Abstract
Compliance with therapy is the single most important factor in Helicobacter pylori (H. pylori) eradication. Poorer levels of compliance with therapy are associated with significantly lower levels of eradication. Numerous factors can contribute to achieving good levels of compliance. These include the complexity and duration of treatment. It is also important that the physician is motivated to ensure eradication is confirmed and the patient is sufficiently informed to empower him or her to achieve high levels of compliance. Compliance is also contingent on medication regimes that are simple, safe, tolerable and efficacious. The opportunity to improve compliance exists at every point of contact between the patient and the medical services. Experts and opinion leaders in the field can play a role by ensuring that physicians are educated and motivated enough to encourage and support compliance with H. pylori eradication therapy. Both patients and physicians need to be aware of the importance of the bacterium in causing disease. The importance of the doctor—patient relationship is paramount. Pragmatic strategies that may be of assistance may come in the form of polypills, combined Blister Packs, adjuvant therapies and modified release compounds. Colleagues such as pharmacists and nurse specialists can also play an important role and should be actively engaged. Structured aftercare and follow up offers the best chance for ensuring compliance and subsequent eradication of the H. pylori pathogen.
Keywords: gastric cancer, Helicobacter pylori, compliance
Introduction
Keep watch also on the faults of the patients which often make them lie about the taking of things prescribed — Hippocrates, 4th century BC
Compliance with therapy is the single most important factor in Helicobacter pylori (H. pylori) eradication. Compliance with therapy has a considerable influence on treatment failures in antibiotic-sensitive patients and in the subsequent development of antibiotic resistance. This has major implications. Eradication rates with standard proton pump inhibitor-amoxicillin-clarithromycin are 87.8% when strains are clarithromycin-sensitive and 18.3% when clari-thromycin-resistant [Megraud, 2004]. It has been proven that 10% of patients prescribed H. pylori eradication therapy will fail to take even 60% of medications [Lee et al. 1999]. It has also been proven that progressively poorer levels of compliance with therapy are associated with significantly lower levels of eradication. In one study eradication levels of 96% were observed for patients who took 60% or more of medications compared to 69% for those taking less than 60% of prescribed medications [Graham et al. 1992].
Compliance is a topic of much recent discussion in the medical literature. An analysis revealed that the number of times the word itself has been cited in the medical literature has grown at an exponential rate over the last 40 years [Düsing et al. 2001]. There has even been criticism of the term itself as it suggests a didactic medical model which stresses obedience and is not patient centred, while some opinions have suggested a newer model of ‘adherence’ which emphasises the patient as an autonomous partner in treatment [Lutfey and Wishner, 1999]. The problems involved in ensuring compliance or adherence with medical therapy are far from new, as the above quotation from Hippocrates illustrates.
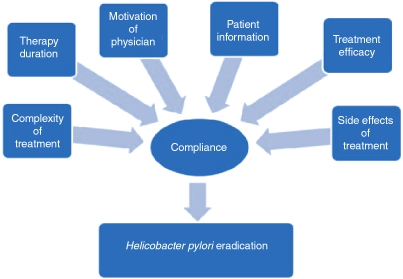
Compliance with H. pylori eradication regimens is a multifactorial process. Current evidence and published guidelines recommend complex and prolonged eradication regimens, using a number of antibiotics and involving manipulation of gastric pH as well. This complexity provides challenges for both the physician and the patient. For the physician, it demands clear understanding of the pathogenesis, sequelae and complications of H. pylori infection and a motivation to test for and treat the infection where appropriate. The motivated physician can then provide information to the patient which will lead to his or her empowerment to play an active role in their treatment by complying with therapy. To do this demands the knowledge outlined above on the physician's part. Trust, which is implicit in the doctor–patient relationship, is also of paramount importance here. The benefit gained from the eradication of H. pylori such as reducing long-term risk of gastric cancer may not be obvious to the patient. The patient's expectations of therapy need to be explored as this benefit is often much more subtle than that gained from the prescription of oral antibiotics for an uncomplicated respiratory or urinary tract infection. The challenges for the patient wishing to comply with therapy may also be financial and social, incorporating factors such as literacy. The combination of all of these factors, however, is immaterial unless efficacious and evidence-based treatments are available with adverse event profiles that are acceptable to both physician and patient. As outlined in Figure 1, these factors converge to influence compliance which leads to H. pylori eradication.
