Abstract
Aim: To analyze the relationship between pretreatment clinical or histological features and the levels of soluble platelet-endothelial cell adhesion molecule-1 (sPECAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1), to determine their serum concentration in responders and nonresponders, to evaluate the behavior under antiviral therapy, to explain their relationship in response to therapy and to assess the association between these two molecules in chronic hepatitis C (CHC).
Methods: The study analyzed 65 CHC patients, including 50 patients (Group 1) with marked fibrosis treated with peginterferon plus ribavirin, 15 patients without fibrosis (Group 2) and 13 healthy volunteers (the control group, Group 3). sPECAM-1 and sVCAM-1 levels were assessed by an immunoenzymatic method (ELISA) before and after therapy.
Results: sVCAM-1 and sPECAM-1 serum concentrations increased significantly in CHC patients (p<001). sPECAM-1 levels corresponded to inflammatory grade (p = 0.03) and fibrosis stage (p =0.01). sVCAM-1 increased only in advanced fibrosis. After therapy, sPECAM-1 levels decreased significantly (p<001) with no difference between responders and nonre-sponders. sPECAM-1 correlated positively with inflammatory activity (p = 0.02), fibrosis stage (p<001), sVCAM-1 (r=0.56, p<001) and alanine aminotransferase activity (r = 0.30, p = 0.05). Receiver operating characteristic curve analysis showed a good discriminant power of serum sPECAM-1 concentrations for detection of liver fibrosis – stage 0 versus stage 1–3, AUC 0.81; cut-off 221.0 ng/ml and a fair discriminant power for distinguishing bridging fibrosis, AUC 0.78; cut-off 237.1 ng/ml.
Conclusions: Hepatitis C virus (HCV) infection results in upregulation of sPECAM-1 and sVCAM-1. sPECAM-1 levels are related to necroinflammatory activity and may also identify patients with advanced fibrosis. The sPECAM-1 value was decreased by therapy but its measurement cannot predict therapy outcome and confirm HCV persistence. sPECAM-1 may influence VCAM-1 expression.
Keywords: soluble platelet-endothelial cell adhesion molecule-1, soluble vascular cell adhesion molecule-1, chronic hepatitis C, fibrosis, inflammatory activity, antiviral therapy
Introduction
Direct virus influence on hepatocytes and host immune response play an essential role in the pathogenesis of liver injury [Ballardini et al. 1995; Cerny et al. 1995; Brillanti et al. 1993]. The normal liver contains an enormous number of lymphocytes such as specialized NK, NKT, CD4 and CD8 cells [Lalor et al. 2002]. During infectious liver disease [Lalor et al. 2002] and nonalcoholic steatohepatitis (NASH) [Ziólkowski et al. 2005] the number of lymphocytes in the liver increases. The extravasation of leukocytes to the inflammatory tissue is the phenomenon that involves several sequential steps and requires a wide spectrum of adhesion molecules [Petri and Bixel, 2006; Engelhardt and Wolburg, 2004; Ley, 1996; Gonzalez-Amaro et al. 1998]. The migration requires not only molecular interaction involved in firm adhesion of leukocytes interfered by function antigen-1/ intercellular adhesion molecule-1 (LFA-1/ ICAM-1) and very late antigen-4/vascular cell adhesion molecule-1 (VLA-4/VCAM-1) complexes, but also receptors located at the endothelial cells (EC) junctions, such as platelet endothelial cell adhesion molecule-1 (PECAM-1) [Petri and Bixel, 2006; Hordjik, 2006].
PECAM-1 (CD 31) is constitutively expressed on platelets, monocytes, neutrophils, NK and CD8T cells. High expression exists on continuous endothelial cells at cell-cell borders [Prager and Stockinger, 2000; Newman, 1997] and weak expression on sinusoidal endothelial cells (SEC) [Katz et al. 2004; Xu et al. 2003; Neubauer et al. 2000].
PECAM-1 plays a putative role in the inflammatory process and leukocyte-endothelial interaction, especially in transmigration of leukocytes through intercellular junctions. PECAM-1 is capable of mediating both homophilic adhesion and heterophilic binding to other molecules [Prager and Stockinger, 2000; DeLisser et al. 1993]. PECAM-1 has been implicated in cell survival, angiogenesis [Newman and Newman, 2003; Graesser, 2002] and in NASH progression [Goel et al. 2007]. In addition PECAM-1 affects activation and regulates trafficking of integrins [Newman and Newman, 2003]. PECAM-1 enhances T lymphocyte ability to bind to ß1 integrin substrates, such as VCAM-1 [Newman and Newman, 2003].
VCAM-1 (CD 106) expression on EC is not constitutive, but can be caused by tumor necrosis factor alpha, interleukin-1 (IL-1), IL-4 and interferon gamma [Pober and Cotran, 1990]. There is low basal expression of VCAM-1 in the normal liver, both on sinusoidal and Kupfer cells [Lang et al. 1995]. As well as the membrane-bound forms, soluble forms - sVCAM-1 and sPECAM-1 exist in human plasma [Prager and Stockinger, 2000; Gearing and Newman, 1993].
Nowadays, liver biopsy seems to be the most suitable method to assess CHC progression. As an invasive procedure, it is associated with high costs and clinical complications. An accurate prediction of the response to antiviral therapy is difficult. Therefore, additional criteria for precise, noninvasive diagnosis should be investigated, including adhesion molecules involved in pathogenesis of viral hepatitis. The exact role of adhesion molecule in viral hepatitis immuno-pathogenesis is still uncertain. To our knowledge, there is very little data regarding sPECAM-1 in CHC available and there is no data with respect to its relationship with other adhesion molecules and antiviral therapy.
The purposes of the study were to analyze the relationship between pretreatment clinical or histological features and the levels of sPECAM-1 and sVCAM-1, to determine their serum concentration in responders and nonresponders, to evaluate the behavior of these adhesins under antiviral therapy, to explain their relationship in response to combined antiviral therapy and to assess the association between these two molecules in CHC.
