Abstract
Treatment of multiple sclerosis (MS) is still unsatisfactory and essentially non-existing for the progressive course of the disease. Recombinant human erythropoietin (EPO) may be a promising neuroprotective/neuroregenerative treatment of MS. In the nervous system, EPO acts anti-apoptotic, antioxidative, anti-inflammatory, neurotrophic and plasticity-modulating. Beneficial effects have been shown in animal models of various neurological and psychiatric diseases, including different models of experimental autoimmune encephalomyelitis. EPO is also effective in human brain disease, as shown in double-blind placebo-controlled clinical studies on ischemic stroke and chronic schizophrenia. An exploratory study on chronic progressive MS yielded lasting improvement in motor and cognitive performance upon high-dose long-term EPO treatment.
Keywords: hematopoietic growth factor, chronic progressive multiple sclerosis (MS), motor function, cognition, experimental autoimmune encephalomyelitis (EAE), clinical trial, neuro-degeneration, erythropoietin receptor (EPOR)
Introduction
With a prevalence of more than 100 per 100,000 in Northern and Central Europe (annual incidence of 3.5–5 per 100,000 in Germany), and a disease onset predominantly in individuals aged between 20 and 40 years, multiple sclerosis (MS) is the most common cause of neurological disability in young and middle-aged adults, and of premature retirement in 33% of affected subjects [Flachenecker et al. 2008, 2005; Pugliatti et al. 2006]. MS is etiologically as well as patho-genetically heterogeneous and despite intensive research efforts still poorly understood.
To date, MS is not curable and even worse there are no means of stopping the disease process lastingly. Immunomodulatory, immunosuppressive and anti-inflammatory treatment approaches have at best led to a reduction of the relapse rate and temporary improvement of clinical severity in relapsing-remitting MS, but it is still unclear if newer compounds such as natalizumab or rituximab will deliver any persisting amelioration of the clinical syndrome [Confavreux and Vukusic, 2006; Feldmann and Steinman, 2005; Giovannoni, 2004; Hohlfeld and Wekerle, 2004; Noseworthy, 2003; Wingerchuk and
Noseworthy, 2002]. In chronic progressive MS, treatment approaches are essentially all ineffective. Importantly, no neuroprotective/neuro-regenerative strategy has been developed so far for MS despite the fact that disease progression is mainly driven by neurodegenerative pathological changes [e.g. Confavreux and Vukusic, 2006; Hauser and Oksenberg, 2006; Compston and Coles, 2002]. Therefore, identification of an efficient add-on treatment targeting axonal repair and remyelination, in addition to a perhaps disease subtype-oriented and more effective immunomodulatory therapy, will be the pivotal challenge for MS therapy research over the next decade [Hauser and Oksenberg, 2006; Rovaris et al. 2006; Brück, 2005; Giovannoni, 2004; Compston and Coles, 2002].
Erythropoietin, a hematopoietic growth factor with potent neuroprotective/neuro-regenerative properties in the nervous system
Erythropoietin (EPO) is a hematopoietic growth factor that has long been known to be produced in kidney and fetal liver, and has more recently been described to be expressed in the brain [Masuda et al. 1994, 1993; Konishi et al. 1993]. The EPO system plays an important role during normal brain development, where it is associated with physiological apoptosis as well as the production and differentiation of neuronal precursor cells [Knabe et al. 2005, 2004; Shingo et al. 2001; Juul et al. 1998]. Postnatal and, even more pronounced during adulthood, the EPO system is downregulated, resulting in low-level expression of EPO and EPO receptor (EPOR) in the normal adult brain [Ehrenreich et al. 2005, 2004; Marti et al. 1996]. In situations of distress, ranging from metabolic to inflammatory, from ischemic to neurodegenerative conditions, EPO/EPOR appear to act as part of an endogenous neuro-protective ‘stand by’ system. In these situations, EPO and/or EPOR are strongly upregulated [Ehrenreich et al. 2004; Eid et al. 2004; Chung et al. 2004; Sirén et al. 2001b]. Interestingly, extraction of endogenous EPO during experimental stroke by intracerebroventricular application of soluble EPOR was found to induce a dramatic increase in ischemic damage [Sakanaka et al. 1998]. Knock-out of the brain EPOR, in turn, provokes higher rates of neuronal apoptosis and enhanced vulnerability to hypoxia [Yu et al. 2002]. Reduced concentrations of EPO in the cerebrospinal fluid (CSF) in amyotrophic lateral sclerosis (ALS) may point to a relative deficiency of endogenous EPO production in neurodegenerative disease. A more efficient extraction of any free molecule of EPO by brain tissue due to high reactive EPOR expression would perhaps explain this phenomenon [Brettschneider et al. 2006].
As in other organs, expression of the brain EPO system appears to be mainly stimulated via low tissue oxygen concentration. In this condition, hypoxia-inducible factor-1 (HIF-1) is activated, leading to increased production of EPO and EPOR together with various other hypoxia-inducible genes, for example vascular endothelial growth factor (VEGF) [Jelkmann, 2007; Ehrenreich et al. 2005; Beleslin-Cokic et al. 2004; Sharp and Bernaudin, 2004; Chikuma et al. 2000; Lewczuk et al. 2000; Bauer and Kurtz, 1989].
EPO acts on cells of the nervous system by binding to its specific receptor, EPOR, which belongs to the cytokine type-1 receptor super-family [Jelkmann, 2007, 1992; Fisher, 2003]. Dimerizing of EPOR upon ligand binding leads to autophosphorylation of the receptor-associated Janus tyrosine kinase 2 and activation of distal signal transduction cascades: phosphatidylinositol-3-kinase (PI3-K)/Akt (protein kinase-B), RAS/mitogen-activated protein kinases (MAPK), signal transducers and activators of transcription-5 (STAT-5), as well as NF-?B-dependent transcription [Byts et al. 2006; Park et al. 2006; Kilic et al. 2005; Digicaylioglu and Lip ton, 2001; Sirén et al. 2001a]. Up to now, it is unclear whether EPOR in the nervous system consists of a homodimer, homopolymer or, as claimed by Brines and coworkers, is composed of an EPOR monomer, dimerizing with the so-called beta-common receptor (ßCR), to form a heterodimer [Tsai et al. 2006; Brines et al. 2004; Livnah et al. 1999; Jubinsky et al. 1997]. Among others, a modified brain EPOR could explain why certain EPO analogs, for example carbamylated erythropoietin (CEPO), still possess the neuroprotective properties of EPO but are essentially devoid of its hematopoietic effects [Leist et al. 2004]. The group of nonhematopoietic EPO analogs/derivatives or EPOR stimulants may provide interesting future pharmacological tools for long-term treatment of brain diseases [for review see Jelkmann, 2008].
EPO has been described both in cell culture work and animal models of neurological and psychiatric diseases to exert potent anti-apoptotic, anti-inflammatory, and antioxidative properties. In addition, its neurotrophic, axon-protective, angiogenetic and neurogenetic properties make EPO an attractive candidate for neuroprotection/ neuroregeneration. It stimulates axonal sprouting and synaptogenesis under certain circumstances and modulates plasticity [Adamcio et al. 2008; Byts et al. 2008; Jelkmann, 2007; Erbayraktar et al. 2006; Kaiser et al. 2006; Tsai et al. 2006; Diem et al. 2005; Bianchi et al. 2004; Chang et al. 2004; Csete et al. 2004; Kertesz et al. 2004; Keswani et al. 2004; Sattler et al. 2004; Weishaupt et al. 2004; Villa et al. 2003; Bocker-Meffert et al. 2002; Celik et al. 2002; Genc et al. 2002; Gorio et al. 2002; Grasso et al. 2002; Grimm et al. 2002; Springborg et al. 2002; Campana and Myers, 2001; Genc et al. 2001; Shingo et al. 2001; Sirén et al. 2001a; Brines et al. 2000; Bernaudin et al. 1999; Sadamoto etal. 1998; Sakanaka et al. 1998; Konishi et al. 1993]. Interestingly, EPO has very potent effects on learning and memory-associated readouts of hippocampal neuronal plasticity (e.g. long-term and short-term potentiation) [Adamcio et al. 2008].
