Abstract
The recent years have witnessed great efforts in establishing new therapeutic options for multiple sclerosis (MS), especially for relapsing–remitting disease courses. In particular, the application of monoclonal antibodies provide innovative approaches allowing for blocking or depleting specific molecular targets, which are of interest in the pathogenesis of MS. While natalizumab received approval by the US Food and Drug Administration and the European Medicines Agency in 2006 as the first monoclonal antibody in MS therapy, rituximab, alemtuzumab, and daclizumab were successfully tested for relapsing-remitting MS in small cohorts in the meantime. Here, we review the data available from these recent phase II trials and at the same time critically discuss possible pitfalls which may be relevant for clinical practice. The results of these studies may not only broaden our therapeutic options in the near future, but also provide new insights into disease pathogenesis.
Keywords: immunotherapy, T-cell, B-cell, blood brain barrier, clinical trial
Introduction
The first generation of monoclonal antibodies (mAbs) by Nobel laureates Cesar Milstein and Georges Köhler marked a milestone in immunology [Kohler and Milstein, 1975]. mAbs are produced from a specific immortalized clonal cell line which is generated by immunization of rodents (typically mice) with a purified antigen (or even epitope) of interest. Yet, this standard process results in allogenic (mouse) antibodies which – especially after repeated application – will likely evoke a severe allergic immune response in patients thus limiting the clinical application. To overcome this problem, more refined protocols use molecular cloning techniques to generate chimeric antibodies [Riechmann et al. 1988]. To this end, the murine antigen-binding domains are fused by recombinant DNA technologywithahumanIgGframeworkwhichcan be complement fixing (subclasses IgG1, IgG2, and IgG3) or noncomplement fixing (subclass IgG4). Today, an even more elaborated approach combines only the murine complementary determining regions and the human variable chain backbone thus generating humanized mAbs. Although immu-nogenicity decreases with these refinements, chi-meric or humanized antibodies can still provoke an immune response against its murine part which can result in the generation of neutralizing antibodies or allergic reactions. Even fully human mAbs can harbor such immunogenic potential.
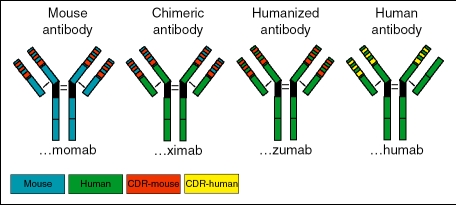
According to their way of generation, mAbs can be classified by a standardized nomenclature (Figure 1:
Figure 1.
According to their way of generation, mAbs can be classified by a standardized nomenclature, which discriminates completely murine monoclonal antibodies (-‘momab’, left), chimeric monoclonal antibodies (-‘ximab’, left middle), humanized monoclonal antibodies (-‘zumab’, or -‘humos’ right middle), and purely human monoclonal antibodies (-‘mumab’, extreme right). Murine antibody parts are depicted in blue, murine complementarity-determining regions (CDR) in red. Human structures are shown in green and human CDR parts in yellow.
‘- momab’: mouse monoclonal antibody
‘- ximab’: chimeric monoclonal antibody
‘- zumab’: humanized monoclonal antibody
‘- humab’: completely humanized monoclonal antibody
In the 1990s, first selective molecular approaches in multiple sclerosis (MS) therapy comprised strategies to neutralize tumor necrosis factor-a (TNF-a). Although these clinical studies were not successful [van Oosten et al. 1996], the recent years have witnessed great efforts in the generation of new selective treatment approaches mainly comprising the application of mAbs. mAb are custom-made against desired targets (against which other small molecule drugs may be not available) and can thus be selectively directed against single molecules involved in the immuno-pathogenesis of MS. Although mAbs can thus exert very specific effects, their application also brings some limitations, like allergic reactions or development of neutralizing antibodies (see above). Moreover, binding of mAbs to their target can evoke a systematic inflammatory response by activation of immune cells and cyto-kine secretion. Symptoms may include fever, headache, nausea, chills, hypotension, and myalgia which can usually be handled by slowing down infusion rates or premedication with nonsteroidal anti-inflammatory drugs. In addition, recent trials with mAbs showed that despite a high specificity for their target structure, the systemic net effect in a complex living organism remains hardly predictable. Thus unexpected side effects including infections and neoplasms (see below) or as recently reported, a cytokine storm in patients treated with the superagonistic CD28 antibody TGN1412 [Suntharalingam et al. 2006] may occur. These observations call for the highest possible awareness of both physicians and patients when administering such therapies.
mAbs may act through specific mechanisms that comprise:
-
–
the depletion of target cells by antibody-dependent cellular cytotoxicity, complement mediated lysis, or apoptosis
-
–
blockade of target antigens
-
–
activation of specific receptors and signaling pathways.
At present, natalizumab is the only mAb approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) for MS treatment while rituximab, alemtuzumab, and daclizumab recently completed phase II studies.