Figure 1.
Factors influencing compliance with therapy and subsequent eradication of Helicobacter pylori.
Complexity of treatment
Complexity of treatment has been proven to be a factor in reducing compliance with medications in other clinical areas such as the management of type 2 diabetes [Mateo et al. 2006]. The same is true in H. pylori eradication where the number of medications which need to be consumed daily in is daunting. Depending on local practices the number of pills taken in standard proton pump inhibitor-amoxycillin-clarithromycin therapy is anywhere between 6 and 12 each day. There is considerable potential for confusion and omissions due to the multiplicity of medications. In other fields such as the management of hypertension and diabetes, the introduction of combination drugs or ‘polypills’ has helped to achieve better rates of compliance [Dickson and Plauchinat, 2008; Pan et al. 2008]. It has been suggested that enhanced compliance programmes can also help improve compliance and eradicate H. pylori. A study on a bismuth-based regimen proved increased levels of compliance when an enhanced compliance programme was enforced. This included both written and oral communication from a pharmacist, a medication calendar, a mini pillbox and a follow-up phone call [Lee et al. 1999]. This led to a significant increase in the numbers of patients taking more than 90% of medications, but the proportion of patients taking 60% or less of medications was similar in both groups. The findings here would suggest that those patients who are at greatest risk of treatment failure due to poor compliance (i.e. taking less than 60% of prescribed medications) are likely to remain noncompliant even with such assistance. Results from other studies however have been mixed. An Australian study suggested that attempts to increase compliance had no impact on outcome or compliance [Henry and Batey, 1999]. Enhanced compliance programmes are certainly worth considering as a means of improving adherence. It is a low-risk and often low-cost intervention, albeit labour intensive for the physician. The opportunity here exists to enlist other healthcare professionals such as pharmacists and nurse specialists to help improve compliance. Although there is certainly evidence to suggest that levels of compliance may be improved, it remains to be proven whether or not enhanced compliance programmes can actually lead to greater eradication rates.
Duration of therapy
The duration of therapy in H. pylori eradication regimens has also been postulated as a reason for poor compliance. This is increasingly important as 10- or 14-day regimens begin to supplant 7-day regimens. Published guidelines in United States have come to reflect this with the 2007 American College of Gastroenterology guidelines recommending longer treatment courses [Chey and Wong, 2007]. The basis for this is somewhat disputed however. One meta-analysis in 2000 suggested a 14-day course of therapy showed 7–9% better cure regimens than 7-day regimes [Calvet et al. 2000]. However, another meta-analysis differed, stating no clinical benefit from longer courses of treatment, although the quality of some of the studies included in this second meta-analysis has been questioned [Fuccio et al. 2007]. Little data exists regarding the side effects of longer regimens or the possible impact prolonged courses will have on antibiotic resistance levels. Current evidence would suggest compliance is affected by longer courses of therapy but not to a very large extent. A study comparing 7—14-day courses of proton pump inhibitor-amoxicillin-clarithromycin showed that the rates of compliance less than 75% of 18.6% for the longer course compared to 17.3% for standard 7-day triple therapy [Zagari et al. 2007]. Opinion is somewhat divided however on the clinical efficacy of this strategy. However, a major limitation to all research into compliance is that no single, consistent definition of compliance can be employed as most estimates are limited by patient manipulation (pill count etc.).
