Abstract
Nonalcoholic steatohepatitis (NASH) is increasing in prevalence and is related to underlying insulin resistance. The aim of this study was to assess the efficacy of metformin on the characteristic histopathologic lesions of NASH. This was a 12-month prospective, randomized, placebo-controlled trial comparing diet and exercise alone to diet, exercise and metformin in nondiabetic patients with insulin resistance and NASH. Patients were randomized to either group A or B. Group A received placebo, dietary counseling, recommendations for weight loss and exercise four times per week. Group B received long-acting metformin 500 mg daily (titrated to 1000 mg daily) plus dietary counseling, recommendations for weight loss and exercise four times per week. Histopathology was assessed at 12 months and biopsies were scored by two pathologists who were blinded to all data. Twenty-three subjects were screened and 19 were randomized to either group A (n ¼10) or group B (n¼ 9). Seven of the 10 subjects in group A completed the study including repeat liver biopsy while all patients in group B completed the study. Body mass index improved in both groups decreasing by 1.7 kg/m2 in group A and 0.9 kg/m2 in group B (not significant, control versus treatment). Homeostasis model assessment of insulin resistance scores improved in both groups decreasing by 1.14 in group A and 1.58 in group B (not significant, control versus treatment). No significant difference in histopathology was seen between groups on follow-up liver biopsy. Metformin appeared to have little effect in improvement in liver function tests or liver histology in nondiabetic patients with insulin resistance and NASH. Decrease in BMI through diet and exercise significantly improved HOMA-IR scores, serum aminotransferases and liver histology.
Keywords: NASH, insulin resistance, metformin, histopathology
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized condition of excess fat deposition within the liver [Angulo, 2002]. NAFLD includes a spectrum of liver pathology ranging from bland hepatic steatosis to steatohepatitis and cirrhosis [Sanyal, 2002]. Nonalcoholic steatohepatitis (NASH) is an inflammatory and fibrosing condition of the liver thought to be an intermediate stage of NAFLD that may progress to end-stage liver disease, liver-related death and hepatocellular carcinoma [Sanyal, 2003; Bugianesi et al. 2002; McCullough, 2002]. The pathologic criteria are now well established and the diagnosis can only be made once the absence or limited use of alcohol is confirmed. The estimated prevalence of NAFLD may be as high as one third in the general population [Browning et al. 2004]. Bland hepatic steatosis seems to have a relatively benign clinical course whereas up to 20% of NASH individuals may develop cirrhosis [McCullough, 2002]. The metabolic syndrome, characterized by increased waist circumference, hypertriglyceridemia, reduced high density lipo-protein (HDL) levels, elevated blood pressure, and/or fasting hyperglycemia, is a strong risk factor for NAFLD and insulin resistance plays a pivotal role in the pathophysiology of the disease [Hamaguchi et al. 2005; Choudhury et al. 2004]. The optimal therapy for NAFLD has not been established. Current recommendations are for dietary modifications, aerobic exercise, and moderate weight loss in order to decrease insulin resistance. Other recommendations would include avoiding offending agents such as alcohol and controlling associated medical disorders such as diabetes mellitus and hyperlipidemia [Reid, 2006]. Pharmacotherapy focusing on improving or reversing insulin resistance is a target of research. Metformin received approval by the FDA for clinical use in the United States in 1995 and revolutionized the treatment of diabetes mellitus type 2 and insulin resistance. Rather than increase insulin levels it works to improve insulin sensitivity and therefore decrease insulin requirements [Bailey and Turner, 1996]. Previous studies of metformin have shown biochemical improvement in NAFLD patients and suggest histologic improvement [Loomba et al. 2009; Buglianesi et al. 2005; Uygun et al. 2004; Coyle and Delaney, 1999], but have been limited to uncontrolled trials. We hypothesized that 12 months of metformin therapy would improve liver histology compared to diet and exercise in nondiabetic, insulin-resistant patients with biopsy proven NASH.