Natalizumab
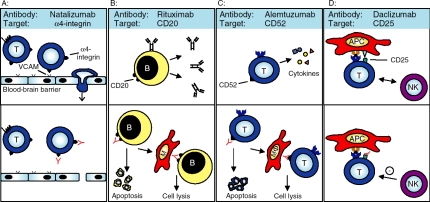
Licensed in 2006, natalizumab (Tysabri, BiogenIdec) is the first commercially available mAb for the treatment of relapsing-remitting MS [Ransohoff, 2007]. In January 2008, natalizumab was also approved for Crohn's disease. Natalizumab is a humanized monoclonal IgG4 antibody directed against the “4 subunit of “4/1 and oc4ß7 integrins. In particular, natalizumab targets the very late antigen-4 (VLA-4), a “4/1 integrin (CD49d/CD29), which has a crucial role in the transmigration of immune cells across the blood brain barrier [Rice et al. 2005]. VLA-4 is expressed on several leukocyte subtypes like eosi-nophilic and basophilic granulocytes, monocytes, and in particular T-cells [Baron et al. 1993]. VLA-4 interacts with different binding partners. Among them in the focus of interest is on vascular cell adhesion molecule-1 (VCAM-1) that is expressed on endothelial cells. Experimental models revealed that VLA-4 is of pivotal importance for the adhesion and subsequent migration of encephalitogenic T-cell clones into the central nervous system (CNS). In fact, studies from in vitro and in vivo models revealed that blocking of VLA-4 can inhibit the interaction of immune cells with VCAM-1 (for review see von Andrian and Engelhardt [2003], Figure 2). Thus, most studies in animal models of MS showed that blockade of VLA-4 limited the infiltration of autoreactive as well as bystander T-cells thereby significantly ameliorating the course of the disease. Despite this extensive body of knowledge on the role of VLA-4 in experimental models, it still remains elusive whether these mechanisms are also the key players for the effect of natalizumab in humans.
Figure 2.
Schematic depiction of the different mechanisms of action of therapeutic monoclonal antibodies: (a) The a4-integrin is critically involved in the process of cell migration across the blood-brain barrier (top panel). Natalizumab interferes with this integrin resulting in a blockade of the interaction between VLA-4 and VCAM and thus inhibits the transmigration of immunocompetent cells (especially of T-cells, T) out of the blood vessel into the central nervous system (bottom panel), (b) B lymphocytes (B), key players in the immunopathogenesis of MS, carry the surface molecule CD20 on their surface (top panel). Rituximab targets the CD20 antigen resulting in a depletion of B cells by antibody-dependent cellular cytotoxicity, complement mediated lysis or apoptosis (bottom panel; MF: macrophage), (c): The surface molecule CD52 is expressed on a large variety of immune cells, especially of T-cells. Alemtuzumab (top panel) induces a profound depletion of CD52 positive leukocytes, among them T-cells by the same mechanisms as described before (bottom panel), (d) The IL-2 receptor (CD25) is expressed on T-cells and critically involved as a co-stimulatory molecule in the process of T-cell activation via antigen presenting cells (APC; top panel). The monoclonal antibody daclizumab (bottom panel) is directed against the alpha chain of CD25 and blocks T-cell activation resulting in a loss of function of T lymphocytes. Evidence is increasing that this immunoregulation is triggered in part by activated natural killer (NK) cells that inhibit T-cell survival.
After first successful phase II and phase IIb studies [Miller et al. 2003; Tubridy et al. 1999], natalizumab was tested at a monthly dosage of 300 mg in two large multicenter randomized controlled phase III studies [Polman et al. 2006; Rudick et al. 2006]. Upon comparison with placebo in the AFFRIM trial, 76% of natalizumab-treated but only 53% of placebotreated patients remained relapse free. In summary, treatment with natalizumab resulted in a relapse rate reduction of 68%, which correlated to a number needed to treat of 1.8. Moreover, more than 40% of patients in the natalizumab group remained free of disease progression.
These clinical data were corroborated by MRI results with an over 90% reduction in gadolinium-enhancing lesions, well in line with the previous results from the phase IIb study. The efficacy of natalitzumab was also shown in further secondary endpoints like improved quality of life, a reduction of concomitant gluco-corticosteroids pulse treatment or MS-related hospitalizations. After an interim analysis, these data led to FDA approval of the drug after study duration of only one year. In the SENTINEL trial, treatment with natalizumab in combination with interferon-beta-1a (IFN-beta-1a) resulted in a relapse rate reduction of 53% in comparison to treatment with IFN-beta-1a alone. Yet, under combination therapy severe side effects were observed (progressive multifocal leukencephalopathy (PML), see below) and today natalizumab is licensed as monotherapy only in MS. So far, conclusive data on the role of natalizumab for the treatment of progressive courses of the disease are not available.