Methods
Patients selection and serological assays
The study was performed on 65 patients with chronic hepatitis C (CHC), infected with HCV genotype 1b. This included 50 patients (25F/25M) with marked fibrosis and persistently elevated alanine aminotransferase (ALT) activity for at least 6 months, aged between 32 and 65 years [average 52.0 (11.2) years, BMI 24.6 (3.2) kg/m2] – Group 1, and 15 patients (8F/7M) with minimal inflammatory activity and without fibrosis, with normal ALT activity [average 48.9 (9.9) years, BMI 24.9 (2.8) kg/m2] – Group 2. The duration (i.e. time since first diagnosis of HCV infection) ranged from 6 to 15 years, an average of 9.3 (4.3) years. Exclusion criteria included other virus genotypes, drug or alcohol abuse and autoimmune, neoplastic, thyroid or psychiatric diseases, hepatitis B or HIV coinfec-tion, diabetes, renal and heart failure.
The diagnosis of CHC was confirmed by presenceofserumHCV RNA assayed with reverse transcription polymerase chain reaction (RT-PCR) method (Amplicor Roche/Promega v.2 Diagnostic Test, New Jersey, USA). Virus genotype was assessed by a reverse-hybrydization line probe assay (LiPA Versant Test Milwauke, USA). The control group (Group 3) comprised 13 healthy volunteers (7F/6M) without anti-HCV antibodies and with normal ALT.
sVCAM-1 and sPECAM-1 serum concentrations were assessed in duplicate by immunoenzymatic method with commercially available ELISA kits (Quantikine Immunoassay, Bender MedSystem, Vienna, Austria, human sVCAM-1 ELISA, catalog number BMS232, sensitivity 0.9 ng/ml and human sPECAM-1 ELISA, catalog number BMS229, sensitivity 0.1 ng/ml).
A blood sample was withdrawn (fasting in the morning) from all subjects at the beginning of the study. The second blood collection was carried out in CHC patients upon completion of antiviral therapy. The samples were centrifuged and serum was frozen at —70°C until assay. The remaining biochemical parameters were measured with routine methods. The study was approved by the Ethical Committee of the Medical University of Silesia in Katowice and conformed to the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained for the whole study series.
Treatment protocol
All patients from Group 1 were administered pegylated interferon α2b subcutaneously (Pegasys, Roche, New Jersey, USA) at a dose of 180μg/week, together with ribavirin (Copegus, the same producer) orally at a dose dependent on body weight (1000-1200 mg/day) for 48 weeks. Patients were considered to be responders if serum ALT activity had reached normal values and HCVRNA was not detectable at the end of treatment - end of treatment response (ETR).
Liver histology
All studied patients had liver biopsy performed with Hepafix kit (B.Braun, Melsungen AG, Germany) before antiviral therapy, as part of the diagnostic routine. Biopsy samples included at least six portal tracts and were examined by two pathologists. Histopathological features were assessed according to modified Scheuer's scale [Gabriel and Ziólkowski, 1997; Scheuer, 1995].
Statistical analysis
The values were expressed as the mean and standard deviation (±SD). The Shapiro-Wilk test was used to evaluate the distribution. Because of non-Gaussian distribution, nonparametric methods were used. Differences in studied variables between groups were tested using U Mann–Whitney and ANOVA rang Kruskal–Wallis tests for independent groups and by means of Wilcoxon's matched-pairs signed-rank test for the dependent groups. Correlations were analyzed with the Spearman rank correlation coefficient. A p value of less than 0.05 was considered to be statistically significant. Analysis of receiver operating characteristic curves (ROC) was performed for assessing the discriminant power of sVCAM-1 and sPECAM-1 for fibrosis stage and inflammatory activity.
Results
Comparison of clinical and biochemical parameters among analyzed groups
The comparison of analyzed clinical and biochemical parameters between investigated groups is shown in Table 1.
Table 1.
The comparison of cLinicaL and biochemical, parameters among analyzed groups before therapy.
| Parameter | Group 1 (n=50) | Group 2 (n=15) | p∗ | Group 3 (n= 13) | p∗ | p∗∗ |
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Age (years) | 52.0 (11.2) | 48.9 (9.9) | NS | 47.9 (11.2) | NS | NS |
| Weight (kg) | 70.6 (9.5) | 71.1 (10.3) | NS | 69.4 (10.1) | NS | NS |
| Height (cm) | 174 (6.5) | 171.8 (9.9) | NS | 173.6 (8.4) | NS | NS |
| BMI (kg/m2) | 24.6 (3.2) | 24.9 (2.8) | NS | 23.0 (3.3) | NS | NS |
| AST (IU/1) | 122.6 (68.2) | 26.7 (6.1) | <0.001 | 24.9 (6.0) | <0.001 | NS |
| ALT (IU/1) | 136.6 (58.2) | 24.7 (7.3) | <0.001 | 22.8 (8.0) | <0.001 | NS |
| BIL (μmol/l) | 21.1 (4.9) | 15.8 (4.2) | NS | 10.1 (4.8) | <0.001 | NS |
| PLT (G/l) | 218.9 (79.9) | 257 (69.5) | 0.02 | 278.1 (71.2) | 0.025 | NS |
| WBC (G/l) | 6.7 (1.8) | 7.1 (1.3) | NS | 7.0 (1.6) | NS | NS |
| RBC (T/l) | 4.5(0.3) | 4.3 (0.4) | NS | 4.4 (0.5) | NS | NS |
| FA (IU/1) | 91.4 (24.1) | 82.1 (20.8) | NS | 78.1 (19.8) | 0.057 | NS |
| TP (g/l) | 76.0 (4.2) | 75 (4.5) | NS | 74 (5.1) | NS | NS |
| ESR (mm/h) | 11.6 (8.7) | 6.7 (4.3) | 0.02 | 5.9 (3.8) | <0.001 | NS |
| HGB (mmol/l) | 8.1 (0.5) | 8.6 (0.5) | NS | 8.7 (0.5) | NS | NS |
p, according to Mann Whitney U-Test,
values versus Group 1;
Group 2 versus Group 3; BMI, Body Mass Index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BIL, total serum bilirubin; FA, alkaline phosphatase; TP, total protein; HGB, hemoglobin, RBC, red blood cells; WBC, white blood cells; PLT, platelets; ESR, erythrocyte sedimentation rate; NS, not significant.
Histopathologic examination of liver tissue samples
Results revealed by histopathologic examination of biopsy specimens of CHC patients belonging to Group 1 and Group 2 are shown in Table 2.
Table 2.
