Abstract
Substantial therapeutic progress has been made in myasthenia gravis (MG) even before the era of molecular medicine. Here we characterize modern treatment algorithms that are adapted to disease severity and introduce the principle of escalating treatment strategies for MG. In very mild cases and in some ocular forms of MG, treatment with acetylcholinesterase inhibitors may be sufficient, at least temporarily, but commonly some kind of immunologically active treatment is needed. In generalized MG, a wide array of immunosuppressive treatments has been established through observational studies, some prospective, but most of them have never been tested in a double-blind, prospective and randomized trial. Within the immunologically active drugs, glucocorticosteroids (GCS) and the immunosuppressive drug azathioprine (Aza) have been studied the longest. Aza is still the standard base-line treatment, in particular in cases where high doses of GCS would be needed to maintain remission. If Aza is not tolerated, several alternatives are available including cyclosporine A (Cic A), mycophenolate mofetil, cyclophosphamide, and methotrexate, all of them off-label in most western countries. Tacrolimus is under investigation. More severe cases may profit from drug combinations in which compounds with more rapidly acting drugs (GCS, Cic A) are combined with others showing a more delayed action (Aza). All such combination therapies need to be supervised by an experienced neuroimmunological center because of potentially serious adverse reactions. Serial measurements of anti-acetylcholine receptor antibodies, once these are elevated, is a useful adjunct for monitoring long-term treatment success and may help in weaning from higher to lower doses or to single drugs rather than combinations. For very severe and treatment-resistant cases, co-treatment with intravenous immunoglobulins or different modalities of plasmapheresis may be considered on the short term while the humanized monoclonal anti-CD 20 antibody (rituximab) is a candidate for the long term. In highly refractory cases also immuno-ablation via high-dose cyclophosphamide, followed by hematologic trophic factors such as G-CSF, has been tried successfully. Future developments may include other immunologically active monoclonal antibodies (e.g., anti-CD 52, Campath-1). Up to 10% of patients with MG are associated with a malignant thymoma, often referred to as paraneoplastic MG, as detected by CT scan or MRI, and these patients require thymomectomy and sometimes postsurgical chemotherapy and radiation treatment. In nonthymoma patients with generalised MG, including older children and adults up to the 5th decade, a complete transsternal thymectomy is recommended based on available open trials and expert opinion, preferentially during the first year of disease. Endoscopic surgery may also be effective. Before surgery, pretreatment with immunosuppressive medication or plasmapheresis is usually recommended to ameliorate MG and subsequently reduce perioperative morbidity and mortality which is now near zero in experienced centers. Myasthenic crisis is the life-threatening exacerbation of MG and is best treated by plasmapheresis, mostly combined with immunoadsorption techniques. Intravenous immunoglobulins are a reasonable alternative, but a shortage in supplies and high prices limit its use.
Keywords: immunosuppression, immunoglobulins, plasmapheresis, immuno-adsorption, acetylcholine receptor, muscle specific kinase, thymoma
Definition
Acquired autoimmune myasthenia gravis (MG) is the commonest disease that affects the neuromuscular junction. Autoantibodies in combination with local deposition of complement reduce the number of available postsynaptic nicotinic acetylcholine receptors (AChR) and microdestruction of the motor endplate, and thereby impair neuromuscular transmission. The cardinal features of MG are fluctuating weakness and fatigability of skeletal muscles, usually in a characteristic distribution. The weakness increases with activity and improves with rest. [Hohlfeld et al. 2003; Drachman, 1994]
Pathogenesis and clinical testing
The basic defect in the commonest form of acquired autoimmune MG is a loss of available postsynaptic AChRs at the neuromuscular junction. The pathogenetic cascade that leads to impairment of neuromuscular transmission have been well understood for three decades [Hohlfeld and Wekerle, 1999; Drachman, 1994], Circulating anti-AChR autoantibodies impair AChR function by three different mechanisms: (1) antibody binding and cross-linking of receptors, which accelerates internalization and degradation of AChR; (2) local activation of the complement cascade, eventually leading to complement-mediated destruction of the folds of the postsynaptic membrane; (3) blocking of the binding site for acetylcholine [Bufler et al. 1996]. The thymus that contains all the elements required to initiate and sustain an autoimmune response against the AChR is profoundly involved in the pathogenesis of MG which may explain the therapeutic benefit from thymectomy [Hohlfeld and Wekerle, 1999; Kirchner et al. 1986; Wekerle and Ketelsen, 1977].
The annual incidence of MG is 1 to 2 per 100,000 while the prevalence can be as high as 20 to over 50 per 100,000 in the population, with higher figures in countries where all modern treatments are available and hence patients live longer with the disease. The distribution is age and sex-related with the first peak in the second and third decades affecting mostly women, and a second peak in the sixth and seventh decades affecting more men. It is rare in children less than ten years of age.
Recently, another type of autoimmune MG was described that is characterized by antibodies to a muscle-specific kinase (MuSK) [Vincent et al. 2003]; this subgroup forms about half of the about 10% of patients hitherto termed seronegative MG.
The clinical diagnosis is based on typical clinical findings including fluctuating weakness and fatigue of extraocular muscles, producing ptosis and diplopia – this is termed pure ocular MG if presenting in isolation for more than 12 months. Generalized MG shows widespread skeletal muscle weakness with, or less commonly without, ocular signs. If, in the course of disease exacerbation, weakness of respiration or swallowing becomes so severe as to require mechanical support, the patient is in myasthenic crisis. With advanced modern immunotherapy less than 2% of MG patients will suffer from a myasthenic crisis during their disease course.
Several clinical and electrophysiological tests are available to establish a diagnosis of MG which is not discussed here except for the pharmacological testing because it immediately bears on symptomatic treatment [Hohlfeld et al. 2003; Drachman, 1994]. As a bedside diagnostic test, edrophonium chloride is used as a short-acting cholinesterase inhibitor (duration of action 3 to 10 min). Atropine (0.5–1 mg) should be available to antagonize possible muscarinic side effects. The rapid action after intravenous administration allows repeated interaction between ACh and the reduced number of still functional AChR, and partially compensates for the functional deficit of receptors. This test should be carried out with objective assessment (scoring, photography) of myasthenic weakness in muscle groups that are unequivocally affected; ocular-bulbar muscles are certainly the most appropriate targets for this test. A useful alternative to the edrophonium test in outpatients is the oral pyridostigmine test. The patient receives 60 mg orally and reports back after 60 and 90 min for quantitative testing. The edrophonium test is not entirely specific for MG, and equivocal or false-positive responses, especially of ocular symptoms, have been observed in a variety of disorders including brain stem glioma or vascular malformations, cranial neuropathies, and orbital tumors which should be looked for in the initial diagnostic work up. Laboratory testing is essential for establishing the diagnosis and includes the search for specific autoantibodies and for ancillary disorders that may affect neuromuscular transmission.
Based on the clinical presentation and prognostic factors, different classifications have been proposed (Table 1). Further evidence for disease heterogeneity is based on differences in age at onset (<45 or >45 years), thymic abnormalities, immunological parameters (HLA-association, AChR-antibody titer or other targets such as MuSK [Vincent et al. 2003] and response to therapy. Before any invasive treatment is started, one needs to consider MG in the differential diagnosis of a variety of disorders presenting with muscle weakness, especially in those cases where no specific antibodies are detected. The term of seronegative MG should be replaced by double-seronegativeMG to indicate absenceof antibodies to AChR and MuSK.
Table 1.