This unusual assortment of properties, likely derived from a prominent multifaceted role of the EPO system during brain development, may explain the broad neuroprotective action of EPO in animal models of diseases as different as hypoxia/ischemia, traumatic brain or spinal cord injury, subarachnoid hemorrhage or epilepsy, retina degeneration, peripheral neuropathies and radiation-induced brain damage [Jelkmann, 2007; Erbayraktar et al. 2006; Sirén et al. 2006; Tsai et al. 2006; Bianchi et al. 2004; Chang et al. 2004; Keswani et al. 2004; Weishaupt et al. 2004; Villa et al. 2003; Bocker-Meffert et al. 2002; Celik et al. 2002; Gorio et al. 2002; Grasso et al. 2002; Grimm et al. 2002; Springborg et al. 2002; Campana and Myers, 2001; Sirén et al. 2001a; Brines et al. 2000; Bernaudin et al. 1999; Sadamoto et al. 1998; Sakanaka et al. 1998]. Furthermore, reports on beneficial effects of EPO exist in animal models of Parkinson's disease [Csete et al. 2004; Genc et al. 2002, 2001] ALS [Grignaschi et al. 2007; Grunfeld et al. 2007; Koh et al. 2007], or cerebral malaria [Wiese et al. 2008; Kaiser et al. 2006]. Interestingly, regarding the neurological consequences of cerebral malaria, the amount of endogenous EPO has recently been found to play an important role for disease severity of children, once more pointing to the importance of EPO as an endogenous neuro-protective system [Casals-Pascual et al. 2008].
Both in vitro and in vivo, EPO has been shown to have a bell-shaped dose–response curve regarding its neuroprotective properties [Ehrenreich et al. 2005; Weishaupt et al. 2004; Sakanaka et al. 1998]. This bell-shaped curve makes the prediction of the adequate dose for optimal translation from animal models to man difficult. Perhaps the most suitable determinant of the ‘right dose’ is the amount of EPO reaching the brain via the blood–brain barrier. While EPO, given systemically at high doses, can indeed penetrate an intact blood–brain barrier sufficiently to exert neuroprotective effects (0.1–1% of the peripherally applied dose), the exact mechanism of penetration of this over 30,000 Dalton molecular weight protein via the blood–brain barrier is still unclear [Xenocostas et al. 2005; Banks et al. 2004; Ehrenreich et al. 2004; Martinez-Estrada et al. 2003; Brines et al. 2000].
Taken together, EPO possesses a broad spectrum of properties suitable to address several of the pathophysiological mechanisms involved in neuropsychiatric diseases. Since many of these mechanisms also play a major role in MS, it was most logical to explore the potential of EPO in relevant MS animal models.
Effects of EPO in animal models of neuroinflammatory disease
With respect to the clinical application of EPO as neuroprotective/neuroregenerative treatment in MS, several preclinical studies have shown potential efficacy of EPO as modulator of disease severity, using experimental autoimmune ence-phalomyelitis (EAE) as an animal model of MS (see Table 1 for overview).
Table 1.
Effects of erythropoietin (EPO) in rodent models of experimental autoimmune encephalomyelitis (EAE).
| Reference | Model | Readouts | Beneficial EPO effects on | No EPO effect on | Remarks |
| Brines et al. 2000 | Lewis rats MBP – acute model |
Clinical rating | Delayed symptom onset and reduced symptom severity |
— | EPO (5000 IU/kg/day i.p.) from day 3 after MBP for 15 days |
| Agnello et al. 2002 |
Lewis rats MBP – acute model |
Clinical rating Histology TNF + IL-6 bioassays |
Reduced gliosis and cell infiltration in spinal cord; TNF increase delayed and IL-6 increase reduced |
— | EPO (500-5000 IU/kg/day i.p.) from day 3 after MBP for 15 days |
| Li et al. 2004 | C57BL/6 mice MOG - chronic progressive model |
Clinical rating Histology |
Reduction of symptom severity, axonal injury, demyelination, blood-brainbarrier leakage and glial expression of MHC II in spinal cord |
— | EPO (range 0.5–5000 IU/kg/day i.p.) from 36 to 48
h after symptom onset for 14 days |
| Sattler et al. 2004 |
Brown Norway rats MOG - hyperacute model of optic neuritis |
Clinical rating Electrophysiology Histology |
Increased survival of retinal ganglion cells and improvement in electroretinogram |
No change of clinical outcome, demyelination, inflammatory infiltration, axon density, optic nerve function |
Research into intracellular neuroprotective pathways; EPO (2000-10000 IU/kg/day i.p.) for 1 or 8 days after MOG |
| Diem et al. 2005 |
Brown Norway rats MOG - hyperacute model of optic neuritis |
Clinical rating Electrophysiology Histology |
Increased survival of retinal ganglion cells and improvement in electroretinogram |
No significant change of clinical outcome, demyelination, inflammatory infiltration, axon density and function of optical nerve |
Combined treatment of methylprednisolone (20 mg/kg/day i.p.) on day 1-3 and EPO (5000 IU/kg/ day i.p.) for 8 days after MOG revealed best neuron and axon protection |
| Zhang et al. 2005 | SJL/J mice Myelin PLP - relapsing-remitting model |
Clinical rating Histology |
Reduction of symptoms, demyelination and inflammatory infiltration; increase in oligodendrocyte progenitors and BDNF positive cells in brain |
Relapses despite EPO-treatment |
EPO (5000 IU/kg/day i.p.) for 7 days after symptom onset |
| Savino et al. 2006 |
C57BL/6 mice MOG - chronic progressive model |
Clinical rating Histology Cytokine expression |
Reduction of clinical symptoms and cytokine expression in spinal cord and peripheral mononuclear cells |
— | Investigation also of non-nonhematopoietic EPO derivatives (CEPO, ASIALO EPO); EPO (0.5 or 50 mg/kg i.p.) for up to 29 days; three times per week: 3 days after MOG or directly after symptom onset or 15 days after symptom onset |
| Yuan et al. 2008 |
C57BL/6 mice MOG - chronic progressive model |
FACS of EAE-derived cells Cytokine levels of T-cell cultures Histology |
Modulation of immune balance in periphery and spinal cord; blocking of T-cell proliferation and dendritic cell expansion in lymph nodes |
— | EPO (5000 IU/kg/day i.v. for 3 days+1000 IU/kg/day i.v. for the next 3 days) after MOG or until 7 days post-MOG |
MBP, myelin basic protein; MHC, major histocompatibility complex; MOG, myelin oligodendrocyte glycoprotein; myelin PLP, myelin proteolipid protein; BDNF, brain-derived neurotrophic factor.
