Abstract
Helicobacter pylori infection is the main known cause of gastritis, gastroduodenal ulcer disease and gastric cancer. After more than 20 years of experience in H. pylori treatment, however, the ideal regimen to treat this infection has still to be found. Nowadays, apart from having to know well first-line eradication regimens, we must also be prepared to face treatment failures. Therefore, in designing a treatment strategy we should not focus on the results of primary therapy alone, but also on the final (overall) eradication rate. The choice of a ‘rescue’ treatment depends on which treatment is used initially. If a first-line clarithromycin-based regimen was used, a second-line metronidazole-based treatment (quadruple therapy) may be used afterwards, and then a levofloxacin-based combination would be a third-line ‘rescue’ option. Alternatively, it has recently been suggested that levofloxacin-based ‘rescue’ therapy constitutes an encouraging second-line strategy, representing an alternative to quadruple therapy in patients with previous PPI-clarithromycin-amoxicillin failure, with the advantage of efficacy, simplicity and safety. In this case, quadruple regimen may be reserved as a third-line ‘rescue’ option. Finally, rifabutin-based ‘rescue’ therapy constitutes an encouraging empirical fourth-line strategy after multiple previous eradication failures with key antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline, and levofloxacin. Even after two consecutive failures, several studies have demonstrated that H. pylori eradication can finally be achieved in almost all patients if several ‘rescue’ therapies are consecutively given. Therefore, the attitude in H. pylori eradication therapy failure, even after two or more unsuccessful attempts, should be to fight and not to surrender.
Keywords: eradication, Helicobacter pylori, levofloxacin, rescue, rifabutin, salvage
Introduction
Helicobacter pylori infection is the main known cause of gastritis, gastroduodenal ulcer disease and gastric cancer. After more than 20 years of experience in H. pylori treatment, however, the ideal regimen to treat this infection has still to be found [Vakil, 2009]. Consensus conferences have recommended therapeutic regimens that achieve H. pylori cure rates higher than 80% on an intention-to-treat basis [Malfertheiner et al. 2007, 2002; Howden et al. 1998]. However, several large clinical trials and meta-analyses have shown that the most commonly used first-line therapies — including proton-pump inhibitors (PPIs) plus two antibiotics — may fail in up to 20% of patients [Gisbert et al. 2007d, 2000b], and in the clinical routine setting, the treatment failure rate might be even higher. Moreover, during the last few years, the efficacy of PPI-based regimens seems to be decreasing, and several studies have reported intention-to-treat eradication rates lower than 75% [Paoluzi et al. 2006; Calvet et al. 2005; Gisbert et al. 2005b; Vakil et al. 2004; Hawkey et al. 2003; Veldhuyzen Van Zanten et al. 2003; Laine et al. 2000, 1998] and even lower than 50% [Altintas et al. 2004; Gumurdulu et al. 2004; Della Monica et al. 2002]. Antibiotic resistance to clarithromy-cin has been identified as one of the major factors affecting our ability to cure H. pylori infection, and the rate of resistance to this antibiotic seems to be increasing in many geographical areas [Egan et al. 2008; Megraud, 2004; Vakil et al. 1998].
Papers dealing with retreatment of H. pylori after failure are difficult to analyze due to several reasons [Axon, 2000]. Firstly, patients who fail with their first treatment probably include a higher percentage of individuals who are unreliable tablet takers, others who have resistant organisms and also the ‘constitutional’ group, where failure will be inevitable. On the other hand, some patients submitted for ‘rescue’ therapy have already had more than one previous treatment for H. pylori, and this circumstance is not always clarified in the protocols. Furthermore, the original primary treatments vary among the different studies, not only with respect to the antibiotic type, but also in respect to the dose and duration of the regimen. Finally, only a few studies have directly compared, in the same protocol, two or more second-line therapies [Gisbert, 2008; Gisbert and Pajares, 2002, 2005].
Several ‘rescue’ therapies have been recommended, but they still fail to eradicate H. pylori in more than 20% of the cases [Gisbert and Pajares, 2002], and these patients constitute a therapeutic dilemma [Gisbert, 2008; Gisbert and Pajares, 2005]. Patients who are not cured with two consecutive treatments including clari-thromycin and metronidazole will have at least single, and usually double, resistance [Romano et al. 2008; Megraud, 2004]. Furthermore, bismuth salts are not available worldwide anymore and, therefore, management of first-line eradication failures is becoming challenging. Currently, a standard third-line therapy is lacking, and European guidelines recommend culture in these patients to select a third-line treatment according to microbial sensitivity to antibiotics [Malfertheiner et al. 2007, 2002]. However, cultures are often carried out only in research centers, and the use of this procedure as ‘routine practice’ in patients who failed several treatments seems not to be feasible [Gisbert, 2008; Gisbert and Pajares, 2005; Zullo et al. 2003b; Gisbert and Pajares, 2002]. Therefore, the evaluation of drugs without cross-resistance to nitroimidazole or macrolides as components of retreatment combination therapies would be worthwhile [Graham, 2009].
Probiotics have been proposed as a useful adjunct. Some studies prescribing probiotics with H. pylori eradication therapy had no effect on the side-effect profile but did increase the rates of eradication [Kim et al. 2008]. However, other studies on concurrent probiotic administration suggested the inverse with better side-effect profiles but no increase in eradication or rates of compliance with therapy [Cremonini et al. 2002].
All these issues are important at the present time, but they will be even more relevant in a near future, as therapy for H. pylori infection is becoming more and more frequently prescribed. Therefore, the evaluation of second or third ‘rescue’ regimens for these problematic cases seems to be worthwhile. In designing a treatment strategy we should not focus on the results of primary therapy alone; an adequate strategy for treating this infection should use several therapies which, if consecutively prescribed, come as close to the 100% cure rate as possible [Calvet et al. 2001; De Boer and Tytgat, 2000; Gisbert, 2008; Gisbert et al. 2005a; Gisbert and Pajares, 2002].
The aim of the present manuscript will be to review the experience dealing with ‘nonrespon-ders’ to H. pylori eradication therapy. As, at present, the current most prescribed first-line regimens include a combination of PPI plus two antibiotics, the present review will focus only in ‘rescue’ regimen when these triple combinations fail. Bibliographical searches were performed in the PubMed (Internet) database including studies available until July 2009, looking for the following words (all fields): pylori AND (retreatment OR re-treatment OR rescue OR failure OR salvage OR second-line OR third-line OR fourth-line). References of reviews on H. pylori eradication treatment, and from the articles selected for the study, were also examined in search of articles meeting inclusion criteria (i.e. dealing with H. pylori ‘rescue’ therapies).
Is it necessary to perform culture after failure of the first eradication treatment?
Pretreatment antibiotic resistance is the most important factor in nonresponse to initial treatment [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Van Der Wouden et al. 1999; Tompkins et al. 1997]. Thus, the choice of a second-line treatment depends on which treatment was used initially, as it would appear that retreatment with the same regimen cannot be recommended [Parente et al. 2003]. If a clari-thromycin-based regimen was used, a metronida-zole-based treatment (or at least a clarithromycin-free regimen) should be used afterwards, and vice versa [Battaglia et al. 1998]. This recommendation is based on the observation that acquired bacterial resistance to metro-nidazole or clarithromycin results primarily from the previous treatment failure [Kobayashi et al. 2006; Peitz et al. 1999; Deltenre et al. 1998], and therefore ‘rescue’ therapies should avoid these antibiotics and use different combinations.
An antimicrobial susceptibility test for H. pylori before second-line treatment is sometimes performed, although whether the test is truly necessary remains unknown. Some authors have evaluated the efficacy of susceptibility-guided versus empiric retreatment for H. pylori after a treatment failure. In the study by Yahav et al. [2006a], patients in whom at least one treatment regimen for H. pylori eradication had failed underwent gastric biopsy and culture, and were retreated according to the in vitro susceptibility results. Findings were compared with those for control patients (where culture was unavailable). Susceptibility-guided retreatment was associated with better eradication rates (86%) than empiric treatment (63%). However, several methodological drawbacks exist in this study. Firstly, more than 50% of the patients received first-line eradication treatment with both clarithromycin and metronidazole (instead of including clarithromy-cin and amoxicillin), which is not the generally recommended combination; consequently, no logical empirical treatment remained afterwards (levofloxacin-based regimens were not available at that time). In this respect, when only the eradication rates in control (culture unavailable) patients treated with a first regimen of PPI-amoxicillin-clarithromycin followed by a second empirical quadruple regimen were considered (the generally recommended first- and second-line strategies), the success figures were not significantly different from those reported in patients receiving susceptibility-guided retreat-ment. Secondly, because this study was nonran-domized, there might have been heterogeneity among the two groups with respect to the treatment regimens prescribed by the treating physicians. Finally, this study was limited by the lack of susceptibility data for the controls, which restricted the ability to analyze the reasons why empiric therapy did not work as well as the susceptibility-guided protocol.
In a French multicenter study [Lamouliatte et al. 2003], patients in whom one previous H. pylori eradication therapy (mainly with PPI-amoxicillinclarithromycin) has failed were randomized to receive one of three empirical triple therapy regimens or a strategy based on antibiotic susceptibility. The empirical regimens were PPI-amoxicillin-clarithromycin (for 7 or 14 days) or PPI-amoxicillin-metronidazole (for 14 days).
In the susceptibility-based strategy, patients with clarithromycin-susceptible strains received PPI-amoxicillin-clarithromycin, whilst the others received PPI-amoxicillin-metronidazole. The eradication rates for empirical therapies were low, while the cure rate was higher (74%) for the susceptibility-based treatment. If the H. pylori strain was clarithromycin-susceptible (which occurred in approximately one-third of the cases), a high success rate was obtained with the PPI-clarithromycin-amoxicillin ‘rescue’ regimen. The study, however, was done in France, where bismuth is banned, so that the use of quadruple therapy with a PPI, bismuth, tetracycline and metronidazole, as recommended by the updated Maastricht Consensus Report [Malfertheiner et al. 2007], was not tested. In fact, as will be reviewed later, several studies have obtained relatively good results with this quadruple regimen empirically prescribed, with mean eradication rate of 77% (i.e. a similar figure than the 74% achieved for the susceptibility-based treatment in the present study). Thus, in this study, instead of not readministering any of the antibiotics against which H. pylori has probably become resistant, the authors insist on prescribing again clarithromycin (or metronida-zole) for the second-line treatment. Furthermore, statistically significant differences were not demonstrated when comparing the efficacy of the empirical PPI-amoxicillin-metronidazole and the susceptibility-based strategy, suggesting that the metronidazole-based combination may be an effective empirical alternative after failure of a clarithromycin-based combination.
In the updated Maastricht Consensus Report [Malfertheiner et al. 2007], it was recommended that culture and antimicrobial sensitivity testing should be routinely performed only after two treatment failures with different antibiotics. According to this statement, some studies have suggested that an antimicrobial susceptibility test for H. pylori before administering second-line treatment is not necessary. In this respect, in the study by Avidan et al. [2001], after failure of first-line eradication treatment, half of the patients were randomly assigned to treatment with a different PPI-based triple regimen regardless of the culture obtained, and the other half were assigned to treatment with PPI and two antibacterial agents chosen according to a susceptibility test; the authors found that the culture results did not influence the treatment protocol employed. Similarly, in the study by Miwa et al. [2003], patients with H. pylori infection for whom first-line treatment with a PPI-amoxicillin-clarithromycin regimen had failed were randomly assigned to two groups: those having or not having the susceptibility test before retreatment. For those patients in the susceptibility-test group, the authors used what they considered the best regimen based on susceptibility testing; while for those patients in the group with no susceptibility testing, PPI-amoxicillin-metronidazole was prescribed. The cure rates in the groups with and without susceptibility testing were not different.
Second-line H. pylori ‘rescue’ therapy after failure of one eradication treatment
‘Rescue’ regimen after PPI-clarithromycin-amoxicillin failure
PPI, amoxicillin and metronidazole. After failure of a combination of PPI, amoxicillin and clari-thromycin, a theoretically correct alternative would be the use, as second option, of other PPI-based triple therapy including amoxicillin (that does not induce resistance) and metronida-zole (an antibiotic not used in the first trial), and several authors have reported encouraging results with this strategy [Ueki et al. 2009; Murakami et al. 2008, 2006; Shirai et al. 2007; Matsuhisa et al. 2006; Nagahara et al. 2004; Shimoyama et al. 2004; Isomoto et al. 2003; Miwa et al. 2003]. However, in our experience, when this therapy has been administered twice-daily for 1 week, eradication rates lower than 50% have been obtained [Gisbert et al. 1999a]; the subsequently use of higher (three times per day) antibiotic doses, was followed only by a mild increase in eradication rate (58%), which was still unacceptable [Gisbert et al. 1999a]. However, if ranitidine bismuth citrate (RBC) is used instead of PPI, also plus amoxicillin and nitroimidazole, encouraging results have been reported (81% cure rate), although in this protocol antibiotics were administered for 14 instead of 7 days [Perri et al. 2001b]. In this same study, the readministration of clarithromycin, even when coprescribed with RBC, was associated with poor eradication rates. In the same way, Nagahara et al. [2001] studied a group of patients who, after failure of first-line PPI-clarithromycin-amoxicillin therapy, had received second-line therapy with the same regimen (for 14 days) or had received PPI-amoxicillin-metronidazole (for 10 days). The eradication rates for second-line therapy with the same regimen (thus readminis-tering clarithromycin) was of only 53%, while it was of 81% with PPI-amoxicillin-metronidazole. These observations underlie the idea that antibiotics, and specifically clarithromycin, should not be readministered in successive treatments.
Quadruple therapy. Another alternative, the use of a quadruple regimen (i.e. PPI, bismuth, tetra-cycline and metronidazole), has been generally used as the optimal second-line therapy after PPI-clarithromycin-amoxicillin failure, and has been the recommended ‘rescue’ regimen in several guidelines [Malfertheiner et al. 2007; Gisbert et al. 2000a; Lam and Talley, 1998]. Several studies have obtained relatively good results with this quadruple regimen, the results being summarized in Table 1 [Usta et al. 2008; Uygun et al. 2008; Chung et al. 2007; Navarro-Jarabo et al. 2007; Choung et al. 2006, Wu et al. 2006; Lee et al. 2005, 1999; Marko et al. 2005; Bilardi et al. 2004; Nista et al. 2003; Orsi et al. 2003; Perri et al. 2003, 2001a; Wong et al. 2003; Baena Diez et al. 2000; Michopoulos et al. 2000; Sicilia et al. 2000; Gasbarrini et al. 2000b; Gomollon et al. 1999; Gisbert et al. 1999a, 1999b; Elizalde et al. 1998]. Thus, the weighted mean eradication rate with this ‘rescue’ therapy, calculated from the studies included in the table, is of 77%. In this combination regimen, PPI should be prescribed in the usual dose and twice a day, colloidal bismuth subcitrate 120 mg four times per day, tetracycline 500 mg four times per day, and metronidazole is probably best prescribed at high doses (i.e. 500 mg three times per day). Precisely, the study with the lowest efficacy [Gisbert et al. 1999b] administered metronida-zole at low doses (250mg four times per day). Limited experience suggests that quadruple therapy may also be effective when the first (failed) regimen included RBC instead PPI. Thus, Beales [2001] reported that four of the five patients initially failing RBC-clarithromycin-amoxicillin therapy were successfully treated with quadruple therapy. A 7-day treatment duration seems to be sufficient when quadruple therapy is used after a failed first regimen, as quite similar eradication rates with 7, 10 and 14 days have been reported (mean figures, calculated from Table 1, of 74%, 72% and 81%, respectively). Furthermore, in a recent retrospective study, patients who failed to the standard triple therapy (PPI, amoxicillin, clarithromycin) received 1 or 2 weeks quadruple therapy, and the eradication rate was similar between the two regimens [Choung et al. 2006].