Osserman and Genkins classification of myasthenia gravis, modified by the MGFA/Task Force (modified after [Schneider-Gold and Toyka, 2007]).
| Class | Clinical form(s) | Signs |
| I*/MGFA I | Ocular | Ptosis and diplopia |
| II a*/MGFA II | Mild generalized | Mild generalized weakness |
| II b*/MGFA IIb | Faciopharyngeal | IIa and bulbar weakness |
| III* | Severe acute generalized | Acute and severe general weakness and bulbar symptoms and respiratory insufficiency |
| MGFA III | Medium severity generalized | Medium severity generalized weakness with more |
| MGFA IIIa | involvement of the extremities/trunk muscles than the faciopharyngeal musculature | |
| MGFA IIIb | Faciopharyngeal/respiratory musculature more than extremities/trunk musculature | |
| IV* | Severe chronic generalized | Severe, often progressive generalized weakness |
| MGFA IV | Severe generalized | |
| MGFA IVa | Extremities/trunk musculature more than faciopharyngeal musculature | |
| MGFA IVb | Faciopharyngeal/respiratory musculature more than extremities/trunk musculature | |
| V* | Myasthenia with severe residual deficits | Severe chronic form with muscle atrophy |
| MGFA V | Severe MG requiring intubation |
MGFA, Myasthenia Gravis Foundation Association; the entries marked * refer to the Osserman and Genkins classification.
For the subgroup of MG with antibodies to MuSK, it has been proposed that the clinical spectrum and severity differs from the predominant type of MG with antibodies to AChR, in that MuSK-positive cases more often have a bulbar distribution with atrophy of the respective muscles [Farrugia et al. 2006], standard treatment modalities may be less effective (see subsequently) and the typical thymic abnormalities are absent or less pronounced [Leite et al. 2005].
Treatment principles*
Myasthenic patients have an increased incidence of several associated disorders that require treatments in addition to and potentially different from the standard regimen in isolated MG. Malignant thymic tumors occur in 10 to 15% of patients [Muller-Hermelink and Marx, 2000]. A subclinical or overt autoimmune thyroid disorder may occur in 3 to 8% of myasthenic patients, and either hyper- or hypo-thyroidism may aggravate myasthenic weakness. Tests for thyroid function and thyroid autoantibodies should be obtained routinely. Disorders that may interfere with immunosuppressive therapy include unsuspected infections such as tuberculosis, diabetes, peptic ulcer, occult gastrointestinal bleeding, renal disease, hypertension, and occult malignancies.
With the treatment options available today, the great majority of patients can lead essentially normal lives. However, most patients must take immunosuppressive medication for many years or even indefinitely, despite of the risk of associated adverse effects. Sudden deterioration with respiratory failure (myasthenic crisis) is now rare (<2%) in patients adequately treated with long-term immunosuppression and monitored by expert neurologists. In thymoma patients, the prognosis is related to the course and histological stage of the tumor [Strobel et al. 2004]. Acetylcholinesterase (AChE) inhibitors are the basic symptomatic treatment, which partially compensate for the reduced safety margin at the neuromuscular junction. This may rarely be sufficient in cases of mild MG and purely ocular involvement [Schneider-Gold and Toyka, 2007]. In patients with generalized autoimmune MG, immunosuppression with steroids, azathioprine (Aza) or other immunosuppressive drugs is usually required. Immediate removal of autoanti-bodies by plasma exchange or immunoadsorption (semi-selective columns [Heininger et al. 1987] or Protein-A adsorption columns [Flachenecker et al. 1998] is generally very effective as a short-term treatment in crisis, in cases with rapid deterioration, or in unstable patients before thymectomy. Following the guidelines below, among various therapeutic options the best available therapy has to be selected for the individual patient.
Acetylcholinesterase inhibitors
The clinically useful anticholinesterase reagents inhibit the synaptic specific AChE reversibly (Figure 1). The AChE inhibitors such as pyridostigmine and neostigmine are also hydrolyzed by AChE, but at a much more slower rate than ACh. In vivo, the duration of inhibition by these carbamylating agents is in the range of hours.
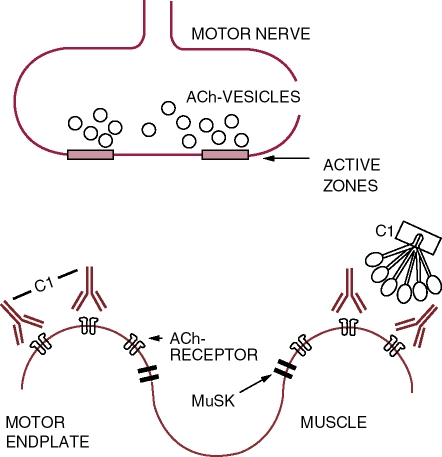
Figure 1.
Neuromuscular junction in myasthenia gravis and in the Lambert Eaton Myasthenic Syndrome: schematic drawing of a motor endplate and a nerve terminal. Acetylcholine (ACh) upon release into the synaptic cleft is rapidly degraded by the specific acetylcholinesterase located at the bottom of the postsynaptic fold and in the basement membrane; MuSK, muscle specific kinase [Hoch et al. 2001]; VGCaChannels, voltage-gated calcium channels; C1, first component of classical pathway the complement cascade binding to the Fc portion of the binding and cross-linking IgG antibodies. Adapted from Toyka and Gold, 2007.
In general, the toxic side effects of AChE inhibitors are caused by excess of ACh and actions on muscarinic AChR. These adverse reactions may affect the gastrointestinal system (abdominal cramps, diarrhea, nausea, vomiting), the respiratory system (bronchoconstriction and increased bronchial secretion), the eyes (miosis, conjunctival congestion), glandular secretion (lacrimation, salivation, profuse sweating), and the heart (bradycardia, hypotension). Atropine chloride effectively antagonizes these muscarinic side effects, but is ineffective at the neuromuscular junction, and therefore has no influence on the muscle weakness due to excessive ACh. The oral antimuscarinic drug, longer-acting ipatropium chloride can be administered twice daily.
Very high doses of AChE inhibitors lead to failure of neuromuscular transmission due to toxic concentrations of ACh at the motor end-plate. Generalized fasciculations or cramps can be observed with overdose, and the remaining functional AChR are desensitized, resulting in increased weakness, fatigability, eventually leading to cholinergic crisis. In myasthenic crisis it may be a problem to discriminate between weakness induced by cholinergic intoxication versus myasthenic weakness with insufficient efficacy of anti-AChE drugs, especially for less experienced neurologists. Here the response to short-lived edrophonium may help to further elucidate these the two options: if weakness improves with a single dose myasthenic weakness is likely to be the predominant problem.
Immunosuppressive and immunomodulating treatments
All long-term treatments usually include a combination of drugs, i.e., glucocorticosteroids (GCS) and one immunosuppressive agent, primarily Aza. [Gold et al. 2003; Hohlfeld et al. 2003].
Glucocorticosteroids
The anti-inflammatory and immunosuppressive effects of GCS have several different components. A gradual effect of prednisone (or any equivalent GCS) at an oral dose of around 1 mg/kg bodyweight is expected to start as early as after a few days but is usually obvious after 2–4 weeks, and the maximum benefit may take months [Hohlfeld et al. 2003]. Short courses of large intravenous doses (1–2g) were employed with good results to manage exacerbations [Arsura et al. 1985], but these high doses carry a considerable risk of steroid-induced worsening, and, if plasmapheresis is not part of the regime, even assisted mechanical ventilation may be required when bulbar symptoms are predominant.
The few published trials of prednisone alone or in combination with other drugs versus placebo all support their therapeutic efficacy [Schneider-Gold et al. 2005].
Up to 10% of patients may show a transient GCS-induced worsening of myasthenic weakness even at maintenance doses. This adverse effect may come from a direct action on neuromuscular transmission [Dudel et al. 1979]. This may be avoided by gradually increasing the dose of the steroid medication over the course of weeks. Alternatively, in more severe MG, a preceding course of plasma exchanges or intravenous immunoglobulin may reduce the likelihood of early steroid-induced exacerbation.