Among the available animal models, various different EAE models most suitably resemble different basic mechanisms and specific features of the histopathology and neurobiology of MS and therefore provide relevant and established tools to investigate emerging therapeutic approaches. EAE in rodents can be induced; for example, via active immunization with central nervous system tissue, myelin oligodendrocyte glycoprotein (MOG), myelin or myelin basic protein (MBP), and results in a high incidence of disease with a reproducible clinical course [Gold et al. 2006].
In a pivotal paper, screening the effect of EPO in various different animal models of neurological and psychiatric diseases, Brines and colleagues noted that the onset of acute MBP-induced EAE was delayed upon EPO and that the severity of symptoms was significantly reduced [Brines et al. 2000]. The same group showed later that EPO has an effect on the inflammatory component of EAE, with delayed increase in tumor necrosis factor and blunted elevation of IL-6, together with reduced gliosis and cell infiltration in the spinal cord of EPO-treated EAE rats [Agnello et al. 2002]. Because EAE in the Lewis rat is considered a short-term transient monophasic disease model, another model was subsequently used by Li and coworkers that apparently shares more clinical and neuropathological features with MS [Li et al. 2004]. In the progressive model of MOG-induced EAE in mice, a reduction of symptom severity was found even upon EPO treatment start after disease onset. EPO blocked axonal injury, demyelination and blood–brain barrier leakage, as well as reduced glial expression of major histocompatibility complex (MHC) class II, interpreted as modulation by EPO of inflammation in EAE [Li et al. 2004]. In a MOG-induced optic neuritis model in Brown Norway rats, improved survival and function of retinal ganglion cells in EPO-treated animals were described [Sättler et al. 2004]. In the same model, a combined treatment of EPO and methylprednisolone was superior to each component alone in targeting the inflammatory as well as the neurodegenerative aspects of optic neuritis [Diem et al. 2005]. Finally, EPO was found to increase oligodendrocyte progenitor cell proliferation and to elevate brain-derived neurotrophic factor (BDNF)-expressing cells in a relapsing-remitting proteolipid protein (PLP) induced murine EAE model [Zhang et al. 2005]. EPO as well as nonhematopoietic EPO derivatives, CEPO [Leist et al. 2004] and ASIALO EPO [Erbayraktar et al. 2003], decreased production of inflammatory cytokines in spinal cord and peripheral lymphocytes in a chronic MOG-induced mouse EAE model [Savino et al. 2006]. Very recently, Yuan and coworkers focused on the immunological aspects of MOG-induced EAE in mice and found that EPO induces substantial long-term tissue protection in the host through signaling to several critical subsets of immune cells that reside in the peripheral lymphatic system as well as through modulating their corresponding cyto-kines [Yuan et al. 2008].
To summarize, various different groups showed with different EAE models and different treatment schedules that EPO reduces clinical severity, improves electrophysiological and histological readouts of EAE, and reduces inflammatory cytokine expression. These stimulating results from preclinical MS models obtained by ourselves and others, together with the beneficial effects of EPO in our human studies in stroke and schizophrenia, have strongly motivated us to perform a first exploratory study on EPO in chronic progressive MS (see below).
Human studies on EPO effects in brain disease
Stroke
Before performing exploratory work in human MS patients using high-dose EPO treatment, we collected evidence of beneficial effects of EPO in other human brain diseases. The first study of its kind was initiated in 1997 and included patients suffering from acute ischemic stroke in the territory of the middle cerebral artery [Ehrenreich et al. 2002]. This study was set up as a small proof-of-concept (phase IIb) trial and comprised a safety and a double-blind placebo-controlled part. EPO was applied over 3 days daily at a dose of 33,000IU as intravenous infusion to result in a total dose of 100,000 IU.
The first application was performed as rapidly as possible after the onset of stroke with a time window of maximally 8h. The second and third dose was given 24 h and 48 h later, respectively. EPO was well tolerated in this group of patients and led to an improvement compared with the placebo group with respect to clinical outcome, imaging results and serum levels of the circulating glial damage marker S100B. Based on these very encouraging results, the German Multicenter EPO Stroke Trial (ClinicalTrial.gov Identifier: NCT00604630) was started in 2003, and aimed to include over 500 patients. This trial, performed in a multicenter setting including Göttingen, Hanover, Bremen, Celle, Erlangen, Leipzig, Essen, Dresden, Braunschweig, Aachen and Berlin has been concluded in summer 2008, as expected. As compared with the first EPO stroke trial, the ‘entire stroke landscape’ has changed due to the approval of recombinant tissue plasminogen activator (rtPA) for treatment of stroke patients, which took place in Germany in 2000 [Weimar et al. 2006]. As a result, over 60% in total of the patients included in this trial received thrombolytic therapy. Therefore, for analysis of a neuroprotective EPO effect, patients will be divided into an rtPA and a non-rtPA group.
Schizophrenia
After the promising results of short-term highdose EPO treatment in human stroke as a classic example of an acute brain disease, we investigated the effect of long-term high-dose EPO treatment in chronic brain diseases. In the first disease that we chose, schizophrenia, neuroprotection was an important challenge to meet. In preparation of this trial, we had to first test the capability of EPO to penetrate an intact blood–brain barrier [Ehrenreich et al. 2004]. We did not only prove that EPO enters the brain in healthy rats, achieving a peak level in the CSF after 3.5h of intraperitoneal injection, but also demonstrated, using indium 111-labeled EPO, that even in healthy human subjects, EPO enriched within brain tissue [Ehrenreich et al. 2004]. The accumulation of labeled compound within the brain, however, was higher in schizophrenic patients as compared with healthy controls, most likely explained by a higher density of EPOR expression in frontal cortex and hippocampus. Importantly, EPO is able to improve cognitive functioning in mice and to enhance hippocampal long-term potentiation and various features of neuronal plasticity, essential for learning and memory processes [Ehrenreich et al. 2004, Adamcio et al. 2008]. Intriguingly, we found that EPO prevents the development of slowly progressing global brain atrophy in a mouse model of chronic neuro-degeneration [Sirén et al. 2006]. Furthermore, EPO reduces haloperidol-induced cell death in primary hippocampal neuronal cultures [Ehrenreich et al. 2004].
Based on these grounds, we performed a doubleblind placebo-controlled multicenter trial on EPO add-on treatment in chronic schizophrenic men [Ehrenreich et al. 2007b]. Participating centers were Göttingen, Kiel, Homburg, Cologne and Marburg. Treatment over 12 weeks with high-dose weekly (40,000IU intravenously) EPO led to significant improvement of cognitive performance compared to placebo controls [Ehrenreich et al. 2007b]. Employing voxel-based morphometrical magnetic resonance imaging (MRI) analysis, we obtained first evidence that EPO treatment of chronic schizophrenic patients delays progressive cortical gray matter loss (manuscript in preparation), similar to the prevention by EPO of brain atrophy, observed in our mouse model of chronic neuro-degeneration [Sirén et al. 2006]. In contrast, we did not see any effect on psychopathology or psychosocial outcome parameters within the 3-month observation time of the human study [Ehrenreich et al. 2007b]. The fact that EPO is the first compound ever that appears to exert a beneficial effect on cognition in schizophrenia will definitely encourage further work along these lines. A treatment trial including patients with a first episode of a schizophrenic psychosis has been initiated.