The results of histopathologic examinations (according to Scheuer's scale) of liver biopsy specimens of CHC patients (Group 1 and Group 2).
| Inflammatory activity | Group 1 | Group 2 |
| Number of patients (%) | Number of patients (%) | |
| Grade 1 (minimal) | 0 | 15 (100) |
| Grade 2 (mild) | 29 (58) | 0 |
| Grade 3 (moderate) | 21 (42) | 0 |
| Fibrosis | ||
| Stage 1 (portal) | 6(12) | NF |
| Stage 2 (periportal) | 20 (40) | NF |
| Stage 3 (septal/bridging) | 24 (48) | NF |
NF: no fibrosis was found in histopathologic examination.
Assessment of sVCAM-1 and sPECAM-1 serum concentrations before antiviral therapy and the association with liver histology
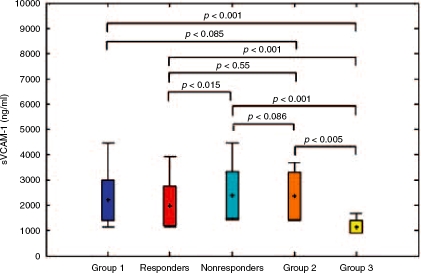
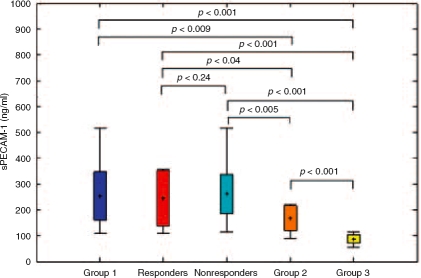
sVCAM-1 serum concentration in Group 1 was significantly higher only in comparison with Group 3 (2197.2 versus, 1144.9 ng/ml, p<0.001) (Table 3; Figure 1). sPECAM-1 serum concentration in Group 1 was significantly increased when compared with Group 2 (252.7 versus 166.1 ng/ml, p=0.009) and Group 3 (252.7 versus 86.4 ng/ml, p≤0.001) (Table 3, Figure 2). Both parameters were significantly elevated in Group 2 when compared with Group 3 (Table 3).
Table 3.
The comparison of sVCAM-1 and sPECAM-1 between analyzed groups before antiviral therapy.
| Parameter | Group 1 (n=50) | Group 2 (n=15) | p∗ | Group 3 (n=13) | p∗ | p∗∗ |
| Mean (SD) | Mean (SD) | Mean (SD) | ||||
| sVCAM-1 (ng/ml) | 2197.2 (793.1) | 2358.8 (958.2) | 0.27 | 1144,9 (253.2) | <0.001 | 0.004 |
| sPECAM-1 (ng/ml) | 252.7 (92.) | 166.1 (48.0) | 0.009 | 86.4 (17.3) | <0.001 | <0.001 |
p, according to Mann-Whitney U test,
, values versus Group 1;
, Group 2 versus Group 3.
Figure 1.
The comparison of sVCAM-1 serum levels before therapy in all analyzed groups.
Figure 2.
The comparison of sPECAM-1 serum levels before therapy in all analyzed groups.
Patients from Group 1 and Group 2 were connected in one group and subsequently divided into subgroups according to grade of inflammatory activity. Minimal activity was found in 15 patients, mild in 29 and moderate in 21. A significant difference was found with respect to sPECAM-1 values (166.1 versus 253.9 versus 252.1 ng/ml, p =0.03), AST and ALT (p50.001) activity, but not sVCAM-1 (p=0.9) (Table 4).
Table 4.
The comparison of sPECAM-1, sVCAM-1 and aminotransferase activity in patients with chronic hepatitis C (Group 1 + Group 2) before therapy with respect to the inflammatory activity grade.
| Parameter | Inflammatory activity (number of patients) | p | ||
| Grade 1 (n = 15) | Grade 2 (n = 29) | Grade 3 (n = 21) | ||
| sPECAM-1 (ng/ml) | 166.1 (48.0) | 253.1 (92.3) | 252.1 (95,5) | 0.03 |
| sVCAM-1(ng/ml) | 2358.8 (958.2) | 2182 (821.2) | 2217.1 (999.1) | 0.9 |
| AST (IU/1) | 26.7 (6.1) | 102.2 (59.9) | 146.8 (72.4) | <0.001 |
| ALT (IU/1) | 24.7 (7.3) | 119.5 (57.3) | 157.0 (76.7) | <0.001 |
, according to ANOVA Kruskal-Wallis test.
According to the fibrosis stage, Group 1 was divided into two subgroups: subgroup 1 included stage 1 and 2 (26 patients) while subgroup 2 included stage 3 (24 patients). The concentrations of sVCAM-1 and sPECAM-1 were noticeably higher in patients with more advanced fibrosis (2070.7 versus, 2308.0 ng/ml, p= 0.47; 224.1 versus 280.0 ng/ml, p= 0.01, respectively) but the difference was significant only for sPECAM-1.
Comparison of sVCAM-1 and sPECAM-1 serum concentrations before and after antiviral therapy and the association with therapy effectiveness
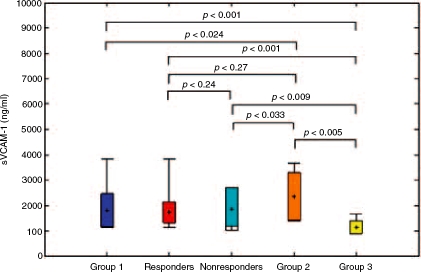
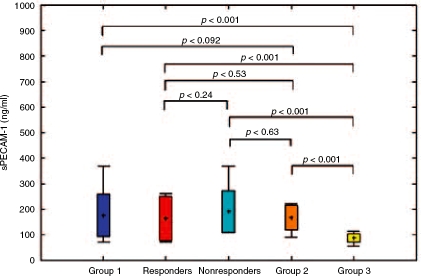
In Group 1 sVCAM-1 serum concentrations decreased insignificantly after therapy (2197.2 versus, 1803.4ng/ml, p=0.17), while sPECAM-1 decreased significantly (252.7 versus 175.9 ng/ml, p<0.001) (Table 5). ETR was achieved in 27 patients. Thus, according to therapy outcome Group 1 was divided into two subgroups: responders (27 patients) and nonresponders (23 patients). sVCAM-1 and sPECAM-1 serum levels between responders and nonresponders both before (1967.9 versus, 2397.9ng/ml, p=0.15 and 243.1 versus 262.2 ng/ml, p = 0.24, respectively) (Table 6, Figures 1 and 2) and after therapy (1747.1 versus, 1869.2 ng/ml, p = 0.70 and 164.1 versus 190.1 ng/ml, p = 0.46, respectively) differed insignificantly (Table 7, Figures 3 and 4). Nevertheless, mean values appeared to be higher in nonresponders. The comparison of soluble adhesion molecule levels in Group 1, responders and nonresponders to untreated patients with minimal inflammatory activity and healthy controls is presented in Figures 3 and 4. The significant difference both in responders (243.1 versus 164.1 ng/ml, p=0.004) and nonre-sponders (262.2 versus 190.1 ng/ml, p<0.001) for values before and after therapy was observed only for sPECAM-1 (Table 8).