Side effects of long-term treatment with GCS include all features of Cushing's syndrome for which patients should be closely monitored and treated, including osteoporosis, hypertension, exacerbation or precipitation of diabetes, obesity, gastrointestinal ulcers, cataracts, opportunistic infections and sepsis, hypertension, and serum electrolyte derangement (in particular potassium loss). Regular slit lamp examinations (every six months) help to detect GCS-induced cataracts early. Gastrointestinal discomfort is best dealt with by drinking skim or low-fat milk during the day. If a patient has a history of recurrent ulcers, histamine H2-receptor antagonists such as ranitidine, or H+, K+-ATPase inhibitors such as omeprazole may be given especially in the evening.
In order to minimize steroid-induced osteoporosis, the single most effective measure is reduction of dosage to below the individual threshold for developing Cushing-like features (usually between 4 and 10 mg orally per day) and, eventually, complete discontinuation of GCS. All patients, especially the elderly, should be given calcium supplements and vitamin D (50,000 units 1–2x per week), based on the level of urinary calcium excretion. Bisphosphonate agents appear to be useful for the treatment and prevention of steroid-induced osteoporosis, and especially in postmenopausal women, estrogens may be given to reduce the risk of fractures. Potassium supplements are necessary only in patients who are known to develop hypokalemia.
Immunosuppressive drugs
Aza acts as a purine analogue primarily on proliferating lymphocytes and induces both Band T-cell lymphopenia dose-dependently. Antigen- and mitogen-induced in vitro proliferative responses of T cells are also inhibited in Aza-treated patients but less so than in patients treated with alkylating cytotoxic drugs such as cyclophosphamide. Aza also has mild anti-inflammatory properties probably due to the inhibition of promonocyte cell division.
Aza is seen most commonly as an adjunct, to reduce the dose of steroids required, but it may be used alone as a long-term maintenance treatment as originally introduced by Mertens [Mertens et al. 1981]. Indeed, Aza is one of the best tolerated therapeutic agents to use but two aspects should be considered. First, patients may show an acute idiosyncratic reaction, with general malaise, fever, skin reactions, and gastrointestinal symptoms of nausea and vomiting, even after a single testing dose of 50 mg orally Some of these adverse reactions may be based on a genetic defect in the enzyme thiopurine methyltrans-ferase which can be analyzed in peripheral blood cells (see review in [Gold et al. 2003]). Yet heterozygote individuals often show borderline enzyme values, and tolerability can best be tested by slow tapering of Aza to tolerable doses. In the situation of severe gastrointestinal symptoms, the drug should be discontinued immediately. Second, its beneficial effects in MG begin slowly, typically requiring many months for an adequate trial.
In a randomized, placebo-controlled doubleblind study, Aza in combination with prednisone was tested versus Aza and placebo [Palace et al. 1998] and Aza was found to be effective over and above prednisone alone, even though the trial was prematurely terminated. None of this and other long-term trials had a placebo arm because this was felt to be unethical based on the results of many open trials. [Schneider-Gold et al. 2005]. In some countries such as USA Aza is formally still ‘off label’ for treatment of MG.
The incidence of serious side effects of Aza is surprisingly low, although it has to be continued as long-term treatment in many patients [Hohlfeld et al. 1985]. In one study, the most frequent adverse reactions encountered were in decreasing order of frequency: reversible bone marrow depression with leukopenia, gastrointestinal complications, infections and transient elevation of liver enzymes [Hohlfeld et al. 1988; Witte et al. 1984]. The most serious long-term adverse effect is the development of a lymphoma which one patient developed after six years of Aza treatment in this series of over 100 patients [Hohlfeld et al. 1988]. Mild intestinal discomfort can usually be prevented or alleviated by starting treatment with a first 50mg bedtime dose (testing dose), splitting the dose into three or more divided doses, taking the drug after meals, and reducing the dose temporarily. Elevation of liver enzymes up to three times baseline may be tolerated since it is usually reversible after the dose has been reduced. By contrast to what might be expected, serious infections are rarely a problem. Aza is potentially teratogenic and mutagenic. Male and female patients should be advised to use contraceptive measures during treatment and for at least several months after its completion whenever this is possible. Data available from mothers treated with Aza for kidney transplant or autoimmune disorders have not shown an increased rate of birth defects in their children, but no data on the actual risk are available.
Patients should be monitored carefully for side effects during treatment. Complete blood counts should be obtained at least biweekly during the first two months, and monthly thereafter. If the total white blood count (WBC) is reduced to <3000/μL, the medication should be discontinued for a few days and treatment continued at a lower dose after the WBC returns to more than 3500/μL. The long-term dose can be adjusted to maintain the WBC between 4000 and 5000/μL, and lymphocyte counts ranging between 800 and 1000/μL. However, it is not certain whether the immunosuppressive efficacy of Aza therapy in autoimmune diseases is directly correlated to the WBC or lymphocyte count.
In patients receiving Aza and GCS in combination, the total WBC is usually elevated because of steroid-induced neutrophilia with concomitant lymphopenia (as described earlier). Therefore, the above suggestions for monitoring treatment by total WBC do not apply. In this type of situation, it is therefore recommended to adjust the dose according to a WBC at 6000 to 8000μL as the lower range during combined treatment.
An important drug interaction occurs with allopurinol. The inhibition of xanthine oxidase by allopurinol impairs the conversion of Aza to the immunosuppressive metabolite 6-thiouric acid which accumulates and eventually leads to potentially deleterious bone marrow suppression. If allopurinol must be administered concurrently, the dose of Aza must be reduced empirically to 25% or less of the regular dose (~0.5mg/kg body weight) and the WBC should be closely monitored.
In cases of pronounced leucopenia, continuously elevated liver enzymes (over three times upper limits) or of other intolerable side effects of Aza, the daily drug dose needs to be reduced to 10-25% or less of the recommended regular dose and, if this fails to solve the problem, it should be stopped and replaced by another immunosuppressive compound (Table 2, see subsequently) or. In patients on allopurinol, a switch to uricosuric medication may be tried.
Table 2.
Treatment of myasthenia gravis (MG) (modified after [Schneider-Gold and Toyka, 2007]).
| 1 - Acetylcholinesterase inhibitors |
| Pyridostigmine 60 mg every 4 h orally, optionally 180mg of a time-span (slow release form) at bedtime* (empirical evidence) |
| 2 - Glucocorticosteroids (GCS) |
| 60-100 mg methylprednisolone per day orally (or prednisone/prednisolone); caveat initial deterioration; optionally slowly increasing doses; after reaching near remission: gradual dose reduction (Evidence class 1)† |
| 3 - Long-term Immunosuppressive Treatment |
| Azathioprine, 2-4 doses of 50 mg per day (2-3 mg per kg BW)** |
| (initially combined with GCS; Evidence class 1)+ |
| Cyclosporine A, 100-200 mg per day *** |
| (Evidence class 1)+ |
| 4. Off-label options |
| (for non-responders or in very severe MG, or with intolerable side effects) |
| - Mycophenolate mofetil, 1000-2000 mg per day orally **** |
| - Methotrexate, 7.5 mg to 15mg/week orally **** |
| - Cyclophosphamide, 500mg/m2 every 4 to 12 weeks i.v. or 1-2mg/kg BW perday orally ****, § |
| - Tacrolimus 2 × 2-5 mg/day orally §§ |
| (each Evidence class 2 or 3) |
| - Rituximab §§ |
| (Evidence class 3) |
Treatment trials designed as class 1 studies but with limited power due to early termination and small numbers of patients.
late evening time span preparations indicated only with marked myasthenic weakness in the morning.
An initial trial dose of 1 × 50mg may help to discover primary hypersensitivity. Divided daily doses are better tolerated than single doses.
monitoring by measuring blood trough levels available. Off-label status despite evidence class 1 study.
off-label, well documented single case studies available; publication of a randomized clinical trial pending.
off-label, available for most severely affected and treatment refractory patients. ‘immune ablation’ with higher doses and subsequent G-CSF rescue is also available (Drachman et al. 2003)
off-label, very limited experience.