Table 1.
Eradication rates with quadruple therapy (proton-pump inhibitor, bismuth, tetra-cycline and a nitroimidazole) as ‘rescue’ therapy for proton-pump inhibitor-clarithromycin-amoxicillin failure.
| Author | Number of patients | Duration (d) | Eradication rate (%) |
| Baena et al. [2000] | 31 | 14 | 90 |
| Bilardi et al. [2004] | 46 | 7 | 37 |
| Elizalde et al. [1998] | 31 | 7 | 87 |
| Choung et al. [2006] | 56 | 7 | 77 |
| Choung et al. [2006] | 99 | 14 | 88 |
| Chung et al. [2007] | 87 | 7 | 84 |
| Gasbarrini et al. [2000b] | 9 | 7 | 88 |
| Gisbert et al. [1999b] | 30 | 7 | 57 |
| Gisbert et al. [1999a] | 9 | 7 | 78 |
| Gomollon et al. [1999] | 21 | 7 | 95 |
| Lee et al. [1999] | 20 | 7 | 68 |
| Lee et al. [2005] | 63 | 7 | 75 |
| Marko et al. [2005] | 27 | 7 | 63 |
| Michopoulos et al. [2000] | 38 | 14 | 76 |
| Navarro-Jarabo et al. [2007] | 54 | 7 | 70 |
| Nista et al. [2003] | 70 | 7 | 63 |
| Nista et al. [2003] | 70 | 14 | 68 |
| Orsi et al. [2003] | 50 | 12 | 88 |
| Perri et al. [2001a] | 45 | 10 | 67 |
| Perri et al. [2003] | 60 | 7 | 83 |
| Sicilia et al. [2000] | 21 | 10 | 83 |
| Usta et al. [2008] | 89 | 14 | 67 |
| Uygun et al. [2008] | 100 | 14 | 82 |
| Wong et al. [2003] | 53 | 7 | 91 |
| Wu et al. [2006] | 47 | 7 | 77 |
Eradication rates by intention-to-treat analysis when available. H. pylori eradication rate (weighted mean) with quadruple therapy: 77%.
These results are in agreement with those reported previously with quadruple therapy as first-line regimen, where 1-week therapy appeared sufficient, and prolonging treatment did not increase efficacy [De Boer et al. 1994]. Finally, although PPIs are generally prescribed as the anti-secretors in quadruple therapy, some authors have shown, in a randomized study, that omeprazole 20 mg twice daily. and ranitidine 300 mg twice daily. were equally effective as antisecretory agents combined in a second-line quadruple eradication regimen after failure with previous regimens without metronidazole (although the power of the study to find statistically significant differences was limited) [Michopoulos et al. 2000]. Nevertheless, these regimens were administered during 14 days and, therefore, remains to be demonstrated if the equivalence between both antisecretors — PPIs and H2-blockers — is also observable with 7-day regimens.
The question may be suggested whether treatment with PPI-clarithromycin-amoxicillin followed by ‘rescue’ with quadruple therapy if failure is preferable to the inverse strategy. To analyze this interesting aspect, Gomollon et al. [2000b] randomized consecutive patients to one of two strategies: (1) treatment during 7 days with quadruple therapy, and if failure second-line treatment with omeprazole-clarithromycin-amoxicillin during 7 days; and (2) initial treatment with omeprazole-clarithromycin-amoxicillin and if failure treatment with quadruple therapy. Direct and indirect costs were estimated, and a cost-effectiveness analysis using a decision-tree model was undertaken after real clinical data. Eradication was obtained (intention-to-treat) in 73% with the first strategy, versus 92% with the second one. Furthermore, cost per case eradicated was lower in the second group (320 versus 296 euros). However, in a similar but more recent study, Marko et al. [2005] assessed the usefulness and the cost-effectiveness of these two treatment strategies, performing a decision analysis. The effectiveness of ‘triple first’ and ‘quadruple first’ strategies was similar, although the latter seemed slightly more cost-effective.
RBC, tetracycline and metronidazole. More recently, it has been reported that replacing the PPI and the bismuth compound of the quadruple therapy by RBC also achieves good results as ‘rescue’ regimen [Gisbert et al. 2007a, 2005c, 1999; Koksal et al. 2005; Hojo et al. 2001, Zullo et al. 2001a; Rinaldi et al. 1999]. RBC is a compound that has, on one hand, the antise-cretory activity of ranitidine, and, on the other, the mucosal protective and anti-H. pylori effects of certain other bismuth salts [Gisbert et al. 2005d, 1999c, Van Oijen et al. 2000]. To date, several studies have evaluated 7—14 day RBC-based second-line regimens after PPI-based triple therapy failures, achieving encouraging results, with eradication rates of 67% [Gisbert et al. 2005c], 68% [Gisbert et al. 2007a], 76% [Thong-Ngam and Mahachai, 2006], 82% [Rinaldi et al. 1999], 83% [Gisbert et al. 1999b], 86% [Koksal et al. 2005] and 96% [Zullo et al. 2001a]. Furthermore, one randomized study has demonstrated that triple RBC-based therapy, when prescribed to patients with previous PPI-clarithromycin-amoxicillin failure, achieves even a higher efficacy than quadruple therapy, with additional advantages of a lower number of drugs and a simpler dose scheme [Gisbert et al. 1999b]. Nevertheless, the eradication rate with quadruple regimen in this last study was remarkably low, which was possibly explained by the low dose of metronidazole prescribed. The favorable results obtained with RBC in aforementioned studies are probably explained, at least in part, by the fact that RBC-based therapies may overcome the impact of metronidazole-resistant and clarithromycin-resistant strains on H. pylori eradication treatment [Gisbert et al. 2005d, 1999c; Megraud, 2004; Van Oijen et al. 2001]. In summary, due to the aforementioned encouraging results, quadruple therapy (as well as RBC-based regimens) may be considered as the preferred regimen after initial treatment failure with PPI-clarithromycin-amoxicillin [Malfertheiner et al. 2007; Hojo et al. 2001; Kearney, 2001; Gisbert and Pajares, 2002]. However, bismuth salts, including RBC, are no longer available worldwide, and some National Guidelines have been accordingly changed [Caselli et al. 2007].
PPI, amoxicillin and levofloxacin. As previously mentioned, after failure of a combination of a PPI-based triple regimen, the use of the quadruple therapy has been generally recommended as the optimal second-line therapy based on the relatively good results reported by several authors [Malfertheiner et al. 2007; Gisbert and Pajares, 2002; Hojo et al. 2001; Kearney, 2001]. However, this quadruple regimen requires the administration of four drugs with a complex scheme (bismuth and tetracycline usually prescribed every 6 hours, and metronidazole every 8 hours) and is associated with a relatively high incidence of adverse effects [Gisbert and Pajares, 2002]. Furthermore, this quadruple regimen still fails to eradicate H. pylori in approximately 20—30% of the patients, and these cases constitute a therapeutic dilemma, as patients who are not cured with two consecutive treatments including clarithromycin and metronidazole will usually have double resistance [Gisbert and Pajares, 2002].
Levofloxacin is a fluoroquinolone antibacterial agent with a broad spectrum of activity against Gram-positive and Gram-negative bacteria and atypical respiratory pathogens [Croom and Goa, 2003]. Recently, some studies have evaluated the efficacy of new fluoroquinolones, such as levofloxacin, that could prove to be a valid alternative to standard antibiotics not only as first-line therapies but, more interesting, as second-line regimens [Vaira et al. 2007; Gisbert and Morena, 2006c; Gisbert and Pajares, 2005; Saad et al. 2006]. In this respect, levofloxacin-based second-line therapies represent an encouraging strategy for eradication failures, as some studies have demonstrated that levofloxacin has, in vitro, remarkable activity against H. pylori [Sanchez et al. 2000], and that primary resistances to such antibiotic are (still) relatively infrequent (when compared with metronidazole or clarithromycin) [Chang et al. 2009, Chisholm and Owen, 2009; Antos et al. 2006; Kumala and Rani, 2006; Gatta et al. 2005; Zou et al. 2003; Xia et al. 2002]. A recent in vitro study also showed a synergistic effect of quinolone antimicrobial agents and PPIs on strains of H. pylori [Tanaka et al. 2002]. Furthermore, it has been shown in vitro that levofloxacin retains its activity when H. pylori strains are resistant to clarithromycin and metronidazole [Antos et al. 2006; Yahav et al. 2006b; Watanabe et al. 2003]. These favorable results have been confirmed in vivo, indicating that most of the patients with both metronidazole and clarithromycin resistance are cured with the levofloxacin-based regimen [Gatta et al. 2005; Matsumoto et al. 2005; Bilardi et al. 2004].
A combination of a PPI, amoxicillin and levofloxacin, as first-line regimen, has been associated with favorable results, with mean eradication rates of about 90% [Rispo et al. 2007; Gisbert et al. 2007b; Antos et al. 2006; Lee et al. 2006; Marzio et al. 2006; Di Caro et al. 2002; Cammarota et al. 2000]. Later, other authors studied this same regimen in patients with one previous eradication failure, also reporting exciting results, with H. pylori cures rates ranging from 60% to 94% [Di Caro et al. 2009; Eisig et al. 2009; Kuo et al. 2009; Schrauwen et al. 2009; Gisbert et al. 2007c; Page et al. 2007; Giannini et al. 2006; Lee et al. 2006; Wong et al. 2006; Matsumoto et al. 2005; Bilardi et al. 2004; Nista et al. 2003; Orsi et al. 2003; Perri et al. 2003; Watanabe et al. 2003; Wong et al. 2003]. A recent systematic review showed a mean eradication rate with levofloxacin-based ‘rescue’ regimens (combined with amoxicillin and a PPI in most studies) of 80%, which represents a relatively high figure when considering that this regimen was evaluated as a ‘rescue’ therapy [Gisbert et al. 2006c]. This systematic review found higher H. pylori cure rates with 10-day than with 7-day regimens, both in general (81% versus 73%) and also with the levofloxacin-amoxicillin-PPI combination in particular (80% versus 68%), suggesting that the longer (10-day) therapeutic scheme should be chosen.
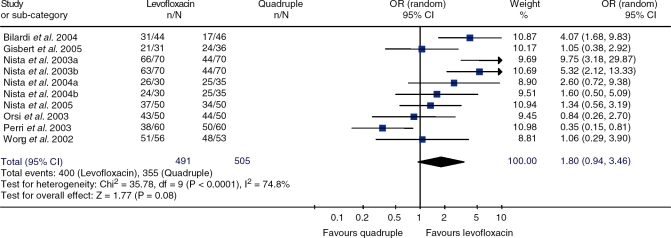
Furthermore, two recent meta-analyses have suggested that after H. pylori eradication failure, levofloxacin-based ‘rescue’ regimen is more effective than the generally recommended quadruple therapy [Saad et al. 2006; Gisbert et al. 2006c]. In one of these meta-analyses [Gisbert et al. 2006c], higher H. pylori cure rates with the levo-floxacin-based triple regimens than with the quadruple combinations were found (81% versus 70%), but with borderline statistical significance (Figure 1). Nevertheless, results were heterogeneous, mainly due to the discordant results of the study by Perri et al. [2003], who reported a cure rate of only 63% with the levofloxacin regimen, the lowest reported in the literature, a figure that contrasts with the mean eradication rate of 80% calculated in a systematic review [Gisbert et al. 2006c]. Nevertheless, when that single outlier study [Perri et al. 2003] was excluded from the meta-analysis, the difference between cure rates with both regimens reached statistical significance and heterogeneity markedly decreased. Furthermore, when only high-quality studies were considered, the advantage of the levofloxa-cin regimen over the quadruple regimen increased (88% versus 64%), also achieving statistical significance, and heterogeneity among studies almost disappeared [Gisbert et al. 2006c].
Figure 1.
Meta-analysis comparing H. pylori eradication efficacy with levofloxacin-based triple regimens versus quadruple therapy, as second-line ‘rescue’ regimen after failure of a proton-pump inhibitor (PPI)-amoxicillin-clarithromycin.
As previously mentioned, the quadruple regimen requires the administration of a complex scheme [Gisbert et al. 2002]. On the contrary, levoflox-acin-based regimens (with amoxicillin and PPIs administered twice daily, and levofloxacin every 12 or 24 hours) represents an encouraging alternative to quadruple therapy, with the advantage of simplicity. Furthermore, the quadruple regimen is associated with a relatively high incidence of adverse effects [Gisbert et al. 2002].
In contrast, levofloxacin is generally well tolerated, and most adverse events associated with its use are mild to moderate in severity and transient [Croom and Goa, 2003]. The most frequent adverse effects affect the gastrointestinal tract [Croom and Goa, 2003]. Occasional cases of tendinitis and tendon rupture have been reported in the literature with levofloxacin therapy [Bilardi et al. 2004; Croom and Goa, 2003]. However, data derived from more than 15 million prescriptions in the US indicated the rate is fewer than 4 per million prescriptions [Kahn, 2001]. Clostridium difficile infection may be associated with the use of this broad spectrum activity antibiotic [Croom and Goa, 2003]. In the aforementioned systematic review [Gisbert et al. 2006c], adverse effects were reported, overall, by 18% of the patients treated with levofloxacin-based therapies, and these adverse effects were severe (defined so by the authors or explaining treatment discontinuation) in only 3% of the cases. Furthermore, the incidence of adverse effects was not different when levofloxacin-amoxicillin-PPI was administered for 7 or 10 days, supporting the aforementioned recommendation of prescribing the more effective 10-day regimen. Moreover, two meta-analyses have demonstrated a lower incidence of adverse effects with levofloxacin-based treatments than with the quadruple combinations [Gisbert et al. 2006c; Saad et al. 2006].
Unfortunately, it has been shown that resistance to quinolones is easily acquired, and in countries with a high consumption of these drugs, the resistance rate is increasing and is already relatively high [Carothers et al. 2007; Cattoir et al. 2007; Glocker et al. 2007; Perna et al. 2007; Zullo et al. 2007; Bogaerts et al. 2006; Kim et al. 2006; Marzio et al. 2006; Miyachi et al. 2006; Wong et al. 2006; Coelho et al. 2005; Gatta et al. 2005; Cammarota et al. 2004; Cabrita et al. 2000]. More importantly, it has been demonstrated that the presence of levofloxacin resistance significantly reduce the eradication rate following a therapy with this antibiotic [Perna et al. 2007; Marzio et al. 2006; Gatta et al. 2005; Krakowka et al. 1996]. Therefore, it has been suggested to reserve levofloxacin for ‘rescue’ treatment to avoid the increase of the resistance phenomenon.