Materials and methods
Patients
The study subjects were obtained as consecutive patients referred to the adult gastroenterology clinic at the Naval Medical Center, San Diego with a histologic diagnosis of NASH defined by the presence of cytologic ballooning and inflammation in addition to steatosis. Study subjects must have had a liver biopsy within 18 months of enrollment to be eligible for the study. Subjects were included if they had biopsy-confirmed NASH and one of the following: a body mass index (BMI) 427 kg/m2; a fasting blood sugar between 110 and 125 mg/dl; a diagnosis of poly-cystic ovarian syndrome; or the metabolic syndrome. Additional inclusion criteria included age greater than 17 years, geographic stability for 1 year from study inclusion and an unremarkable serologic evaluation for chronic liver diseases including viral hepatitis studies, autoimmune studies, anti-mitochondrial antibodies, iron indices and, when deemed appropriate, alpha-1 antitrypsin studies and ceruloplasmin levels. Patients were excluded if they had known diabetes mellitus (type 1 or 2), a fasting blood sugar>125 mg/dl, prior history of alcoholic liver disease, any other known chronic liver disease, renal insufficiency (defined in this study as a serum creatinine >1.2), a known allergic reaction to metformin, prior use of an insulin-sensitizing agent such as metformin or a thiazolidinedione, gastric bypass within 2 years, untreated thyroid disease, coagulopathy, chronic thrombocytopenia, or significant alcohol use defined as consumption of >20 g/day or 80 g/week during the 2 years prior to study enrollment. All subjects signed an informed consent for participation in the study. The study was approved by our Department of Clinical Investigation and Institutional Review Board.
Study design
Subjects who met eligibility requirements were randomized to group A or B by the pharmacy using a computer-generated program. Patients randomized to group A (control group) received placebo. Group B (treatment group) received long-acting metformin 500 mg daily. In addition, both groups were referred to a dietician for counseling regarding a DASH (Dietary Approaches to Stop Hypertension) diet emphasizing fruit, vegetables and lowering saturated fat and cholesterol. Both groups were also advised to attempt to complete 30 minutes of aerobic exercise four times per week. Study participants were seen 2 weeks after enrollment and at 6-week intervals thereafter where BMI was measured and compliance with exercise regimen was assessed. At the 3-month follow-up visit, if serum aminotrans-ferases did not show improvement then the placebo or metformin dose was doubled (to a maximum dose of metformin of 1000mg/d). The treatment period was 12 months in duration and repeat liver biopsy was performed after completion of the therapy.
Methods
All subjects had a complete clinical, anthropo-metric and laboratory evaluation at the time of enrollment. The clinical data included age, race, sex, blood pressure, height, weight and BMI. Baseline laboratory measurements included fasting insulin and glucose measurement, fasting lipid profile with total cholesterol, triglycerides and HDL levels and liver enzymes including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase. A home-ostasis model assessment of insulin resistance (or HOMA-IR) score was calculated using the formula: fasting insulin (mIU/ml) × fasting glucose (mg/dl)/405 [Matthews et al. 1985]. A liver biopsy was performed as entry requirement for the study and repeated in 12 months at study conclusion. All biopsies were evaluated separately in a blinded fashion by two study pathologists who scored the histology using the scoring system proposed by Brunt et al. [1999] (Box 1). At study conclusion, scores were assessed. Scores differing by 1 or less between the two pathologists were averaged. Scores differing by more than 1 were than re-evaluated by both pathologists and a final score was given. In addition, all biopsies were given a NAFLD activity score as proposed by Kleiner et al. [2005].
Box 1. Nonalcoholic steatohepatitis histopathoLogic scoring system [Brunt et al. 1999].
NecroinfLammatory Grade:
1-MiLd
2-Moderate
3-Severe
Steatosis (% of hepatocytes in specimen):
1-one
2-up to 33%
3-33-66%
4->66%
Hepatocellar Ballooning and Disarry:
1-minimal, focally involving
2-mild, obvious ballooning
3-marked, obvious ballooning and disarry
Intra-acinar (lobular) inflammation (inflammatory foci per 20x):
0-none
1-1-2/20x
2-3-4/20x
3->4/20x
Portal Tract Inflammation:
0-none
1-mild
2-moderate
3-severe
Fibrosis Stage:
0-none
1-zone 3 perisinusoidal/pericellular fibrosis
2-zone 3 perisinusoidal/pericellular fibrosis with periportal fibrosis
3-zone 3 perisinusoidal/pericellular fibrosis with periportal fibrosis with bridging
4-cirrhosis
Endpoints
The primary objective of this pilot study was improvement in histopathology in metformin-treated patients compared with placebo. Secondary endpoints included overall improvement in BMI, HOMA-IR and serum amino-transferases.