Multiple sclerosis
Encouraged by the manifold neuroprotective effects of EPO in experimental models of MS [Chopp et al. 2008, Yuan et al. 2008; Savino et al. 2006; Diem et al. 2005; Zhang et al. 2005; Li et al. 2004; Sättler et al. 2004; Agnello et al. 2002; Brines et al. 2000] as well as by the success of our human trials on stroke and schizophrenia [Ehrenreich et al. 2007b; Ehrenreich et al. 2002], we initiated an investigator-driven, exploratory open label study (phase IIa) addressing patients suffering from either primary or secondary chronic progressive MS [Ehrenreich et al. 2007a]. The main objectives of this study were as follows: (1) evaluation of the safety of long-term high-dose intravenous EPO treatment in MS, and (2) collecting first evidence of potential efficacy of EPO on clinical outcome parameters most relevant for MS. We included a total of eight MS patients [five randomly assigned to high-dose (48,000 IU), three to low-dose (8,000 IU) EPO treatment] and, as disease controls, two drug-naive Parkinson's disease patients also receiving high-dose EPO. The study design comprised a 6-week lead-in phase, a 12-week treatment phase with weekly intravenous applications of EPO, another 12-week treatment phase with biweekly EPO application and a 24-week post-treatment phase (with an individual study duration of up to 1 year in total). After 12 weeks of weekly EPO treatment, a decision was made for each individual patient on continuing or terminating study participation. Qualification for study continuation required that the patient had improved upon treatment in at least three independent test items (e.g. walking distance, fine motor and bladder function). It turned out that all high-dose EPO patients fulfilled the criteria of study continuation whereas none of the low-dose patients did. Clinical and electrophysiological improvement of motor function, reflected even by a reduction in the Expanded Disability Status Score (EDSS), and improvement of cognitive performance, in particular working memory, was found upon high-dose EPO treatment in MS patients, persisting for 3–6 months after cessation of EPO application. In contrast, low-dose EPO MS patients as well as drug-naive Parkinson's disease patients receiving high-dose EPO did not improve in any of the parameters tested.
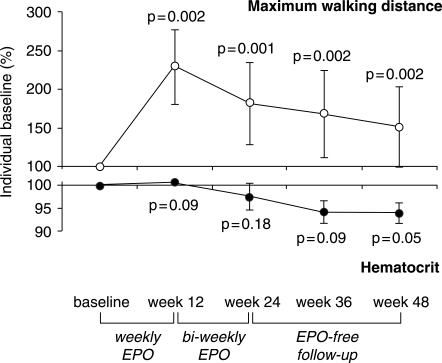
In this small exploratory trial there were no safety concerns, no study drug-related adverse events reported or observed, and a surprisingly low need for blood lettings. The infrequent requirement of blood lettings in MS upon EPO is interesting since it might reflect a relative hyporesponsiveness of the hematopoietic system to EPO as shown in other conditions of systemic latent inflammatory disease [Kwack and Balakrishnan 2006]. Altered cytokine patterns in chronic MS may well modulate the bone marrow response causing an ‘EPO nonresponder profile’. Since clear diagnosis of flaring inflammation in MS and thus the prediction of imminent disease progression are essentially impossible up to now, we suggest developing the degree of hematopoietic response of MS patients to EPO as a biological readout of occult inflammation. Estimation of required additional immunosuppression may be derived from such a test. Most interestingly, the hypo-responsiveness of the hematopoietic system as observed in our group of high-dose EPO MS patients supports the notion that the therapeutic efficacy of EPO in MS is not simply related to improved oxygen supply via increased red blood cell mass (Figure 1).
Figure 1.
Course of maximum walking distance and of hematocrit in five nonanemic, chronic progressive multiple sclerosis patients receiving high-dose erythropoietin (EPO). The mean of all available measurements of maximum walking distance of each patient obtained during the whole lead-in period was set to 100% (to provide a reliable baseline value) and used for calculating individual improvement over time. Mean change of all patients at denoted timepoints of follow-up upon treatment or during the treatment-free period was calculated from percentage individual baseline. Respective hematocrit values are presented in parallel. Note the difference in scales. The slight decrease in hematocrit was explained by strong encouragement of patients to adhere to high fluid intake. Meanis.e.m given. Statistical analysis: Friedman test.
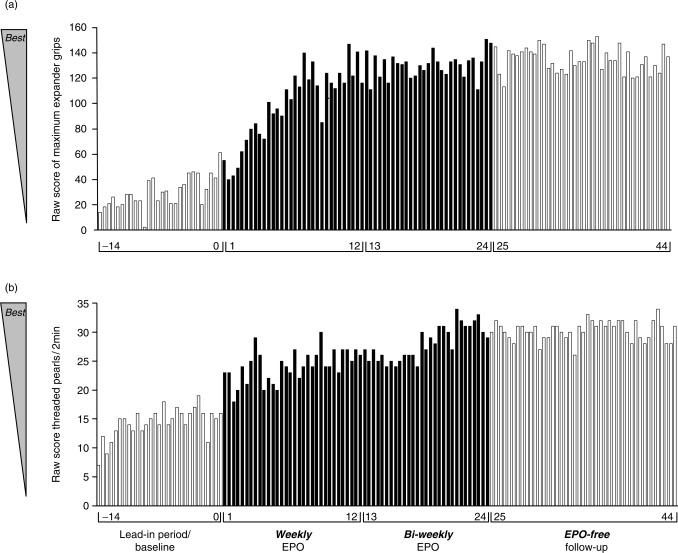
In addition to the above described results of our exploratory phase IIa trial in chronic progressive MS, we have accumulated further evidence of beneficial effects of EPO in MS over the years, based on long-term follow up of individual patients. In Figure 2, the effect of high-dose long-term EPO treatment on strength and fine motor function of paretic upper extremities is illustrated. The data were obtained from a single patient with relapsing-remitting MS who presented with considerable residual dysfunction of the sensory system (up to level C8/Th1) and severe bilateral pareses of both upper extremities, mainly affecting his hands. Despite treatment with corticosteroids, plasmapheresis, mitoxantrone and glatiramer acetate (the latter continuing over the EPO treatment period), no improvement of his situation was observed. The clinical state had been stable over half a year before EPO treatment was initiated. As illustrated in Figure 2, the EPO-free lead-in period shows a slight training effect on strength and endurance as well as on fine motor performance. Upon weekly and biweekly EPO treatment, a dramatic further improvement became obvious that persisted over the EPO-free follow-up period, similar to the results obtained in maximum walking distance in the exploratory chronic progressive MS trial [Ehrenreich et al. 2007a].
Figure 2.
Case report: Follow-up of parameters of motor function upon high-dose erythropoietin (EPO) treatment in a right-handed multiple sclerosis (MS) patient with pareses of both upper extremities. (a) motor strength/endurance of the left (nondominant, more severely affected) hand, presented as number of maximum expander grips for every test timepoint; (b) fine motor performance of the left hand, presented as number of threaded pearls over 2 min for every test timepoint. Improvement of the right hand in both tests was similar. Light columns on the left (—14 to 0 weeks) show performance during lead-in/baseline period. Dark columns (1 to 24 weeks) show performance during active-treatment phase. Light columns (25 to 44 weeks) show performance during EPO-free follow-up.