‘Rescue’ regimen after PPI-amoxicillin-nitroimidazole failure
After PPI-amoxicillin-nitroimidazole failure, retreatment with PPI-amoxicillin-clarithromycin has proved to be very effective, and it seems to be a logical strategy, as while amoxicillin is maintained (which does not induce resistance), clari-thromycin is substituted for metronidazole. Furthermore, the absence of cross-resistance among nitroimidazoles and clarithromycin favors this position. With this therapy, some authors [Gisbert et al. 1999a] have achieved H. pylori eradication in 85% of cases, while others have reported success rates of 86% [Reilly et al. 1995] or even 100% [Lerang et al. 1997]. In favor of this strategy is the study by Magaret et al. [2001], who studied a group of 48 patients after failure of previous H. pylori therapy with a metronidazole-containing regimen, and randomized them to either lansoprazole, amoxicillin and clarithromycin twice daily for 14 days (i.e. the logical approach with triple therapy not repeating metronidazole), or to lansopra-zole, bismuth, metronidazole and tetracycline for 14 days (i.e. the quadruple therapy repeating metronidazole). Intention-to-treat efficacies were 75% for triple regimen and 71% for quadruple. Although this difference did not reach statistical significance, the small sample size of this study does not preclude the possibility of a small but clinically significant difference in efficacy between the regimens. Finally, preliminary studies have suggested that RBC may be used instead of PPI in this triple second-line strategy (i.e. RBC-clarithromycin-amoxicillin), with similar or even better results [Beales, 2001]
‘Rescue’ regimen after PPI-clarithromycin-nitroimidazole failure
As previously mentioned, acquired bacterial resistance to metronidazole or clarithromycin results primarily from the previous treatment failure [Peitz et al. 1999; Deltenre et al. 1998], and therefore the first choice probably should not be a regimen that combines these two antibiotics in the same regimen [Calvet et al. 2001; Axon, 2000; De Boer et al. 2000b]. Although this regimen is very effective [Gisbert et al. 2000b], patients who are not cured will probably have double resistance [Peitz et al. 2002, 1999], and no logical empirical treatment remains afterwards (although, more recently, the levofloxacin-based regimens may represent an option). Thus, some authors have demonstrated that initial regimens containing both clarithromycin and nitroimida-zole are associated with significantly worse results overall, with lower eradication rates after logically chosen second-line therapy and sensitivity-directed third-line therapy; these poor results were due to the emergence of multiply resistant strains as evidenced by the results of culture testing after the second failed course [Beales, 2001]. In summary, due to problems with resistance it could be suggested that both key antibiotics — clarithromycin and metronidazole — should not be used together until a valid empirical back up regimen is available [De Boer et al. 2000b].
Nevertheless, if culture is not performed after failure of PPI-clarithromycin-metronidazole, and hence antibiotic susceptibility is unknown, several ‘rescue’ options may be suggested. Firstly, omeprazole plus amoxicillin, with a high dose of both the antibiotic and the antisecretor, could in theory be recommended [Miehlke et al. 2006; Axon, 2000]; however, we must remember that this ‘old-fashioned’ dual combination has achieved disappointing results in many countries. Therefore, a second antibiotic should be added, and at this point a difficult decision appears, as both antibiotics used in the first trial (clarithromycin and metronidazole) are capable of inducing secondary resistance to H. pylori, playing a negative role in future efficacy [Saad and Chey, 2008; Dore et al. 2000; Pilotto et al. 2000; Houben et al. 1999; Peitz et al. 1999; Van Der Wouden et al. 1999; Tompkins et al. 1997]. Nevertheless, the following possibilities exist.
Readministering metronidazole. Due to the fact that metronidazole resistance is frequent and clinically relevant [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Van Der Wouden et al. 1999], if this antibiotic is readministered, it should be used within bismuth-based quadruple regimen (thus PPI might reduce the negative effect of metronidazole resistance [Graham et al. 2000; Gomollon et al. 1999, Van Der Wouden et al. 1999; De Boer et al. 1995]). With this regimen, eradication rates up to 80% have been achieved [Gisbert et al. 1999a]. RBC, which may overcome the impact of resistance to metronidazole [Gisbert et al. 1999c], may also play a role in this regimen. Thus, some authors have reported an 88% cure rate with 2-week regimen or RBC-tetracycline-tinidazole in patients who had previously failed a clarithromycin-tinidazole based triple therapy [Zullo et al. 2001a].
Readministering clarithromycin. Several studies have underlined the relevance of clarithromycin resistance [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Tompkins et al. 1997], which advise against readministering this antibiotic. Therefore, a further option which has been proposed is to add (e.g. to PPI-amoxicillin-clarithromycin) a fourth medication (as bismuth) with bactericidal effect against H. pylori, with which a 70% eradication rate has been achieved [Gisbert et al. 1999a].
Readministering no antibiotic. A final alternative, obviously, consists of no readministering either metronidazole or clarithromycin. Although only published in abstract form, one study has prescribed RBC, tetracycline and amoxicillin for 2 weeks and has reported the eradication in 89% of the cases who had previously failed PPI, clarithromycin and tinidazole [Cudia et al. 1997]. These encouraging results may be due, at least in part, to the use of RBC instead of bismuth in this regimen, as ‘classic’ triple therapy with bismuth, tetracycline and amoxicillin has been previously considered relatively ineffective. Finally, although not specifically evaluated in PPI-clarithromycin-metronidazole failures, rifabutin or levofloxacin-based regimens (e.g. PPI, amoxicillin and either levofloxacin or rifabutin) could play a role in this difficult situation.
Patients allergic to penicillin. When penicillin allergy is documented, replacing amoxicillin with metronidazole has been recommended in PPI-based triple combinations. H. pylori eradication is a challenge in patients allergic to penicillin, especially in those who have failed a first-eradication trial with these two key antibiotics, clarithro-mycin and nitroimidazoles. To date, only few studies have evaluated the efficacy of H. pylori eradication treatment specifically in those patients allergic to penicillin, which constitutes a relatively common subgroup [Gisbert et al. 2009, 2005e; Rodriguez-Torres et al. 2005]. In our previous study, H. pylori infected patients allergic to penicillin failing first-line treatment with PPI-clarithromycin-metronidazole therapy received a second-line treatment with RBC, tetracycline and metronidazole, but this rescue regimen cured the infection in only 47% of the patients [Gisbert et al. 2005e]. In this same study, it was shown that rifabutin-clarithromycin-PPI regimen was ineffective and poorly tolerated. However, a final rescue regimen including levofloxacin cured the infection in all treated patients [Gisbert et al. 2005e]. In a more recent study, it has been confirmed that a levofloxacin-containing regimen (together with omeprazole and clarithromycin) represents an encouraging second-line alternative in the presence of penicillin allergy [Gisbert et al. 2009].
Is it necessary to perform culture after failure of the second eradication treatment?
As previously mentioned, it has been generally recommended that performing culture after a first eradication failure is not necessary and therefore assessing H. pylori sensitivity to antibiotics only after failure of the second treatment may be suggested in clinical practice [Malfertheiner et al. 2007; De Boer et al. 2000b; European Helicobacter pylori Study Group 1997]. However, the utility of the culture (with consequent antibiotic susceptibility testing) and the moment when it must be performed after eradication failure are both controversial [Gisbert, 2008a, 2005b]. It is evident that, as pretreatment antibiotic resistance is the most important factor in nonresponse to initial treatment [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Van Der Wouden et al. 1999; Tompkins et al. 1997], knowledge of the organism's antibiotic susceptibility may represents an aid in selecting the therapy regimen. However, performing culture systematically after the second eradication failure also has some limitations, which are summarized as follows:
-
(1)
Culture implies, obviously, the performance of endoscopic exploration, which has several disadvantages: it is not free of risk, and, since endoscopy centers have been meeting increasing demand, usually involves prolonged waiting times.
-
(2)
H. pylori culture is expensive, due to the cost of the procedure itself, but mainly the costs of the associated endoscopy, which is necessary to obtain biopsy samples.
-
(3)
Culture is time-consuming, as H. pylori is a rather ‘fastidious’ bacterium at culture, especially when a low bacterial load is present, as generally occurs after eradication failure [Zullo et al. 2003b].
-
(4)
Culture is not always available on a routine basis.
-
(5)
The sensitivity of bacterial culture is not 100%, and therefore the antimicrobial susceptibility cannot be obtained in all cases. Indeed, even in the optimal conditions usually encountered in therapeutic trials — when both gastroenterologist and microbiologist are thoroughly motivated — a culture sensitivity of ‘only’ approximately 90% has been achieved in patients not previously treated [Zullo et al. 2003b]. Furthermore, in several studies enrolling patients who had failed one or more eradication treatments, the bacterium was isolated in less than 80% of cases [Zullo et al. 2003b]. Therefore, an even lower probability of isolating the bacterium is to be expected in routine clinical practice. This indicates that, even in the hands of experts, antimicrobial sensitivity would not be obtained in several eradication failure patients, who had undergone an upper endos-copy solely for bacterial culture [Zullo et al. 2003b].
-
(6)
Antibiotic susceptibility testing in clinical practice yields useful information only regarding a few antibiotics. Antibiotics effective and generally used against H. pylori are mainly amoxicillin, clarithro-mycin, metronidazole, and tetracycline. Resistance to amoxicillin has been estimated to be less than 1% in most studies [Megraud, 2004; Zullo et al. 2003b]. Hence, its role in clinical practice may even be marginalized. Similarly, resistance to tetracycline is also very low, or even absent, in most countries [Megraud, 2004; Zullo et al. 2003b]. Therefore, it may even be assumed that antibiotic susceptibility testing in clinical practice yields useful information only regarding the latter two antibiotics, namely clarithromycin and metronidazole [Zullo et al. 2003b].
-
(7)
In vitro antibiotic susceptibility does not necessarily lead to eradication in vivo. Even knowing the susceptibility of H. pylori, eradication rates do not achieve 100%, as the results observed in vivo by following in vitro susceptibility to anti-H. pylori antibiotics are often disappointing [Guslandi, 2001]. Some discrepancies between antibiotic susceptibility and H. pylori eradication may occur, due, for example, to the possibility of coinfection with different H. pylori strains [Kim et al. 2003]. Thus, a variable proportion of noneradicated patients is made of subjects who harbor strains sensitive to the administered drugs, and in these patients the reasons for treatment failure are unclear [Ali et al. 1999]. For example, Gomollon et al. [2000a] reported how third-line treatment often (in 50% of the cases) failed to eradicate H. pylori infection, in spite of giving 14-day, full-dose, quadruple culture-guided combination, showing that in vitro susceptibility did not predict eradication success. In the same way, Vicente et al. [2002] determined the effectiveness of a third, culture-guided, treatment of H. pylori infection after two unsuccessful attempts. Patients received a 2-week quadruple culture-guided therapy, and overall eradication was achieved in only 60% of the patients. In fact, paradoxically, the lowest eradication rate was obtained in patients with H. pylori strains sensitive to all antibiotics. In summary, it seems that despite the use of culture-guided combinations of drugs, a third treatment is frequently unsuccessful, indicating that other factors different from in vitro antibiotic susceptibility influence eradication rates. On the other hand, the reverse situation is also possible, as H. pylori eradication may nonetheless be achieved in the presence of metronidazole- or clarithromy-cin-resistant strains even with a drug combination including these antibiotics. Therefore, in vitro resistance to either clarithromycin or metronidazole could be overcome in vivo in a significant proportion of patients by prescribing the same antibiotics [Zullo et al. 2003b].
-
(8)
When a repeat (rescue) therapy must be selected, we have several data that will aid us in suspecting resistance to a particular antibiotic, without the necessity of a culture, based on the observation that acquired bacterial resistance to metronidazole or clari-thromycin results primarily from the previous treatment failure [Peitz et al. 1999; Deltenre et al. 1998]. Thus, when a therapy with clari-thromycin fails, resistance to this antibiotic appears in most cases, and the same is true when a nitroimidazole is the antibiotic first used [Megraud, 2004; Pilotto et al. 2000; Adamek et al. 1998; Tompkins et al. 1997]. Even if resistance to these antibiotics does not appear, it remains uncertain whether their readministration is adequate, as they were not efficacious (for unknown reasons) for the first time. Although some studies suggest that retreatment of H.pylori infection with the same combination is still a choice when the status of bacterial resistance to antibiotics is unknown, however, full doses and a longer treatment duration must be used and a poor eradication rate has usually been reported [Huang and Hunt, 1999]. Therefore, the position in the case of therapy failure would be clear: do not readminister any of the antibiotics against which H. pylori has probably become resistant [Howden and Hunt, 1998; Lam and Talley, 1998].
-
(9)
Finally, relatively high eradication rates have been obtained with empirical third-line treatment after two consecutive failures in several studies [Gisbert et al. 2008b, 2006a, 2006b, 2004, 2003; Dore et al. 2003; Zullo et al. 2003a; Treiber et al. 2002a; Canducci et al. 2001; Zullo et al. 2001a; Bock et al. 2000, Chan et al. 2000; Perri et al. 2000; Seppala et al. 2000].
However, limited experience suggests that endo-scopy with culture and susceptibility testing may be appropriate after failure of two eradication therapies; in this situation, a nonrandomized retrospective study suggests that third-line therapy directed by the results of sensitivity testing improve eradication compared to further empirical antibiotics, demonstrating that the success rate of sensitivity-directed therapy is superior to PPI-amoxicillin-rifabutin triple therapy, and therefore suggesting that endoscopy and sensitivity testing at this point may be worthwhile rather than more widespread use of rifabutin-based regimens [Beales, 2001]. Cammarota et al. [2004] assessed the efficacy of a third-line, culture-guided treatment approach for the eradication of H. pylori. After the first two eradication attempts, all patients were resistant to metroni-dazole, and 95% were resistant to clarithromycin. Consequently, most patients (89 out of 94) received quadruple regimen including PPI, bismuth, tetracycline and amoxicillin, and H. pylori eradication was obtained in 90% of the cases. Although the authors conclude that a culture-guided, third-line therapeutic approach is effective for the eradication of H. pylori, it would seem more appropriate to conclude, in fact, that the tetracycline- and amoxicillin-based quadruple regimen may be a good empirical third-line ‘rescue’ treatment option (as to choose such a regimen, which implies not readministering metronidazole or clarithromycin, it would not be necessary to know antibiotic susceptibilities).
In summary, when critically reviewing the role of culture in the management of H. pylori infection in clinical practice it may be concluded, in coincidence with other authors, that H. pylori culture is an invasive, time-consuming method, offering quite low sensitivity, requiring significant cost and which, in practice, tests very few antibiotics, with a questionable contribution to the management of nonresponder patients [Zullo et al. 2003b, Cianci et al. 2006]. Obviously, the importance of H. pylori culture remains unaltered both in epidemiological and pharmacological research fields. However, whether patients should undergo an upper endoscopy for bacterial culture after second-line therapy failure remains a debatable matter, and the role of culture in clinical practice requires a critical reappraisal [Cianci et al. 2006; Zullo et al. 2003b]. As it has been brilliantly expressed by Zullo et al. [2003b], regrettably, gastroenterologists need to accept that gastric biopsy culture is not as simple as filling a sample bottle!
Nevertheless, it is recommended that those prescribing H. pylori eradication therapies continually assess their success rate and adjust the relevant local practices and policies in line with the results and local bacterial resistance patterns. Thus, it would be recommendable that culture should be routinely performed after eradication failure in some specialized centers with special interest in H. pylori research and treatment, with the intention to study the incidence of resistances after failures and also to evaluate the influence of such resistances on the efficacy of ‘rescue’ regimens [Megraud and Lamouliatte, 2003]. Data coming from this experience on H. pylori resistance will be used as reference for the corresponding population. This preventive approach has been recommended to avoid an increase in refractory H. pylori infection in the future [Megraud and Lamouliatte, 2003].