Cyclosporine A (Cic A) belongs to the group of immunophilin-binding drugs. The cyclosporine-cyclophilin complexes inhibit the phosphatase calcineurin and its substrate, the transcription factor NFAT, thus preventing the transcription of messenger RNAs for key cytokines, such as interleu-kin-2. Cic A was the first effective drug in MG to be studied in a prospective, double-blind and placebo-controlled trial [Tindall et al. 1987]. It is about as effective as Aza, but its onset of action appears more like GCS (2-4 weeks).
The more serious potential side effects of Cic A include dose-dependent nephrotoxicity, and hepatic disorders. Further adverse reactions include arterial hypertension, tremor, weight gain, and hirsutism and these are the main factors conflicting with treatment adherence and more extensive use. Most of the adverse effects correlate with the dose and duration of treatment. Optimal dosage is monitored by measuring trough drug levels, 12 h after the last dose (best in the morning). The starting dose is 5 mg/ kg body weight divided into two daily doses which can be tapered to lower doses after amelioration of myasthenic weakness. A helpful predictor of nephrotoxicity is the decline of the creatinine clearance which is recommended at offset of Cic A treatment. If the creatinine level increases by 50% over baseline levels or to more than 1.5mg per 100μL during treatment, the dose should be reduced or the drug discontinued and replaced by other compounds (Table 2). Cic A must be discontinued if idiosyncratic or allergic reactions develop. The risk of late malignancies is not established, but in practical terms the same rules as for Aza may apply. With overt malignancy including thymic carcinoma, Cic A is not recommended because of its multiple and potentially serious side effects, and its higher cost – it is considered to be a second-line drug.
Tacrolimus is a more recent development that aims at the same pathways as Cic A, with greater efficacy, lower side effects but higher costs [Schneider-Gold et al. 2006].
Other immunosuppressive drugs
Cyclophosphamide and methotrexate are potentially useful in very severe MG not responding to the basic treatments but have serious long-term side effects. They may have a place in treatment-resistant patients after all other options have been tried (treatment escalation).
The most recent drug evaluated for refractory MG in open trials is the much less toxic compound mycophenolate mofetil (MMF) [Schneider-Gold et al. 2006]. Like Aza, MMF is an immunosuppressive agent acting on DNA metabolism. In transplantation medicine, MMF has proved useful and appears to be more effective than Aza. In patients with neuroimmunological diseases such as MG, MMF has been used as an alternative to Aza. MMF inhibits inosine monophosphate dehydrogenase and thereby depletes guanine nucleotides, leading to inhibition of DNA synthesis in lymphocytes, but not in other cells (which have an alternative ‘salvage pathway’ of purine synthesis). Its reported adverse effects include gastrointestinal symptoms, gastrointestinal hemorrhage, leukopenia and infection. Compared to Aza, its hepatotoxicity is low, but its risk of secondary lymphoma may be slightly higher. In contrast to Aza, the combination of MMF and allopurinol is not a problem. Thus far, a number of case reports [Schneider et al. 2000] and open trials indicate that MMF may be beneficial in MG to some degree. Despite published observations where Azanonresponders improved with MMF, a recent prospective, randomized multicenter trial (Muscle Study Group, 2008) was reported that failed to show that treatment with MMF is steroid-sparing. In general, clinical benefit from MMF occurs as late as with Aza, i.e., 3–12 months, or even longer. Since its main action is to prevent proliferation of lymphocytes, the preexisting populations of AChR-reactive lymphocytes and their signaling to plasma cells must gradually die off before a beneficial clinical effect is apparent. Overall, MMF may be worth trying as an off-label compound as one of the alternative immunosuppressive drugs in severe refractory MG.
Immunomodulating and antibody-depletion treatments
Intravenous polyvalent immunoglobulin G (IVIG) The potential mechanisms of action include, amongst many others, interactions with inhibitory Fc receptors on phagocytic and antigen presenting cells. Moreover, they can directly neutralize the blocking effects of various autoan-tibodies including AChR antibodies [Buchwald et al. 1998, Buchwald and Toyka, unpublished]. There is now convincing evidence that i.v. immu-noglobulin treatment is effective in MG [Gajdos et al. 2008; Gajdos et al. 1997]. IVIG has a potential role as an acute intervention in rapidly progressive weakness or as a chronic maintenance therapy when all other treatment modalities have failed or are contraindicated. The clinical response to IVIG is similar to but slower than the response to plasma exchange, but it offers an alternative in myasthenic crisis when therapeutic plasmapheresis is contraindicated, or when vascular access is problematic.
In the first randomized trial in patients with severe myasthenic exacerbation, IVIG treatment showed slightly weaker efficacy but was better tolerated than plasmapheresis [Gajdos et al. 1997]. In a subsequent trial 1 g/kg of IVIG was as effective as 2g/kg for the treatment of MG exacerbations [Gajdos et al. 2005]. Fifty-one patients were randomized to infusion with 2gIgG/kg or placebo and scored with the quantitative MG score at baseline and at days 14 and 28 after therapy [Zinman et al. 2007]. In IVIG-treated patients, a significant improvement in muscle strength was observed at both timepoints. The highest benefit was reported in patients with a more pronounced muscle weakness.
If patients respond, the onset is usually within 4 to 5 days. The effect lasts for several weeks. Once IVIG is deemed necessary patients should be started on immunosuppressive medication, simultaneously, because of the short-lived efficacy – usually up to three weeks. Adverse reactions occur in <10% of patients and include headache, fluid overload, aseptic meningitis, and rarely with replacement of osmotic stabilizers, renal failure. Patients with selective IgA deficiency (about 1 in 300) can develop anti-IgA antibodies causing anaphylactic reaction on repeated treatment. Disadvantages of IVIG therapy are the inconsistency of the response, high cost and shortage of supplies.
Plasmapheresis
There are two principal techniques of standard therapeutic plasmapheresis: on-line plasma separation by a cell separator (centrifuge) or by a plasma separator, i.e., membrane filtration. A typical plasmapheresis protocol employs 4 to 5 exchanges of one or 1.5 plasma volumes over one week or longer until the patient shows satisfactory improvement. Usually, plasma exchange therapy is of limited efficacy due to resynthesis or even rebound production of the respective autoantibo-dies and therefore it is combined with immuno-suppressive medication, most commonly a combination of corticosteroids and Aza.
Plasmapheresis aims at the removal of circulating autoantibodies and inflammatory mediators, and is in itself a strong immunomodulator [Heininger et al. 1986]. In MG, early clinical effects of plasmapheresis are occasionally observed in <24h. Such immediate improvement is probably due to the removal of a fraction of IgG autoanti-bodies that have a direct blocking effect on the ACh binding site on the alpha-subunit of the ACh-Receptor [Bufler et al. 1996]. Often the effects of plasmapheresis are more delayed and become apparent only after two or more days. This more delayed improvement is usually due to the removal of antibodies that act by increased receptor turnover or by complement-mediated lysis of the postsynaptic membrane.
Although, there is now practically no age limit for this treatment, it is the elderly patient with multi-organ disease who carries an increased risk for developing severe complications. Cardiovascular systemic reactions, electrolyte disturbances, sepsis, thrombosis and thrombophlebitis, pulmonary embolism, and subacute bacterial endocarditis have been observed, particularly in patients who have had arteriovenous shunts or grafts placed for vascular access.
In order to increase the efficiency and selectivity of standard plasmapheresis, these techniques have been combined in series (online) with (semi-)selective immunoadsorption columns with tryptophan-linked polyvinylalcohol gels or columns containing a gel to which the staphylo-coccal protein A has been linked [Flachenecker et al. 1998; Heininger et al. 1987]. These more selective procedures seem to be at least as effective as standard plasmapheresis in the treatment of MG with antibodies to AChR. It is not clear to which extent the Tryptophane-PVA columns are also effective in patients with MuSK antibodies.