Table 5.
The comparison of serum concentrations of sPECAM-1, sVCAM-1 and other analyzed parameters in Group 1 before and after therapy.
| Parameter | Before therapy | After therapy | p |
| Mean (SD) | Mean (SD) | ||
| sVCAM-1 (ng/ml) | 2197.2 (793.1) | 1803.4 (648,3) | 0.17 |
| sPECAM-1 (ng/ml) | 252.7 (92.6) | 175.9 (83.7) | <0.001 |
| AST (IU/1) | 122.6 (68.2) | 49.0 (25.1) | <0.001 |
| ALT (IU/1) | 136.6 (58.2) | 53.0 (32.4) | <0.001 |
| Bilirubin (μmol/l) | 21.1 (4.9) | 19.8 (5.8) | 0.6 |
| HGB (mmol/l) | 8.1 (0.5) | 7.1 (0.4) | <0.001 |
| RBC (T/l) | 4.5 (0.3) | 3.7 (0.3) | 0.02 |
| WBC (G/l) | 6.7 (1.8) | 3.9 (0.9) | 0.003 |
| PLT (G/l) | 218.9 (79.9) | 133.1 (46.5) | 0.001 |
, according to Wilcoxon's matched-pairs signed-rank test.
Table 6.
The comparison of sVCAM-1 and sPECAM-1 serum concentrations as well as some analyzed biochemical parameters in responders and non-responders before therapy.
| Parameter | Responders (n=27) | Nonresponders (n=23) | p |
| Mean (SD) | Mean (SD) | ||
| sVCAM-1 (ng/ml) | 1967.9 (787.8) | 2397.9 (955.0) | 0.15 |
| sPECAM-1 (ng/ml) | 243.1 (107.6) | 262.2 (75.3) | 0.24 |
| AST (IU/1) | 95.9 (52.1) | 147.8 (74.2) | 0.13 |
| ALT (IU/1) | 114.6 (55.2) | 157.4 (74.7) | 0.28 |
| FA (IU/1) | 84.7(20.1) | 98.0 (27.4) | 0.30 |
, according to Mann-Whitney U test.
Table 7.
The comparison of sVCAM-1 and sPECAM-1 serum concentrations in responders and nonresponders after therapy.
| Parameter | Responders | Nonresponders | p |
| Mean (SD) | Mean (SD) | ||
| sPECAM-1 (ng/ml) | 164.1 (86.6) | 190.1 (80.8) | 0.46 |
| sVCAM-1 (ng/ml) | 1747.1 (422.3) | 1869.2 (857.8) | 0.70 |
, according to Mann-Whitney U test.
Figure 3.
The comparison of sVCAM-1 serum levels after therapy in Group 1, responders and non-responders with Group 2 and the control group.
Figure 4.
The comparison of sPECAM-1 serum levels after therapy in Group 1, responders and nonresponders with Group 2 and the control group.
Table 8.
sVCAM-1 and sPECAM-1 serum concentrations in responders and nonresponders before and after therapy.
| Parameter | Before therapy | After therapy | p |
| Mean (SD) | Mean (SD) | ||
| Responders | |||
| sVCAM-1 (ng/ml) | 1967.9 (787.8) | 1747.1 (422.3) | 0.34 |
| sPECAM-1 (ng/ml) | 243.1 (107.6) | 164.1 (86.6) | 0.004 |
| Nonresponders | |||
| sVCAM-1 (ng/ml) | 2397.9 (955.0) | 1869.2 (857.8) | 0.3 |
| sPECAM-1 (ng/ml) | 262.2 (75.3) | 190.1 (80.8) | <0.001 |
, according to Wilcoxon's matched-pairs signed-rank test.
Analysis of ROC curve of sVCAM-1 and sPECAM-1 for inflammatory activity and fibrosis
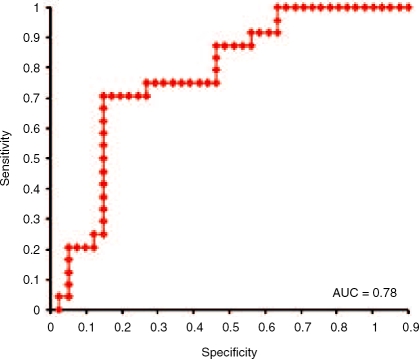
sPECAM-1 specificity and sensitivity for the detection of liver fibrosis stage – stage 0 versus stage 1–3 is shown in Figure 5. ROC curve analysis showed a good discriminant power of serum sPECAM-1 concentrations for liver fibrosis detection with an area under curve (AUC) 0.81. The best cut-off was identified as 221.0 ng/ml (sensitivity 62.8% and specificity 100%). Figure 6 shows sPECAM-1 ROC curve for groups with fibrosis stages 0–2 versus stage 3. AUC 0.78 indicated a fair discriminant power for liver fibro-sis differentiation, with cut-off set for 237.1 ng/ml (sensitivity 77.7% and specificity 87.5%). Unfortunately, ROC curves analysis showed no discriminant power of sVCAM-1 concentrations for liver fibrosis (stage 1 versus stage 2-3, AUC 0.52, sensitivity 66% and specificity 50%) and both analyzed molecules for inflammation (data not shown).
Figure 5.
Receiver operating characteristic curve analysis for sPECAM-1. Sensitivity and specificity for the detection of liver fibrosis - stage 0 versus stage 1-3. Area under the curve = 0.81. The best cut-off was set as 221.0 ng/ml with sensitivity 62.8% and specificity 100%.
Figure 6.
Receiver operating characteristic curve analysis for sPECAM-1. Sensitivity and specificity for the detection of liver fibrosis stage – stage 0–2 versus stage 3. Area under the curve=0.78. The best cut-off was set as 237.1 ng/ml with sensitivity 77.7% and specificity 87.5%.