Physician factors
The motivation and attitude of the physician is critical to patient compliance and successful eradication. Chronic gastritis induced by H. pylori is the strongest known risk factor for gastric adenocarcinoma and as far back as 1994 the World Health Organisation through its International Agency for Research on Cancer designated H. pylori as a class I carcinogen [International Agency for Research on Cancer, 1994]. As such, successful strategies for H. pylori eradication and the confirmation thereof are of paramount importance in cancer prevention. A study of the knowledge of the most important aspects of H. pylori infection carried out amongst internal medicine residents in the US reveals gaps in knowledge. Most of the responding doctors claimed that they routinely test for H. pylori infection in appropriate circumstances, they may not always treat when indicated. Although treatment regimens used were found to be appropriate, awareness of issues regarding antibiotic resistance was poor [Sharma et al. 2000]. A more recent study of a broad pool of US doctors revealed that around one-third do not adhere to the ‘test and treat’ policy which forms the cornerstone of H. pylori management [Howden et al. 2007]. A study of internists and surgeons in Israel revealed similar concerns about physician knowledge of H. pylori and specific concerns were raised here regarding the knowledge of the bacterium as a causative agent in gastric adenocarcinoma and lymphoma [Niv and Abuksis, 2003]. If practitioners had greater awareness of these matters it would lead to greater adherence to a test-and-treat policy. Similarly, this knowledge could be transmitted to a newly informed and empowered patient who would be better motivated and likely to achieve greater compliance. The impact of motivated physicians on ensuring compliance may reflect the setting in which the prescription is issued. The opportunity to educate the patient on the importance of compliance is surely greater in a physician's office setting than in the case of prescribing eradication therapy for a patient possibly still under the effect of sedation in an endoscopy unit where a positive rapid urease test has been recorded.
Patient information
Patient information is another factor that can greatly influence compliance. In Japan, where the incidence of gastric adenocarcinoma is greatest, 51% of the general population are aware of causal link between infectious agents and stomach cancer [Inoue et al. 2006]. No data are available to measure if awareness in patients in the western hemisphere is as good. However, if one is to consider the knowledge of western physicians as a proxy for patient knowledge, based on the surveys outlined in the previous paragraph, it could be presumed that the level of awareness of the importance of H. pylori and its link to gastric adenocarcinoma is much lower. Referring back to the frequent scenario outlined previously of prescriptions being issued to patients recovering from sedation in an endos-copy suite, this surely does not permit a conversation of sufficient depth to motivate and empower the patient to achieve better compliance. A solid therapeutic relationship is the key to this. It may reasonably be suggested that public awareness programmes about H. pylori and gastric cancer may assist in improving compliance rates. This is likely to be true and is probably proven by the impressive levels of public awareness in Japan where a mass screening programme is widely advertised in the popular media. The issue is less straightforward in low-prevalence countries in the Western world where gastric cancer is less prevalent. In this case, a balance needs to be struck between providing education sufficient to encourage compliance while avoiding raising unnecessary public anxiety. The method of ensuring with the most realistic prospect of success is by communicating on a one-to-one basis and educating the individual patient on their illness and reinforcing the primacy of compliance with therapy in reducing cancer risk. The onus here lies with every physician who treats H. pylori infection. A study carried out in Northern Ireland compared such an approach including informing the patient of the risks of H. pylori infection, structured counselling and follow up to a ‘prescription and discharge’ strategy. This revealed significantly increased levels of both compliance and eradication in the cohort who had been given greater knowledge of their illness and the importance of compliance stressed [Al-Eidan et al. 2002].
Efficacious and beneficial treatment
Comprehensive patient information should include a discussion of treatment options and to this end patients need to know that efficacious and beneficial treatment is available to them should they comply. Again, this is considerably easier in a setting where structured follow up is available. Following the discovery of H. pylori as the causative agent in peptic ulcer disease, numerous treatment regimens had been proposed. However, it was not until a rigorously controlled clinical trial that the currently most accepted combination of two antibiotics and a proton pump inhibitor became standard treatment [Lind et al. 1996]. This has been widely adopted around the world and has gained acceptance as the first choice in published guidelines in both Europe and North America and the Asia Pacific region [Chey and Wong, 2007; Malfertheiner et al. 2007; Lam and Talley, 1998]. Initial eradication rates using such regimens were greater than 90% [Kearney and Brousal, 2000], but over the intervening two decades these have fallen alarmingly. For instance, in Turkey the rates of eradication from 7-day triple therapy fell from 83.7% in 1997 to 61.1% in 2005 [Kadayifci et al. 2006]. This has led to the use of newer antibiotics such as levofloxacin and rifabutin and efforts to devise newer regimens such as sequential therapy with amoxicillin, metronidazole, clarithromycin and a proton pump inhibitor [Jafri et al. 2008; Rispo et al. 2007; Borody et al. 2006]. However, it would appear that the problem lies more in ensuring compliance than the actual combinations of agents employed. For example, a study in a Finnish tertiary referral centre revealed 100% eradication in 644 consecutive patients where compliance was ensured and patients were treated with standard first and second-line therapies as per the Maastricht guidelines and third-line rescue therapy tailored to antibiotic susceptibility [Seppälä et al. 2008]. Another study in Greece found 98.1% eradication rates when the Maastricht-III guidelines were implemented [Rokkas et al. 2009]. Although the role of antibiotic resistance is undoubtedly a major factor in treatment failures, achieving better compliance rates within existing guidelines will have a greater effect on minimising resistance and ensuring eradication than devising new protocols.