In the phase III studies, the application of natalizumab resulted in a low drop-out rate of below 10% thus underscoring the good tolerability of the drug. Side effects include headache, and a so far poorly understood fatigue. Moreover, in 0.1% of treated patients, elevations of serum liver enzymes and bilirubin have been observed occurring as early as six days following the start of natalizumab therapy. As a humanized antibody with small murine residues, allergic reactions can occur in up to 4% of natalizumab-treated patients. Usually allergic reactions comprise a type I pattern with urticaria, headache, flush, and hypotonia, most likely occurring after the second infusion [Phillips et al. 2006]. Yet, delayed hypersensitivity reactions (type III reactions) are also possible [Krumbholz et al. 2007; Leussink et al. 2007]. Indeed, many of these allergic reactions are linked to the production of anti-natalizumab antibodies, which can be tested by an easily performed and highly specific ELISA. Data from the AFFRIM trial revealed that 68% of patients with infusion-related reactions were anti-natalizumab antibody positive. Upon analysis of patients from all trials, 10% of patients were transiently and 6% persistently antibody positive, over 90% of antibodies were detected within the first twelve weeks of treatment [Polman et al. 2006; Rudick et al. 2006]. Pragmatically, testing for anti-natalizumab antibodies is recommended once during the first six months of treatment and in all cases of suspected allergic reaction. Antibody-positive patients are at a particular risk of allergic reactions and thus should not receive re-infusions. Since persistent antibodies may also neutralize the action of natalizumab and thus limit efficacy of this expensive therapy, testing for antibodies may also have socio-economic implications.
Only three months after the first introduction into the market, two cases of progressive PML alarmed the neurologic community and led to voluntary, transient withdrawal of the FDA approval for natalizumab [Langer-Gould et al. 2005; Kleinschmidt-DeMasters and Tyler 2005]. Both patients were included in the SENTINEL study and received a combination therapy with IFN- ß1a and natalizumab. Subsequently, another fatal case from a natalizumab trial in inflammatory bowel disease was identified [Van Assche et al. 2005]. This patient had received preceding therapies with TNF-a blockade and azathioprine. While two of these patients died, the surviving individual remained severely disabled until end of follow-up. These PML cases led to a comprehensive work-up of a total of 3417 patients who received natalizumab in the different studies. After review of clinical data, MRI and JCV-DNA in the CSF, no new PML cases were identified resulting in a PML risk in these studies of 1 : 1000 patients treated with natalizumab for a mean of 17.9 months [Yousry et al. 2006]. So far, a conclusive explanation for the occurrence of PML under combination therapy with natalizumab was not provided. In all three cases, a profoundly disturbed immu-noregulation can be suspected. Recently, it has been shown that natalizumab may mobilize stem cells from the bone marrow thus opening the possibility for reactivation of the PML-associated JC virus from this reservoir [Zohren et al. 2008]. Moreover, a selective impairment of the immunosurveillance in the CNS is conceivable. This notion is underscored by a reduction of CD4 and CD8 positive T-cells, B-cells, plasma cells, and also the CD4/CD8 ratio in the CSF of natalizumab-treated patients thus mimicking the situation seen in HIV patients [Stuve et al. 2006]. As of March 2008, over 36,000 patients have been treated with natalizumab in the TOUCH and TYGRIS surveillance programs. In these cohorts, 9900 patients have received natalizumab for at least one year while 3600 patients have been treated for at least 18 months and no further PML cases or other opportunistic infections were identified. Yet, the described PML cases under natalizumab did not occur until 24 months of treatment. Thus, only extended surveillance over the next two years will allow final assessment of the formal PML risk. However, a recent report described cases of melanoma under natalizumab treatment [Mullen et al. 2008]. In the meantime, PML was also described under therapy with rituximab and more recently mycophenolate mofetil thus pointing at rather unspecific mechanisms of PML pathogenesis under therapies altering the immune system. Recently, recommendations for patient selection and monitoring as well as guidelines for the use of natalizumab in MS patients previously treated with other immunomodulators have been published [Kappos et al. 2007; Gold et al. 2007]. In parallel, extensive pharmacovigilance programs have been implemented to increase patients’ safety under natalizumab therapy.
Future therapeutic directions, focusing on adhesion molecules and integrins, comprise the blockade of the related integrin VLA-2 [Tsunoda et al. 2007] as well as the development of small, orally available antagonists thus circumventing the immunogenicity of an antibody based approach [Huryn et al. 2004]. One such compound is firategrast, which is currently being tested in a phase II study in relapsing–remitting MS (TIME Study, Sponsor: GlaxoSmithKline).
Rituximab
Rituximab is a human-mouse chimeric mAb directed against the CD20 antigen (Figure 2, which is present on pre-B cells and B-cells but not on antibody-producing plasma cells or stem cells in the bone marrow. Treatment with rituximab results in depletion of CD20 positive cells via multiple mechanisms, including complement-mediated cytotoxicity, antibody-dependent cytotoxicity and apoptosis [Voso et al. 2002]. Rituximab was the first mAb to receive FDA approval for hematologic disorders and is currently mainly used for non-Hodgkin's lymphoma [Maloney et al. 1997]. In this indication, rituximab is given in four, weekly infusions at 375 mg/ m2 and has been used in more than 300,000 patients thus resulting in a well-characterized safety profile. Rituximab is now also tested in autoimmune disorders like rheumatoid arthritis, immune thrombocytopenic purpura, autoimmune hemolytic anemia, and systemic lupus erythematosus [Rastetter et al. 2004]. Here, two subsequent infusions at an interval of two weeks and a fixed dosage of 1000mg are applied; this scheme has also been adopted for recert MS trials, resulting in B-cell depletion for the following 6–9 months.