Since there is negligible adsorption of albumin with immunoadsorption columns, protein substitution is not required. In a large series, 72 patients with MG crisis were treated by plasma-pheresis, immunoadsorption or a combination of both. Immunoadsorption, given alone or in combination, was associated with faster improvement of patients with myasthenic crisis, a shorter stay in hospital, and it was better tolerated (Gold, Toyka in preparation).
In the rare situation where a patient has severe clinical signs of typical autoimmune MG yet no autoantibodies are detected, plasmapheresis can be tried both for treatment and to help establishing a diagnosis of a putative antibody-mediated MG.
Thymectomy
Thymectomy has not yet been investigated in a prospective randomized controlled clinical trial in MG (a NIH supported multicenter trial is pending). However, this form of treatment has been found useful empirically, supported by several meta-analyses, and is widely applied. Thymectomy is recommended for patients with non-thymomatous autoimmune MG as an option to increase the probability of remission or improvement [Gronseth and Barohn, 2000]. Most studies report better responses when thymectomy is performed in the first years into the disease and a trans-sternal surgical approach is preferred over a transcervical incisure. Minimally invasive, endoscopically guided thymectomy is now advocated by some surgeons but its benefits on MG are not established in a formal clinical study.
There is no general consensus about the lower and upper age limits for thymectomy, the indication for thymectomy in pure ocular myasthenia, or the benefit of early versus late thymectomy as compared with the natural course of MG. Thymectomy is usually recommended in patients between 10 and 50 years of age with relatively recent onset of MG, i.e., within 3–5 years after the first manifestation. Between ages 6 and 10, the indication for thy-mectomy is controversial. It is usually not recommended to operate on pure ocular myasthenia although this may be an effective treatment [Hohlfeld et al. 2003]. Patients older than 60–65 years are usually not thymectomized, except for thymoma (see section on thymoma). If the severity of MG is marked or severe, pre-treatment with immunosuppressive drugs and plasmapheresis is recommended. Thymectomy is not recommended in patients with doubleseronegative MG and also not in patients with antibodies to MuSK, because retrospective analyses indicate a lack the typical thymus pathology [Leite et al. 2005] which is clearly different from the more common type of MG. Formal clinical trials are lacking to substantiate these claims. A malignant thymoma is generally considered as an absolute indication for radical thymomectomy at any age.
When properly performed, thymectomy has a low mortality rate that is essentially that of any operation with general anesthesia. However, it should be performed in a center with extensive experience and a neuromuscular consultant available. In patients with stable disease and after appropriate pretreatments (as described earlier), severe perioperative complications are very uncommon (<1%). There is no need to discontinue Aza before or after surgery. During the immediate pre- and postoperative period, oral pyridostigmine can be replaced by continuous flow i.v. neostigmine (formerly prostigmine).
Practical treatment recommendations
Generalized myasthenia gravis
Therapy should aim at complete or nearly complete remission which can be achieved in over 90% of patients.
A gradual (stepwise) treatment regimen is recommended with gradual escalation according to disease severity and treatment effects (Table 2). Restrictions by national health authorities and health insurances may exist for offlabel treatments.
All patients should first be treated with an AChE-inhibiting drug, usually pyridostigmine or, alternatively, ambenonium chloride. The optimum dose of any of these drugs and the timing of repeated doses must be determined for each patient. The patient is advised to use a monitoring flow sheet every 4–5h where the major items of the Modified Clinical MG Score (Table 3; [Besinger et al. 1983] or of the MGFA/Task Force Score [Gronseth and Barohn, 2000] are listed.
Table 3.
Myasthenia score modified after Besinger and Toyka (5).*
|
No weakness (0) |
Mild weakness (1) |
Moderate weakness (2) |
Marked weakness (3) |
|
| Arm outstretched time† | >180s | 60-180 s | 10-60 s | <10s |
| Leg outstretched time‡ | >45s | 30-45 s | 5-30 s | 55 s |
| Head holding time§ | >90s | 30-90 s | 5-30 s | 55 s |
| Vital capacity¶ | >4.0 L (m) | 2.5-4 L (m) | 1.5-2.5 L (m) | <1.5 L (m) |
| >3.0L (w) | 2.0–3.0L (w) | 1.2–2L (w) | <1.2L (w) | |
| FEV1¶ | >90% | 60-90% | 40-60% | <40% |
| Chewing/swallowing | Normal | Fatiguing (with solid food) | Soft foods only | Gastrostomy needed |
| Facial expression | Normal | Lid closure weak | Incomplete lid closing | No facial expression |
| Diplopia | >60s | 10-60 s | >0-10 s | Spontaneous diplopia |
| Ptosis | >60s | 10-60 s | >0-10 s | Spontaneous ptosis |
This original score has been adapted and extended for clinical trials by the MGFA.
Dominant arm, outstretched horizontal during sitting; in semiprone, severely ill patients, lift arm by about 30-45° (the outstretched times are only approximate measures).
Supine, dominant leg, lifted 45°.
Supine, head lifted 45°.
Vital capacity measured while seated; m, men, w, women; FEV1 = forced expiratory volume in one second. Originally, vital capacity has been assessed as the standard bedside procedure. Complete testing of pulmonary function is recommended at the initial examination to check for nonMG-related respiratory disorders.
Especially when treatment is initiated, patients should be carefully observed for side effects. In adult patients, treatment begins with 30–60 mg pyridostigmine every 4h during the daytime and should not exceed 90mg per dose and a maximal total daily dose of about 600 mg. The action of pyridostigmine begins about 30 min after ingestion and lasts for about 4–6 h, although the half life is much longer. In infants and children the starting oral dose is 0.5–1.0 mg/kg pyr-idostigmine. The need for a steady increase in the dose of AChE inhibitors indicates progressive disease and should alert the physician to the possibility that myasthenic crisis may be imminent and that further increasing the dose of AChE inhibitors may cause toxic cholinergic effects. Rather, at this point additional treatment modalities, such as plasma exchange treatment, must be considered.
The most common side effects (as described earlier) are gastrointestinal symptoms. Persistent diarrhea can be treated with atropine, 0.125–0.25mg (rarely 0.5 mg), probanthine, 7.5–15 mg, or other analogues, occasionally with every dose of pyridostigmine, if the AChE inhibitor cannot be reduced. Antimuscarinic agents should not be given routinely from the start.
If remaining symptoms are mild, thymectomy can be planned (as described earlier). For patients with moderate or severe MG, surgery should be postponed until the symptoms, especially pulmonary function and swallowing, are properly controlled by medical treatment, plasma exchange or IVIG.
In mild-to-moderate MG, after thymectomy immunosuppressive treatment is advised in patients whose symptoms persist for more than 3–6 months after the operation and in those who deteriorate after thymectomy. In patients for whom thymectomy is rejected because of high surgical risk or in the elderly, immunosuppressive medication is the treatment of choice from the start. If symptoms are not disabling but sufficiently severe to interfere significantly with daily activities it seems justified to offer the possibility of treatment with GCS alone at tolerable maintanance doses for a few months after thymectomy in young patients and women who wish to have children, but the combination with Aza is preferred in all other patients.
Immunosuppression may be initiated either (a) with corticosteroids alone, followed later with Aza as needed, or (b) with a combination of corticosteroids and Aza from the beginning. The rationale for (a) is that it permits evaluation of the beneficial and adverse effects of each drug separately. The use of combined immunosup-pression (b) takes advantage of the more rapid clinical benefit of corticosteroids, while allowing extra time for Aza to take effect. It may be possible to taper the steroids somewhat earlier with this regimen. There are two different approaches to initiate GCS treatment, as follows.