Side effects
Adverse events relating to H. pylori therapy obviously vary from regimen to regimen but may also be a factor that influences compliance. It has been shown that side effects were significantly associated with treatment failure and decreased compliance [Henry and Batey, 1999]. In many of the randomised controlled trials in the field, various different scoring systems are used. A standard side-effect scoring system has been proposed but has not been universally used [de Boer et al. 1996]. Although side effects of standard therapies are common, they rarely result in severe adverse events mandating discontinuation of therapy. For standard first-line triple therapy the overall rate of adverse events was 53.3% in a multicentre study but these were rarely serious with only one patient experiencing pseudomembranous colitis [Misiewicz, 1997]. No deaths were recorded. However the most common adverse events recorded are bothersome symptoms such as diarrhoea, nausea and vomiting which have significant physical and social impacts. Again, the central themes of physician motivation and patient information assume an importance here. The patient is more likely to tolerate ‘minor’ adverse events if he or she is clear in their mind as to the goals of therapy. For second-line and subsequent therapies, concerns have been expressed about more serious adverse events as well as patient fatigue having taken several courses of treatment. In a study of 46 000 users of fluoroquinolones, achilles tendo-nitis occurred in 704 subjects [van der Linden, 2002]. Case reports of hepatotoxicity have also been published [Spahr et al. 2001]. Myelosuppression has been noted in patients taking rifabutin [Coelho et al. 2005]. It had been hoped that the concurrent administration of probiotics would lessen the side-effect profile of triple therapy; however, this may also increase the complexity of the treatment regimen. The evidence on this has been equivocal. In one study, prescribing probiotics with H. pylori eradication therapy had no effect on the side-effect profile but did increase rates of eradication [Kim et al. 2008]. However, another study on concurrent probiotic administration suggested the inverse with better side-effect profiles but no increase in eradication or rates of compliance with therapy [Cremonini et al. 2002]. Safer, tolerable regimes will undoubtedly be easier to ‘sell’ to patients and will encourage greater compliance.
Conclusion
The opportunity to improve compliance exists at every point of contact between the patient and the medical service. Experts and opinion leaders in the field can play a role by ensuring that physicians are educated and motivated enough to encourage and support compliance with H. pylori eradication therapy. Both patients and physicians need to be aware of the importance of the bacterium in causing disease. This can be achieved to some extent by improving public awareness but the opportunity primarily lies in the individual doctor–patient relationship. The practicalities of improving compliance in the willing patient also need to be addressed. This can be helped by devising regimes that are easy to remember, prescribe and use for patient and physician alike. In the developing world especially, where the burden of H. pylori is often greatest, costs must also be kept low. Research efforts should continue to be focussed on the development of simple, safe, tolerable and efficacious treatments. Pragmatic strategies which may be of assistance may come in the form of polypills, combined blister packs, adjuvant therapies and modified release compounds. Colleagues such as pharmacists and nurse specialists can also play an important role and should be actively engaged. Compliance and all other problems associated with H. pylori eradication treatment could potentially be avoided were an effective vaccine to be developed. Pharmacoeconomic studies suggest that a prophylactic vaccine would be cost effective in developed countries and, based on its anticipated cost effectiveness, development of a prophylactic H. pylori vaccine has been designated a high priority by the US Institute of Medicine [Stratton et al. 2000; Rupnow et al. 1999]. Finally, compliance with therapy is about much more than swallowing pills at regular intervals, it involves partnership between physician and patient with a plan for eradication. As such, the importance of structured aftercare and follow up of patients is of critical significance.
Conflict of interest statement
None declared.
Contributor Information
John P. Anthony O'Connor, Adelaide and Meath Hospital incorporating the National Children's Hospital, and Trinity College, Dublin, Eire jpanthonyoconnor@hotmail.com.