Several clinical trials investigated the use of rituximab in demyelinating diseases of the CNS. First experiences included the application in neuromyelitis optica (NMO), where humoral disease mechanisms are increasingly recognized [Lennon et al. 2005]. A first open-label study included eight NMO patients and revealed a reduction in attacks of optic neuritis or myelitis resulting in a clearly improved EDSS score by 2 points [Cree et al. 2005]. In the US, another 20-patient single arm study in NMO with comparison to a historical control group is currently ongoing.
In the recent years, B-cell-dependent patho-mechanims (pattern II according to the classification by Lassmann, Brück, and Lucchinetti) also came into the focus of interest in MS, thus opening the way for B-cell-directed therapies [Lassmann et al. 2007]. So far, several cases of successful rituximab treatment were reported in relapsing–remitting MS with B-cell depletion in the blood as well as the CSF, while oligoclonal bands persisted [Chan et al. 2007; Petereit and Rubbert, 2005; Stuve et al. 2005]. In a first study, rituximab was applied in patients with relapsing MS that had not responded optimally to standard immunomodulatory therapies. B-cells were found to be depleted from the cerebrospinal fluid at 24 weeks after initial treatment [Cross et al. 2006]. Further information on safety and tolerability were reported in a recently published multicenter US/Canadian, phase I/II trial that enrolled 26 patients [Bar-Or et al. 2008]. In this open-label study, rituximab was given every six months and patients were followed for a total of 72 weeks. Although all patients experienced some mild to moderate, mostly infusion-related side effects, no serious advents were noted. On frequent MRI scans, fewer new MRI lesions were seen accompanied by an apparent reduction in relapses as compared to the year before therapy. These results were corroborated in another recently published phase II, double-blind, controlled trial involving 104 patients. This study revealed a highly significant reduction in the amount of gadolinium-enhancing lesions beginning already at week 12 with a sustained effect over the complete treatment period of 48 weeks [Hauser et al. 2008]. In comparison with the placebo group, the proportion of patients with relapses was significantly reduced after rituximab treatment. These striking and rapidly commencing effects cannot be attributed to a rituximab action on antibody production. Rather, rituximab indirectly impacts on T-cell function by altering the delicate interplay between the T-cell and B-cell compartment as recently suggested in an animal model of rheumatoid arthritis [Yanaba et al. 2007]. Such effects of rituximab may comprise an influence on the antigen presenting function of B cells.
Rituximab was also tested in a phase II/III, two-year, placebo-controlled study in primary progressive multiple sclerosis (PPMS) where humoral mechanisms may also play a role [Silber et al. 2002; Sadatipour et al. 1998]. Here, patients received rituximab or placebo every six months in a 2 : 1 randomization. In a first press release from April 2008, this study was reported negative, but data were not published so far.
Side effects of rituximab primarily include infusion reactions like headache, fever, chills, mild hypotonia as well as fatigue, and can usually be easily managed by slowing down the infusion rate and premedication with acetaminophen and antihistamines. Rare, but potentially severe adverse events comprise a tumor lysis syndrome, neutropenic fever [Gross et al. 2001], and single cases of fatal infusion reactions as well as PML. These events seem more likely in tumor patients or after preceding intense immuno-suppression and were not described in MS patients so far.
Future B-cell-directed approaches comprise the application of a completely human anti-CD20 antibody (Ofatumumab) or inhibition of B-cell survival factors with atacicept. Atacicept is a ‘TNF family receptor transmembrane activator and calcium-modulator and cyclophilin ligand interactor’, immunoglobulin fusion protein (TACI-Ig), which sequesters the B-cell survival factors, ‘a proliferation-inducing Ligand’ (APRIL) and ‘B-lymphocyte stimulator of the TNF family’ (BLys) and thus blocks later stages of B-cell development [Gross et al. 2001]. A phase II trial of atacicept (sponsor: Merck Serono) in relapsing-remitting MS is awaited with great interest.
Alemtuzumab
Alemtuzumab is a mAb directed against the CD52 antigen expressed on the cell surface of both T and B lymphocytes (Figure 2, mono-cytes, macrophages, and eosinophils, but not stem cells. Alemtuzumab is a humanized derivative of the rat mAb Campath-1, named after the Cambridge University Department of Pathology (for review see Flynn and Byrd [2000]). Like rituximab, alemtuzumab depletes target antigen-carrying cells through complement-mediated lysis, antibody-dependent cell toxicity, and induction of apoptosis, although the exact mechanisms of action in humans have not yet been clarified. At present, alemtuzumab is FDA approved for the treatment of refractory chronic lymphocytic leukemia, it may also have a role in T-prolymphocytic leukemia, mycosis fungoides, and Sézary syndrome [Dearden 2002].