Management of hematopoietic effects and prevention of potential side effects of high-dose EPO treatment in MS
Employing EPO as neuroprotective/neuro-regenerative treatment of chronic progressive MS (particularly when using the high dose of EPO required to obtain sufficient EPO levels within the brain in a situation of predominantly intact blood–brain barrier), requires careful and comprehensive safety management. A most elaborated follow-up at all times is mandatory, including clinical examination as well as routine laboratory screening attached to each EPO application. The hematocrit (hemoglobin) has to stay within clearly defined limits. Although the necessity of blood letting in our patients was very low due to the above-described reduced response of the hematopoietic system to EPO, it is still of the utmost importance to carefully follow each and every individual and strictly initiate blood lettings in a situation of hematocrit increase over 48% in females and over 50% in males. Along the same lines, no iron substitution is allowed at any time. Iron substitution, as performed in studies targeting anemia, pushes hematopoiesis and therefore would definitely induce an undesired side-effect in non-anemic patients suffering from diseases of the nervous system. Indeed, we make use of the observation that the need for blood lettings will not perpetuate but undergo self-limitation in the absence of iron substitution. Interestingly, the fact that EPO treatment leads to temporary shifts in iron stores as delineated in our exploratory trial in chronic progressive MS [Ehrenreich et al. 2007a], might provide an additional beneficial effect in chronic progressive MS patients. Accelerated and intensified integration of iron into new red blood cells and thereby withdrawal of iron from its stores, leads to a picture similar to that of true iron deficiency. This picture is corrected after termination of EPO treatment without iron substitution. In the absence of appreciable blood loss and upon normal nutrition, iron deficiency will not occur. Binding more and more of the freely available iron, on the other hand, might additionally reduce inflammatory processes in MS and thereby contribute to beneficial effects of high-dose long-term EPO treatment. In fact, iron chelators have been proposed for treatment of MS [Lynch et al. 2000]. Disturbed iron metabolism has been described in MS patients [Sfagos et al. 2005] and iron-deficient mice fail to develop EAE [Weilbach et al. 2004; Grant et al. 2003]. Taken together, the temporary relative iron depletion induced by EPO might add to the panel of neuroprotective effects of this growth factor.
Thrombocyte (platelet) counts have also to be carefully and constantly observed. Patients with thrombocytosis have to be excluded at any time, be it upon inclusion or during treatment with EPO, which in some patients can lead to a pronounced increase in circulating platelets above normal limits. Risk patients with past thromboembolic complications have to be strictly excluded. Exsiccosis has to be prevented at any time. Immobility is contraindicated, precluding the use of this treatment in patients who are prone to bed. Furthermore, patients with cardiovascular disease or cardiovascular risk factors (diabetes, therapy-resistant hypertension, smoking, contraceptive medication) have to be excluded. Potential side-effects of EPO include increases in blood pressure as observed in some patients suffering from anemia due to renal failure [Miyashita et al. 2004; Quaschning et al. 2003; Vaziri et al. 1995; Carlini et al. 1993a; 1993b]. Although in our studies no such increases have been noted in any of our patients, blood pressure monitoring is essential and increases in blood pressure upon EPO treatment have to either be pharmacologically counteracted or should lead to exclusion of patients.
Although the exact effects of EPO on tumor growth are still unclear, patients suffering from any kind of malignancy, treated or untreated, should be strictly excluded [for review see (Jelkmann et al. 2008)].
The temporary problem of antibody formation upon EPO has essentially resolved due to changes in EPO preparations/packaging introduced by manufacturers and more careful adherence to the ‘cooling chain’ upon EPO transportation and storage. Nevertheless, antibody determinations should be performed before EPO treatment in all MS patients due to the known strong autoimmune predisposition in this population. Furthermore, potential formation of EPO antibodies has to be controlled at any time when reticulocyte counts below the normal limit are observed, and at the end of each treatment period.
To conclude, results of further proof-of-concept trials on EPO in MS should be awaited before treatment of MS patients with EPO can be recommended. In any case, careful risk and side-effect management is mandatory. Even upon potential future approval of EPO for the indication MS, these safety rules have to be carefully followed. High-dose EPO, although very well tolerated, will never be a ‘laissez-faire’ treatment in any of the neuropsychiatric patient populations.
Planning a proof-of-concept (phase IIb) trial on EPO in chronic progressive MS
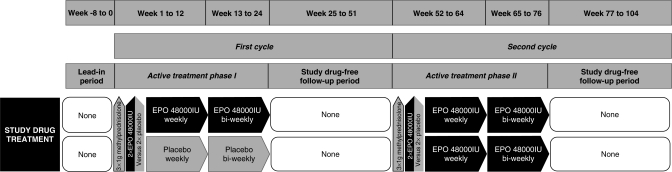
Based on the auspicious results obtained in our exploratory study on high-dose long-term EPO treatment in chronic progressive MS [Ehrenreich et al. 2007a], a pivotal trial for proof-of-concept and potential preparation of approval of EPO for treatment of MS is under planning. The design of the trial resembles in many ways the design used for the exploratory study (Figure 3). In this double-blind, placebo-controlled, randomized proof-of-concept (phase IIb) trial, EDSS will be used as a primary outcome measure, supplemented by motor and cognitive parameters as well as readouts of neurophysiology, neuro-ophthalmology and MRI as secondary outcome measures. The total duration of this study will extend to 3–4 years with an individual duration of two years, comprising two EPO treatment cycles. The second EPO cycle is planned as ‘all patients switch to verum’.
Figure 3.
Investigational drug treatment design of ‘recombinant human erythropoietin as a neuroprotective/-regenerative treatment of chronic progressive multiple sclerosis (EPO-MS): a double-blind, placebo-controlled, randomized phase IIb trial’. Patients start with an 8-week lead-in period, allowing extensive, repeated rating of baseline performance (estimation of fluctuations) ended by a short, dense ‘start-up assessment’ (including clinical, neurophysiological, neuroophthalmological, MRI, cognitive, laboratory assessments). Treatment initiation with 3 × 1000 mg methylprednisolone given on 3 consecutive days, and EPO (48,000 IU; in additional dose-finding treatment arms alternative dosing with 32,000 and 16,000 IU, respectively) vs placebo on days 2 and 3 of methylprednisolone treatment follows. Patients then receive EPO/placebo weekly for 12 weeks, biweekly for another 12 weeks and undergo a 6-month post-treatment observation period with visits every 6 weeks. In the second year all patients are switched to verum following the same schedule until individual study end after 26 months (2 + 12 + 12).
Concluding remarks
Development of EPO, EPO derivatives/analogs, EPO mimetics or EPO inducers (all together EPO variants), for improving motor function or cognitive performance in patients suffering from chronic progressive MS or other neuropsychiatric diseases, is a novel and promising field of clinical neuroscience that is still in its infancy. It will not only require further human trials but also a return to rodent studies designed to better understand the mechanisms of action of EPO. These mechanisms in turn will help to design even better treatment schedules; for example, with respect to timing of applications, duration and combination therapies to just name a few, and perhaps also to work on new EPO mimetics. Main result of the translational EPO work in the field of neuroscience at this point is that the concept of neuroprotection works in man and that its introduction into clinical practice is feasible.
Conflict of interest statement/ acknowledgment
The clinical studies reported here have been funded by the Max-Planck-Society (MPG) and by research grants from Johnson & Johnson – Ortho Biotech (Stroke). Basic research received grant support predominantly from MPG, the German Research Foundation (DFG-CMPB), Johnson & Johnson – Ortho Biotech, Lundbeck, and Roche.
Contributor Information
Claudia Bartels, Division of Clinical Neuroscience, Max-Planck-Institute of Experimental Medicine, Göttingen, Germany.
Kira Späte, Division of Clinical Neuroscience, Max-Planck-Institute of Experimental Medicine, Göttingen, Germany.
Henning Krampe, Division of Clinical Neuroscience, Max-Planck-Institute of Experimental Medicine, Göttingen, Germany.