Correlations among analyzed variables
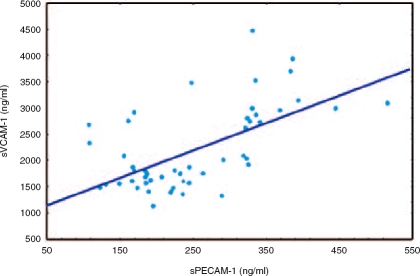
Significant mutual correlation appeared between serum concentrations of sVCAM-1 and sPECAM-1 before therapy r=0.56, p=0.001) (Figure 7). sPECAM-1 serum concentrations correlated positively with fibrosis stage r=0.35, p=0.02), inflammatory grade r=0.53, p<0.001), AST r=0.31, p=0.048) and ALT activity r=0.30, p=0.049), whereas sVCAM-1 correlated with the duration of CHC r=0.34; p=0.047). AST and ALT serum activities correlated with fibrosis stage (r = 0.42, p=0.006; r=0.36, p=0.02, respectively) and inflammation grade r=0.60, p<0.001; r=0.53, p<0.001, respectively).
Figure 7.
Correlation between sVCAM-1 and sPECAM-1 serum concentrations in all patients with chronic hepatitis C (Group 1 and Group 2) before antiviral therapy.
Discussion
HCV infection leads to inflammatory processes of different grades that involve activation of adhesion molecules and cytokines which facilitate recruitment of leukocytes to inflammatory areas. The evidence supporting this suggestion is the increase of sVCAM-1 and sPECAM-1 serum concentrations, as observed from the performed study. Consistent with other authors [Dejica et al. 2002; Fukuda et al. 1998; Lo Iacono et al. 1998; Kapllanski et al. 1997; Marui et al. 1996], we observed significantly higher serum sVCAM-1 levels in CHC patients. Dejica et al. showed a weak expression of VCAM-1 on cell borders of SEC in normal livers [Dejica et al. 2002]. Volpes et al. [1992], using immunohistochemical methods, demonstrated the expression of VCAM-1 during hepatitis on endothelial cells of portal tracts, part of SEC and some Kupffer cells. sVCAM-1 may be released from cytokine-activated endothelial cells and the serum levels may reflect activity of these cells [Bruno et al. 2005; Dejica et al. 2002].
Another possible source of sVCAM-1 may be activated dendritic cells localized in inflamed portal tracts [Garcia-Monzon et al. 1996]. Moreover, CD8 lymphocytes infiltrating the liver during CHC, express VLA-4 on its cell border, confirming participation of this molecule in cellular adhesion [Garcia-Monzon et al. 1996]. Binding T cells by VLA-4/VCAM-1 complex may cause T-cell activation and proliferation leading in consequence to damage of the surrounding hepatocytes [Dejica et al. 2002].
Unfortunately, our study showed the similar concentrations of sVCAM-1 in CHC patients with more advanced and minimal inflammatory activity. Bruno et al. [2005] affirmed that sVCAM-1 levels were significantly higher in a case of severe inflammatory activity and liver cirrhosis when compared to mild inflammatory activity and portal fibrosis. Moreover, sVCAM-1 concentration was marginally higher in patients with mild CHC activity when compared with healthy subjects. Fukuda et al. [1998] did not observe significant sVCAM-1 difference between healthy volunteers and asymptomatic CHC carriers with minimal inflammatory grade. Nevertheless, differences between patients with minimal inflammatory activity but elevated aminotransferases and those with normal aminotransferase activity were showed. Kapllanski et al. [1997] did not observe association between inflammatory activity and sVCAM-1 concentration.
Higher sVCAM-1 concentration in patients with minimal inflammatory grade without fibrosis compared with healthy subjects observed in our study suggests that sVCAM-1 increases may reflect an ongoing inflammatory process and persistence of viremia. Nevertheless, Lo Iacano et al. [1998] and Radu et al. [2003] did not establish a relationship between sVCAM-1 concentration and viral load. Our results correspond to other authors [Bruno et al. 2005; Dejica et al. 2002; Granot et al. 2001] and demonstrate the lack of association between sVCAM-1 concentration and aminotransferase activity, which may be an intermediate marker of inflammatory activity in the liver. Only Lo Iacono et al. [1998] demonstrated the significant correlations between ALT, gammaglutamyltranspeptydase and sVCAM-1 concentrations.
Until now, unequivocal results that defined the role of PECAM-1 in pathogenesis of inflammatory process during CHC had not been obtained. Some authors [Katz et al. 2004; Xu et al. 2003; Neubauer et al. 2000; Garcia-Monzon et al. 1996] observed expression of PECAM-1 in mononuclears and SEC from healthy human or rat livers. The marked differences in cell adhesion molecules expression on EC in the liver appeared to be due to the differential structure of the liver vasculature [Volpes et al. 1992].
Significantly higher sPECAM-1 concentrations in patients with more advanced hepatitis alongside positive association between sPECAM-1 levels and grade of inflammatory activity suggests that PECAM-1 may reflect disease progression and its expression can be induced by an active inflammatory process. Correlation between sPECAM-1 and aminotransferase activity – a suitable marker of inflammatory process – also supports this suggestion. Moreover, the lowest concentrations in patients with minimal inflammatory activity were still significantly higher than in healthy subjects, which indicates that sPECAM-1 also points to the ongoing inflammatory process. Unfortunately, ROC curve analysis showed the uselessness of sPECAM-1 and distinguish various grades of inflammation (data not shown).