In a preliminary study in MS patients, treatment with alemtuzumab resulted in profound lymphopenia and led to reduction of disease activity in MRI scans [Moreau et al. 1994]. This observation nourished the hope that during subsequent reconstitution of the T-cell repertoire, autoreactive T-cell clones may be excluded. In an open-label study, alemtuzumab was then tested in 25 patients with secondary progressive MS. After a single dose of alemtuzumab, patients were followed for 18 months with serial MRI scans. Although there was a significant decline in the number and volume of contrast-enhancing lesions over 18 months [Paolillo et al. 1999], most patients suffered from disease progression and an increase in brain atrophy despite the clear suppression of inflammatory activity. Alemtuzumab was then investigated in a second open-label study, mostly with treatment naive patients [Coles et al. 1999b]. The relapsing– remitting MS patients in this cohort were characterized by a very active disease (annualized relapse rate of 2.2) and a relatively high EDSS score. After treatment with alemtuzumab, the relapse rate decreased by 94% from 2.2 relapses to 0.14 relapses per year during the two years of follow up. In parallel, the mean EDSS score decreased from 4.8 to 2.1. The striking discrepancy between the relapsing-remitting and progressive MS patient cohorts also provided clues for the pathophysiology of the disease implying that in chronic MS courses, ongoing disease progression is rather not caused by active inflammation, but mediated by a neuro-degenerative process independent of active inflammation [Coles et al. 2006]. Thus chronic progressive MS patients may rather benefit from protective strategies than anti-inflammatory medications alone.
In view of these preliminary results, a phase II, randomized, open-label, randomized study was initiated. In three arms including 334 patients, this study designed for an observational period of three years compared high and low-dose alemtuzumab versus high-dose IFN-beta in patients with early, active relapsing-remitting MS. The primary outcome measure was defined as time to sustained accumulation of disability; secondary read-outs include relapse rate and MRI markers of disease activity. Preliminary results were reported at the American Academy of Neurology 2007 [Coles and the CAMMS223 Study Group, 2007]. In comparison to IFNbeta-1a, two treatment cycles of alemtuzumab with an annual interval led to a highly significant 75% reduction in relapse rate and a 60% reduction in sustained disability. These data including a more comprehensive safety analysis (see below) were also presented at the American Academy of Neurology 2008. Currently, two large multi-center phase III trial are initiated (CARE I/II trial, sponsor: Bayer Schering Pharma/Genzyme).
Although alemtuzumab was generally well tolerated in the MS studies, some documented side effects exist. These include a possible infusion reaction characterized by fever, rigors, rash, nausea, and hypotension, which may be mediated by cytokine release during lymphocyte lysis, e.g., via TNF-a and interleukin (IL)-6 [Wing et al. 1996] and may lead to transient worsening of MS symptoms [Moreau et al. 1996]. This infusion reaction can be prevented by pretreat-ment with glucocorticosteroids, diphenhydra-mine, and acetaminophen. Moreover, the lymphocyte depletion puts alemtuzumab-treated individuals at risk for infections. In the initial MS studies, possibly treatment-related infections included cases of measles, spirochetal gingivitis, herpes zoster, varicella zoster, recurrent oral aphthous ulcers, and pyogenic granuloma. Unexpectedly, in the secondary progressive MS cohort, alemtuzumab treatment resulted in 15 cases of autoimmune hyperthyroidism (Grave's disease, [Coles et al. 1999a]). Development of Grave's disease was associated with a faster reappearance of CD8 positive lymphocytes, which are implicated in the pathogen-esis of autoimmune thyroiditis [Watanabe et al. 1997]. Interestingly, Grave's disease was not reported after alemtuzumab treatment in hematologic patients thus suggesting a special disposition for this condition in MS patients. In the subsequent phase II study, no further cases of Grave's disease were reported. However, alemtuzumab treatment resulted in six individuals in autoimmune idiopathic thrombocytopenic purpura leading to fatal intracranial hemorrhage in one patient. Another patient suffered acute glomerulonephritis finally requiring kidney transplantation. In view of the occurrence of these autoimmune diseases, it seems conceivable that alemtuzumab-mediated lymphocyte depletion may result in disturbed regulatory circuits, e.g., via depletion of regulatory T-cells. Thus, future studies with alemtuzumab are of high interest given its outstanding efficacy, but also need special attention regarding unexpected (autoimmune) side effects.
Daclizumab
Daclizumab is a mAb directed against the CD25 surface antigen which is identical with the alpha chain of the receptor for IL-2 (Figure 2. IL-2 is secreted by activated lymphocytes and induces secretion of other proinflammatory cytokines and stimulates lymphocytic proliferation in an autocrine loop. Daclizumab was the first humanized mAb to be FDA approved and is currently in clinical use in transplantation medicine to prevent rejection of kidney transplants. A recent Cochrane review disclosed a significant effect of daclizumab in acute transplant rejection with a number needed to treat being 7 [Webster et al. 2004].
Initially, two preliminary open-label studies with daclizumab in MS were performed. In the first study, five patients with secondary progressive MS and six patients with relapsing-remitting MS who had ongoing clinical disease activity despite treatment with IFN-beta were treated with the addition of daclizumab (seven infusions at a dose of 1 mg/kg). In comparison to baseline, the total number of contrast-enhancing lesions decreased by 78% and the exacerbation rate decreased by 81% [Bielekova et al. 2004].