Hannelore Ehrenreich, Ehrenreich Division of Clinical Neuroscience, Max-Planck-Institute of Experimental Medicine, Göttingen, Germany, ehrenreich@em.mpg.de.
References
- Adamcio B., Sargin D., Stradomska A., Medrihan L., Gertler C., Theis F.et al. (2008)Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnello D., Bigini P., Villa P., Mennini T., Cerami A., Brines M.L.et al. (2002)Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 952:128–134 [DOI] [PubMed] [Google Scholar]
- Banks W.A., Jumbe N.L., Farrell C.L., Niehoff M.L., Heatherington A.C.(2004)passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol 505:93–101 [DOI] [PubMed] [Google Scholar]
- Bauer C., Kurtz A.(1989)Oxygen sensing in the kidney and its relation to erythropoietin production. Annu Rev Physiol 51:845–856 [DOI] [PubMed] [Google Scholar]
- Beleslin-Cokic B.B., Cokic V.P., Yu X., Weksler B.B., Schechter A.N., Noguchi C.T.(2004)Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104:2073–2080 [DOI] [PubMed] [Google Scholar]
- Bernaudin M., Marti H.H., Roussel S., Divoux D., Nouvelot A., Mackenzie E.T.et al. (1999)A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 19:643–651 [DOI] [PubMed] [Google Scholar]
- Bianchi R., Buyukakilli B., Brines M., Savino C., Cavaletti G., Oggioni N.et al. (2004)Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA 101:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker-Meffert S., Rosenstiel P., Rohl C., Warneke N., Held-Feindt J., Sievers J.et al. (2002)Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci 43:2021–2026 [PubMed] [Google Scholar]
- Brettschneider J., Widl K., Ehrenreich H., Riepe M., Tumani H.(2006)Erythropoietin in the cerebrospinal fluid in neurodegenerative diseases. Neurosci Lett 404:347–351 [DOI] [PubMed] [Google Scholar]
- Brines M., Grasso G., Fiordaliso F., Sfacteria A., Ghezzi P., Fratelli M.et al. (2004)Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA 101:14907–14912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M.L., Ghezzi P., Keenan S., Agnello D., De Lanerolle N.C., Cerami C.et al. (2000)Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97:10526–10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W.(2005)Clinical implications of neuro-pathological findings in multiple sclerosis. J Neurol 252(Suppl. 3):iii10–iii14 [DOI] [PubMed] [Google Scholar]
- Byts N., Samoylenko A., Fasshauer T., Ivanisevic M., Hennighausen L., Ehrenreich H.et al. (2008)Essential role for Stat5 in the neurotrophic but not in the neuroprotective effect of erythropoietin. Cell Death Differ 15:783–792 [DOI] [PubMed] [Google Scholar]
- Byts N., Samoylenko A., Woldt H., Ehrenreich H., Siren A.L.(2006)Cell type specific signalling by hematopoietic growth factors in neural cells. Neurochem Res 31:1219–1230 [DOI] [PubMed] [Google Scholar]
- Campana W.M., Myers R.R.(2001)Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. Faseb J 15:1804–1806 [DOI] [PubMed] [Google Scholar]
- Carlini R., Obialo C.I., Rothstein M.(1993a)Intravenous erythropoietin (rhuepo) administration increases plasma endothelin and blood pressure in hemodialysis patients. Am J Hypertens 6:103–107 [DOI] [PubMed] [Google Scholar]
- Carlini R.G., Dusso A.S., Obialo C.I., Alvarez U.M., Rothstein M.(1993b)Recombinant human erythropoietin (rhuepo) increases endothelin-1 release by endothelial cells. Kidney Int 43:1010–1014 [DOI] [PubMed] [Google Scholar]
- Casals-Pascual C., Idro R., Gicheru N., Gwer S., Kitsao B., Gitau E.et al. (2008)High levels of erythropoietin are associated with protection against neurological sequelae in african children with cerebral malaria. Proc Natl Acad Sci USA 105:2634–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik M., Gokmen N., Erbayraktar S., Akhisaroglu M., Konakc S., Ulukus C.et al. (2002)Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99:2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.H., Tam M., Stevenson M.M.(2004)Modulation of the course and outcome of blood-stage malaria by erythropoietin-induced reticulocytosis. J Infect Dis 189:735–743 [DOI] [PubMed] [Google Scholar]
- Chikuma M., Masuda S., Kobayashi T., Nagao M., Sasaki R.(2000)Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am J Physiol Endocrinol Metab 279:1242–1248 [DOI] [PubMed] [Google Scholar]
- Chopp M., Li Y., Zhang J.(2008)Plasticity and remodeling of brain. J Neurol Sci 265:97–101 [DOI] [PubMed] [Google Scholar]
- Chung Y.H., Joo K.M., Kim Y.S., Lee K.H., Lee W.B., Cha C.I.(2004)Enhanced Expression of erythropoietin in the central nervous system of Sod1(G93a) transgenic mice. Brain Res 1016:272–280 [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A.(2002)Multiple sclerosis. Lancet 359:1221–1231 [DOI] [PubMed] [Google Scholar]
- Confavreux C., Vukusic S.(2006)Accumulation of irreversible disability in multiple sclerosis: from epidemiology to treatment. Clin Neurol Neurosurg 108:327–332 [DOI] [PubMed] [Google Scholar]
- Csete M., Rodriguez L., Wilcox M., Chadalavada S.(2004)Erythropoietin receptor is expressed on adult rat dopaminergic neurons and erythropoietin is neurotrophic in cultured dopaminer-gic neuroblasts. Neurosci Lett 359:124–126 [DOI] [PubMed] [Google Scholar]
- Diem R., Sattler M.B., Merkler D., Demmer I., Maier K., Stadelmann C.et al. (2005)Combined therapy with methylprednisolone and erythropoietin in a model of multiple sclerosis. Brain 128:375–385 [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M., Lipton S.A.(2001)Erythropoietin-mediated neuroprotection involves cross-talk between JAK2 and NF-kappab signalling cascades. Nature 412:641–647 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H., Degner D., Meller J., Brines M., Behe M., Hasselblatt M.et al. (2004)Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Molecular Psychiatry 9:42–54 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H., Fischer B., Norra C., Schellenberger F., Stender N., Stiefel M.et al. (2007a)Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 130:2577–2588 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H., Hasselblatt M., Dembowski C., Cepek L., Lewczuk P., Stiefel M.et al. (2002)Erythropoietin therapy for acute stroke is both safe and beneficial. Molecular Medicine 8:495–505 [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H., Hasselblatt M., Knerlich F., Von Ahsen N., Jacob S., Sperling S.et al. (2005)A hematopoietic growth factor, thrombopoietin, has a proapoptotic role in the brain. Proc Natl Acad Sci USA 102:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H., Hinze-Selch D., Stawick S., Aust C., Knolle-Veentjer S., Wilms S.et al. (2007b)Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Molecular Psychiatry 12:206–220 [DOI] [PubMed] [Google Scholar]
- Eid T., Brines M.L., Cerami A., Spencer D.D., Kim J.H., Schweitzer J.S.et al. (2004)Increased expression of erythropoietin receptor on blood vessels in the human epileptogenic hippocampus with sclerosis. J Neuropathol Exp Neurol 63:73–83 [DOI] [PubMed] [Google Scholar]