Increasing daily dose mode: If GCS are used alone to initiate treatment, the beginning daily dose may be as low as 15 to 20mg of prednisone [Drachman, 1987]. It is increased by about 5mg every 2 to 3 days, while observing the patient closely. The rate of increase should be guided by the patient's clinical response, and the endpoint is either a satisfactory clinical response, or a maximum dose of 50 to 60mg/ day (whichever occurs first). This slowly increasing dose regimen can be recommended in mild cases and the outpatient situation. Note that this mode of treatment was evaluated and proposed before the advent of modern intensive care medicine and plasmapheresis (1973), in view of the high risks involved in treating exacerbations. Aza may be added to the treatment regimen after steroid-induced improvement is established. A test dose of Aza (50 mg/day) is given for one or up to several days. If tolerated, it is increased to 2 to 3 mg/kg/day.
Maintenance dose mode: If the patient's condition is mild to moderate and stable, or after plasma-pheresis has been carried out to rapidly improve the clinical status, the prednisone dosage may be started at a higher level (1–1.5 mg prednisone or equivalent GCS per kg bodyweight per day, i.e., 60–100 mg/day). Aza is then added; if the patient tolerates a test dose (50 mg/day) of Aza, this drug may be added to the regimen at a dosage of 3mg/kg body weight/day with subsequent tapering to 2 or 2.5 mg/kg once remission has been achieved.
GCS should be tapered very slowly and gradually after reaching (near) remission. AChR-(or MuSK-) antibody levels are monitored bimonthly in the beginning. The majority of patients require continued immunosuppression at some level for many years. The goal of tapering the dose is to find the minimum effective amounts required for maintenance of a satisfactory clinical status.
Duration of treatment: After clinical remission has been achieved, and after an additional period of stable clinical remission or near remission and with stable antibody titers for about 1 year, an attempt can be made to taper and eventually discontinue Aza gradually over 6–12 months. After withdrawal of Aza, patients are monitored monthly for the first six months and every 2–3 months thereafter, for signs of clinical relapse and rising antibody titers. If there are signs of clinical deterioration while off any drug, immuno-suppressive treatment is re-instituted immediately. [Hohlfeld et al. 1985].
There is evidence that the risk of late malignancies is dependent on duration of treatment. The general consensus appears to be that a total treatment duration of up to ten years should not be exceeded for Aza [Hohlfeld et al. 1988]. Yet, lymphoma has been observed even after shorter periods. In view of the various options for immunosuppressive therapy, it is recommended to switch treatment modalities after some years or even escalate treatment if relevant weakness is still present.
Ocular myasthenia
Ptosis responds toAChE inhibitors (30–60mg per dose) much more favorably than does diplopia. If AChE inhibitors do not correct the symptoms at moderate to high doses, they should be discontinued at that time but may become more effective after successful immunosuppressive treatment. If AChE inhibitors fail, various mechanical devices may be considered. Occlusion of one eye with an adhesive patch applied to a spectacle may be helpful as a means to relieve transient diplopia. In an occasional patient, the diplopia may be sufficiently stable so that prisms may restore binocular vision. Self-adhesive plastic prisms are relatively inexpensive, and may be helpful. Ptosis may be relieved by a custom-made lid ‘crutch’ soldered to the inner side of a metal spectacle frame, or temporarily by the use of a strip of transparent adhesive tape. If AChE inhibitors fail, and if mechanical devices are either insufficient or not acceptable to the patient (driving, professional activities), GCS should be tried. Often, a relatively low dose of GCS (20–30mg per day) may result in resolution of ptosis and diplopia [Hohlfeld et al. 2003].
The indication for Aza in ocular myasthenia is controversial. However, Aza or Cic A may be considered for patients who have permanent ocular symptoms interfering with their professional activities despite maintenance GCS or if GCS at low doses are not tolerated.
Neonatal transient myasthenia
All children born to a mother with MG should be watched carefully for myasthenic signs during the first 3 to 6 days of life. If no symptoms have occurred by then, they are unlikely to occur later. Myasthenic weakness develops in about 15% of infants born to myasthenic mothers mediated by maternal antibodies circulating in the infant's blood. Neither the mother's anti-AChR antibody titer nor her clinical state is predictive of transient neonatal MG. Usually, the symptoms last for 2–4 weeks but elimination of maternal IgG antibodies may take months. Patients should be advised to schedule delivery in a specialized center with experience in MG. In afflicted children, myasthenic symptoms usually become apparent 3 to 72 h after birth. Apart from acute problems in the first week, the prognosis of neonatal MG is excellent. Recovery is usually complete within 2–4 months after birth. When symptoms are mild, small feedings and careful surveillance are sufficient. When symptoms are more severe, with weakness of suckling and swallowing, a feeble cry, general muscle hypo-tonia, or respiratory difficulties, neostigmine methylsulfate should be given immediately by subcutaneous or intramuscular injection (usually 0.04–0.05 mg/kg), or neostigmine bromide can be given orally through a nasogastric tube (0.5 mg/kg). Alternatively, pyridostigmine bromide intramuscularly (0.05–0.15 mg/kg) or orally (1–2 mg/kg) may be used. In cases of severe respiratory problems due to neonatal myasthenia, exchange transfusion or discontinuous plasma exchange may be considered. In rare cases children with arthrogryposis have been born to mothers suffering from MG.
Myasthenic crisis
Myasthenic crisis is defined as a severe and acute exacerbation of myasthenic weakness often precipitated by systemic infections, major surgery, severe emotional distress, insufficient long-term immunosuppressive treatment or too rapid tapering of medication. Clinical signs include the inability to maintain adequate respiratory function, to swallow, and to keep airways patent and free of secretions. Myasthenic crisis is a neurological emergency requiring immediate mesures and adequate treatment. The warning signs of an imminent crisis may develop over days and are often not interpreted as warning signs or simply denied by the patient. Shortness of breath and slurred speech, swallowing difficulties, progressive respiratory and neck weakness with orthop-nea, and profound sweating all qualify as warning sign which are prone to escalate to overt crisis within minutes. Pale or cyanotic skin indicate life-threatening hypoxemia, Blood gases show rising CO2 and falling oxygen levels. In this setting of progressive weakness, cholinergic crisis may be superimposed on myasthenic weakness because of increasing and eventually excessive doses of AChE inhibitors (usually more than 600mg pyr-idostigmine per day) inappropriately given to a myasthenic patient with progressive disease, but this is now rare (see above for diagnostic procedures). If deterioration of muscle strength cannot be improved by a single dose of neostigmine (0.5– 1mg), or pyridostigmine (1–2 mg) combined with atropine (0.5 mg) i.v., the full set of emergency measures has to be initiated including ventilatory support. Patients with imminent or overt crisis should be hospitalized in a specialized intensive care unit. Ventilatory support must be available during transfer.
Respiratory assistance should be provided if the forced vital capacity is less than 15 μL/kg bodyweight, the tidal volume drops to below 5–6 μL/kg bodyweight, or if arterial oxygen decreases to <85mm Hg (in patients with chronic lung disease baseline oxygen values may be much lower) and carbon dioxide increases to >45mm Hg. It should be noted that arterial blood gas determinations may give a false assurance and cannot substitute for close and careful clinical observation of the patient. Patients in myasthenic crisis receive the same respiratory support as do patients with other neuromuscular respiratory disorders. After the respiratory problems are under control the cause of the crisis should be investigated.
During assisted ventilation, AChE inhibitors may be maintained by continuous i.v. infusion, e.g., 0.15 to 0.5 mg neostigmine per hour, i.e., a total maximal dose of about 8–12mg per day, sometimes even up to 20mg per day in the short term. Along with improvement, AChE should be temporarily reduced in order to judge the clinical response to the immunologic therapies.
