Abstract
Nonalcoholic fatty liver disease (NAFLD) has become increasingly recognized as the most common cause of abnormal liver enzymes in the last few decades and is among the most common forms of chronic liver disease in the Western world and across the globe. With the growing epidemic of obesity and diabetes, NAFLD is estimated to affect about one-quarter of the US population. Although most patients with NAFLD have nonprogressive bland steatosis, a minority of patients develop the histological subtype of nonalcoholic steatohepatitis (NASH), which may progress to cirrhosis, hepatocellular carcinoma, and liver-related death. This is especially true when NASH patients have type 2 diabetes. Treatment of NAFLD should therefore be directed towards patients with established NASH. Sustained weight loss seems to improve insulin resistance and associated NASH. In fact, weight loss with bariatric surgery leads to biochemical and histological improvement in morbidly obese patients with NASH. Several pharmacologic agents have been studied in an effort to improve insulin resistance and pro-inflammatory mediators potentially responsible for the development and progression of NASH. While some studies have shown initial promise, none has established long-term efficacy using randomized clinical trials. This paper briefly reviews the epidemiology, natural history, and pathophysiology of NAFLD and NASH and then focuses on the clinical trials of various therapeutic modalities for NAFLD. These include weight loss agents, bariatric surgery, insulin-sensitizing agents, lipid-lowering agents, antioxidants, probiotics, anti-tumor necrosis factor agents, cytoprotective and other novel agents.
Keywords: NAFLD, NASH, treatment
Background
Nonalcoholic fatty liver disease (NAFLD) is one of the most common forms of liver disease across the world. NAFLD represents a spectrum ranging from bland steatosis to nonalcoholic steatohepatitis (NASH), and is closely associated with obesity and metabolic syndrome [Ludwig et al. 1980]. Owing to the increasing prevalence of NAFLD and the potential for NASH to progress to cirrhosis and liver-related mortality, more research has been focused on this important liver disease over the last two decades. NAFLD has become a common diagnosis with the increasing prevalence of obesity, diabetes mellitus, and other components of the metabolic syndrome. The Adult Treatment Panel III defines metabolic syndrome as having three of the following: visceral obesity, hypertension, elevated fasting blood glucose, hypertriglyceridemia, or low high-density lipoprotein (HDL) levels. The criterion for visceral obesity is a waist circumference greater than 40 inches in men and greater than 35 inches in women. Hypertension is defined as blood pressure greater than 130/85 mm Hg. Glucose levels above 100 and triglyceride levels of 150 or greater are considered elevated. HDL levels below 40 for men and below 50 for women are considered low [National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002]. Because as many as 90% of NAFLD cases have at least one component of the metabolic syndrome and as many as 33% have three or more components, NAFLD is characterized as the hepatic manifestation of the metabolic syndrome [Almeda-Valdés et al. 2009; Rafiq and Younossi, 2009].
While the presence of metabolic syndrome is highly predictive of NAFLD, metabolic syndrome is not always accompanied by NAFLD, nor is NAFLD always accompanied by a component of metabolic syndrome. Rarely, patients with NAFLD may have a body mass index (BMI) that is within acceptable limits, and liver function enzymes may also be normal. Nevertheless, the prevalence of NASH and fibrosis increases with increasing number of metabolic conditions (diabetes, hypertension, visceral obesity)[Hossain et al. 2009].
It is important to note that NAFLD is a diagnosis of exclusion that is finally reached after evaluation for asymptomatic liver enzyme elevation. Alternatively, a NAFLD diagnosis is reached inadvertently after hepatic steatosis is incidentally noted on abdominal imaging. Nonetheless, several dilemmas accompany attempts to diagnose NAFLD or its subtype of NASH. Serological tests, panels, and biomarkers are not yet validated and routine radiographic modalities have unavoidable limitations. Currently, liver biopsy remains the ‘imperfect’ gold standard for diagnosis and staging of NAFLD [Wieckowska and Feldstein, 2008; Adams and Angulo, 2007; Charatcharoenwitthaya and Lindor, 2007].
Epidemiology
It is difficult to estimate the true incidence and prevalence of NAFLD and NASH because of the absence of accurate and noninvasive diagnostic measures. Currently no studies have examined the incidence of NAFLD and NASH in North America, and there are no prospective studies evaluating prevalence except in patients undergoing bariatric surgery. Two population-based studies have attempted to estimate the prevalence of NAFLD in general United States population [Szczepaniak et al. 2005; Clark et al. 2002]. Analysis of the third US National Health and Nutrition Examination Survey (1988–1994) found that 23% of the population had unexplained elevation of alanine transaminase (ALT) [Clark et al. 2002]. Another study, which used proton magnetic resonance spectroscopy to measure hepatic steatosis in Dallas County, found the prevalence of NAFLD to be 33.6% [Szczepaniak et al. 2005]. Notably, ALT was normal in most (79%) of these patients with hepatic steatosis [Browning et al. 2004]. Autopsy studies on reportedly nonalcoholic patients have suggested that steatosis occurs in 36% of lean patients and in 72% of obese patients. Furthermore, steatohepatitis is present in 2.7% of lean patients and in 18.5% of obese patients [Wanless and Lentz, 1990]. Overall, in the general United States population, estimates of NAFLD prevalence range from 23% to 33.6% [Szczepaniak et al. 2005; Browning et al. 2004; Clark et al. 2002], while estimates of NASH prevalence range from 2% to 5.7% [Wanless and Lentz, 1990]. Among patients undergoing bariatric surgery, as many as 96% have NAFLD and up to 25% have NASH [Ong et al. 2005; Dixon et al. 2001]. Of the 47 million people in the United States with metabolic syndrome, up to 80% may have NAFLD [Ong et al. 2008].