- Erbayraktar S., De Lanerolle N., De Lotbiniere A., Knisely J.P., Erbayraktar Z., Yilmaz O.et al. (2006)Carbamylated erythropoietin reduces radiosurgically-induced brain injury. Mol Med 12:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbayraktar S., Grasso G., Sfacteria A., Xie Q.W., Coleman T., Kreilgaard M.et al. (2003)Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA 100:6741–6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Steinman L.(2005)Design of effective immunotherapy for human autoimmunity. Nature 435:612–619 [DOI] [PubMed] [Google Scholar]
- Fisher J.W.(2003)Erythropoietin: physiology and pharmacology update. Exp Biol Med 228:1–14 [DOI] [PubMed] [Google Scholar]
- Flachenecker P., Stuke K., Elias W., Freidel M., Haas J., Pitschnau-Michel D.et al. (2008)Multiple-Sklerose-Register in Deutschland: Ausweitung des Projektes 2005/2006. Deutsches Ärzteblatt 105:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachenecker P., Zettl U.K., Gotze U., Haas J., Schimrigk S., Elias W.et al. (2005)[Ms registry in Germany - design and first results of the pilot phase]. Nervenarzt 76:967–975 [DOI] [PubMed] [Google Scholar]
- Genc S., Akhisaroglu M., Kuralay F., Genc K.(2002)Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyri-dine-induced neurotoxicity in C57bl mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett 321:73–76 [DOI] [PubMed] [Google Scholar]
- Genc S., Kuralay F., Genc K., Akhisaroglu M., Fadiloglu S., Yorukoglu K.et al. (2001)Erythropoietin exerts neuroprotection in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/Bl mice via increasing nitric oxide production. Neurosci Lett 298:139–141 [DOI] [PubMed] [Google Scholar]
- Giovannoni G.(2004)Management of secondary-progressive multiple sclerosis. CNS Drugs 18:653–669 [DOI] [PubMed] [Google Scholar]
- Gold R., Linington C., Lassmann H.(2006)Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129:1953–1971 [DOI] [PubMed] [Google Scholar]
- Gorio A., Gokmen N., Erbayraktar S., Yilmaz O., Madaschi L., Cichetti C.et al. (2002)Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA 99:9450–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S.M., Wiesinger J.A., Beard J.L., Cantorna M.T.(2003)Iron-deficient mice fail to develop autoimmune encephalomyelitis. J Nutr 133:2635–2638 [DOI] [PubMed] [Google Scholar]
- Grasso G., Buemi M., Alafaci C., Sfacteria A., Passalacqua M., Sturiale A.et al. (2002)Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc Natl Acad Sci USA 99:5627–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignaschi G., Zennaro E., Tortarolo M., Calvaresi N., Bendotti C.(2007)Erythropoietin does not preserve motor neurons in a mouse model of familial ALS. Amyotroph Lateral Scler 8:31–35 [DOI] [PubMed] [Google Scholar]
- Grimm C., Wenzel A., Groszer M., Mayser H., Seeliger M., Samardzija M.et al. (2002)HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med 8:718–724 [DOI] [PubMed] [Google Scholar]
- Grunfeld J.F., Barhum Y., Blondheim N., Rabey J.M., Melamed E., Offen D.(2007)Erythropoietin delays disease onset in an amyotrophic lateral sclerosis model. Exp Neurol 204:260–263 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Oksenberg J.R.(2006)The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron 52:61–76 [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Wekerle H.(2004)Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc Natl Acad Sci USA 101(Suppl. 2):14599–14606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W.(1992)Erythropoietin: structure, control of production, and function. Physiol Rev 72:449–489 [DOI] [PubMed] [Google Scholar]
- Jelkmann W.(2007)Erythropoietin after a century of research: younger than ever. Eur J Haematol 78:183–205 [DOI] [PubMed] [Google Scholar]
- Jelkmann W.(2008)Developments in the therapeutic use of erythropoiesis stimulating agents. Br J Haematol 141:287–297 [DOI] [PubMed] [Google Scholar]
- Jelkmann W., Bohlius J., Hallek M., Sytkowski A.J.(2008)The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol 67:39–61 [DOI] [PubMed] [Google Scholar]
- Jubinsky P.T., Krijanovski O.I., Nathan D.G., Tavernier J., Sieff C.A.(1997)The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood 90:1867–1873 [PubMed] [Google Scholar]
- Juul S.E., Anderson D.K., Li Y., Christensen R.D.(1998)Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res 43:40–49 [DOI] [PubMed] [Google Scholar]
- Kaiser K., Texier A., Ferrandiz J., Buguet A., Meiller A., Latour C.et al. (2006)Recombinant human erythropoietin prevents the death of mice during cerebral malaria. J Infect Dis 193:987–995 [DOI] [PubMed] [Google Scholar]
- Kertesz N., Wu J., Chen T.H., Sucov H.M., Wu H.(2004)The role of erythropoietin in regulating angiogenesis. Dev Biol 276:101–110 [DOI] [PubMed] [Google Scholar]
- Keswani S.C., Buldanlioglu U., Fischer A., Reed N., Polley M., Liang H.et al. (2004)A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol 56:815–826 [DOI] [PubMed] [Google Scholar]
- Kilic E., Kilic U., Soliz J., Bassetti C.L., Gassmann M., Hermann D.M.(2005)Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and AKT pathways. Faseb J 19:2026–2028 [DOI] [PubMed] [Google Scholar]
- Knabe W., Knerlich F., Washausen S., Kietzmann T., Siren A.L., Brunnett G.et al. (2004)Expression patterns of erythropoietin and its receptor in the developing midbrain.Anat Embryol (Berl) 207:503–512 [DOI] [PubMed] [Google Scholar]
- Knabe W., Siren A.L., Ehrenreich H., Kuhn H.J.(2005)Expression patterns of erythro-poietin and its receptor in the developing spinal cord and dorsal root ganglia.Anat Embryol (Berl) 210:209–219 [DOI] [PubMed] [Google Scholar]
- Koh S.H., Kim Y., Kim H.Y., Cho G.W., Kim K.S., Kim S.H.(2007)Recombinant human erythro-poietin suppresses symptom onset and progression of G93a-Sod1 mouse model of ALS by preventing motor neuron death and inflammation. Eur J Neurosci 25:1923–1930 [DOI] [PubMed] [Google Scholar]
- Konishi Y., Chui D.H., Hirose H., Kunishita T., Tabira T.(1993)Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res 609:29–35 [DOI] [PubMed] [Google Scholar]
- Kwack C., Balakrishnan V.S.(2006)Managing erythropoietin hyporesponsiveness. Semin Dial 19:146–151 [DOI] [PubMed] [Google Scholar]
- Leist M., Ghezzi P., Grasso G., Bianchi R., Villa P., Fratelli M.et al. (2004)Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305:239–242 [DOI] [PubMed] [Google Scholar]
- Lewczuk P., Hasselblatt M., Kamrowski-Kruck H., Heyer A., Unzicker C., Siren A.L.et al. (2000)Survival of hippocampal neurons in culture upon hypoxia: effect of erythropoietin. Neuroreport 11:3485–3488 [DOI] [PubMed] [Google Scholar]
- Li W., Maeda Y., Yuan R.R., Elkabes S., Cook S., Dowling P.(2004)Beneficial effect of erythro-poietin on experimental allergic encephalomyelitis. Ann Neurol 56:767–777 [DOI] [PubMed] [Google Scholar]
- Livnah O., Stura E.A., Middleton S.A., Johnson D.L., Jolliffe L.K., Wilson I.A.(1999)Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283:987–990 [DOI] [PubMed] [Google Scholar]