It is a cardinal rule that infections in MG should be treated with antibiotics early and vigorously. Bacterial respiratory tract infections are a common problem in myasthenic crisis. A common error in the management of myasthenic crisis is to wait too long before initiating antibiotic therapy, thus allowing spread of infection and an associated immune response which in turn aggravates symptoms of myasthenia. Appropriate cultures should be obtained as quickly as possible, and empirical antibiotic treatment started immediately, even before the results of these cultures are available. A good principle is to apply the antibiotic regimen that is used for immunocompromised oncology patients with acute infections. If indicated, third-generation cephalosporins can be given without worsening effects on myasthenic symptoms. Certain antibiotics, such as the aminoglycosides, may have adverse effects on neuromuscular transmission and are generally avoided. However, in an intensive care setting with the patient being ventilated, the primary consideration is the successful treatment of the infection, while potential neuromuscular effects of antibiotics are clearly less important.
If myasthenic crisis cannot be controlled within a few hours and weakness further progresses, plasmapheresis with or without immunadsorption is indicated. This is often combined with GCS, although the use of corticosteroids is controversial in patients with bacterial infection. High dose IVIG (up to 25g per day) may be given once or twice immediately after an exchange treatment, both as an adjunct treatment modality and in order to substitute for losses during plasmapher-esis. Aza may be added to the regimen if long-term immunosuppressive therapy is indicated. This is usually the case in patients whose symptoms are severe enough to evolve into crisis. It is important to note that the removal of clotting factors during plasmapheresis, in particular fibrinogen, may result in impairment of hemostasis for about 24 h. This should result in waiting for a day or two before the next plasmapheresis is done.
If there are contraindications to plasmapheresis, high-dose IVIG is indicated instead (as described earlier), with recent studies suggesting a plateau effect at a dose of 1 g/kg bodyweight. The infusion time should be 4–6h to reduce the risk of adverse effects in a compromised patient (Table 4).
Table 4.
Medications relatively contraindicated in myasthenia gravis.*
| Drug group | Examples |
| Analgesics | Morphine, flupirtine |
| Antibiotics | Aminoglycosides, macrolides, ketolides, gyrase inhibitors, sulphonamides, tetracyclines, penicillins (high doses) |
| Antidepressants | Tricyclics |
| Rheumatological agents | D penicillamine, chloroquine |
| Beta-blockers | Pindolol, propranolol, timolol |
| Calcium antagonists | Verapamil, diltiazem, nifedipine |
| Magnesium | High dose magnesium, e.g., in tocolysis |
| Muscle relaxants | Curare derivatives (max. 10–50% of the normal dose), succinylcholine |
These restrictions apply for patients with clinically visible signs, despite treatment with cholinesterase inhibitors; they may be used otherwise with observation during first treatment. (Table modified after [Schneider-Gold and Toyka, 2007]).
Pregnancy and myasthenia gravis
The influence of pregnancy on myasthenic symptoms is variable and unpredictable. Frequent adjustments of the anti-AChE medications may be required. During pregnancy, AChE inhibitors should not be given intravenously except in emergencies because they may cause uterine contractions. Infants born to mothers who have taken GCS during pregnancy should be monitored for adrenal insufficiency during the neonatal period. Also, since GCS are excreted in breast milk, inhibition of endogeneous steroid production as well as growth suppression can occur in infants who are breastfed by mothers receiving GCS. Myasthenic crisis during pregnancy should be treated essentially as described for nonpregnant patients, yet IVIG should be preferred over plasmapheresis because of the potential adverse reactions. Sedatives and narcotics may be given in half the usual doses used for nonmyasthenic patients. Consultation with the obstetrician-pediatrician team is recommended to discuss early delivery.
Drugs with adverse effects on neuromuscular transmission
Many drugs may compromise neuromuscular transmission and exacerbate myasthenic weakness. This is clinically relevant in MG patients once they have marked systemic weakness. The following agents should be used only if absolutely necessary, and the patient should be closely monitored for any exacerbation of myasthenic symptoms: neuromuscular blocking agents (e.g., curare-like compounds); local anesthetics (prefer amide- over ester- type) and anti-arrhythmic drugs (quinine, quinidine, procainamide, verapa-mil); aminoglycosides, quinolone, and macrolide antibiotics; beta blockers; calcium channel blocking agents). D-penicillamine must not be used in myasthenic patients because it can itself induce autoimmune MG, which is completely reversible after the drug is discontinued. Many other drugs have been reported to produce worsening or unmasking of myasthenic symptoms. In general, all myasthenic patients should be informed of these risks and observed for clinical worsening when any new medication is begun.
Thymoma and thymic carcinoma
Prior to surgery, the patient's clinical status should be optimized, exactly as for thymectomy without thymoma (as described earlier). After removal of the tumor, the principles of treatment for MG, described above, also apply for patients with thymoma. Further treatment of the thy-moma depends on the intraoperative staging of tumor invasion, on the histopathological findings, and on the clinical response after surgery. The prognosis of medullary and mixed thymo-mas (A stages; benign thymoma) seems better than that of the other tumors. [Strobel et al. 2004; Graeber and Tamim, 2000].
For noninvasive, encapsulated thymomas, radical thymectomy is considered curative. Nonetheless, patients should be followed with regular chest CT or MRI scans. Postoperative radiotherapy is usually not necessary in noninvasive thymomas. Patients with invasive thymomas are commonly treated with surgery, radiotherapy, and chemotherapy in varying combinations and sequences. It should be noted that previous radiation may be a complicating factor if the tumor recurs and a second operation is needed. Several experimental protocols of adjuvant chemotherapy have been evaluated in invasively growing thymomas.
Acknowledgments
Parts of this article have been adapted from previously published review articles and book chapters by the same group of authors, including two recent review articles [Schneider-Gold and Toyka, 2007] (English translation) and [Toyka and Gold, 2007].
We thank our many colleagues who helped in managing the patients that are included in this review. The research of the authors has been continuously supported by German Federal granting agencies including DFG and BMBF (to all authors), by the Myasthenia Gravis Foundation (USA, to KVT), The Muscular Dystrophy Association (USA, to KVT), the Hertie Foundation (to all authors), by University Research Funds and by educational grants from pharmaceutical companies.
Conflict of interest statement
All three authors have been members on panels installed by national and international public health authorities and agencies and by pharmaceutical companies in treatment issues related to neuroimmunological disorders including myasthenia gravis and have received remuneration and consultation fees under the supervision of the respective State and Hospital authorities following State and Federal guidelines.
Footnotes
This review does not formally address the different regulations for drug licensing by national health authorities: Every treating physician needs to check the status of any drug (on-label or off-label) before prescribing treatments discussed in this manuscript. All drug dosages have been double-checked but readers should be aware that they are liable to check the individual drug labels as to numerical values before actually treating patients.
Contributor Information
Ralf Gold, Neurologische Klinik St. Josef-Spital Gudrunstrasse 56 44791 Bochum, Germany ralf.gold@ruhr-uni-bochum.de.
Reinhard Hohlfeld, Institute of Clinical Neuroimmunology Klinikum Großhadern University of Munich Germany.