The combination of daclizumab and IFN-beta was well tolerated, although there appeared to be an increase in urinary tract and upper respiratory tract infections. In another open-label trial, 21 patients mostly with ongoing disease activity under treatment with IFN-ß were treated with daclizumab. From 19 patients who completed the study, 10 patients appeared to improve while the other 9 remained stable [Rose et al. 2004]. On average, the EDSS score decreased by 1.5 points and MRI lesion load substantially decreased under treatment with daclizumab. Daclizumab was also effective in a further phase II trial in reducing contrast-enhancing lesions and improving clinical scores in patients with relapsing and remitting MS with active disease not controlled by IFN therapy [Rose et al. 2007]. Immunological analyses in daclizumab-treated patients revealed a significant expansion of CD56 positive natural killer (NK) cells in vivo [Bielekova et al. 2006], which highly correlated with the treatment response. In contrast, depletion of CD25 positive regulatory T cells did not seem to play role. This is of special interest, since antibodies against the beta chain of the IL-2 receptor rather exacerbated disease and affected CD8 positive regulatory T-cells in an animal model of MS [Lee et al. 2008]. In a recently completed phase II trial of daclizumab (CHOICE study), a total of 230 (mostly relapsing-remitting) MS patients were enrolled and randomized to three treatment arms, all in combination with IFN-beta. Treatment comprised either placebo or the subcutaneous application of daclizumab, either at a low (1 mg/kg) or high dose (2 mg/kg) for 24 weeks with a further observation of another 24 weeks. Preliminary week 44 results were presented at the ECTRIMS meeting in Prague 2007 [Montalban et al. 2007] and a more detailed analysis at the American Academy of Neurology 2008. In comparison to placebo, the high-dose daclizumab treatment resulted in a significant reduction in the mean number of new or enlarging gadolinium-enhancing lesions by 72%. In the low-dose group, there was a 25% reduction, which was not statistically significant. Both the low- and high-dose daclizumab groups also showed some reduction in annualized relapse rates as compared to placebo; this effect did not reach statistical significance. Yet the study was too short and underpowered to offer insights into potential clinical effects of this drug. Safety analyses revealed a similar incidence of overall infection rates in all groups, although there was higher incidence of cutaneous side effects and severe infections in the daclizumab groups.
Despite its clinical use for over 10 years, there are few data on the long-term safety of daclizumab since it has mainly been used acutely following organ transplantation and not for long-term immunomodulation. In addition, a study in liver transplantation disclosed that 3 of 13 patients developed anti-daclizumab antibodies already under short-term therapy [Koch et al. 2002]. If these antibodies block the interaction of daclizumab with CD25, they may also limit its potential therapeutic efficacy. Further studies will need to address these issues as well as the question whether daclizumab should be used alone or in combination with other MS therapies. For this purpose, another multicenter randomized controlled trial of daclizumab in MS is initiated at present.
Conclusion
The application of mAbs will broaden the therapeutic options in MS treatment opening the way for more refined and individualized therapeutic concepts in the near future. Yet, the increased efficacy of these compounds has also brought new, unexpected side effects of which neurologists as well as patients will have to be aware upon decision for an appropriate, individualized therapy in the future.
Conflict of interest statement
RAL received personal compensation for activities with Bayer Health Care, Biogen Idec, TEVA Pharma. BCK has received honoraria for lecturing, travel expenses for attending meetings and financial support for research from Bayer Health Care, Biogen, Merck Serono, Novartis, Sanofi Aventis, Talecris and TEVA.
Contributor Information
Ralf A. Linker, Department of Neurology St. Josef Hospital Ruhr-University Bochum D-44791 Bochum Germany ralf.linker@rub.de.
Bernd C. Kieseier, Department of Neurology Heinrich-Heine University Düsseldorf, Düsseldorf Germany
References
- Bar-Or A., Calabresi P.A., Arnlod D., Markowitz C., Shafer S., Kasper L.H.et al. (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63: 395–400 [DOI] [PubMed] [Google Scholar]
- Baron J.L., Madri J.A., Ruddle N.H., Hashim G., Janeway C.A., Jr (1993) Surface expression of alpha 4 integrin by CD4T cells is required for their entry into brain parenchyma. J Exp Med 177: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B., Catalfamo M., Reichert-Scrivner S., Packer A., Cerna M., Waldmann T.A.et al. (2006) Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 103: 5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B., Richert N., Howard T., Blevins G., Markovic-Plese S., McCartin J.et al. (2004) Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci USA 101: 8705–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A., Lee D.H., Linker R., Mohr A., Toyka K.V., Gold R. (2007) Rescue therapy with anti-CD20 treatment in neuroimmunologic breakthrough disease. J Neurol 254: 1604–1606 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Cox A., Le P.E., Jones J., Trip S.A., Deans J.et al. (2006) The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 253: 98–108 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Wing M., Smith S., Coraddu F., Greer S., Taylor C.et al. (1999a) Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 354: 1691–1695 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Wing M.G., Molyneux P., Paolillo A., Davie C.M., Hale G.et al. (1999b) Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 46: 296–304 [DOI] [PubMed] [Google Scholar]