Liver fibrosis follows hepatic inflammation and results from hepatocyte injury [Gressner and Weiskirchen, 2006]. Interactions between hepatic stellate cell (HSC) membrane receptors and the extracellular matrix proteins is regulated by adhesion molecules [Friedmann, 2003; Rachwal and Brigstock, 2003]. These interactions may influence on many signal pathways in cells important for collagen synthesis and metalloproteinases activation, leading to the increase of synthesis and/or the decrease of degradation of liver connective tissue. It is thought that VCAM-1 can play a certain role in these processes [Friedmann, 2003]. Consistent with Granot et al. [2001], in our investigation no association between fibrosis stage and sVCAM-1 concentration was observed, although slightly higher levels were found in patients with bridging fibrosis. In the above-mentioned study, the highest sVCAM-1 levels occurred in patients with liver cirrhosis. On the other hand, a positive relationship between sVCAM-1 concentration and fibrosis stage was found by Kapllanski et al. [1997] and Lo Iacono et al. [1998] with significantly higher sVCAM-1 values in case of bridging fibrosis/cirrhosis compared with portal/periportal fibrosis. This association may result from VCAM-1 synthesis by activated endothelial [Steinhoff et al. 1993] and dendritic cells [Garcia-Monzon et al. 1996]. Additionally, Lo Iacono showed correlation between sVCAM-1 and procollagen type-III amino-terminal propeptide, the biochemical marker of fibrosis progression [Lo Iacono et al. 1998]. Bruno et al. [2005] found elevated sVCAM-1 concentration only in cirrhotic patients. Moreover, sVCAM-1 increased significantly in patients with Child-Pugh C liver cirrhosis when compared with class B. A notable fact also was that the lowest sVCAM-1 concentration in patients with liver cirrhosis was above the highest one for those with low fibrosis progression. It can be assumed that serum sVCAM-1 levels increase is rather associated with stage of liver fibrosis than liver inflammation. The above observations may also suggest that sVCAM-1 levels markedly increase when fibrosis has already become irreversible. Unfortunately, the study by Bruno et al. [2005] did not include patients with periportal/bridging fibrosis, whereas all studies except for ours included cirrhotic patients. Thus, the complete interpretation and comparison with our results is difficult and the diversity in results may be due to selection of patients.
The evaluation of usefulness of sVCAM-1 determination, with respect to differentiation of fibro-sis stage by the assessment of area under ROC curve for sVCAM-1 concentrations in our study, gave negative results. Contrary to our findings analysis of area under ROC curve by Lo Iacono et al. [1998] demonstrated usefulness of sVCAM-1 determinations to differentiate septal fibrosis/cirrhosis from lower fibrosis stages, with the cut-off point set at, 1280 ng/ml.
There is very little data available with respect to the relationship between sPECAM-1 and liver fibrosis in CHC. Weak immunoreactivity for PECAM-1 observed on SEC and endothelial cells of vessels in portal tracts in normal liver increases significantly in the case of cirrhosis [Shah et al. 1999]. SEC isolated from fibrotic rat livers demonstrated increased expression of PECAM-1 on cell borders and in cytoplasm [DeLeve et al. 2004]. The expression was increased during neoangiogenesis in portal tracts [Garcia-Monzon et al. 1996] the phenomenon, which is markedly intensified in CHC [Kapllanski et al. 1997] and contributes to fibro-sis progression [Garcia-Monzon et al. 1996; Bhunchet and Fujieda, 1995]. The pattern of PECAM-1 on endothelial cells was always similar in enlarged portal tracts acquiring a characteristic form of capillary tube formation [Garcia-Monzon et al. 1996]. PECAM-1 expression on endothelial cell–cell borders seems to play a fundamental role in leukocyte migration [Scalia and Lefer, 1998]. The above-mentioned changes suggest a difference in leukocyte migration between normal and fibrotic liver [DeLeve et al. 2004; Katz et al. 2004]. Our results showed the highest sPECAM-1 concentrations in patients with the most advanced fibrosis stage. Moreover, the lowest concentrations in patients with portal fibrosis were significantly increased in comparison with patients without fibrosis. These observations corresponded to data by DeLeve et al. [2004], who revealed significant PECAM-1 expression increase on cell–cell borders and in cytoplasm of SEC isolated from rat livers with advanced fibrosis. Unfortunately, on the basis of our study, it was difficult to unequivocally assess the association between fibrosis stage and sPECAM-1 concentration due to the lack of cirrhotic patients. Nevertheless, more distinct variation in sPECAM-1 levels compared with sVCAM-1 may result from changes in expression pattern of PECAM-1 not only in leukocytes but also in endothelial cells of constituent and new-formed vessels.
Our study showed sPECAM-1 concentration was positively associated with fibrosis stage. ROC curve analysis confirmed the feasibility of sPECAM-1 serum determinations in CHC to differentiate patients with respective, histopathologically confirmed fibrosis stages. The value 0.81 of area under the ROC curve for sPECAM-1 concentrations confirms the test's usefulness in differentiating patients with and without fibrosis. A sPECAM-1 concentration of 221.0 ng/ml should be taken as the cut-off point. Similarly, the value 0.78 of area under the ROC curve suggests feasibility of this parameter to differentiate patients with bridging fibrosis from lower stages, with the cut-off point set at 237.1 ng/ml. Antiviral therapy reduces extent of inflammatory process and therefore contributes to downregulation of PECAM-1 and VCAM-1 expression and subsequent significant decrease of sPECAM-1, and lesser degree of sVCAM-1, both in responders and nonresponders.
In our study sVCAM-1 concentration did not change significantly after therapy, which was in accordance with results of Lo Iacono et al. [1998] and Simpson et al. [1994]. Nevertheless, sVCAM-1 concentrations in both responders and nonresponders after therapy were slightly lower compared with initial values. Interestingly, Dejica et al. [2002] observed unchanged or even increased sVCAM-1 levels in some nonresponders after therapy. In contrast, Marui et al. [1996] showed that after antiviral monotherapy, serum sVCAM-1 concentrations in responders decreased significantly in comparison with non-responders. However, the analyzed group was small and sVCAM-1 decreases were obvious only when initial levels were very high, which may suggest that results were ambiguous.
On admission, sVCAM-1 values did not differ between responders and nonresponders and these results correspond with data by Dejica et al. [2002] and Granot et al. [2001]. Similarly, sPECAM-1 concentrations did not differ between these groups. The results indicate that initial adhesion molecules measurement is useless in predicting therapy outcome. However, after antiviral therapy sVCAM-1 and sPECAM-1 levels, although insignificantly, seemed to be lower in responders. Nevertheless, the lack of significant difference between responders and nonresponders indicates that the persistence of viremia has no influence on PECAM-1 and VCAM-1 expression and therefore they are not a feasible indicator of virus persistence. With respect to sVCAM-1, the results are comparable to Dejica et al. [2002] and Granot et al. [2001]. Moreover, our study confirmed observations of Granot et al. [2001] which revealed the lack of association between sVCAM-1 concentrations and ALT activity during antiviral therapy.
It is thought that PECAM-1, as a signal transducer in cells, can be responsible for upregulation of integrins expression, their binding to other molecules and the formation of VLA-4/VCAM-1 complex [Engelhardt and Wolburg, 2004; Newman and Newman, 2003; RayChaudhury et al. 2001]. A positive correlation between both these molecules, observed in our research, additionally suggests that PECAM-1 may influence VCAM-1 expression.