Empirical third-line H. pylori‘rescue’ therapy after failure of two eradication treatments
If we have decided, finally, not to perform culture before the administration of the third-line ‘rescue’ treatment after failure of the first two trials (generally including clarithromycin and metronidazole), different possibilities of empirical treatment may be suggested. As eradication regimens may be less efficacious for retreatment as compared to their efficacy when used as primary treatment, it may be suggested that the course of the ‘rescue’ therapy should be extended to 10—14 days, at least when ‘rescue’ therapy fails and third-line regimens are therefore prescribed [De Boer and Brody, 2000]. As several studies have underlined the relevance of metronidazole [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Van Der Wouden et al. 1999] and clarithromycin [Dore et al. 2000; Houben et al. 1999; Peitz et al. 1999; Tompkins et al. 1997] resistance, these two antibiotics should not be readministered, and several regimens have been evaluated in this scenario, outlined as follows.
Amoxicillin ± tetracycline-based regimens
In a recent study, patients with at least one treatment failure and infected with H. pylori resistant to both metronidazole and clarithromycin, were treated with high doses of omeprazole (4 × 40 mg) and amoxicillin (4 × 750 mg) for 14 days, and the infection was cured in 76% of the cases [Miehlke et al. 2003]. This study suggests that, although the ‘old-fashioned’ dual combination of omeprazole plus amoxicillin is generally considered quite ineffective as first-line regimen, it may be associated with relatively good results if prescribed at high doses, even for H. pylori infection resistant to both metronidazole and clari-thromycin in patients who experienced previous treatment failures. Another possibility to avoid retreatment with clarithromycin or metronida-zole is to prescribe a quadruple combination of PPI, bismuth, tetracycline and amoxicillin (instead of metronidazole), which has been used by some authors with favorable results [Chi et al. 2003]. Nevertheless, this regimen has been tested only as second-line (and not third-line) therapy, and only after failure of PPI-clarithromycin-amoxicillin (and not after metronidazole-based therapy), emphasizing that the experience should be extended to patients with two previous eradication failures containing both clarithromy-cin and metronidazole. Finally, as previously mentioned, one study prescribed RBC, tetracy-cline and amoxicillin for 2 weeks and achieved eradication in 89% of the cases who had previously failed PPI, clarithromycin and tinidazole [Cudia et al. 1997].
Levofloxacin-based ‘rescue’ regimens
It has been suggested that levofloxacin-based therapies may also represent an alternative when two (or more) consecutive eradication treatments have failed to eradicate the infection [Nishizawa et al. 2009; Hsu et al. 2008; Rokkas et al. 2006; Gisbert et al. 2006a, 2006b, 2005a; Coelho et al. 2005; Gatta et al. 2005; Bilardi et al. 2004; Zullo et al. 2003a, 2001a]. As an example, a recent study by Zullo et al. [2003a] aimed to evaluate the efficacy of a levofloxacin-amoxicillin-PPI combination in patients who previously had failed two or more therapeutic attempts, and eradication rate was 83% (intention-to-treat analysis). More recently, Gisbert et al. [2006b] evaluated, in a multicenter study including 100 patients, the efficacy of a third-line levofloxacin-based regimen in patients with two consecutive H. pylori eradication failures. An intention-to-treat eradication rate was 66%, which represents a relatively high figure when considering that this regimen was evaluated as a third-line therapy. Other alternatives of ‘rescue’ therapies, different from levofloxacin-based regimens, have been suggested. Rifabutin-based ‘rescue’ therapy, as will be reviewed in the following section, also constitutes a possible strategy after previous eradication failures, although it has been recently shown that 10-day triple levofloxacin-based regimen is more effective than the same combination with rifabutin as a ‘rescue’ regimen [Gisbert et al. 2006b]. In summary, levofloxacin-based ‘rescue’ therapy constitutes an encouraging empirical third-line strategy after multiple previous H. pylori eradication failures with key antibiotics (such as amoxicillin, clarithromycin, metronida-zole and tetracycline).
Rifabutin-based ‘rescue’ regimens
As previously mentioned, the evaluation of drugs without cross-resistance to nitroimidazole or macrolides as components of retreatment combination therapies seem to be worthwhile. H. pylori has been proved to be highly susceptible in vitro to rifabutin, a rifamycin derivate of the established tuberculostatic drug [Akada et al. 1999; Heep et al. 1999; Brogden and Fitton, 1994]. Moreover, rifabutin is chemically stable at a wide pH range and its antibacterial activity is likely not to be hampered by the acidic environment of the stomach [Rossi, 1999]. Furthermore, selection of resistant H. pylori strains has been low in experimental conditions. Thus, until now, no rifabutin-resistant strain has been isolated from patients who were either treated or untreated for H. pylori infection [Heep et al. 1999].
As summarized in Table 2, rifabutin-based ‘rescue’ therapy constitutes an encouraging strategy after multiple previous eradication failures [Gonzalez Carro et al. 2007; Navarro-Jarabo et al. 2007; Van Der Poorten and Katelaris, 2007; Borody et al. 2006; Miehlke et al. 2006; Gisbert et al. 2006b, 2003; Toracchio et al. 2005; Beales, 2001; Canducci et al. 2001; Bock et al. 2000; Perri et al. 2000]. As an example, Perri et al. [2000, 1998] used a 1-week regimen of PPI, amoxicillin and rifabutin in patients who were still H. pylori infected after two or more courses of PPI-based triple therapies, and achieved a eradication rate of 71% by intention-to-treat analysis. Gisbert et al. [2003], in a prospective multicenter study, included patients in whom a first eradication trial with PPI, clarithro-mycin and amoxicillin and a second trial with PPI, bismuth, tetracycline and metronidazole had failed. A third 14-day eradication regimen with rifabutin, amoxicillin and a PPI was effective in 79% of the patients (intention-to-treat analysis). However, these encouraging results were not confirmed in a more recent study by these same authors [Gisbert et al. 2006b]. In the largest study up to now with rifabutin [Gonzalez Carro et al. 2007], 92 consecutive patients diagnosed with H. pylori infection resistant to two previous treatment regimens were treated with a PPI, rifabutin and amoxicillin for 10 days, and the intention-to-treat eradication rate was 61%. In summary, the weighted mean eradication rate with rifabutin-based ‘rescue’ therapy, calculated from the studies included in the Table 2, is of 69%.
Table 2.
Rifabutin-based ‘rescue’ therapies (rifabutin-amoxicillin PPI) in patients with previously failed eradication treatments and/or resistance to clarithromycin and nitroimidazoles.
| Author | Number of patients | Drugs and doses | Duration of treatment (d) | Eradication rate (%) |
| Beales [2001] | 10 | Rifabutin 300mg o.d. Amoxicillin 1 g b.i.d. Omeprazole 20mgb.i.d. | 14 | 60 |
| Bock et al. [2000] | 25 | Rifabutin 150mgb.i.d. Amoxicillin 1 g b.i.d. Lansoprazole 30mgb.i.d. | 7 | 72 |
| Borody et al. [2006] | 67 | Rifabutin 150 mg b.i.d. Amoxicillin 1—1.5 g t.i.d. | ||
| Pantoprazole 60 mg t.i.d | 12 | 90 | ||
| Canducci et al. [2001] | 10 | Rifabutin 300mg o.d. Amoxicillin 1 g b.i.d. Omeprazole 20mgb.i.d. | 10 | 70 |
| Gisbert et al. [2003] | 14 | Rifabutin 150 mg b.i.d. Amoxicillin 1 g b.i.d. Omeprazole 20 mg b.i.d. | 14 | 79 |
| Gisbert et al. [2006b] | 20 | Rifabutin 150mgb.i.d. Amoxicillin 1 g b.i.d. Omeprazole 20mgb.i.d. | 10 | 45 |
| Gonzalez Carro [2007] | 92 | Rifabutin 150mgb.i.d. Amoxicillin 1 g b.i.d. Pantoprazole 40mgb.i.d | 10 | 61 |
| Miehlke et al. [2006] | 73 | Rifabutin 150 mg b.i.d. Amoxicillin 1 g b.i.d. Esomeprazole 20 mg b.i.d. | 7 | 74 |
| Navarro-Jarabo et al. [2007] | 45 | Rifabutin 150mgb.i.d. Amoxicillin 1 g b.i.d. Omeprazole 20mgb.i.d. | 7 | 44 |
| Perri et al. [2000] | 41 | Rifabutin 300mg o.d. Amoxicillin 1 g b.i.d. Pantoprazole 40mgb.i.d. | 7 | 71 |
| Toracchio et al. [2005] | 65 | Rifabutin 150 mg b.i.d. Amoxicillin 1 g b.i.d. Pantoprazole 40 mg b.i.d. | 10 | 78 |
| Van der Poorten et al. [2007] | 44 | Rifabutin 150 mg b.i.d. Amoxicillin 1 g b.i.d. PPI b.i.d. | 10 | 68 |
PPI, proton-pump inhibitor (omeprazole, pantoprazole, rabeprazole or esomeprazole) at the usual dose. Eradication rates by intention-to-treat analysis when available. H. pylori eradication rate (weighted mean) with rifabutin-based ‘rescue’ therapy: 69%. o.d., once daily; b.i.d., twice daily; t.i.d., three times per day.
These findings suggest that new rifabutin-based combinations are effective for H. pylori strains resistant to antibiotics, and specifically to clari-thromycin or metronidazole [Moayyedi et al. 2006; Miehlke et al. 2008]. Furthermore, rifabu-tin-based therapies have been compared with the widely used ‘classic’ quadruple therapy. Perri et al. [2001a] performed a randomized study where three groups of patients were treated for 10 days with pantoprazole, amoxicillin and rifa-butin 150mg once daily, or 300mg once daily, and quadruple therapy. On intention-to-treat analysis, eradication rates were 67% in the rifa-butin 150mg and quadruple groups, and higher (87%) in the rifabutin 300 mg group. Finally, in this comparative study, side effects were less frequent in rifabutin-treated patients than in those on quadruple therapy [Perri et al. 2001a].
Several concerns still remain, however, regarding rifabutin treatment. Firstly, this drug is very expensive. Secondly, severe leucopoenia and trombocytopenia have been reported in one patient treated with rifabutin, with myelotoxicity demonstrated by bone marrow aspirate [Canducci et al. 2001]. Although blood cell count returned to normal at day 15 after discontinuation of therapy, physicians should be aware of the risk of major side effects arising during rifabutin-based regimen [Gisbert et al. 2006b, 2003]. Finally, there is some concern about a widespread use of rifabutin, a member of a class of established antimycobacterial drugs, in patients with H. pylori infection. Because multi-resistant strains of Mycobacterium tuberculosis increase in numbers, indications for these drugs should be chosen very carefully to avoid further acceleration of development of resistance [Bock et al. 2000]. At present, therefore, rifabutin should be considered only as the last option (e.g. restricted to infected patients even after several eradication regimens including, among them, levofloxacin).
Furazolidone-based ‘rescue’ regimens
Furazolidone is an antimicrobial drug that belongs to synthetic nitrofurans and is active against a broad spectrum of gram-negative and gram-positive bacteria and protozoa. This antibiotic has demonstrated a high antimicrobial activity against H. pylori if given as a single drug [Xiao et al. 1990], and the majority of first-line furazolidone-based combination therapies revealed eradication rates above 80% [Xia et al. 2002]. Primary resistance to furazolidone is virtually absent [Megraud et al. 2003; Kwon et al. 2001; Haas et al. 1990], and its potential to develop resistanceisas lowasfor bismuth compoundsoramoxicillin [Treiber et al. 2002b]. Moreover, this drug has no cross-resistance potential to metronidazole [Haas et al. 1990]. Triple therapy in which furazolidone is used instead of metronidazole achieves high eradication rates, even in populations with a high prevalence of nitroimidazole resistance [Eisig et al. 2005; Nijevitch et al. 2005; Xiao et al. 2001; Ebrahimi-Dariani et al. 2003]. In this respect, a recent study has evaluated furazoli-done-based triple therapy (combined with bismuth and tetracycline) in the eradication of H. pylori resistant to metronidazole, with favorable results (86% eradication rate) [Isakov et al. 2002]. A few years ago, some authors tested a quadruple combination of furazolidone, bismuth, tet-racycline and PPI as second-line eradication therapy, and reported encouraging results [Sotoudehmanesh et al. 2001]. More recently, Treiber et al. [2002a] and Sanches et al. [2008] investigated whether this quadruple regimen containing furazolidone could be effective as third-line therapy in patients with H. pylori treatment failure after first-line (clarithromycin-metro-nidazole±amoxicillin) and/or second-line (PPI-bismuth-tetracycline-metronidazole) regimens, and H. pylori infection was cured in >90% of the cases. Furthermore, 7-day triple regimen comprising furazolidone, amoxicillin and a PPI achieved an eradication rate of60%in10 patients who failed first-line, second-line and even rifabutin-based triple therapy [Qasim et al. 2005].
A recent systematic review and meta-analysis of the effect of furazolidone- and nitrofurantoin-based regimens in the eradication of H. pylori infection has been performed [Buzas and Jozan, 2007]. The pooled eradication rate of primary PPI-based regimens containing furazolidone was 76%. Second-line schedules containing furazolidone obtained eradication rates of 76%. Finally, third-line ‘rescue’ therapies were effective in 65% of the cases. In summary, a quadruple regimen including furazolidone, bismuth, tetra-cycline and PPI seems to represent a promising alternative after two consecutive failures with regimens including both metronidazole and clari-thromycin. However, some aspects related with the use of furazolidone as a rescue therapy for H. pylori infection should be remarked, especially regarding its potential oncologic risk [De Francesco et al. 2009]. Furthermore, furazoli-done is not available worldwide.
Cumulative eradication rates with three (or more) consecutive eradication treatments
In patients with conditions where the indication for H. pylori eradication is definitively accepted, as is the case of peptic ulcer disease (or gastric MALT lymphoma), ‘rescue’ treatment after firstline failure is clearly advisable. Furthermore, if the second therapy fails, a third or even a fourth regimen should be prescribed, as infected patients continue to have high risk of ulcer recurrence and ulcer complications and are in an obviously disadvantageous situation in view of the enormous benefits that follow H. pylori eradication in peptic ulcer disease: increased ulcer healing, less ulcer recurrence and less ulcer complication. However, multiple repeated antibiotic treatment of patients where benefits of H. pylori eradication has not been so clearly established, such as those with functional dyspepsia [Moayyedi et al. 2006; Laine et al. 2001], may not be completely justified.