- Coles A.the CAMMS223 Study Group (2007) Efficacy of Alemtuzumab in treatment-naive relapsing remitting multiple sclerosis: analysis after two yeares of study CAMMS223. Neurology Suppl 1: A100 [Google Scholar]
- Cree B.A., Lamb S., Morgan K., Chen A., Waubant E., Genain C. (2005) An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64: 1270–1272 [DOI] [PubMed] [Google Scholar]
- Cross A.H., Stark J.L., Lauber J., Ramsbottom M.J., Lyons J.A. (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden C.E. (2002) Alemtuzumab in lymphoproli-ferate disorders. Rev Clin Exp Hematol 6: 435–448 [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Byrd J.C. (2000) Campath-1H monoclonal antibody therapy. Curr Opin Oncol 12: 574–581 [DOI] [PubMed] [Google Scholar]
- Gold R., Jawad A., Miller D.H., Henderson D.C., Fassas A., Fierz W.et al. (2007) Expert opinion: guidelines for the use of natalizumab in multiple sclerosis patients previously treated with immunomo-dulating therapies. J Neuroimmunol 187: 156–158 [DOI] [PubMed] [Google Scholar]
- Gross J.A., Dillon S.R., Mudri S., Johnston J., Littau A., Roque R.et al. (2001) TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity 15: 289–302 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T., Antel J., Fox R.J.et al. (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676–688 [DOI] [PubMed] [Google Scholar]
- Huryn D.M., Konradi A.W., Ashwell S., Freedman S.B., Lombardo L.J., Pleiss M.A.et al. (2004) The identification and optimization of orally efficacious, small molecule VLA-4 antagonists. Curr Top Med Chem 4: 1473–1484 [DOI] [PubMed] [Google Scholar]
- Kappos L., Bates D., Hartung H.P., Havrdova E., Miller D., Polman C.H.et al. (2007) Natalizumab treatment for multiple sclerosis: recommendations for patient selection and monitoring. Lancet Neurol 6: 431–441 [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters B.K., Tyler K.L. (2005) Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 353: 369–374 [DOI] [PubMed] [Google Scholar]
- Koch M., Niemeyer G., Patel I., Light S., Nashan B. (2002) Pharmacokinetics, pharmaco-dynamics, and immunodynamics of daclizumab in a two-dose regimen in liver transplantation. Transplantation 73: 1640–1646 [DOI] [PubMed] [Google Scholar]
- Kohler G., Milstein C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497 [DOI] [PubMed] [Google Scholar]
- Krumbholz M., Pellkofer H., Gold R., Hoffmann L.A., Hohlfeld R., Kumpfel T. (2007) Delayed allergic reaction to natalizumab associated with early formation of neutralizing antibodies. Arch Neurol 64: 1331–1333 [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Atlas S.W., Green A.J., Bollen A.W., Pelletier D. (2005) Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 353: 375–381 [DOI] [PubMed] [Google Scholar]
- Lassmann H., Bruck W., Lucchinetti C.F. (2007) The immunopathology of multiple sclerosis: an overview. Brain Pathol 17: 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.H., Ishida Y., Rifa'i M., Shi Z., Isobe K., Suzuki H. (2008) Essential role of CD8+CD122+ regulatory T cells in the recovery from experimental autoimmune encephalomyelitis. J Immunol 180: 825–832 [DOI] [PubMed] [Google Scholar]
- Lennon V.A., Kryzer T.J., Pittock S.J., Verkman A.S., Hinson S.R. (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202: 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussink V.H., Lehmann, Hartung H., Gold, Kieseier B. (2007) Type III systemic allergic reaction to natalizumab. Arch Neurol 64: 1331–1333 [DOI] [PubMed] [Google Scholar]
- Maloney D.G., Grillo-Lopez A.J., White C.A., Bodkin D., Schilder R.J., Neidhart J.A.et al. (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90: 2188–2195 [PubMed] [Google Scholar]
- Miller D.H., Khan O.A., Sheremata W.A., Blumhardt L.D., Rice G.P., Libonati M.A.et al. (2003) A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 348: 15–23 [DOI] [PubMed] [Google Scholar]
- Montalban X.D., Wynn M., Kaufmann M., Wang Fong A. (2007) Preliminary CHOICE results: a phase 2, randmomized, double-blind, placebo con-trollled multicentre study of subcutaneous daclizumab in patients with active, relapsing forms of multiple sclerosis on interferon beta. Mult Scler 13: S18 [Google Scholar]
- Moreau T., Coles A., Wing M., Isaacs J., Hale G., Waldmann H.et al. (1996) Transient increase in symptoms associated with cytokine release in patients with multiple sclerosis. Brain 119(Pt 1):225–237 [DOI] [PubMed] [Google Scholar]
- Moreau T., Thorpe J., Miller D., Moseley I., Hale G., Waldmann H.et al. (1994) Preliminary evidence from magnetic resonance imaging for reduction in disease activity after lymphocyte depletion in multiple sclerosis. Lancet 344: 298–301 [DOI] [PubMed] [Google Scholar]
