost-effectiveness of screening the average-risk population for colorectal cancer, Gastrointest Endosc Clin N Am 12(1):93–109 [DOI] [PubMed] [Google Scholar]
- Ransohoff D.F. (2005) Colon cancer screening in 2005: status and challenges, Gastroenterology 128(6):1685–1695 [DOI] [PubMed] [Google Scholar]
- Schroy P.C., 3rd, Wilson S., Afdhal N. (1996) Feasibility of high-volume screening sigmoidoscopy using a flexible fiberoptic endoscope and a disposable sheath system, Am J Gastroenterol 91(7):1331–1337 [PubMed] [Google Scholar]
- Schwartz L.M., Woloshin S., Black W.C., Welch H.G. (1997) The role of numeracy in understanding the benefit of screening mammography, Ann Intern Med 127(11):966–972 [DOI] [PubMed] [Google Scholar]
- Shapiro J.A., Seeff L.C., Nadel M.R. (2001) Colorectal cancer-screening tests and associated health behaviors, Am J Prev Med 21(2):132–7 [DOI] [PubMed] [Google Scholar]
- Smith R.A., Cokkinides V., Eyre H.J. (2006) American Cancer Society guidelines for the early detection of cancer, 2006, CA Cancer J Clin 56(1):11–25 [DOI] [PubMed] [Google Scholar]
- Taupin D., Chambers S.L., Corbett M., Shadbolt B. (2006) Colonoscopic screening for colorectal cancer improves quality of life measures: a population-based screening study, Health Qual Life Outcomes 4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon S.W. (1999) Risk perception and risk communication for cancer screening behaviors: a review, Monogr Natl Cancer Inst 25:101–119 [DOI] [PubMed] [Google Scholar]
- Weinstein N.D. (1980) Unrealistic optimism about future life events, J Pers Soc Psychol 39:806–820 [Google Scholar]
- Weinstein N.D. (1988) The precaution adoption process, Health Psychol 7:355–386 [DOI] [PubMed] [Google Scholar]
- Weitz J., Koch M., Debus J., Hohler T., Galle P.R., Buchler M.W. (2005) Colorectal cancer, Lancet 365:153–165 [DOI] [PubMed] [Google Scholar]
- World Health Organisation (2003) International Agency for Research in Cancer. World Cancer Report. Lyon: IARC Press
- Worthley D.L., Cole S.R., Esterman A., Mehaffey S., Roosa N.M., Smith A.et al. (2006) Screening for colorectal cancer by faecal occult blood test: why people choose to refuse, Int Med J 36(9):607–610 [DOI] [PubMed] [Google Scholar]
- Worthley D.L., Cole S.R., Mehaffey S., Roosa N.M., Smith A., Turnbull D.et al. (2007) Participant satisfaction with fecal occult blood test screening for color-ectal cancer, J Gastroenterol Hepatol 22(1):142–143 [DOI] [PubMed] [Google Scholar]
- Zikmund-Fisher B.J., Fagerlin A., Ubel P.A. (2005) What's time got to do with it? Inattention to duration in interpretation of survival graphs, Risk Anal 25(3):589–595 [DOI] [PubMed] [Google Scholar]