- Lynch S.G., Fonseca T., Levine S.M.(2000)A multiple course trial of desferrioxamine in chronic progressive multiple sclerosis.Cell Mol Biol (Noisy-le-grand) 46:865–869 [PubMed] [Google Scholar]
- Marti H.H., Wenger R.H., Rivas L.A., Straumann U., Digicaylioglu M., Henn V.et al. (1996)Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci 8:666–676 [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada O.M., Rodriguez-Millan E., Gonzalez-De Vicente E., Reina M., Vilaro S., Fabre M.(2003)Erythropoietin protects the in vitro blood-brain barrier against vegf-induced permeability. Eur J Neurosci 18:2538–2544 [DOI] [PubMed] [Google Scholar]
- Masuda S., Nagao M., Takahata K., Konishi Y., Gallyas F., Tabira T.et al. (1993)Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem 268:11208–11216 [PubMed] [Google Scholar]
- Masuda S., Okano M., Yamagishi K., Nagao M., Ueda M., Sasaki R.(1994)A Novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem 269:19488–19493 [PubMed] [Google Scholar]
- Miyashita K., Tojo A., Kimura K., Goto A., Omata M., Nishiyama K.et al. (2004)Blood pressure response to erythropoietin injection in hemodialysis and predialysis patients. Hypertens Res 27:79–84 [DOI] [PubMed] [Google Scholar]
- Noseworthy J.H.(2003)Management of multiple sclerosis: current trials and future options. Curr Opin Neurol 16:289–297 [DOI] [PubMed] [Google Scholar]
- Park M.H., Lee S.M., Lee J.W., Son D.J., Moon D.C., Yoon D.Y.et al. (2006)ERK-mediated production of neurotrophic factors by astrocytes promotes neuronal stem cell differentiation by erythropoietin. Biochem Biophys Res Commun 339:1021–1028 [DOI] [PubMed] [Google Scholar]
- Pugliatti M., Rosati G., Carton H., Riise T., Drulovic J., Vecsei L.et al. (2006)The epidemiology of multiple sclerosis in Europe. Eur J Neurol 13:700–722 [DOI] [PubMed] [Google Scholar]
- Quaschning T., Ruschitzka F., Stallmach T., Shaw S., Morawietz H., Goettsch W.et al. (2003)Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. Faseb J 17:259–261 [DOI] [PubMed] [Google Scholar]
- Rovaris M., Confavreux C., Furlan R., Kappos L., Comi G., Filippi M.(2006)Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol 5:343–354 [DOI] [PubMed] [Google Scholar]
- Sadamoto Y., Igase K., Sakanaka M., Sato K., Otsuka H., Sakaki S.et al. (1998)Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun 253:26–32 [DOI] [PubMed] [Google Scholar]
- Sakanaka M., Wen T.C., Matsuda S., Masuda S., Morishita E., Nagao M.et al. (1998)In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA 95:4635–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sättler M.B., Merkler D., Maier K., Stadelmann C., Ehrenreich H., Bahr M.et al. (2004)Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ 11(Suppl. 2):S181–192 [DOI] [PubMed] [Google Scholar]
- Savino C., Pedotti R., Baggi F., Ubiali F., Gallo B., Nava S.et al. (2006)Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalo-myelitis. J Neuroimmunol 172:27–37 [DOI] [PubMed] [Google Scholar]
- Sfagos C., Makis A.C., Chaidos A., Hatzimichael E.C., Dalamaga A., Kosma K.et al. (2005)Serum ferritin, transferrin and soluble trans-ferrin receptor levels in multiple sclerosis patients. Mult Scler 11:272–275 [DOI] [PubMed] [Google Scholar]
- Sharp F.R., Bernaudin M.(2004)Hif1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448 [DOI] [PubMed] [Google Scholar]
- Shingo T., Sorokan S.T., Shimazaki T., Weiss S.(2001)Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 21:9733–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén A.L., Fratelli M., Brines M., Goemans C., Casagrande S., Lewczuk P.et al. (2001a)Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 98:4044–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén A.L., Knerlich F., Poser W., Gleiter C.H., Brück W., Ehrenreich H.(2001b)Erythropoietin and erythropoietin receptor in human ischemic/ hypoxic brain. Acta Neuropathol 101:271–276 [DOI] [PubMed] [Google Scholar]
- Sirén A.L., Radyushkin K., Boretius S., Kämmer D., Riechers C.-C., Natt O.et al. (2006)Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain 129:480–489 [DOI] [PubMed] [Google Scholar]
- Springborg J.B., Ma X., Rochat P., Knudsen G.M., Amtorp O., Paulson O.B.et al. (2002)A single subcutaneous bolus of erythropoietin normalizes cerebral blood flow autoregulation after subarachnoid haemorrhage in rats. Br J Pharmacol 135:823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P.T., Ohab J.J., Kertesz N., Groszer M., Matter C., Gao J.et al. (2006)A critical role of erythropoietin receptor in neurogenesis and post—stroke recovery. J Neurosci 26:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri N.D., Zhou X.J., Smith J., Oveisi F., Baldwin K., Purdy R.E.(1995)In vivo and in vitro pressor effects of erythropoietin in rats. Am J Physiol 269:F838–845 [DOI] [PubMed] [Google Scholar]
- Villa P., Bigini P., Mennini T., Agnello D., Laragione T., Cagnotto A.et al. (2003)Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med 198:971–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbach F.X., Chan A., Toyka K.V., Gold R.(2004)The cardioprotector dexrazoxane augments therapeutic efficacy of mitoxantrone in experimental autoimmune encephalomyelitis. Clin Exp Immunol 135:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar C., Kraywinkel K., Maschke M., Diener H.C.(2006)Intravenous thrombolysis in German stroke units before and after regulatory approval of recombinant tissue plasminogen activator. Cerebrovasc Dis 22:429–431 [DOI] [PubMed] [Google Scholar]
- Weishaupt J.H., Rohde G., Polking E., Siren A.L., Ehrenreich H., Bahr M.(2004)Effect of erythropoietin axotomy-induced apoptosis in ratretinal ganglion cells. Invest Ophthalmol Vis Sci 45:1514–1522 [DOI] [PubMed] [Google Scholar]
- Wiese L., Hempel C., Penkowa M., Kirkby N., Kurtzhals J.A.(2008)Recombinant human erythro-poietin increases survival and reduces neuronal apoptosis in a murine model of cerebral malaria. Malar J 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk D.M., Noseworthy J.H.(2002)Randomized controlled trials to assess therapies for multiple sclerosis. Neurology 58:S40–48 [DOI] [PubMed] [Google Scholar]
- Xenocostas A., Cheung W.K., Farrell F., Zakszewski C., Kelley M., Lutynski A.et al. (2005)The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol 61:189–195 [DOI] [PubMed] [Google Scholar]
- Yu X., Shacka J.J., Eells J.B., Suarez-Quian C., Przygodzki R.M., Beleslin-Cokic B.et al. (2002)Erythropoietin receptor signalling is required for normal brain development. Development 129:505–516 [DOI] [PubMed] [Google Scholar]
- Yuan R., Maeda Y., Li W., Lu W., Cook S., Dowling P.(2008)Erythropoietin: a potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS ONE 3:e1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li Y., Cui Y., Chen J., Lu M., Elias S.B.et al. (2005)Erythropoietin Treatment Improves Neurological Functional Recovery in EAE Mice. Brain Res 1034:34–39 [DOI] [PubMed] [Google Scholar]