Some authors have evaluated, in the same study, different regimens after failure of two eradication treatments, which provide interesting information about cumulative, and not only absolute, eradication rates [Gisbert et al. 2008a, 2005b]. For example, in the study by Gasbarrini et al. [2000a], a total of 2606 patients were administered a PPI, tinidazole and clarithromycin for 1 week. Patients with continuing infection were then given a second 1-week course of amoxicillin, clarithromycin and RBC. Finally, patients still infected after the second course underwent upper gastrointestinal endoscopy with H. pylori culture, and then received a 1-week quadruple scheme established on antibiotic sensitivity. Eradication rates after the first, second and third treatment, were 79%, 77% and 52%, respectively. This algorithm led to overall per-protocol eradication rates of 99%. Chan et al. [2000] prescribed quadruple therapy to a group of patients who had failed to respond to RBC-based regimens (as first regimen) and PPI-clarithromycin-amoxicillin combination (as second regimen), and achieved successful eradication in 83% of the cases receiving quadruple regimen, finally achieving a 99% cumulative eradication rate. Beales [2001] evaluated 469 patients receiving eradication therapy in routine clinical practice. Second-line therapy was chosen empirically, using whichever of clarithromycin or metronidazole was not used initially. All patients requiring third-line therapy underwent endo-scopy, choice of therapy being guided by sensitivities. Overall success after one, two and three courses of therapy were 73%, 94% and 98% respectively. Zullo et al. [2001a] reported an 83% cure rate in patients who had previously failed two courses of clarithromycin-amoxicillin and clarithromycin-tinidazole-based triple therapies. Gomollon et al. [2000a] studied the effectiveness of third-line treatment of H. pylori infection with 2-week quadruple, culture-guided regimens. The combination of omeprazole, tetra-cycline, bismuth and clarithromycin showed an eradication rate of only 36%, but if amoxicillin was used the rate was 67%. In the study by Vicente et al. [2002], after two unsuccessful attempts at eradication, all patients underwent endoscopy and culture, and patients received a quadruple culture-guided therapy. Cumulative H. pylori eradication rate with this strategy was as high as 99.6%. Treiber et al. [2002a] investigated whether a quadruple regimen containing furazolidone could be effective as a third-line therapy in patients with two previous H. pylori treatment failures. Cure of H. pylori was achieved in 90% of the patients nonrespon-sive to second eradication trial, which gave a final eradication rate of 99%. In the study by Qasim et al. [2005], 3280 patients received standard first-line eradication therapy, which was successful in 77% of the cases. Second-line therapy (bismuth-based quadruple) or triple therapy (altering constituent antibiotics) was successful in 56% of treated patients. Subsequent eradication attempts using rifabutin-based regimen was successful in 38% of patients, giving a cumulative eradication rate of 94%. Gisbert et al. [2004] included consecutive patients in whom two eradication regimens had failed to eradicate H. pylori, prescribed empirical third-line ‘rescue’ regimens, and achieved H. pylori eradication in 71% of the cases (intention-to-treat analysis). Based on these results, with estimated efficacy of 85%, 75% and 71%, respectively with first, second and third regimens, H. pylori eradication could finally be achieved in 99% of the patients. Gisbert et al. [2008b] evaluated the efficacy of different ‘rescue’ therapies empirically prescribed during 10 years to 500 patients in whom at least one eradication regimen had failed to cure H. pylori infection. Antibiotic susceptibility was unknown (therefore ‘rescue’ regimens were chosen empirically). Overall, H. pylori cure rates with the second and third-line ‘rescue’ regimens were 70% and 74%, giving a cumulative eradication rate as high as 98%. Finally, Rokkas et al. [2009] treated initially their patients with a triple regimen (omeprazole, amoxicillin and clari-thromycin), and subsequently with a second-line quadruple. After two previous H. pylori eradication failures, patients received omeprazole, amoxicillin and levofloxacin, as a third-line empirical strategy. Out of 540 patients initially included in the study, H. pylori was finally eradicated in 90% (intention-to-treat) and in 98% (per-protocol) of them.
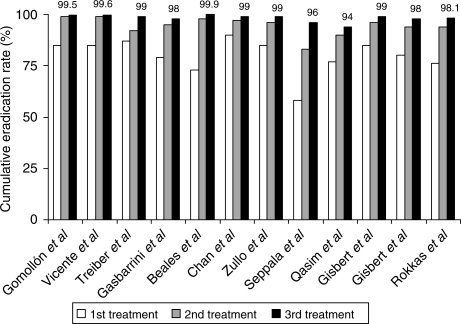
Therefore, a wider perspective of the benefits of retreating H. pylori infection can be obtained if cumulative eradication rates with successive treatments are taken into account. Thus, as represented in Figure 2, it can be concluded that H. pylori eradication can finally be achieved in almost 100% of the patients if three ‘rescue’ therapies are consecutively given [Rokkas et al. 2009; Gisbert et al. 2008b, 2004; Qasim et al. 2005; Vicente et al. 2002; Treiber et al. 2002a; Beales, 2001; Zullo et al. 2001a; Chan et al. 2000; Seppala et al. 2000; Gasbarrini et al. 2000a; Gomollon et al. 2000a].
Figure 2.
Cumulative H. pylori eradication rates with three consecutive eradication treatments.
Furthermore, these encouraging (cumulative) results have been obtained when more than three consecutive treatments have been prescribed [Gisbert et al. 2008a, 2005b]. As an example, Seppala et al. [2000] reported a cumulative eradication rate of 93% (intention-to-treat analysis) and even 100% (per-protocol analysis) after four empirical retreatments. We have recently confirmed that levofloxacin-based regimen can also be administered with good results after three previous eradication failures with antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline and even rifabutin [Gisbert, 2005a]. Thus, we prospectively evaluated 10 patients with three consecutive H. pylori eradication failures (first treatment with PPI-clarithromycin-amoxicillin, second treatment with RBC-tetracycline-metronidazole, and third treatment with PPI-amoxicillin-rifabutin). A fourth eradication regimen with 10-day levofloxacin, amoxicillin and PPI was prescribed, and intention-to-treat eradication rates were 70%. When we reviewed our experience with different ‘rescue’ therapies empirically prescribed during 10 years to 500 patients, the cumulative H. pylori eradication rate with four successive treatments was 99.5% [Gisbert et al. 2008b].
Finally, reports of ‘ineradicable’ H. pylori infection after more than four eradicating treatments failed have been recently published. Dore et al. [2003] prescribed a quadruple combination of PPI, bismuth, tetracycline, and metronidazole to patients who had failed two or more treatment courses of H. pylori eradication therapy (33 patients had failed prior treatment twice, 19 had failed three times, and 16 had failed four or more times); despite this a priori difficult task, H. pylori eradication was finally achieved in 93% of the patients. Tucci et al. [1999] reported their experience of 13 patients with at least five eradication failures and H. pylori strains resistant to both clarithromycin and nitroimidazoles. The treatment was organized into three sequential schedules employing partially different drug combinations (to face the various resistant strains), suspension formulations were preferred to tablets (to improve the dispersal of the drugs into the stomach), antibiotics were administered after meals and a variation on a standard diet exceeding the normal fat composition was given (to increase the time of contact of the antimicrobials with gastric mucosa), and patients were invited to lie down after the meals, changing their position every 5 min (to facilitate the penetration of drugs amid the anfractuosities of fundic mucosa). With this particular therapy, eradication was successful in 70% of the patients. In another example of ‘ineradicable’ H. pylori infection, levofloxacin-amoxicillin combination was successfully employed in a patient with a clari-thromycin- and metronidazole-resistant strain, who previously failed eight consecutive therapeutic attempts [Zullo et al. 2001b].
Conclusion
Even with the current most effective treatment regimens, >20% of patients will fail to eradicate H. pylori infection. This issue seems important at the present time, as therapy for H. pylori infection is becoming more and more frequently prescribed. Nowadays, apart from having to know well first-line eradication regimens, we must also be prepared to face treatment failures. Therefore, in designing a treatment strategy we should not focus on the results of primary therapy alone, but also on the final (overall) eradication rate.
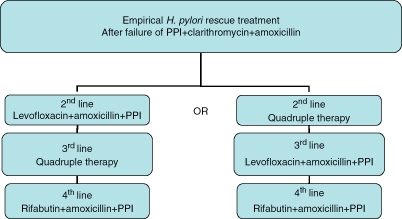
The choice of a ‘rescue’ treatment depends on which treatment is used initially. If a first-line clari-thromycin-based regimen was used, a second-line metronidazole-based treatment (such as the quadruple therapy) may be used afterwards, and then a levofloxacin-based combination would be a third-line ‘rescue’ option. Alternatively, it has recently been suggested that levofloxacin-based ‘rescue’ therapy constitutes an encouraging second-line strategy, representing an alternative to quadruple therapy in patients with previous PPI-clarithromycin-amoxicillin failure, with the advantage of efficacy, simplicity and safety. In this case, quadruple regimen may be reserved as a third-line ‘rescue’ option. Finally, rifabutin-based ‘rescue’ therapy constitutes an encouraging empirical fourth-line strategy after multiple previous eradication failures with key antibiotics such as amoxicillin, clarithromycin, metronidazole, tetracycline and levofloxacin (Figure 3).
Figure 3.
Choice of an empirical retreatment regimen, without culture and antimicrobial sensitivity testing, after failure of proton-pump inhibitor (PPI), amoxicillin and clarithromycin combination.
Note: quadruple therapy: combination of proton-pump inhibitor (PPI), bismuth, tetracycline and nitroimidazole (metronidazole or tinidazole).
Even after two consecutive failures, several studies have demonstrated that H. pylori eradication can finally be achieved in almost all patients if several ‘rescue’ therapies are consecutively given. As a final conclusion, therefore, the attitude in H. pylori eradication therapy failure, even after two or more unsuccessful attempts, should be to fight and not to surrender [Gisbert, 1998].
Acknowledgements
CIBEREHD is funded by the Instituto de Salud Carlos III.
Conflict of interest statement
None declared.
References
- Adamek R.J., Suerbaum S., Pfaffenbach B., Opferkuch W.(1998)Primary and acquired Helicobacter pylori resistance to clarithromycin, metro-nidazole, and amoxicillin - influence on treatment outcome. Am J Gastroenterol 93:386–389 [DOI] [PubMed] [Google Scholar]
- Akada J.K., Shirai M., Fujii K., Okita K., Nakazawa T.(1999)In vitro anti-Helicobacter pylori activities of new rifamycin derivatives, KRM-1648 and KRM-1657. Antimicrob Agents Chemother 43: 1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Menegatti M., Gatta L., Landi F., Ricci C, Acciardi C.et al. (1999)A Second-line anti-Helicobacter pylori therapy in patients with previously failed treatment. Am J Gastroenterol 94:2321–2323 [DOI] [PubMed] [Google Scholar]
- Altintas E., Sezgin O., Ulu O., Aydin O., Camdeviren H.(2004)Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol 10:1656–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antos D., Schneider-Brachert W., Bastlein E., Hanel C, Haferland C, Buchner M.et al. (2006)7-day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter 11:39–45 [DOI] [PubMed] [Google Scholar]
- Avidan B., Melzer E., Keller N., Bar-Meir S.(2001)The effect of culture results for Helicobacter pylori on the choice of treatment following failure of initial eradication. Isr Med Assoc J 3:163–165 [PubMed] [Google Scholar]
- Axon A.(2000)Quadruple therapy should be second-line treatment for Helicobacter pylori infection. In: Hunt R. H., Tytgat G. N. J.Helicobacter pylori Basic Mechanisms to Clinical Cure,Kluwer Academic Publishers:The Netherlands,pp. 631–635 [Google Scholar]
- Baena Diez J.M., Lopez Mompo C., Rams Rams F., Garcia Lareo M., Rosario Hernandez Ibanez M., Teruel Gila J.(2000)Eficacia de una terapia secuencial en la erradicación de Helicobacter pylori: cuádruple terapia con omeprazol, metronidazol, tetraciclina y bismuto tras el fracaso de la combinación de omepra-zol, claritromicina y amoxicilina.Med Clin (Barc) 115:617–619 [DOI] [PubMed] [Google Scholar]
- Battaglia G., Di Mario F., Vigneri S., Vianello F., Benvenuti M.E., Donisi P.M.et al. (1998)Strategy for the retreatment of failed Helicobacter pylori eradication therapy: a case series. ItalJ Gastroenterol Hepatol 30:370–374 [PubMed] [Google Scholar]
- Beales I.L.(2001)Efficacy of Helicobacter pylori eradication therapies: a single centre observational study. BMC Gastroenterol 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilardi C, Dulbecco P., Zentilin P., Reglioni S., Iiritano E., Parodi A.et al. (2004)A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: a controlled trial. Clin Gastroenterol Hepatol 2:997–1002 [DOI] [PubMed] [Google Scholar]
- Bock H., Koop H., Lehn N., Heep M.(2000)Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: preliminary experience. J Clin Gastroenterol 31:222–225 [DOI] [PubMed] [Google Scholar]
- Bogaerts P., Berhin C, Nizet H., Glupczynski Y.(2006)Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter 11:441–445 [DOI] [PubMed] [Google Scholar]
- Borody T.J., Pang G., Wettstein A.R., Clancy R., Herdman K., Surace R.et al. (2006)Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 23:481–488 [DOI] [PubMed] [Google Scholar]
- Brogden R.N., Fitton A.(1994)Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47:983–1009 [DOI] [PubMed] [Google Scholar]
- Buzas G.M., Jozan J.(2007)Nitrofuran-based regimens for the eradication of Helicobacter pylori infection. J Gastroenterol Hepatol 22:1571–1581 [DOI] [PubMed] [Google Scholar]
- Cabrita J., Oleastro M., Matos R., Manhente A., Cabral J., Barros R.et al. (2000)Features and trends in Helicobacter pylori antibiotic resistance in Lisbon area, Portugal (1990-1999). J Antimicrob Chemother 46:1029–1031 [DOI] [PubMed] [Google Scholar]
- Calvet X., Ducons J., Bujanda L., Bory F., Montserrat A., Gisbert J.P.(2005)Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. Am J Gastroenterol 100:1696–1701 [DOI] [PubMed] [Google Scholar]
- Calvet X., Gene E., Sanfeliu I.(2001)Estrategias terapéuticas para la Infección por Helicobacter pylori.Med Clin (Barc) 116:239. [DOI] [PubMed] [Google Scholar]
- Cammarota G., Cianci R., Cannizzaro O., Cuoco L., Pirozzi G., Gasbarrini A.et al. (2000)Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther 14:1339–1343 [DOI] [PubMed] [Google Scholar]