Statistical analysis
The scores were compared across study arms at entry and at end of study using a Wilcoxon signed-rank test. The scores were compared between the two study groups using the two-sample Wilcoxon rank-sum test. The analysis was carried out on an intention-to-treat basis. A p value of <0.05 was considered significant. All data in the text and tables are given as means + standard deviation.
Results
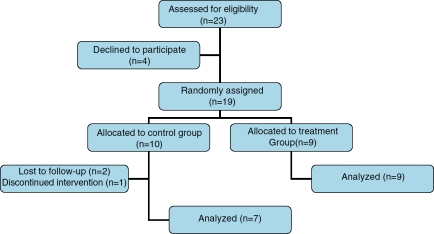
Twenty-three consecutive patients were assessed and fulfilled entry criteria for eligibility in the study. Four patients declined to participate. Nineteen patients were randomly assigned to either group A (n = 10) or group B (n = 9). All patients in group B completed the study including repeat liver biopsy at 12 months. All liver biopsies were deemed adequate for interpretation. Three patients in group A did not complete the study (two were lost to follow up and one declined further participation in the study). Their data are included in the final intention-to-treat analysis. Four of nine patients in group B (treatment group) were titrated up to metformin 1000mg/day Figure 1 illustrates the study flow.
Figure 1.
Study flow.
Baseline data
The baseline clinical and demographic data from the two groups are shown in Table 1. The two groups were similar regarding their laboratory and anthropometric data. The treatment group was older and predominantly male. The majority of patients in both groups were Caucasian, obese (BMI > 30) and had laboratory evidence of insulin resistance and hyperlipidemia. Most showed mild abnormalities in ALT and AST.
Table 1.
Baseline clinical and demographic data.
| Characteristic | Group A: Control (n=10) | Group B: Treatment (n = 9) | P Value |
| Age | 44.4 (+/-12) | 50.2(+/-9.1) | 0.252 |
| Sex(M:F) | 5:5 | 8:1 | 0.091 |
| Race | |||
| Hispanic | 1 | 1 | |
| Caucasian | 5 | 6 | |
| Asian | 4 | 1 | |
| African American | 0 | 1 | |
| BMI (kg/m2) | 32.8(+/-4.9) | 32.2(+/-4.9) | 0.775 |
| HOMA-IR | 4.02(+/-3.99) | 6.14(+/-4.5) | 0.124 |
| Fasting Insulin (mlU/mL) | 14.9(+/-8.4) | 31.4(+/-32.9) | 0.177 |
| Total Cholestero (mg/dl) | 196.2(+/-55.4) | 213.3(+/-49.8) | 0.624 |
| Triglycérides (mg/dL) | 163.2(+/-85.2) | 209.8(+/-114.2) | 0.513 |
| ALT (IU/L) | 97.5(+/-45.6) | 79.4(+/-26.1) | 0.513 |
| AST (IU/L) | 62(+/-21.4) | 52.8(+/-12.6) | 0.595 |
| Alk Phos (IU/L) | 84.5(+/-20.3) | 80.4(+/-25.5) | 0.414 |
Clinical outcomes
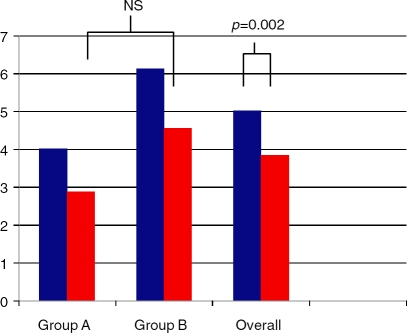
BMI improved in both treatment arms. In group A, the BMI decreased by 1.7kg/m2 whereas in group B the BMI decreased by 0.9 kg/m2. The difference between control group and treatment group was not significant (p = 0.514). In all study subjects, the BMI decreased by 1.3 kg/m2. This was significant (p ¼ 0.007) and is illustrated in Figure 2.
Figure 2.
Body mass index (kg/m2) between study subjects at baseline (blue bars) and end of treatment (red bars).
Insulin resistance was improved in all study subjects. HOMA-IR decreased by 1.14 in group A and by 1.58 in group B. There was no significant difference between the two groups (p¼0.886). Overall, HOMA-IR decreased by 1.18. This was significant (p ¼ 0.002) and is illustrated in Figure 3.
Figure 3.
Insulin resistance (HOMA-IR) between groups at baseline (blue bars) and end of treatment (red bars).