Conclusion
Infection with HCV results in upregulation of sPECAM-1 and sVCAM-1. Our findings are consistent with the importance of PECAM-1 in CHC pathogenesis and suggest that its soluble form is related to necroinflammatory activity as assessed by biochemistry and histological score. Therefore, serum sPECAM-1 may be an additional feasible marker of cytonecrotic activity. Our analysis has shown that serum sPECAM-1 measurement was useful in distinguishing between patients with or without fibrosis, and enabled us to identify patients with advanced fibrosis as well. On the other hand, high sVCAM-1 levels may point to advanced and probably irreversible fibrosis. Antiviral therapy, by reducing the inflammatory activity, decreases markedly sPECAM-1 and to a lesser degree sVCAM-1 concentrations. However, measurement of these adhesion molecules does not allow us to predict therapy outcome and confirm HCV persistence. PECAM-1, as a signal transducer in cells, may influence VCAM-1 expression. Further studies on a greater number of patients are necessary to better determine the role of sPECAM-1 and sVCAM-1 in the pathogenesis, diagnosis and management of CHC.
Conflict of interest statement
None declared.
Contributor Information
Michal Kukla, Department of Physiology, Medical University of Silesia, Zabrze, Poland. kuklamich@poczta.onet.pl.
Krystyna Zwirska-Korczala, Department of Physiology in Zabrze, Medical University of Silesia Katowice, Poland.
Andrzej Gabriel, Department of Pathomorphology in Zabrze, Medical University of Silesia, Katowice Poland.
Ewa Janczewska-Kazek, Outpatients Clinic for Infectious Diseases Chorzów, Poland.
Agnieszka Berdowska, Department of Microbiology and Biotechnology, Jan Dlugosz University Czestochowa, Poland.
Andrzej Wiczkowski, Department of Biology in Zabrze, Medical University of Silesia, Katowice Poland.
Barbara Rybus-Kalinowska, Department of Basic Sciences in Bytom Medical University of Silesia, Katowice, Poland.
Mariusz Kalinowski, Department of Cardiology Silesian Center for Heart Diseases in Zabrze Medical University of Silesia, Katowice, Poland.
Adam Ziolkowski, Department of Pathomorphology in Zabrze, Medical University of Silesia, Katowice Poland.
Elzbieta Wozniak-Grygiel, Department of Physiology in Zabrze, Medical University of Silesia Katowice, Poland.
Marek Waluga, Department of Gastroenterology and Hepatology, Medical University of Silesia Katowice, Poland.
Blazej Nowak, Department of Internal Medicine, Regional Hospital in Nowa Sol Poland.
References
- Ballardini G., Groff P., Pontisso P., Giostra F., Francesconi R., Lenzi M.et al. (1995) Hepatitis C (HCV) virus genotype, tissue HCV antigens, hepatocellular expression of HLA-A, B, C and intercellular adhesion 1 molecules. Clues to pathogenesis of hepatocellular damage and response to interferon treatment in patients with chronic hepatitis C, J Clin Invest 95:2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunchet E., Fujieda K. (1995) Capillarization and venularization of hepatic sinusoids in porcine serum-induced rat liver fibrosis: a mechanism to maintain liver flow, Hepatology 18:1450–1458 [PubMed] [Google Scholar]
- Brillanti S., Foli M., Gaiani S., Masci C., Miglioli M., Barbara L. (1993) Persistent hepatitis C viraemia without liver disease, Lancet 341:464–465 [DOI] [PubMed] [Google Scholar]
- Bruno C.M., Sciacca C., Cilio D., Bertino G., Marchese A.E., Politi G.et al. (2005) Circulating adhesion molecules in patients with virus-related chronic diseases of the liver, World J Gastroenterol 11:4566–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny A., McHutchison J.G., Pasquinelli C., Brown M.E., Brothers M.A., Grabscheid B.et al. (1995) Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif, J Clin Invest 95:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejica D., Grigorescu M., Dejica V., Radu C., Neculoiu D. (2002) Serum levels of soluble intercellular-1 and vascular cell-1 adhesion molecules in chronic hepatitis C and the influence of interferon-alpha ribavirin therapy, Rom J Gastroenterol 11:277–283 [PubMed] [Google Scholar]
- DeLeve L.D., Wang X., Hu L., McCuskey M.K., McCuskey R.S. (2004) Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation, Am J Physiol Gastrointest Liver Physiol 287:G757–763 [DOI] [PubMed] [Google Scholar]
- DeLisser H.M., Yan H.C., Newman P.J., Muller W.A., Buck C.A., Albelda S.M. (1993) Platelet-endothelial cell adhesion molecule-1 (CD31) - mediated cellular aggregation involves cell surface glycosaminoglycans, J Biol Chem 268:16037–16046 [PubMed] [Google Scholar]
- Engelhardt B., Wolburg H. (2004) Trans-endothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol 34:2955–2963 [DOI] [PubMed] [Google Scholar]
- Friedmann S.L. (2003) Liver fibrosis- from banch to bedside, J Hepatol 30:38–53 [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Nakano I., Katano Y., Marui A., Hayakawa T. (1998) Serum levels of soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in asymptomatic carriers of hepatitis C virus, J Int Med Res 26:313–318 [DOI] [PubMed] [Google Scholar]
- Gabriel A., Ziólkowski A. (1997) New terminology of chronic hepatitis, including a scoring scale, Med Sci Monit 3:113–118 [Google Scholar]
- Garcia-Monzon C., Sanchez-Madrid F., Garcia-Buey L., Garcia-Sanchez A., Moreno-Otero R. (1995) Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in potral tracts, Gastroenterology 108:231–241 [DOI] [PubMed] [Google Scholar]
- Gearing A. J. H., Newman W. (1993) Circulating adhesion molecules in disease, Immunol Today 14:506–512 [DOI] [PubMed] [Google Scholar]