- Cammarota G., Martino A., Pirozzi G., Cianci R., Branca G., Nista E.C.et al. (2004)High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment Pharmacol Ther 19:789–795 [DOI] [PubMed] [Google Scholar]
- Canducci F., Ojetti V., Pola P., Gasbarrini G., Gasbarrini A.(2001)Rifabutin-based Helicobacter pylori eradication ‘rescue therapy’. Aliment Pharmacol Ther 15:143. [DOI] [PubMed] [Google Scholar]
- Carothers J.J., Bruce M.G., Hennessy T.W., Bensler M., Morris J.M., Reasonover A.L.et al. (2007)The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin Infect Dis 44:e5–8 [DOI] [PubMed] [Google Scholar]
- Caselli M., Zullo A., Maconi G., Parente F., Alvisi V., Casetti T.et al. (2007)‘Cervia II Working Group Report 2006’: guidelines on diagnosis and treatment of Helicobacter pylori infection in Italy. Dig Liver Dis 39:782–789 [DOI] [PubMed] [Google Scholar]
- Cattoir V., Nectoux J., Lascols C, Deforges L., Delchier J.C., Megraud F.et al. (2007)Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyra polymorphism associated with hypersuscept-ibility. Int J Antimicrob Agents 29:389–396 [DOI] [PubMed] [Google Scholar]
- Chan F.K., Sung J.J., Suen R., Wu J.C., Ling T.K., Chung S.C.(2000)Salvage therapies after failure of Helicobacter pylori eradication with ranitidine bismuth citrate-based therapies. Aliment Pharmacol Ther 14:91–95 [DOI] [PubMed] [Google Scholar]
- Chang W.L., Sheu B.S., Cheng H.C., Yang Y.J., Yang H.B., Wu J.J.(2009)Resistance to metroni-dazole, clarithromycin and levofloxacin of Helicobacter pylo ri before and after clarithromycin-based therapy in Taiwan. J Gastroenterol Hepatol 24(7):1230–1235 [DOI] [PubMed] [Google Scholar]
- Chi C.H., Lin C.Y, Sheu B.S., Yang H.B., Huang A.H., Wu J.J.(2003)Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment Pharmacol Ther 18:347–353 [DOI] [PubMed] [Google Scholar]
- Chisholm S.A., Owen R.J.(2009)Frequency and molecular characteristics of ciprofloxacin-and rifampicin-resistant Helicobacter pylori from gastric infections in the United Kingdom.J Med MicrobiolJul 9 [Epub ahead at print] [DOI] [PubMed]
- Choung R.S., Lee S.W., Jung S.W., Han WS., Kim M.J., Jeen Y.T.et al. (2006)[Comparison of the effectiveness of quadruple salvage regimen for Helicobacter pylori Infection according to the duration of treatment]. Korean J Gastroenterol 47:131–135 [PubMed] [Google Scholar]
- Chung S.J., Lee D.H., Kim N., Jung S.H., Kim J.W, Hwang J.H.et al. (2007)Eradication rates of Helicobacter pylori infection with second-line treatment: non-ulcer dyspepsia compared to peptic ulcer disease. Hepatogastroenterology 54:1293–1296 [PubMed] [Google Scholar]
- Cianci R., Montalto M., Pandolfi F., Gasbarrini G.B., Cammarota G.(2006)Third-line rescue therapy for Helicobacter pylori infection. World J Gastroenterol 12:2313–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho L.G., Moretzsohn L.D., Vieira W.L., Gallo M.A., Passos M.C., Cindr J.M.et al. (2005)New once-daily, highly effective rescue triple therapy after multiple Helicobacter pylori treatment failures: a pilot study. Aliment Pharmacol Ther 21:783–787 [DOI] [PubMed] [Google Scholar]
- Cremonini F., Di Caro S., Covino M., Armuzzi A., Gabrielli M., Santarelli L.et al. (2002)Effect of different probiotic preparations on an ti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol 97:2744–2749 [DOI] [PubMed] [Google Scholar]
- Croom K.F., Goa K.L.(2003)Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs 63:2769–2802 [DOI] [PubMed] [Google Scholar]
- Cudia B., Romano M., Gioè F.P., Barbera N., Lo Gerfo D., Montalto R.(1997)‘Rescue’ therapy including ranitidine bismuth citrate + minocicline + amoxicillin for eradication of Helicobacter pylori in previous h.p. treatment failure. Gut 41(Suppl. 1):A103 [Google Scholar]
- De Boer W, Driessen W, Jansz A., Tytgat G.(1995)Effect of acid suppression on efficacy of treatment for Helicobacter pylori infection. Lancet 345:817–820 [DOI] [PubMed] [Google Scholar]
- De Boer WA., Borody T.J.(2000)Treatment failures and secondary resistance to antibiotics. a growing concern in Helicobacter pylori therapy. Dig Liver Dis 32:673–675 [DOI] [PubMed] [Google Scholar]
- De Boer W.A., Driessen W.M., Potters V.P., Tytgat G.N.(1994)Randomized study comparing 1 with 2 weeks of quadruple therapy for eradicating Helicobacter pylori. Am J Gastroenterol 89:1993–1997 [PubMed] [Google Scholar]
- De Boer WA., Tytgat G.N.(2000)Regular review: treatment of Helicobacter pylori infection. BMJ 320:31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco V., Ierardi E., Hassan C., Zullo A.(2009)Furazolidone therapy for Helicobacter pylori: is it effective and safe? World J Gastroenterol 15:1914–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltenre M., Ntounda R., Jonas C., De Koster E.(1998)Eradication of Helicobacter pylori: why does it fail? Ital J Gastroenterol Hepatol 30(Suppl. 3):S326–328 [PubMed] [Google Scholar]
- Della Monica P., Lavagna A., Masoero G., Lombardo L., Crocella L., Pera A.(2002)Effectiveness of Helicobacter pylori eradication treatments in a primary care setting in Italy. Aliment Pharmacol Ther 16:1269–1275 [DOI] [PubMed] [Google Scholar]
- Di Caro S., Assunta Zocco M., Cremonini F., Candelli M., Nista E.C., Bartolozzi F.et al. (2002)Levofloxacin based regimens for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 14:1309–1312 [DOI] [PubMed] [Google Scholar]
- Di Caro S., Franceschi F., Mariani A., Thompson F., Raimondo D., Masci E.et al. (2009)Second-line levofloxacin-based triple schemes for Helicobacter pylori eradication. Dig Liver Dis 41:480–485 [DOI] [PubMed] [Google Scholar]
- Dore M.P., Leandro G., Realdi G., Sepulveda A.R., Graham D.Y.(2000)Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci 45:68–76 [DOI] [PubMed] [Google Scholar]
- Dore M.P., Marras L., Maragkoudakis E., Nieddu S., Manca A., Graham D.Y.et al. (2003)Salvage therapy after two or more prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter 8:307–309 [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Dariani N., Mirmomen S., Mansour-Ghanaei F., Noormohammadpoor P., Sotodehmanesh R., Haghpanah B.et al. (2003)The efficacy of furazolidone-based quadruple therapy for eradication of Helicobacter pylori infection in Iranian patients resistant to metronidazole-based quadruple therapy. Med Sci Mon it 9:PI105–108 [PubMed] [Google Scholar]
- Egan B.J., Marzio L., O'Connor H., O'Morain C.(2008)Treatment of Helicobacter pylori infection. Helicobacter 13(Suppl. 1):35–40 [DOI] [PubMed] [Google Scholar]
- Eisig J.N., Silva F.M., Barbuti R.C., Rodriguez T.N., Malfertheiner P., Moraes Filho J.P.et al. (2009)Efficacy of a 7-day course of furazolidone, levofloxacin, and lansoprazole after failed Helicobacter pylori eradication. BMC Gastroenterol 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisig J.N., Silva F.M., Rodriguez T.N., Hashimoto C.L., Barbuti R.C.(2005)A furazolidone-based quadruple therapy for Helicobacter pylori retreatment in patients with peptic ulcer disease. Clinics 60:485–488 [DOI] [PubMed] [Google Scholar]
- Elizalde I.R., Borda F., Jara C, Martínez A., Rodríguez C., Jiménez J.(1998)Eficacia de dos tratamientos consecutivos en la erradicación de Helicobacter pylori. Anales Sis San Navarra 21(Suppl. 2):83–88 [Google Scholar]
- European Helicobacter pylori Study Group. The Maastricht Consensus Report.(1997)Current European Concepts in the Management of Helicobacter pylori Infection. Gut 41:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarrini A., Ojetti V., Armuzzi A., Branca G., Canducci F., Torre E.S.et al. (2000a)Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment Pharmacol Ther 14:79–83 [DOI] [PubMed] [Google Scholar]
- Gasbarrini A., Ojetti V., Pitocco D., Armuzzi A., Silveri N.G., Pola P.et al. (2000b)Efficacy of different Helicobacter pylori eradication regimens in patients affected by insulin-dependent diabetes mellitus. Scand J Gastroenterol 35:260–263 [DOI] [PubMed] [Google Scholar]
- Gatta L., Zullo A., Perna F., Ricci C, De Francesco V., Tampieri A.et al. (2005)A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Aliment Pharmacol Ther 22:45–49 [DOI] [PubMed] [Google Scholar]
- Giannini E.G., Bilardi C., Dulbecco P., Mamone M., Santi M.L., Testa R.et al. (2006)A study of 4- and 7-day triple therapy with rabeprazole, high-dose levofloxacin and tinidazole rescue treatment for Helicobacter pylori eradication. Aliment Pharmacol Ther 23:281–287 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P.(1998)[The attitude in a failure to eradicate Helicobacter pylori: surrender or fight?].Med Clin (Barc) 111:778–782 [PubMed] [Google Scholar]
- Gisbert J.P.(2005a)Rescue therapy with levofloxacin after multiple H. pylori treatment failures. Aliment Pharmacol Ther 22:653–654;author reply 654-655 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P.(2008a)‘Rescue’ regimens after Helicobacter pylori treatment failure. World J Gastroenterol 14:5385–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert J.P., Bermejo F., Castro-Fernandez M., Perez-Aisa A., Fernandez-Bermejo M., Tomas A.et al. (2008c)Second-line rescue therapy with levofloxacin after H. pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol 103(1):71–76 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Boixeda D., Bermejo F., Nieves Rincon M., Jesus Higes M., Angeles Arpa M.et al. (1999a)Re-treatment after Helicobacter pylori eradication failure. Eur J Gastroenterol Hepatol 11:1049–1054 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Calvet X., Bujanda L., Marcos S., Gisbert J.L., Pajares J.M.(2003)‘Rescue’ therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter 8:90–94 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Calvet X., Gomollon F., Sainz R.(2000a)[Treatment for the eradication of Helicobacter pylori. Recommendations of the Spanish Consensus Conference].Med Clin (Barc) 114:185–195 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Castro-Fernandez M., Bermejo F., Perez-Aisa A., Ducons J., Fernandez-Bermejo M.et al. (2006a)Third-line rescue therapy with levofloxacin after two H. pylori treatment failures. Am J Gastroenterol 101:243–247 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Dominguez-Munoz A., Dominguez-Martin A., Gisbert J.L., Marcos S.(2005b)Esomeprazole-based therapy in Helicobacter pylori eradication: any effect by increasing the dose of esome-prazole or prolonging the treatment? Am J Gastroenterol 100:1935–1940 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Fernandez-Bermejo M., Molina-Infante J., Perez-Gallardo B., Prieto-Bermejo A.B., Mateos-Rodriguez J.M.et al. (2007b)First-line triple therapy with levofloxacin for Helicobacter pylori eradication. Aliment Pharmacol Ther 26:495–500 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Fuentes J., Carpio D., Tito L., Guardiola J., Tomas A.et al. (2005c)7-Day rescue therapy with ranitidine bismuth citrate after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 21:1249–1253 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Gravalos R.G., Carpio D., Pajares J.M.(1999b)Seven-day ‘rescue’ therapy after Helicobacter pylori treatment failure: omeprazole, bismuth, tetracycline and metronidazole vs. ranitidine bismuth citrate, tetracycline and metronidazole. Aliment Pharmacol Ther 13:1311–1316 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Jimenez-Alonso I., Moreno-Otero R., Pajares J.M.(2008b)Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment Pharmacol Ther 27:346–354 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Moreno-Otero R., Pajares J.M.(2006b)Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther 24:1469–1474 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Moreno-Otero R., Pajares J.M.(2007c)Levofloxacin- vs. raniti-dine bismuth citrate-containing therapy after H. pylori treatment failure. Helicobacter 12:68–73 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Olivares D., Pajares J.M.(2005e)Helicobacter pylori first-line treatment and rescue options in patients allergic to penicillin. Aliment Pharmacol Ther 22:1041–1046 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gisbert J.L., Marcos S., Pajares J.M.(2004)Empirical Helicobacter pylori ‘rescue’ therapy after failure of two eradication treatments. Dig Liver Dis 36:7–12 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gonzalez L., Calvet X.(2005d)Systematic review and meta-analysis: proton pump inhibitor vs. ranitidine bismuth citrate plus two antibiotics in Helicobacter pylori eradication. Helicobacter 10:157–171 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gonzalez L., Calvet X., Garcia N., Lopez T., Roque M.et al. (2000b)Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta-analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther 14:1319–1328 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Morena F.(2006c)Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther 23:35–44 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Pajares J.M.(2002)Review article: Helicobacter pylori ‘rescue’ regimen when proton pump inhibitor-based triple therapies fail. Aliment Pharmacol Ther 16:1047–1057 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Pajares J.M.(2005)Helicobacter pylori ‘rescue’ therapy after failure of two eradication treatments. Helicobacter 10:363–372 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Pajares J.M., Valle J.(1999c)Ranitidine bismuth citrate therapy regimens for treatment of Helicobacter pylori infection: a review. Helicobacter 4:58–66 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Pajares R., Pajares J.M.(2007d)Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter 12(Suppl. 2):50–58 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Perez-Aisa A., Castro-Fernandez M., Barrio J., Rodrigo L., Cosme A.et al. (2009)Helicobacter pylori First-line treatment and rescue option containing levofloxacin in patients allergic to penicillin.Dig Liver DisJul 23 [Epub ahead of print] [DOI] [PubMed]
- Glocker E., Stueger H.P., Kist M.(2007)Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrob Agents Chemother 51:346–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomollon F., Ducons J.A., Ferrero M., Garcia Cabezudo J., Guirao R., Simon M.A.et al. (1999)Quadruple therapy is effective for eradicating Helicobacter pylori after failure of triple proton-pump inhibitor-based therapy: a detailed, prospective analysis of 21 consecutive cases.Helicobacter 4:222–225 [DOI] [PubMed] [Google Scholar]
- Gomollon F., Sicilia B., Ducons J.A., Sierra E., Revillo M.J., Ferrero M.(2000a)Third line treatment for Helicobacter pylori: a prospective, culture-guided study in peptic ulcer patients. Aliment Pharmacol Ther 14:1335–1338 [DOI] [PubMed] [Google Scholar]
- Gomollon F., Valdeperez J., Garuz R., Fuentes J., Barrera F., Malo J.et al. (2000b)Análisis coste-efectividad de dos estrategias de erradicación de Helicobacter pylo ri: resultados de un estudio prospectivo y aleatori-zado en atención primaria.Med Clin (Barc) 115:1–6 [DOI] [PubMed] [Google Scholar]