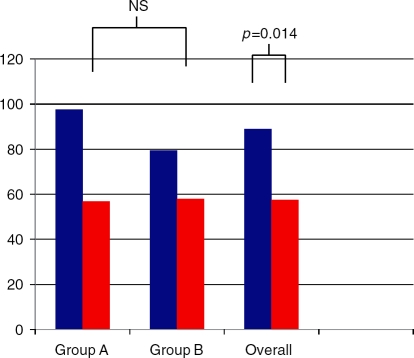
Liver enzymes improved in both treatment groups (Figures 4 and 5). ALT levels decreased by 40.7 IU/l in group A, 21.5 IU/l in group B and 31.6 IU/l overall. AST levels decreased by 20.1 IU/l in group A, 5.7 IU/l in group B and 13.2 IU/l overall. The differences between the two groups were not significant for ALT, AST or alkaline phosphatase. The overall improvements were significant for ALT (p¼0.014) and AST (p¼0.04) and approached significance with alkaline phosphatase (p ¼ 0.056).
Figure 4.
Alanine aminotransferase between groups at baseline (blue bars) and end of treatment (red bars).
Figure 5.
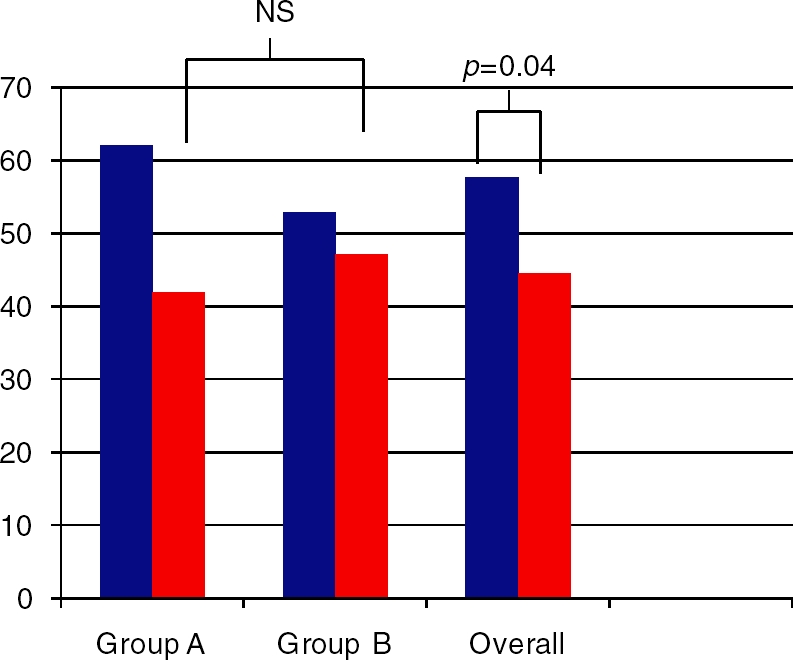
Aspartate aminotransferase between groups at baseline (blue bars) and end of treatment (red bars).
Histologic outcomes are shown in Table 2. There were no statistically significant differences between the two groups in regards to overall NAFLD activity score (NAS), individual components of NAS or fibrosis. There was, however, a significant improvement in steatosis and NAS across all study subjects (p¼ 0.021).
Table 2.
Baseline and end of treatment histology.