- Mullen J.T., Vartanian T.K., Atkins M.B. (2008) Melanoma complicating treatment with natalizumab for multiple sclerosis. N Engl J Med 358: 647–648 [DOI] [PubMed] [Google Scholar]
- Paolillo A., Coles A.J., Molyneux P.D., Gawne-Cain M., MacManus D., Barker G.J.et al. (1999) Quantitative MRI in patients with secondary progressive MS treated with monoclonal antibody Campath 1H. Neurology 53: 751–757 [DOI] [PubMed] [Google Scholar]
- Petereit H.F., Rubbert A. (2005) Effective suppression of cerebrospinal fluid B cells by rituximab and cyclophosphamide in progressive multiple sclerosis. Arch Neurol 62: 1641–1642 [DOI] [PubMed] [Google Scholar]
- Phillips J.T., O'Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H.et al. (2006) Infusion-related hypersensitivity reactions during natalizumab treatment. Neurology 67: 1717–1718 [DOI] [PubMed] [Google Scholar]
- Polman C.H., O'Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H.et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899–910 [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M. (2007) Natalizumab for multiple sclerosis. N Engl J Med 356: 2622–2629 [DOI] [PubMed] [Google Scholar]
- Rastetter W., Molina A., White C.A. (2004) Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med 55: 477–503 [DOI] [PubMed] [Google Scholar]
- Rice G.P., Hartung H.P., Calabresi P.A. (2005) Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64: 1336–1342 [DOI] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. (1988) Reshaping human antibodies for therapy. Nature 332: 323–327 [DOI] [PubMed] [Google Scholar]
- Rose J.W., Burns J.B., Bjorklund J., Klein J., Watt H.E., Carlson N.G. (2007) Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 69: 785–789 [DOI] [PubMed] [Google Scholar]
- Rose J.W., Watt H.E., White A.T., Carlson N.G. (2004) Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol 56: 864–867 [DOI] [PubMed] [Google Scholar]
- Rudick R.A., Stuart W.H., Calabresi P.A., Confavreux C., Galetta S.L., Radue E.W.et al. (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354: 911–923 [DOI] [PubMed] [Google Scholar]
- Sadatipour B.T., Greer J.M., Pender M.P. (1998) Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann Neurol 44: 980–983 [DOI] [PubMed] [Google Scholar]
- Silber E., Semra Y.K., Gregson N.A., Sharief M.K. (2002) Patients with progressive multiple sclerosis have elevated antibodies to neurofilament subunit. Neurology 58: 1372–1381 [DOI] [PubMed] [Google Scholar]
- Stuve O., Cepok S., Elias B., Saleh A., Hartung H.P., Hemmer B.et al. (2005) Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing-remitting multiple sclerosis. Arch Neurol 62: 1620–1623 [DOI] [PubMed] [Google Scholar]
- Stuve O., Marra C.M., Bar-Or A., Niino M., Cravens P.D., Cepok S.et al. (2006) Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 63: 1383–1387 [DOI] [PubMed] [Google Scholar]
- Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D.et al. (2006) Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355: 1018–1028 [DOI] [PubMed] [Google Scholar]
- Tsunoda I., Terry E.J., Marble B.J., Lazarides E., Woods C., Fujinami R.S. (2007) Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain Pathol 17: 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubridy N., Behan P.O., Capildeo R., Chaudhuri A., Forbes R., Hawkins C.P.et al. (1999) The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology 53: 466–472 [DOI] [PubMed] [Google Scholar]
- van Oosten B.W., Barkhof F., Truyen L., Boringa J.B., Bertelsmann F.W., von Blomberg B.M.et al. (1996) Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47: 1531–1534 [DOI] [PubMed] [Google Scholar]
- Van Assche G., Van Ransohoff M., Sciot R., Dubois B., Vermeire S., Noman M.et al. (2005) Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 353: 362–368 [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Engelhardt B. (2003) Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med 348: 68–72 [DOI] [PubMed] [Google Scholar]
- Voso M.T., Pantel G., Rutella S., Weis M., D'Alo F., Urbano R.et al. (2002) Rituximab reduces the number of peripheral blood B-cells in vitro mainly by effector cell-mediated mechanisms. Haematologica 87: 918–925 [PubMed] [Google Scholar]
- Watanabe M., Amino N., Hochito K., Watanabe K., Kuma K., Iwatani Y. (1997) Opposite changes in serum soluble CD8 in patients at the active stages of Graves' and Hashimoto's diseases. Thyroid 7: 743–747 [DOI] [PubMed] [Google Scholar]
- Webster A.C., Playford E.G., Higgins G., Chapman J.R., Craig J. (2004) Interleukin 2 receptor antagonists for kidney transplant recipients.Cochrane Database Syst Rev CD003897. [DOI] [PubMed]
- Wing M.G., Moreau T., Greenwood J., Smith R.M., Hale G., Isaacs J.et al. (1996) Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest 98: 2819–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K., Hamaguchi Y., Venturi G.M., Steeber D.A., St Clair E.W., Tedder T.F. (2007) B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J Immunol 179: 1369–1380 [DOI] [PubMed] [Google Scholar]
- Yousry T.A., Major E.O., Ryschkewitsch C., Fahle G., Fischer S., Hou J.et al. (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354: 924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohren F., Toutzaris D., Klarner V., Hartung H.P., Kieseier B., Haas R. (2008) The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood 111: 3893–3895 [DOI] [PubMed] [Google Scholar]