Klaus V. Toyka, Department of Neurology and Clinical Research Group for Neuroimmunology, University of Würzburg Germany
References
- Arsura E., Brunner N.G., Namba T., Grob D. (1985) High-dose intravenous methylprednisolone in myasthenia gravis. Arch Neurol 42:1149–1153 [DOI] [PubMed] [Google Scholar]
- Besinger U.A., Toyka K.V., Homberg M., Heininger K., Hohlfeld R., Fateh-Moghadam A. (1983) Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 33:1316–1321 [DOI] [PubMed] [Google Scholar]
- Buchwald B., Dudel J., Toyka K.V. (1998) Neuromuscular blockade by immunoglobulin G from patients with Miller Fisher syndrome. Ann NY Acad Sci 841:659–669 [DOI] [PubMed] [Google Scholar]
- Bufler J., Kahlert S., Tzartos S., Toyka K.V., Maelicke A., Franke C. (1996) Activation and blockade of mouse muscle nicotinic channels by antibodies directed against the binding site of the acetylcholine receptor.J Physiol (Lond) 492(Pt 1): 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman D.B. (1987) Present and future treatment of myasthenia gravis. N Engl J Med 316:743–745 [DOI] [PubMed] [Google Scholar]
- Drachman D.B. (1994) Myasthenia gravis. N Engl J Med 330:1797–1810 [DOI] [PubMed] [Google Scholar]
- Drachman D.B., Jones R.J., Brodsky R.A. (2003) Treatment of refractory myasthenia: “Rebooting” with high-dose cyclophosphamide. Ann Neurol 53:29–34 [DOI] [PubMed] [Google Scholar]
- Dudel J., Birnberger K.L., Toyka K.V., Schlegel C., Besinger U. (1979) Effects of myasthenic immunoglobulins and of prednisolone on spontaneous miniature end-plate potentials in mouse diaphragms. Exp Neurol 66:365–380 [DOI] [PubMed] [Google Scholar]
- Farrugia M.E., Robson M.D., Clover L., Anslow P., Newsom-Davis J., Kennett R.et al. (2006) MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 129:1481–1492 [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Taleghani B.M., Gold R., Grossmann R., Wiebecke D., Toyka K.V. (1998) Treatment of severe myasthenia gravis with protein A immunoadsorption and cyclophosphamide. Transfus Sci 19(Suppl):43–46 [DOI] [PubMed] [Google Scholar]
- Gajdos P., Chevret S., Clair B., Tranchant C., Chastang C. (1997) Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Ann of Neurol 41:789–796 [DOI] [PubMed] [Google Scholar]
- Gajdos P., Chevret S., Toyka K. (2008) Intravenous immunoglobulin for myasthenia gravis - art. no. CD002277.Cochrane Database of Systematic Reviews 2277. [DOI] [PubMed]
- Gajdos P., Tranchant C., Clair B., Bolgert F., Eymard B., Stojkovic T.et al. (2005) Treatment of Myasthenia Gravis exacerbation with intravenous immunoglobulin - A randomized double-blind clinical trial. Arch Neurol 62:1689–1693 [DOI] [PubMed] [Google Scholar]
- Gold R., Dalakas M.C., Toyka K.V. (2003) Immunotherapy in autoimmune neuromuscular disorders. Lancet Neurol 2:22–32 [DOI] [PubMed] [Google Scholar]
- Graeber G.M., Tamim W. (2000) Current status of the diagnosis and treatment of thymoma. Semin Thorac Cardiovasc Surg 12:268–277 [DOI] [PubMed] [Google Scholar]
- Gronseth G.S., Barohn R.J. (2000) Practice parameter: Thymectomy for autoimmune myasthenia gravis (an evidence-based review) - Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 55:7–15 [DOI] [PubMed] [Google Scholar]
- Heininger K., Hartung H.-P., Toyka K.V., Gaczkowski A., Borberg H. (1987) Therapeutic plasma exchange in myasthenia gravis: semiselective adsorption of Anti-AChR autoantibodies with trypto-phane-linked polyvinylalcohol gels. Ann N Y Acad Sci 505:898–900 [Google Scholar]
- Heininger K., Toyka K.V., Gaczkowski A., Borberg H., Hartung H.P., Grabensee B. (1986) Semi-selective removal of pathogenic factors in neurologic disease. Plasma therapy 7:351–357 [Google Scholar]
- Hoch W., McConville J., Helms S., Newsom-Davis J., Melms A., Vincent A. (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368 [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Melms A., Schneider C., Toyka K.V., Drachman D.B. (2003) Therapy of myasthenia gravis and myasthenic syndromes. In Brandt T., Caplan L. R., Dichgans J., Diener H. C., Kennard C.Neurological disorders: course and treatment. Elsevier; Stuttgart, 1341–62 [Google Scholar]
- Hohlfeld R., Michels M., Heininger K., Besinger U., Toyka K.V. (1988) Azathioprine toxicity during long-term immunosuppression of generalized myasthenia gravis. Neurology 38:258–261 [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K.V., Besinger U.A., Gerhold B., Heininger K. (1985) Myasthenia gravis: reactivation of clinical disease and of autoimmune factors after discontinuation of long-term azathioprine. Ann Neurol 17:238–242 [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Wekerle H. (1999) The immuno-pathogenesis of myasthenia gravis. In Engel A. G.Myasthenia gravis and myasthenic syndromes. Oxford: Oxford University Press,87–110 [Google Scholar]
- Kirchner T., Schalke B., Melms A., von Kugelgen T., Muller-Hermelink H.K. (1986) Immunohistological patterns of non-neoplastic changes in the thymus in Myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol 52:237–257 [DOI] [PubMed] [Google Scholar]
- Leite M.I., Strobel P., Jones M., Micklem K., Moritz R., Gold R.et al. (2005) Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative, MG. Ann of Neurol 57:444–448 [DOI] [PubMed] [Google Scholar]
- Mertens H.G., Hertel G., Reuther P., Ricker K. (1981) Effect of immunosuppressive drugs (azathioprine). Ann NY Acad Sci 377:691–699 [DOI] [PubMed] [Google Scholar]
- Muller-Hermelink H.K., Marx A. (2000) Thymoma. Curr Opin Oncol 12:426–433 [DOI] [PubMed] [Google Scholar]
- Muscle Study Group.(2008) A trial of mycophenolate mofetil with prednisolone as initial immunotherapy in myasthenia gravis. Neurology 71:394–399 [DOI] [PubMed] [Google Scholar]
- Palace J., Newsom-Davis J., Lecky B.Myasthenia Gravis Study Grp.(1998) A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Neurology 50:1778–1783 [DOI] [PubMed] [Google Scholar]
- Schneider-Gold C., Gajdos P., Toyka K.V., Hohlfeld R.R. (2005) Corticosteroids for myasthenia gravis.Cochrane Database Syst Rev CD002828. [DOI] [PMC free article] [PubMed]
- Schneider C., Gold R., Reiners K., Toyka K.V. (2001) Mycophenolate mofetil in the therapy of severe myasthenia gravis. Eur Neurol 46:79–82 [DOI] [PubMed] [Google Scholar]
- Schneider-Gold C., Hartung H.P., Gold R. (2006) Mycophenolate mofetil and tacrolimus: New therapeutic options in neuroimmunological diseases. Muscle & Nerve 34:284–291 [DOI] [PubMed] [Google Scholar]
- Schneider-Gold C., Toyka K.V. (2007) Myasthenia gravis: Pathogenesis and immunotherapy (English version). Dtsch Arzteblatt 104:A420–A426 [Google Scholar]
- Strobel P., Bauer A., Puppe B., Kraushaar T., Krein A., Toyka K.et al. (2004) Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 22:1501–1509 [DOI] [PubMed] [Google Scholar]
- Tindall R.S., Rollins J.A., Phillips J.T., Greenlee R.G., Wells L., Belendiuk G. (1987) Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med 316:719–724 [DOI] [PubMed] [Google Scholar]
- Toyka K.V., Gold R. (2007) Treatment of myasthenia gravis. Schweiz Arch Neurol Psychiatr 158:309–321 [Google Scholar]
- Vincent A., Bowen J., Newsom-Davis J., McConville J. (2003) Seronegative generalised myasthenia gravis: clinical features, antibodies and their targets. Lancet Neurol 2:99–106 [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U.P. (1977) Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet 1:678–680 [DOI] [PubMed] [Google Scholar]
- Witte A.S., Cornblath D.R., Parry G.J., Lisak R.P., Schatz N.J. (1984) Azathioprine in the treatment of myasthenia gravis. Ann Neurol 15:602–605 [DOI] [PubMed] [Google Scholar]
- Zinman L., Ng E., Bril V. (2007) IV immunoglobulin in patients with myasthenia gravis - A randomized controlled trial. Neurology 68:837–841 [DOI] [PubMed] [Google Scholar]