| Variable | Control | Treatment | Overall | p value (control vs treatment) | p value (overall) | |||
| Baseline | End of treatment | Baseline | End of treatment | Baseline | End of treatment | |||
| Grade | 1.9 | 1.55 | 1.5 | 1.39 | 1.71 | 1.47 | 0.67 | 0.064 |
| Steatosis | 2.23 | 1.58 | 2 | 1.91 | 2.12 | 1.74 | 0.23 | 0.019 |
| Ballooning | 1.78 | 1.5 | 1.69 | 1.47 | 1.74 | 1.49 | 0.967 | 0.09 |
| Intra-acinar inflammation | 1.4 | 1.28 | 1.25 | 1.36 | 1.33 | 1.32 | 0.478 | 0.951 |
| Portal tract inflammation | 1.38 | 1.3 | 0.78 | 0.56 | 1.09 | 0.95 | 0.523 | 0.22 |
| Fibrosis | 1.7 | 1.9 | 1.61 | 1.56 | 1.66 | 1.74 | 0.447 | 0.933 |
| NAS (NAFLD activity score! | 4.6 | 3.4 | 3.9 | 3.8 | 4.3 | 3.6 | 0.108 | 0.021 |
Discussion
Insulin resistance and the metabolic syndrome play a central role in the pathogenesis of NAFLD and NASH [Choudhury, 2004]. At present, the standard of care is aimed at weight loss through aerobic exercise and dietary changes. However, large, randomized controlled trials evaluating the efficacy of weight loss on histopathology in NASH are lacking [Clark, 2006]. Subsequently, multiple pharmacotherapies have been investigated with variable results including lipid lowering agents [Gomez-Dominguez et al. 2006; Basaranoglu et al. 1998], vitamin E [Sanyal et al. 2004], pentoxifylline [Adams et al. 2004] and ursodeoxycholic acid [Lindor et al. 2004]. Small randomized, controlled trials have suggested a benefit using the thiazolidinedione, pioglitazone. Belfort et al. [2006] demonstrated both metabolic and histologic improvement in 55 patients with NASH treated for 6 months with pioglitazone [Belfort et al. 2006]. This has been supported by a more recent study of pioglitazone in 74 NASH patients treated for 1 year with pioglitazone [Aithal et al. 2008]. However, this type of insulin sensitizer is associated with side effects such as weight gain and increased fracture risk that raise questions as to the utility of using this drug long term. Furthermore, not every NASH patient appears to have a favorable histopathologic response. Therefore, it would seem appropriate to assess the utility of other medications that improve insulin sensitivity such as metformin. Metformin is indicated for the treatment of diabetes mellitus and as such would be considered a candidate drug to treat NASH given the underlying association. Improvement in insulin sensitivity is thought to occur via upregulation of AMP-activated protein kinase (AMPK), an integral component of glucose and lipid metabolism that results in decreased hepatic glucose and triacylglycerol production as well as increased peripheral glucose utilization via skeletal muscle. A recent Cochrane Review suggested a favorable response with metformin in two small, randomized trials in improving aminotrans-ferases [Angelico et al. 2007]. Data is limited with regards to changes in histology and trials reviewing metformin lack double-blinding controls and/or control biopsy data. Our study is the first reported prospective, randomized, double blinded, placebo-controlled trial of 12 months of therapy with metformin in the treatment of NASH in nondiabetic patients with primary histologic endpoints. There was no significant difference between patients treated with metformin compared to those randomized to placebo with regards to changes in serum amino-transferases, insulin resistance or in liver histology.
All patients met with a dietician and were given instructions for a DASH diet. In addition, they were asked to comply with an aerobic exercise regimen of 30 minutes at least four times a week. Lifestyle modifications have been the standard of care in the treatment of NAFLD despite limited supportive data. Our patients significantly improved their BMI throughout the 12-month study. This improvement in BMI was associated with an improvement in insulin resistance as HOMA-IR scores were significantly lower at the end of the study in both groups. In addition, there was significant improvement in serum aminotransferase levels and more importantly in liver histology with a significant improvement in steatosis and NAFLD activity scores. There were also trends toward significance in improvement in ballooning degeneration and overall inflammatory grade. Our study provides needed support for continued recommendations of lifestyle modifications in the treatment of NAFLD. Furthermore, our study demonstrates that liver histology can improve with weight loss and exercise alone.
Our study has a number of limitations. First, as a pilot study, it is limited by a small sample size increasing the risk of type II error associated with the statistical results. Second, the potential benefit may be masked by a relatively low dose of metformin (maximum 1000 mg/d). It is important to note, however, that our study specifically excluded patients with a diagnosis of diabetes mellitus type 2. Finally, although our study has histologic endpoints at 12 months, the benefits of inflammation improvement and potential fibrosis reversal may require longer-term histologic data. Clearly, in the field of NASH large-scale trials with long-term histologic endpoints are necessary.
Conclusion
Metformin appears to have little effect compared with placebo in improving liver chemistries or liver histology in nondiabetic, insulin-resistant patients with NASH. Weight loss utilizing the DASH diet and regular aerobic exercise significantly improves insulin resistance, serum amino-transferases and liver histology in NASH.
Conflict of interest statement
The opinion or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the view of the Department of the Army, Navy or the Department of Defense.