- Gonzalez Carro P., Perez Roldan F., De Pedro Esteban A., Legaz Huidobro M.L., Soto Fernandez S., Roncero Garcia Escribano O.et al. (2007)Efficacy of rifabutin-based triple therapy in Helicobacter pylori infected patients after two standard treatments. J Gastroenterol Hepatol 22:60–63 [DOI] [PubMed] [Google Scholar]
- Graham D.Y.(2009)Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol 7:145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.Y., Osato M.S., Hoffman J., Opekun A.R., Anderson S.Y., Kwon D.H.et al. (2000)Metronidazole containing quadruple therapy for infection with metronidazole resistant Helicobacter pylori: a prospective study. Aliment Pharmacol Ther 14:745–750 [DOI] [PubMed] [Google Scholar]
- Gumurdulu Y., Serin E., Ozer B., Kayaselcuk F., Ozsahin K., Cosar A.M.et al. (2004)Low eradication rate of Helicobacter pylori with triple 7-14 days and quadruple therapy in Turkey. World J Gastroenterol 10:668–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guslandi M.(2001)Alternative antibacterial agents for Helicobacter pylori eradication. Aliment Pharmacol Ther 15:1543–1547 [DOI] [PubMed] [Google Scholar]
- Haas C.E., Nix D.E., Schentag J.J.(1990)In vitro selection of resistant Helicobacter pylori. Antimicrob Agents Chemother 34:1637–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey C.J., Atherton J.C., Treichel H.C., Thjodleifsson B., Ravic M.(2003)Safety and efficacy of 7-day rabeprazole- and omeprazole-based triple therapy regimens for the eradication of Helicobacter pylori in patients with documented peptic ulcer disease. Aliment Pharmacol Ther 17:1065–1074 [DOI] [PubMed] [Google Scholar]
- Heep M., Beck D., Bayerdorffer E., Lehn N.(1999)Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob Agents Chemother 43:1497–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M., Miwa H., Nagahara A., Sato N.(2001)Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. ScandJ Gastroenterol 36:690–700 [DOI] [PubMed] [Google Scholar]
- Houben M.H., Van Der Beek D., Hensen E.F., Craen A.J., Rauws E.A., Tytgat G.N.(1999)A systematic review of Helicobacter pylori eradication therapy - the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther 13:1047–1055 [DOI] [PubMed] [Google Scholar]
- Howden C.W., Hunt R.H.(1998)Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 93:2330–2338 [DOI] [PubMed] [Google Scholar]
- Hsu P.I., Wu D.C., Chen A., Peng N.J., Tseng H.H., Tsay F.W.et al. (2008)Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest 38:404–409 [DOI] [PubMed] [Google Scholar]
- Huang J.Q., Hunt R.H.(1999)Treatment after failure: the problem of ‘non-responders’. Gut 45(Suppl. 1):I40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov V., Domareva I., Koudryavtseva L., Maev I., Ganskaya Z.(2002)Furazolidone-based triple ‘rescue therapy’ vs. quadruple ‘rescue therapy’ for the eradication of Helicobacter pylori resistant to metroni-dazole. Aliment Pharmacol Ther 16:1277–1282 [DOI] [PubMed] [Google Scholar]
- Isomoto H., Inoue K., Furusu H., Enjoji A., Fujimoto C., Yamakawa M.et al. (2003)High-dose rabeprazole-amoxicillin versus rabeprazole-amoxicil-lin-metronidazole as second-line treatment after failure of the Japanese standard regimen for Helicobacter pylori infection. Aliment Pharmacol Ther 18:101–107 [DOI] [PubMed] [Google Scholar]
- Kahn J.B.(2001)Latest industry information on the safety profile of levofloxacin in the US. Chemotherapy 47(Suppl. 3):32–37,discussion 44-38 [DOI] [PubMed] [Google Scholar]
- Kearney D.J.(2001)Retreatment of Helicobacter pylori infection after initial treatment failure. Am J Gastroenterol 96:1335–1339 [DOI] [PubMed] [Google Scholar]
- Kim J.J., Kim J.G., Kwon D.H.(2003)Mixed-infection of antibiotic susceptible and resistant Helicobacter pylori isolates in a single patient and underestimation of antimicrobial susceptibility testing. Helicobacter 8:202–206 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Kim J.S., Kim N., Kim S.G., Jung H.C., Song I.S.(2006)Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents 28:6–13 [DOI] [PubMed] [Google Scholar]
- Kim M.N., Kim N., Lee S.H., Park Y.S., Hwang J.H., Kim J.W.et al. (2008)The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 13:261–268 [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Saika T, Muraoka H., Murakami K., Fujioka T.(2006)Helicobacter pylori isolated from patients who later failed H. pylori eradication triple therapy readily develop resistance to clarithromycin. J Med Microbiol 55:737–740 [DOI] [PubMed] [Google Scholar]
- Koksal A.S., Parlak E., Filik L., Yolcu O.F., Odemis B., Ulker A.et al. (2005)Ranitidine bismuth citrate-based triple therapies as a second-line therapy for Helicobacter pylori in Turkish patients. J Gastroenterol Hepatol 20:637–642 [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ringler S.S., Eaton K.A., Green W.B., Leunk R.(1996)Manifestations of the local gastric immune response in gnotobiotic piglets infected with Helicobacter pylori. Vet Immunol Immunopathol 52:159–173 [DOI] [PubMed] [Google Scholar]
- Kumala W., Rani A.(2006)Patterns of Helicobacter pylori isolate resistance to fluoroquinolones, amoxicillin, clarithromycin and metronidazoles. Southeast Asian J Trop Med Public Health 37:970–974 [PubMed] [Google Scholar]
- Kuo C.H., Hu H.M., Kuo F.C., Hsu P.I., Chen A., Yu F.J.et al. (2009)Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother 63:1017–1024 [DOI] [PubMed] [Google Scholar]
- Kwon D.H., Lee M., Kim J.J., Kim J.G., El-Zaatari F.A., Osato M.S.et al. (2001)Furazolidone- and nitrofurantoin-resistant Helicobacter pylori: prevalence and role of genes involved in metronidazole resistance. Antimicrob Agents Chemother 45:306–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L., Fennerty M.B., Osato M., Sugg J., Suchower L., Probst P.et al. (2000)Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol 95:3393–3398 [DOI] [PubMed] [Google Scholar]
- Laine L., Schoenfeld P., Fennerty M.B.(2001)Therapy for Helicobacter pylori in patients with nonul-cer dyspepsia. a meta-analysis of randomized, controlled trials. Ann Intern Med 134:361–369 [DOI] [PubMed] [Google Scholar]
- Laine L., Suchower L., Frantz J., Connors A., Neil G.(1998)Twice-daily, 10-day triple therapy with omeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in duodenal ulcer disease: results of three multicenter, double-blind, United States trials. Am J Gastroenterol 93:2106–2112 [DOI] [PubMed] [Google Scholar]
- Lam S.K, Talley N.J.(1998)Report of the 1997 Asia Pacific Consensus Conference on the Management of Helicobacter pylori Infection. J Gastroenterol Hepatol 13:1–12 [DOI] [PubMed] [Google Scholar]
- Lamouliatte H., Megraud F., Delchier J.C., Bretagne J.F., Courillon-Mallet A., De Korwin J.D.et al. (2003)Second-line treatment for failure to eradicate Helicobacter pylori: a randomized trial comparing four treatment strategies. Aliment Pharmacol Ther 18:791–797 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Cheon J.H., Park M.J., Kim N, Lee D.H., Kim J.M.et al. (2005)[The trend of eradication rates of second-line quadruple therapy containing metronidazole for Helicobacter pylo ri infection: an analysis of recent eight years]. Korean J Gastroenterol 46:94–98 [PubMed] [Google Scholar]
- Lee J.H., Hong S.P., Kwon C.I., Phyun L.H., Lee B.S., Song H.U.et al. (2006a)[The efficacy of levofloxacin based triple therapy for Helicobacter pylori eradication]. Korean J Gastroenterol 48:19–24 [PubMed] [Google Scholar]
- Lee J.M., Breslin N.P., Hyde D.K., Buckley M.J., O'Morain C.A.(1999)Treatment options for Helicobacter pylori infection when proton pump inhibitor-based triple therapy fails in clinical practice. Aliment Pharmacol Ther 13:489–496 [DOI] [PubMed] [Google Scholar]
- Lee Y.C., Wu H.M., Chen T.H., Liu T.Y., Chiu H.M., Chang C.C.et al. (2006b)A community-based study of Helicobacter pylori therapy using the strategy of test, treat, retest, and re-treat initial treatment failures. Helicobacter 11:418–424 [DOI] [PubMed] [Google Scholar]
- Lerang F., Moum B., Haug J.B., Berge T., Tolas P., Sandvei P.K.et al. (1997)Highly effective second-line anti-Helicobacter pylori therapy in patients with previously failed metronidazole-based therapy. Scand J Gastroenterol 32:1209–1214 [DOI] [PubMed] [Google Scholar]
- Magaret N., Burm M., Faigel D., Kelly C, Peterson W., Fennerty M.B.(2001)A randomized trial of lansoprazole, amoxycillin, and clarithromycin versus lansoprazole, bismuth, metronidazole and tetracycline in the retreatment of patients failing initial Helicobacter pylori therapy. Dig Dis 19:174–178 [DOI] [PubMed] [Google Scholar]
- Malfertheiner P., Megraud F., O'Morain C, Bazzoli F., El-Omar E., Graham D.et al. (2007)Current Concepts in the Management of Helicobacter pylori Infection: The Maastricht III Consensus Report. Gut 56:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfertheiner P., Megraud F., O'Morain C, Hungin A.P., Jones R., Axon A.et al. (2002)Current Concepts in the Management of Helicobacter pylori Infection - the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther 16:167–180 [DOI] [PubMed] [Google Scholar]
- Marko D., Calvet X., Ducons J., Guardiola J., Tito L., Bory F.(2005)Comparison of two management strategies for Helicobacter pylori treatment: clinical study and cost-effectiveness analysis. Helicobacter 10:22–32 [DOI] [PubMed] [Google Scholar]
- Marzio L., Coraggio D., Capodicasa S., Grossi L., Cappello G.(2006)Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxi-cillin, and esomeprazole. Helicobacter 11:237–242 [DOI] [PubMed] [Google Scholar]
- Matsuhisa T, Kawai T, Masaoka T, Suzuki H., Ito M., Kawamura Y.et al. (2006)Efficacy of metronidazole as second-line drug for the treatment of Helicobacter pylori infection in the Japanese population: a multicenter study in the Tokyo metropolitan area. Helicobacter 11:152–158 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Miki I., Aoyama N., Shirasaka D., Watanabe Y, Morita Y.et al. (2005)Levofloxacin-versus metronidazole-based rescue therapy for H. pylori infection in Japan. Dig Liver Dis 37:821–825 [DOI] [PubMed] [Google Scholar]
- Megraud F.(2004)H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraud F., Lamouliatte H.(2003)Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 17:1333–1343 [DOI] [PubMed] [Google Scholar]
- Michopoulos S., Tsibouris P., Bouzakis H, Balta A., Vougadiotis J., Broutet N.et al. (2000)Randomized study comparing omeprazole with raniti-dine as anti-secretory agents combined in quadruple second-line Helicobacter pylori eradication regimens. Aliment Pharmacol Ther 14:737–744 [DOI] [PubMed] [Google Scholar]
- Miehlke S., Hansky K., Schneider-Brachert W., Kirsch C., Morgner A., Madisch A.et al. (2006)Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther 24:395–403 [DOI] [PubMed] [Google Scholar]
- Miehlke S., Kirsch C, Schneider-Brachert W., Haferland C, Neumeyer M., Bastlein E.et al. (2003)A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 8:310–319 [DOI] [PubMed] [Google Scholar]
- Miehlke S., Schneider-Brachert W., Kirsch C, Morgner A., Madisch A., Kuhlisch E.et al. (2008)One-week once-daily triple therapy with esomeprazole, moxifloxacin, and rifabutin for eradication of persistent Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter 13:69–74 [DOI] [PubMed] [Google Scholar]
- Miwa H., Nagahara A., Kurosawa A., Ohkusa T, Ohkura R., Hojo M.et al. (2003)Is antimicrobial susceptibility testing necessary before second-line treatment for Helicobacter pylori infection? Aliment Pharmacol Ther 17:1545–1551 [DOI] [PubMed] [Google Scholar]
- Miyachi H., Miki I., Aoyama N., Shirasaka D., Matsumoto Y, Toyoda M.et al. (2006)Primary levofloxacin resistance and gyra/B mutations among Helicobacter pylori in Japan. Helicobacter 11:243–249 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Soo S., Deeks J., Delaney B., Harris A., Innes M.et al. (2006)Eradication of Helicobacter pylori for non-ulcer dyspepsia.Cochrane Database Syst Rev CD002096. [DOI] [PubMed]
- Murakami K, Okimoto T, Kodama M., Sato R., Miyajima H., Ono M.et al. (2006)Comparison of amoxicillin-metronidazole plus famotidine or lansoprazole for amoxicillin-clarithromycin-proton pump inhibitor treatment failures for Helicobacter pylori infection. Helicobacter 11:436–440 [DOI] [PubMed] [Google Scholar]
- Murakami K, Okimoto T, Kodama M., Sato R., Watanabe K., Fujioka T.(2008)Evaluation of three different proton pump inhibitors with amoxicillin and metronidazole in retreatment for Helicobacter pylo ri infection. J Clin Gastroenterol 42:139–142 [DOI] [PubMed] [Google Scholar]
- Nagahara A., Miwa H., Kawabe M., Kurosawa A., Asaoka D., Hojo M.et al. (2004)Second-line treatment for Helicobacter pylori infection in Japan: proton pump inhibitor-based amoxicillin and metronidazole regimen. J Gastroenterol 39:1051–1055 [DOI] [PubMed] [Google Scholar]
- Nagahara A., Miwa H., Ohkura R., Yamada T., Sato K., Hojo M.et al. (2001)Strategy for retreatment of therapeutic failure of eradication of Helicobacter pylori infection. J Gastroenterol Hepatol 16:613–618 [DOI] [PubMed] [Google Scholar]
- Navarro-Jarabo J.M., Fernandez N., Sousa F.L., Cabrera E., Castro M., Ramirez L.M.et al. (2007)Efficacy of rifabutin-based triple therapy as second-line treatment to eradicate Helicobacter pylori infection. BMC Gastroenterol 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijevitch A.A., Shcherbakov P.L., Sataev V.U., Khasanov R., Al Khashash R., Tuygunov M.M.(2005)Helicobacter pylori eradication in childhood after failure of initial treatment: advantage of quadruple therapy with nifuratel to furazolidone. Aliment Pharmacol Ther 22:881–887 [DOI] [PubMed] [Google Scholar]
- Nishizawa T., Suzuki H., Hibi T.(2009)Quinolone-based third-line therapy for Helicobacter pylori eradication. J Clin Biochem Nutr 44:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nista E.C., Candelli M., Cremonini F., Cazzato I.A., Di Caro S., Gabrielli M.et al. (2003)Levofloxacin-based triple therapy vs. quadruple therapy in second-line Helicobacter pylori treatment: a randomized trial. Aliment Pharmacol Ther 18:627–633 [DOI] [PubMed] [Google Scholar]
- Orsi P., Pinazzi O., Aragona G., Di Mario F.(2003)Rabeprazole/levofloxacin based triple therapy as a salvatage treatment after failure of H. pylori eradication with standard regimens. Helicobacter 8:339–493 [Google Scholar]
- Page R.L., Ii, Ferguson D., Cantu M.(2007)An alternative salvage regimen for Helicobacter pylori- resistant patients with heart failure. Cardiology 110:112–115 [DOI] [PubMed] [Google Scholar]
- Paoluzi P., Iacopini F., Crispino P., Nardi F., Bella A., Rivera M.et al. (2006)2-week triple therapy for Helicobacter pylori infection is better than 1-week in clinical practice: a large prospective single-center randomized study. Helicobacter 11:562–568 [DOI] [PubMed] [Google Scholar]