- Goel R., Boylan B., Gruman L., North P.E., Newman D.K. (2007) The proinflammatory phenotype of PECAM-1-deficient mice results in atherogenic diet-induced steatohepatitis, Am J Physiol Gastrointest Liver Physiol 293:G1205–1214 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Amaro R., Diaz-Gonzalaz F., Sanchez-Madrid F. (1998) Adhesion molecules in inflammatory diseases, Drugs 56:977–988 [DOI] [PubMed] [Google Scholar]
- Graesser D., Solowiej A., Bruckner M., Osterweil E., Juedes A., Davis S.et al. (2002) Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice, J Clin Invest 109:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot E., Shouval D., Ashur Y. (2001) Cell adhesion molecules and hyaluronic acid as markers of inflammation, fibrosis and response to antiviral therapy in chronic hepatitis C patients, Mediators Inflamm 10:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A.M., Weiskirchen R. (2006) Modern pathogenesis concepts of liver fibrosis suggest stellate cells and TGF-b as major players and therapeutic targets, J Cell Mol Med 10:76–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordjik P.L. (2006) Endothelial signalling events during leukocyte transmigration, FEBS J 273:4408–4415 [DOI] [PubMed] [Google Scholar]
- Kapllanski G., Farnarier C., Payan M.J., Bongrand P., Durand J.M. (1997) Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosis, Dig Dis Sci 42:2277–2284 [DOI] [PubMed] [Google Scholar]
- Katz S.C., Pillarisetty V.G., Bleier J.I., Shah A.B., DeMatteo R.P. (2004) Liver sinusoidal endothelial cells are insufficient to activate T cells, J Immunol 173:230–235 [DOI] [PubMed] [Google Scholar]
- Lalor P.F., Shields P., Grant A.J., Adams D.H. (2002) Recruitment of lymphocytes to the human liver, Immunol Cell Biol 80:52–64 [DOI] [PubMed] [Google Scholar]
- Lang T., Krams S.M., Villanueva J.C., Cox K., So S., Martinez O.M. (1995) Differential patterns of circulating intercellular adhesion molecule -1 (cICAM-1) and vascular cell adhesion molecule -1 (cVCAM-1) during liver allograft rejection, Transplantation 59:584–589 [PubMed] [Google Scholar]
- Ley K. (1996) Molecular mechanisms of leukocyte recruitment in the inflammatory process, Cardiovasc Res 32:733–742 [PubMed] [Google Scholar]
- Lo Iacono O., Garcia-Monzon C, Almasio P., García-Buey L., Craxí A., Moreno-Otero R. (1998) Soluble adhesion molecules correlate with liver inflammation and fibrosis in chronic hepatitis C treated with interferon -a, Aliment Pharmacol Ther 12:1091–1099 [DOI] [PubMed] [Google Scholar]
- Marui A., Fukuda Y., Koyama Y., Nakano I., Urano F., Hamada M.et al. (1996) Serum levels of soluble intercellular adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in liver disease, and their changes by treatment with interferon, J Int Med Res 24:258–265 [DOI] [PubMed] [Google Scholar]
- Neubauer K., Wilfling T., Ritzel A., Ramadori G. (2000) Platelet-endothelial cell adhesion molecule-1 gene expression in liver sinusoidal endothelial cells during liver injury and repair, J Hepatol 32:921–932 [DOI] [PubMed] [Google Scholar]
- Newman P.J. (1997) The biology of PECAM-1, J Clin Invest 99:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P.J., Newman D.K. (2003) Signal transduction pathways mediated by PECAM-1. New roles for an old molecule in platelet and vascular biology, Arterioscler Thromb Vasc Biol 23:953–964 [DOI] [PubMed] [Google Scholar]
- Petri B., Bixel M.G. (2006) Molecular events during leukocyte diapedesis, FEBS J 273:4399–4407 [DOI] [PubMed] [Google Scholar]
- Pober J.S., Cotran R.S. (1990) The role of endothelial cells in inflammation, Transplantation 50:537–544 [DOI] [PubMed] [Google Scholar]
- Prager E., Stockinger H. (2000) CD31, J Biol Regul Homeost Agents 14:307–310 [PubMed] [Google Scholar]
- Rachwal A.W., Brigstock D.R. (2003) Connective tisssue growth factor (CTGF/CCN2) in hepatic fibrosis, Hepatol Res 26:1–9 [DOI] [PubMed] [Google Scholar]
- Radu C., Dejica D., Grigorescu M., Zaharie T., Neculoiu D. (2003) Correlation of sICAM-1 and sVCAM-1 level with biochemical, histological and viral findings in chronic hepatitis C after interferon-alpha ribavirin therapy, Rom J Gastroenterol 12:91–95 [PubMed] [Google Scholar]
- RayChaudhury A., Elkins M., Kozien D., Nakada M.T. (2001) Regulation of PECAM-1 in endothelial cells during cell growth and migration, Exp Biol Med 226:686–691 [DOI] [PubMed] [Google Scholar]
- Scalia R., Lefer A.M. (1998) In vivo regulation of PECAM-1 activity during acute endothelial dysfunction in the rat mesenteric microvasculature, J Leukoc Biol 64:163–169 [DOI] [PubMed] [Google Scholar]
- Scheuer P.J. (1995) The nomenclature of chronic hepatitis: time for a change, J Hepatol 22:112–114 [DOI] [PubMed] [Google Scholar]
- Shah V., Toruner M., Haddad F., Cadelina G., Papapetropoulos A., Choo K.et al. (1999) Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat, Gastroenterology 117:1222–1228 [DOI] [PubMed] [Google Scholar]
- Simpson K.J., Harrison D.J., Hayes P.C. (1994) Serum and tissue vascular adhesion molecules in hepatitis C infection, Gut 35(Suppl. 5):S68 [Google Scholar]
- Steinhoff G., Behrend M., Schrader B., Duijvestijn A.M., Wonigeit K. (1993) Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3, Am J Pathol 142:481–488 [PMC free article] [PubMed] [Google Scholar]
- Volpes R., Van den Oord J.J., Desmet V.J. (1992) Vascular adhesion molecules in acute and chronic liver inflammation, Hepatology 15:269–275 [DOI] [PubMed] [Google Scholar]
- Xu B., Broome U., Uzunel M., Nava S., Ge X., Kumagai-Braesch M.et al. (2003) Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis, Am J Pathol 163:1275–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziólkowski A., Wylezol M., Kukla M., Zwirska-Korczala K., Berdowska A., Jedrzejowska-Szypulka H.et al. (2005) Factors associated with steatosis and necroinflammatory activity in morbidly obese patients with non-alcoholic fatty liver disease, Pol J Environ Stud 14(Suppl. 2):417–420 [Google Scholar]