Contributor Information
William W. Shields, Department of Gastroenterology, Naval Medical Center San Diego, San Diego, CA, USA william.shields@med.navy.mil
K.E. Thompson, Department of Pathology, Naval Medical Center San Diego, San Diego, CA, USA
G.A. Grice, Department of Pathology, Naval Medical Center San Diego, San Diego, CA, USA
S.A. Harrison, Division of Gastroenterology and Hepatology, Brooke Army Medical Center, Fort Sam Houston, TX, USA
W.J. Coyle, Division of Gastroenterology and Hepatology, Scripps Clinic Torrey Pines, LaJolla, CA, USA
References
- Adams L.A., Zein C.O., Angulo P., Lindor K.D. (2004) A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol 99:2365–2368 [DOI] [PubMed] [Google Scholar]
- Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I.et al. (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135:1176–1184 [DOI] [PubMed] [Google Scholar]
- Angelico F., Burattin M., Alessandri C., Del Ben M., Lirussi F. (2007) Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database of Systematic Reviews, CD005166. [DOI] [PubMed] [Google Scholar]
- Angulo P. (2002) Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231 [DOI] [PubMed] [Google Scholar]
- Bailey C.J., Turner R.C. (1996) Metformin. N Engl J Med 334:574–579 [DOI] [PubMed] [Google Scholar]
- Basaranoglu M., Acbay O., Sonsuz A. (1998) A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol 29:495–501 [DOI] [PubMed] [Google Scholar]
- Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J.et al. (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355:2297–2307 [DOI] [PubMed] [Google Scholar]
- Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D.et al. (2004) The prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40:1387–1395 [DOI] [PubMed] [Google Scholar]
- Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. (1999) Nonalcoholic steatohepatitis: A proposal for grading and staging the histologic lesions. Am J Gastroenterol 94:2467–2474 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N.et al. (2005) A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100:1082–1090 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Leone N., Vanni E., Marchesini G., Brunello F., Coracci et al. (2002) Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 123:134–140 [DOI] [PubMed] [Google Scholar]
- Choudhury J. (2004) Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liv Dis 8:575–594 [DOI] [PubMed] [Google Scholar]
- Clark J.M. (2006) Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol 40:S39–43 [DOI] [PubMed] [Google Scholar]
- Coyle W.J., Delaney N. (1999) Metformin treatment in patients with nonalcoholic steatohepatitis normalizes LFTs and improves histology. Gastroenterology 116:L00883(abstract) [Google Scholar]
- Gomez-Dominguez E., Gisbert J.P., Moreno-Monteagudo J.A., Garcia-Buey L., Moreno-Otero R. (2006) A pilot study of atorvastatin treatment in dyslipidemic, non-alcoholic fatty liver patients. Aliment Pharmacol Ther 23:1643–1647 [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Kojima T., Takeda N., Nakagawa T., Taniguchi H., Fujii K.et al. (2005) The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Int Med 143:722–728 [DOI] [PubMed] [Google Scholar]
- Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W.et al. (2005) Decision and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- Lindor K.D., Kowdley K.V., Heathcote E.J., Harrison M.E., Jorgensen R., Angulo P.et al. (2004) Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39:770–778 [DOI] [PubMed] [Google Scholar]
- Loomba R., Lutchman G., Kleiner D.E., Ricks M., Feld J.J.et al. (2009) Clinical trial: pilot study of metformin for the treatment of non-alcoholic steato-hepatitis. Aliment Pharmacol Ther 29:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- McCullough A.J. (2002) Update on nonalcoholic fatty liver disease. J Clin Invest 34:255–262 [DOI] [PubMed] [Google Scholar]
- Reid A.E. (2006) Nonalcoholic fatty liver disease. In: Feldman M., Freidman L., Sleisenger M.Sleisenger and Fordtran's Gastrointestinal and Liver Disease, 8th edn.Philadelphia: WB Saunders, 1793–1805 [Google Scholar]
- Sanyal A.J. (2002) AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 123:1705–1725 [DOI] [PubMed] [Google Scholar]
- Sanyal A.J., Mofrad P.S., Contos M.J., Sargeant C., Luketic V.A., Sterling R.K.et al. (2004) A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastro Hepatol 2:1107–1115 [DOI] [PubMed] [Google Scholar]
- Uygun A., Kadayifici A., Isik A.T., Ozgurtas T., Deveci S., Tuzun A.et al. (2004) Metformin in the treatment of patients with non-alcoholic steato-hepatitis. Aliment Pharmacol Ther 19:537–544 [DOI] [PubMed] [Google Scholar]