- Parente F., Cucino C., Bianchi Porro G.(2003)Treatment options for patients with Helicobacter pylori infection resistant to one or more eradication attempts. Dig Liver Dis 35:523–528 [DOI] [PubMed] [Google Scholar]
- Peitz U., Hackelsberger A., Malfertheiner P.(1999)A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs 57:905–920 [DOI] [PubMed] [Google Scholar]
- Peitz U., Sulliga M., Wolle K., Leodolter A., Von Arnim U., Kahl S.et al. (2002)High rate of post-therapeutic resistance after failure of macrolide-nitroi-midazole triple therapy to cure Helicobacter pylo ri infection: impact of two second-line therapies in a randomized study. Aliment Pharmacol Ther 16:315–324 [DOI] [PubMed] [Google Scholar]
- Perna F., Zullo A., Ricci C, Hassan C, Morini S., Vaira D.(2007)Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liver Dis 39:1001–1005 [DOI] [PubMed] [Google Scholar]
- Perri F., Festa V., Andriulli A.(1998)Treatment of antibiotic-resistant Helicobacter pylori. N Engl J Med 339:53. [DOI] [PubMed] [Google Scholar]
- Perri F., Festa V., Clemente R., Quitadamo M., Andriulli A.(2000)Rifabutin-based ‘rescue therapy’ for Helicobacter pylori infected patients after failure of standard regimens. Aliment Pharmacol Ther 14:311–316 [DOI] [PubMed] [Google Scholar]
- Perri F., Festa V., Clemente R., Villani M.R., Quitadamo M., Caruso N.et al. (2001a)Randomized study of two ‘rescue’ therapies for Helicobacter pylori- infected patients after failure of standard triple therapies. Am J Gastroenterol 96:58–62 [DOI] [PubMed] [Google Scholar]
- Perri F., Festa V., Merla A., Barberani F., Pilotto A., Andriulli A.(2003)Randomized study of different ‘second-line’ therapies for Helicobacter pylori infection after failure of the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther 18:815–820 [DOI] [PubMed] [Google Scholar]
- Perri F., Villani M.R., Quitadamo M., Annese V, Niro G.A., Andriulli A.(2001b)Ranitidine bismuth citrate-based triple therapies after failure of the standard ‘Maastricht triple therapy’: a promising alternative to the quadruple therapy? Aliment Pharmacol Ther 15:1017–1022 [DOI] [PubMed] [Google Scholar]
- Pilotto A., Franceschi M., Rassu M., Leandro G., Bozzola L., Furlan F.et al. (2000)Incidence of secondary Helicobacter pylori resistance to antibiotics in treatment failures after 1-week proton pump inhibitor-based triple therapies: a prospective study. Dig Liver Dis 32:667–672 [DOI] [PubMed] [Google Scholar]
- Qasim A., Sebastian S., Thornton O., Dobson M., McLoughlin R., Buckley M.et al. (2005)Rifabutin-and furazolidone-based Helicobacter pylori eradication therapies after failure of standard first- and second-line eradication attempts in dyspepsia patients. Aliment Pharmacol Ther 21:91–96 [DOI] [PubMed] [Google Scholar]
- Reilly T.G., Ayres R.C., Poxon V, Walt R.P.(1995)Helicobacter pylo ri eradication in a clinical setting: success rates and the effect on the quality of life in peptic ulcer. Aliment Pharmacol Ther 9:483–490 [DOI] [PubMed] [Google Scholar]
- Rinaldi V, Zullo A., De Francesco V, Hassan C, Winn S., Stoppino V.et al. (1999)Helicobacter pylori eradication with proton pump inhibitor-based triple therapies and re-treatment with ranitidine bismuth citrate-based triple therapy. Aliment Pharmacol Ther 13:163–168 [DOI] [PubMed] [Google Scholar]
- Rispo A., Di Girolamo E., Cozzolino A., Bozzi R., Morante A., Pasquale L.(2007)Levofloxacin in first-line treatment of Helicobacter pylori infection. Helicobacter 12:364–365 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Torres M., Salgado-Mercado R., Rios-Bedoya CF., Aponte-Rivera E., Marxuach-Cuetara A.M., Rodriguez-Orengo J.F.et al. (2005)High eradication rates of Helicobacter pylori infection with first- and second-line combination of esomeprazole, tetracycline, and metronidazole in patients allergic to penicillin. Dig Dis Sci 50:634–639 [DOI] [PubMed] [Google Scholar]
- Rokkas T., Sechopoulos P., Robotis I., Margantinis G., Pistiolas D.(2009)Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am J Gastroenterol 104:21–25 [DOI] [PubMed] [Google Scholar]
- Rokkas T., Sechopoulos P., Robotis J., Pistiolas D.(2006)Triple levofloxacin-based rescue therapy is an accepted empirical third-line treatment. Am J Gastroenterol 101:1938;author reply 1938-1939 [DOI] [PubMed] [Google Scholar]
- Romano M., Iovene M.R., Russo M.I., Rocco A., Salerno R., Cozzolino D.et al. (2008)Failure of first-line eradication treatment significantly increases prevalence of antimicrobial-resistant Helicobacter pylori clinical isolates. J Clin Pathol 61:1112–1115 [DOI] [PubMed] [Google Scholar]
- Rossi G.(1999)[An update on the antibiotic therapy of tuberculosis]. Recenti Prog Med 90:241–243 [PubMed] [Google Scholar]
- Saad R.J., Chey W.D.(2008)Persistent Helicobacter pylori infection after a course of antimicrobial therapy - what's next? Clin Gastroenterol Hepatol 6:1086–1090 [DOI] [PubMed] [Google Scholar]
- Saad R.J., Schoenfeld P., Kim H.M., Chey W.D.(2006)Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol 101:488–496 [DOI] [PubMed] [Google Scholar]
- Sanches B., Coelho L., Moretzsohn L., Vieira Jr G.(2008)Failure of Helicobacter pylori treatment after regimes containing clarithromycin: new practical therapeutic options. Helicobacter 13:572–576 [DOI] [PubMed] [Google Scholar]
- Sanchez J.E., Saenz N.G., Rincon M.R., Martin I.T., Sanchez E.G., Martinez M.J.(2000)Susceptibility of Helicobacter pylori to mupirocin, oxa-zolidinones, quinupristin/dalfopristin and new quino-lones. J Antimicrob Chemother 46:283–285 [DOI] [PubMed] [Google Scholar]
- Schrauwen R.W., Janssen M.J., De Boer W.A.(2009)Seven-day ppi-triple therapy with levofloxacin is very effective for Helicobacter pylori eradication. Neth J Med 67:96–101 [PubMed] [Google Scholar]
- Seppala K., Kosunen T.U., Nuutinen H., Sipponen P., Rautelin H., Sarna S.et al. (2000)Cure of Helicobacter pylori infection after failed primary treatment: one-center results from 120 patients. Scand J Gastroenterol 35:929–934 [DOI] [PubMed] [Google Scholar]
- Shimoyama T., Fukuda S., Mikami T., Fukushi M., Munakata A.(2004)Efficacy of metronidazole for the treatment of clarithromycin-resistant Helicobacter pylori infection in a Japanese population. J Gastroenterol 39:927–930 [DOI] [PubMed] [Google Scholar]
- Shirai N., Sugimoto M., Kodaira C, Nishino M., Ikuma M., Kajimura M.et al. (2007)Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol 63:743–749 [DOI] [PubMed] [Google Scholar]
- Sicilia B., Sierra E., Lago A., Villar M., Garcia S., Gomollon F.(2000)[High eradication rates in Helicobacter pylori infection in patients with duodenal ulcer who failed previous eradication therapy].Med Clin (Barc) 115:641–643 [DOI] [PubMed] [Google Scholar]
- Sotoudehmanesh R., Malekzadeh R., Vahedi H., Dariani N.E., Asgari A.A., Massarrat S.(2001)Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion 64:222–225 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Isogai E., Isogai H., Hayashi S., Hirose K., Kimura K.et al. (2002)Synergic effect of quinolone antibacterial agents and proton pump inhibitors on Helicobacter pylori. J Antimicrob Chemother 49:1039–1040 [DOI] [PubMed] [Google Scholar]
- Thong-Ngam D., Mahachai V.(2006)14-day quadruple therapy with ranitidine bismuth citrate after Helicobacter pylori treatment failure in Thailand. J Med Assoc Thai 89(Suppl. 3):S119–125 [PubMed] [Google Scholar]
- Tompkins D.S., Perkin J., Smith C.(1997)Failed treatment of Helicobacter pylori infection associated with resistance to clarithromycin. Helicobacter 2:185–187 [DOI] [PubMed] [Google Scholar]
- Toracchio S., Capodicasa S., Soraja D.B., Cellini L., Marzio L.(2005)Rifabutin based triple therapy for eradication of H. pylori primary and secondary resistant to tinidazole and clarithromycin. Dig Liver Dis 37:33–38 [DOI] [PubMed] [Google Scholar]
- Treiber G., Ammon S., Malfertheiner P., Klotz U.(2002a)Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylo ri after previous treatment failures. Helicobacter 7:225–231 [DOI] [PubMed] [Google Scholar]
- Treiber G., Wittig J., Ammon S., Walker S., Van Doorn L.J., Klotz U.(2002b)Clinical outcome and influencing factors of a new short-term quadruple therapy for Helicobacter pylori eradication: a randomized controlled trial (Maclor Study). Arch Intern Med 162:153–160 [DOI] [PubMed] [Google Scholar]
- Tucci A., Poli L., Caletti G.(1999)Treatment of the ‘ineradicable’ Helicobacter pylori infection. Am J Gastroenterol 94:1713–1715 [DOI] [PubMed] [Google Scholar]
- Ueki N., Miyake K, Kusunoki M., Shindo T, Kawagoe T, Futagami S.et al. (2009)Impact of quadruple regimen of clarithromycin added to metronidazole-containing triple therapy against Helicobacter pylori infection following clarithromycin-containing triple-therapy failure. Helicobacter 14:91–99 [DOI] [PubMed] [Google Scholar]
- Usta Y., Saltik-Temizel I.N., Demir H., Uslu N., Ozen H., Gurakan F.et al. (2008)Comparison of short- and long-term treatment protocols and the results of second-line quadruple therapy in children with Helicobacter pylori infection. J Gastroenterol 43:429–433 [DOI] [PubMed] [Google Scholar]
- Uygun A., Ozel A.M., Yildiz O., Aslan M., Yesilova Z., Erdil A.et al. (2008)Comparison of three different second-line quadruple therapies including bismuth subcitrate in Turkish Patients with non-ulcer dyspepsia who failed to eradicate Helicobacter pylori with a 14-day standard first-line therapy. J Gastroenterol Hepatol 23:42–45 [DOI] [PubMed] [Google Scholar]
- Vaira D., Ricci C, Lanzini A., Perna F., Romano A., Corinaldesi R.(2007)How to proceed in Helicobacter pylori-positive chronic gastritis refractory to first- and second-line eradication therapy. Dig Dis 25:203–205 [DOI] [PubMed] [Google Scholar]
- Vakil N.(2009)H pylori treatment: new wine in old bottles? Am J Gastroenterol 104:26–30 [DOI] [PubMed] [Google Scholar]
- Vakil N., Hahn B., McSorley D.(1998)Clarithromycin-resistant Helicobacter pylori in patients with duodenal ulcer in the United States. Am J Gastroenterol 93:1432–1435 [DOI] [PubMed] [Google Scholar]
- Vakil N., Lanza F., Schwartz H., Barth J.(2004)Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther 20:99–107 [DOI] [PubMed] [Google Scholar]
- Van Der Poorten D., Katelaris P.H.(2007)The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment Pharmacol Ther 26:1537–1542 [DOI] [PubMed] [Google Scholar]
- Van Der Wouden E.J., Thijs J.C., Van Zwet A.A., Sluiter W.J., Kleibeuker J.H.(1999)The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta- analysis. Am J Gastroenterol 94:1751–1759 [DOI] [PubMed] [Google Scholar]
- Van Oijen A.H., Verbeek A.L., Jansen J.B., De Boer W.A.(2000)Review article: treatment of Helicobacter pylori infection with ranitidine bismuth citrate- or proton pump inhibitor-based triple therapies. Aliment Pharmacol Ther 14:991–999 [DOI] [PubMed] [Google Scholar]
- Veldhuyzen Van Zanten S., Machado S., Lee J.(2003)One-week triple therapy with esomeprazole, clarithromycin and metronidazole provides effective eradication of Helicobacter pylori infection. Aliment Pharmacol Ther 17:1381–1387 [DOI] [PubMed] [Google Scholar]
- Vicente R., Sicilia B., Gallego S., Revillo M.J., Ducons J., Gomollon F.(2002)[Helicobacter pylori eradication in patients with peptic ulcer after two treatment failures: a prospective culture-guided study]. Gastroenterol Hepatol 25:438–442 [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Aoyama N, Shirasaka D., Maekawa S., Kuroda K., Miki I.et al. (2003)Levofloxacin based triple therapy as a second-line treatment after failure of Helicobacter pylori eradication with standard triple therapy. Dig Liver Dis 35:711–715 [DOI] [PubMed] [Google Scholar]
- Wong W.M., Gu Q., Chu K.M., Yee Y.K., Fung F.M., Tong T.S.et al. (2006)Lansoprazole, levofloxacin and amoxicillin triple therapy vs. quadruple therapy as second-line treatment of resistant Helicobacter pylori infection. Aliment Pharmacol Ther 23:421–427 [DOI] [PubMed] [Google Scholar]
- Wong W.M., Gu Q., Lam S.K., Fung F.M., Lai K.C., Hu W.H.et al. (2003)Randomized controlled study of rabeprazole, levofloxacin and rifabutin triple therapy vs. quadruple therapy as second-line treatment for Helicobacter pylori infection. Aliment Pharmacol Ther 17:553–560 [DOI] [PubMed] [Google Scholar]
- Wu D.C., Hsu P.I., Chen A., Lai K.H., Tsay F.W., Wu C.J.et al. (2006)Randomized comparison of two rescue therapies for Helicobacter pylori infection. Eur J Clin Invest 36:803–809 [DOI] [PubMed] [Google Scholar]
- Xia H.H., Yu Wong B.C., Talley N.J., Lam S.K.(2002)Alternative and rescue treatment regimens for Helicobacter pylori eradication. Expert Opin Pharmacother 3:1301–1311 [DOI] [PubMed] [Google Scholar]
- Xiao S.D., Liu WZ., Hu P.J., Ouyang Q., Wang J.L., Zhou L.Y.et al. (2001)A multicentre study on eradication of Helicobacter pylori using four 1-week triple therapies in China. Aliment Pharmacol Ther 15:81–86 [DOI] [PubMed] [Google Scholar]
- Xiao S.D., Liu W.Z., Xia D.H., Jiang S.J., Wang R.N, Zhang Z.H.et al. (1990)The efficacy of fura-zolidone and metronidazole in the treatment of chronic gastritis associated with Helicobacter (Campylobacter) pylori - a randomized double-blind placebo-controlled clinical trial. Hepatogastroenterology 37:503–506 [PubMed] [Google Scholar]
- Yahav J., Samra Z., Niv Y, Evans CT., Passaro D.J., Dinari G.et al. (2006a)Susceptibility-guided vs. empiric retreatment of Helicobacter pylori infection after treatment failure. Dig Dis Sci 51:2316–2321 [DOI] [PubMed] [Google Scholar]
- Yahav J., Shmuely H., Niv Y, Bechor J., Samra Z.(2006b)In vitro activity of levofloxacin against Helicobacter pylori isolates from patients after treatment failure. Diagn Microbiol Infect Dis 55:81–83 [DOI] [PubMed] [Google Scholar]
- Zou J., Yang Z.X., Qin Z.M.(2003)[Laboratory and clinical study of levofloxacin against Helicobacter pylori]. Zhonghua YiXue Za Zhi 83:1778–1781 [PubMed] [Google Scholar]
- Zullo A., Hassan C, Campo S.M., Lorenzetti R., Febbraro I., De Matthaeis M.et al. (2001a)A triple therapy regimen after failed Helicobacter pylori treatments. Aliment Pharmacol Ther 15:1193–1197 [DOI] [PubMed] [Google Scholar]
- Zullo A., Hassan C, De Francesco V, Lorenzetti R., Marignani M., Angeletti S.et al. (2003a)A third-line levofloxacin-based rescue therapy for Helicobacter pylori eradication. Dig Liver Dis 35:232–236 [DOI] [PubMed] [Google Scholar]
- Zullo A., Hassan C, Lorenzetti R., Morini S.(2001b)Helicobacter pylori eradication: do we have another ace up our sleeve? Dig Liver Dis 33:805–806 [DOI] [PubMed] [Google Scholar]
- Zullo A., Hassan C, Lorenzetti R., Winn S., Morini S.(2003b)A clinical practice viewpoint: to culture or not to culture Helicobacter pylor i ? Dig Liver Dis 35:357–361 [DOI] [PubMed] [Google Scholar]
- Zullo A., Perna F., Hassan C, Ricci C, Saracino I., Morini S.et al. (2007)Primary antibiotic resistance in Helicobacter pylori strains isolated in Northern and Central Italy. Aliment Pharmacol Ther 25:1429–1434 [DOI] [PubMed] [Google Scholar]