Ikue Taneike, Adelaide and Meath Hospital incorporating the National Children's Hospital, and Trinity College, Dublin, Eire.
Colm O'Morain, Adelaide and Meath Hospital incorporating the National Children's Hospital, and Trinity College, Dublin, Eire.
References
- Al-Eidan F.A., McElnay J.C., Scott M.G., McConnell J.B. (2002) Management of Helicobacter pylori eradication - the influence of structured counselling and follow-up. Br J Clin Pharmacol 53:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody T.J., Pang G., Wettstein A.R., Cloancy R., Herdman K., Surace R.et al. (2006) Efficacy and Safety of rifabutin-containing ‘rescue therapy' for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 23:481–488 [DOI] [PubMed] [Google Scholar]
- Calvet X., Garćia N., López T., Gisbert J.P., Gené E., Roque M. (2000) A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther 14:603–609 [DOI] [PubMed] [Google Scholar]
- Chey W.D., Wong B.C. (2007) American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102:1808–1825 [DOI] [PubMed] [Google Scholar]
- Coelho L.G., Moretzsohn L.D., Vieira W.L., Gallo M.A., Passos M.C., Cindr J.M.et al. (2005) New once-daily, highly effective rescue triple therapy after multiple Helicobacter pylori treatment failures: a pilot study. Aliment Pharmacol Ther 21:783–787 [DOI] [PubMed] [Google Scholar]
- Cremonini F., Di Caro S., Covino M., Armuzzi A., Gabrielli M., Santarelli L.et al. (2002) Effect of different probiotic preparations on anti-helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 97:2744–2749 [DOI] [PubMed] [Google Scholar]
- de Boer W.A., Thys J.C., Borody T.J., Graham D.Y., O'Morain C.A., Tytgat G.N. (1996) Proposal for use of a standard side effect scoring system in studies exploring Helicobacter pylori treatment regimens. Eur J Gastroenterol Hepatol 8:641–643 [PubMed] [Google Scholar]
- Dickson M., Plauschinat C.A. (2008) Compliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapy. Am J Cardiovasc Drugs 8:45–50 [DOI] [PubMed] [Google Scholar]
- Düsing R., Lottermoser K., Mengden T. (2001) Compliance with drug therapy-new answers to an old question. Nephrol Dial Transplant 16:1317–1321 [DOI] [PubMed] [Google Scholar]
- Fuccio L., Minardi M.E., Zagari R.M., Grilli D., Magrin N., Bazzoli F. (2007) Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med 147:553–562 [DOI] [PubMed] [Google Scholar]
- Graham D.Y., Lew G.M., Malaty H.M., Evans D.G., Evans D.J., Jr, Klein P.D. (1992) Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 102:493–496 [DOI] [PubMed] [Google Scholar]
- Henry A., Batey R.G. (1999) Enhancing compliance not a prerequisite for effective eradication of Helicobacter pylori: the HelP study. Am J Gastroenterol 94:811–815 [DOI] [PubMed] [Google Scholar]
- Howden C.W., Blume S.W., de Lissovoy G. (2007) Practice patterns for managing Helicobacter pylori infection and upper gastrointestinal symptoms. Am J Manag Care 13:37–44 [PubMed] [Google Scholar]
- Inoue M., Iwasaki M., Otani T., Sasazuki S., Tsugane S. (2006) Public awareness of risk factors for cancer among the Japanese general population: A population-based survey. BMC Public Health 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. (1994) Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61:1–241 [PMC free article] [PubMed] [Google Scholar]
- Jafri N.S., Hornung C.A., Howden C.W. (2008) Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med 148:923–931 [DOI] [PubMed] [Google Scholar]
- Kadayifci A., Buyukhatipoglu H., Cemil Savas M., Simsek I. (2006) Eradication of Helicobacter pylori with triple therapy: an epidemiologic analysis of trends in Turkey over 10 years. Clin Ther 28:1960–1966 [DOI] [PubMed] [Google Scholar]
- Kearney D.J., Brousal A. (2000) Treatment of Helicobacter pylori infection in clinical practice in the United States. Dig Dis Sci 45:265–271 [DOI] [PubMed] [Google Scholar]
- Kim M.N., Kim N., Lee S.H., Park Y.S., Hwang J.H., Kim J.W.et al. (2008) The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 13:261–268 [DOI] [PubMed] [Google Scholar]
- Lam S.K, Talley N.J. (1998) Report of the 1997 Asia Pacific consensus conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol 13:1–12 [DOI] [PubMed] [Google Scholar]
- Lee M., Kemp J.A., Canning A., Egan C, Tataronis G., Farraye FA. (1999) A randomized controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med 159:2312–2316 [DOI] [PubMed] [Google Scholar]
- Lind T., Veldhuyzen van Zanten S., Unge P., Spiller R., Bayerdorffer E., O'Moráin C.et al. (1996) Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter 1:138–144 [DOI] [PubMed] [Google Scholar]
- Lutfey K.E., Wishner WJ. (1999) Beyond ‘compliance' is ‘adherence'. Improving the prospect of diabetes care. Diabetes Care 22:635–639 [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Megraud F., O'Morain C, Bazzoli F., El-Omar E., Graham D.et al. (2007) Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 56:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J.F., Gil-Guillén V.F., Mateo E., Orozco D., Carbayo J.A., Merino J. (2006) Multifactorial approach and adherence to prescribed oral medications in patients with type 2 diabetes. Int J Clin Pract 60:422–428 [DOI] [PubMed] [Google Scholar]
- Megraud F. (2004) H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiewicz J.J., Harris A.W, Bardham K.D., Levi S., O'Moráin CA., Cooper B.T.et al. (1997) One week triple therapy for helicobacter pylori: a multocentre comparative study. Gut 41:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y., Abuksis G. (2003) Survey of the opinions, knowledge and practices of surgeons and internists regarding Helicobacter pylori test-and-treat policy. J Clin Gastroenterol 36:139–143 [DOI] [PubMed] [Google Scholar]
- Pan F., Chernew M.E., Fendrick A.M. (2008) Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med 23:611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispo A., Di Girolamo E., Cozzolino A., Bozzi R., Morante A., Pasquale L. (2007) Levofloxacin in first-line treatment of Helicobacter pylori infection. Helicobacter 12:364–365 [DOI] [PubMed] [Google Scholar]
- Rokkas T., Sechopoulos P., Robotis I., Margantis G., Pistiolas D. (2009) Cumulative H. pylori eradication rates in clinical practice by adopting first and second line regimens proposed by the Maastricht III consensus and a third line empirical regimen. Am J Gastroenterol 104:21–25 [DOI] [PubMed] [Google Scholar]
- Rupnow M. F. T., Owens D., Shachter R., Parsonnet J. (1999) Helicobacter pylori vaccine development and use: a cost-effectiveness analysis using the Institute of Medicine Methodology. Helicobacter 4:272–280 [DOI] [PubMed] [Google Scholar]
- Seppälä K., Kosunen T.U., Veijola L., Sipponen P., Arkkila P.E., Rautelin H.et al. (2008) Cure of Helicobacter pylori infection in all compliant patients: report on 644 subjects. ScandJ Gastroenterol 43:1149–1150 [DOI] [PubMed] [Google Scholar]
- Sharma V.K., Bailey D.M., Raufman J.P., Elraie K., Metz D.C., Go M.F.et al. (2000) A survey of internal medicine residents' knowledge about Helicobacter pylori infection. Am J Gastroenterol 95:1914–1919 [DOI] [PubMed] [Google Scholar]
- Spahr L., Rubbia-Brandt L., Marinescu O., Armenian B., Hadengue A. (2001) Acute fatal hepatitis related to levofloxacin. J Hepatol 35:308–309 [DOI] [PubMed] [Google Scholar]
- Stratton K.R., Durch J.S., Lawrence R.S. (2000) Vaccines for the 21st century. A tool for decision making, The National Academies Press: Washington: [PubMed] [Google Scholar]
- van der Linden P.D., Sturkenboom M.C., Herings R.M., Leufkens H.G., Stricker B.H. (2002) Fluoroquinolones and risk of Achilles tendon disorders. BMJ 324:1306–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagari R.M., Bianchi-Porro G., Fiocca R., Gasbarrini G., Roda E., Bazzoli F. (2007) Comparison of 1 and 2 weeks of omeprazole, amoxi-cillin and clarithromycin treatment for Helicobacter pylori eradication: the HYPER Study. Gut 56:475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]