NAFLD and NASH have been noted in all ethnic groups, all regions of the world, all ages, and in both genders. In the United States, the prevalence is greater in the Hispanic population when compared with non-Hispanic Whites or non-Hispanic African Americans [Ong et al. 2008; McCullough, 2005; Weston et al. 2005; Browning et al. 2004; Caldwell et al. 2002]. The Dallas proton magnetic spectroscopy study reported NAFLD prevalence of 33% in non-Hispanic Whites, 45% in Hispanics, and 24% in non-Hispanic African Americans [Browning et al. 2004]. Reports have also suggested that the prevalence of NAFLD among Asian Indians is comparable to that seen in the West [Misra et al. 2009; Amarapurkar et al. 2007; Duseja et al. 2007; Malik et al. 2007; Duseja, 2006; Madan et al. 2006; Misra and Vikram, 2004; Singh et al. 2004]. In East Asia, estimates of NAFLD prevalence vary from 11.5% to 20.8% [Shifflet and Wu, 2009; Chen et al. 2006; Park et al. 2006; Fan et al. 2005; Yiu and Leung, 2004].
As noted previously, NAFLD is seen in all age groups. Prevalence peaks in the fourth decade in men and in the sixth decade in women [Ruhl and Everhart, 2003]. Earlier studies described a greater frequency of NAFLD in women. More recent studies are showing that the prevalence of NAFLD in men is equal to or greater than the prevalence in women [Shifflet and Wu, 2009; Amarapurkar et al. 2007; Weston et al. 2005; Ruhl and Everhart, 2003; Clark et al. 2002]. With the increasing prevalence of obesity in children and adolescents, NAFLD also affects the pediatric population. However, this review focuses primarily on adult the population.
Natural history
The natural history of NAFLD has emerged over the past two decades. Most cases of NAFLD do not progress to advanced liver disease. The risk of progression seems to be determined by histological subtype. In fact most patients with bland steatosis typically follow a benign course, whereas NASH carries the potential to progress. A few reports suggest that bland steatosis may rarely progress to NASH [Ong and Younossi, 2007; Ong et al. 2005, 2008; Dixon et al. 2001; Wanless and Lentz, 1990]. Although most reports suggest that the presence of metabolic syndrome or its components is predictive of NASH and NASH-related fibrosis [Ong and Younossi, 2007; Marchesini et al. 2003], a few indicate that metabolic syndrome is not predictive [Uslusoy et al. 2009; Singh et al. 2004]. Nonetheless, a growing consensus points to progression when NASH occurs in the setting of insulin resistance or type 2 diabetes.
Several studies report that all NAFLD patients are at greater risk for overall mortality when compared with non-NAFLD patients [Ong et al. 2008; Ong and Younossi, 2007; Ekstedt et al. 2006; Adams et al. 2005]. This increase in overall mortality stems from the metabolic syndrome that is often comorbid with NAFLD [Ong and Younossi, 2007; Targher and Arcaro, 2007]. In many long-term follow-up studies of NAFLD, coronary artery disease continues to be the most common cause of overall mortality [Rafiq et al. 2009; Ong et al. 2008; Ong and Younossi, 2007]. Since insulin resistance is a main pathogenic factor, NAFLD patients are also at greater risk for developing diabetes. A recent study by Adams et al. found that patients with NAFLD (diagnosed on the basis of unexplained ALT elevation) were at significantly greater risk for developing diabetes (20/106, 18.9% vs. 15/246, 6.1%; p < 0.001) and metabolic syndrome (27/81, 33.3% vs. 51/226, 22.6%; p = 0.056) than patients with normal ALT [Adams et al. 2005]. Further analysis revealed that the increased risk may have been due to concomitant metabolic risk factors (baseline waist circumference, hypertension, and insulin resistance).
In addition to cardiac mortality, liver-related mortality is also increased in NAFLD patients, especially in those with NASH [Rafiq et al. 2009; Ong et al. 2008]. One recent study noted findings consistent with NASH in 41.6% (N = 173) of patients diagnosed with NAFLD by biopsy [Rafiq et al. 2009]. As reported in previous studies, the two most common causes of death in these NASH patients were coronary artery disease and malignancy [Ong et al. 2008; Ong and Younossi, 2007; Ekstedt et al. 2006; Adams et al. 2005]. Liver-related mortality was the third most common cause of death in this cohort of NASH patients. For comparison, liver-related mortality is typically around the thirteenth most common cause of death in the general population [Ong et al. 2008; Ong and Younossi, 2007]. Again, patients with NAFLD and type II diabetes seem to be at greater risk for liver related mortality [Rafiq et al. 2009]. In another study of biopsy-proven NASH, age and the presence of inflammation on initial biopsy were independent risk factors for progression to advanced fibrosis [Adams et al. 2009]. Although the risk factors for progression remain to be clarified, 15–25% of NASH cases progress to cirrhosis over 10 to 20 years [Ong and Younossi, 2007; Matteoni et al. 1999; Bacon et al. 1994; Powell et al. 1990]. NAFLD patients who have progressed to cirrhosis are at risk for hepatocellular carcinoma, similar to patients with cirrhosis due to other etiologies. In addition to potential for progression, NAFLD patients suffer from impaired health-related quality of life [Afendy et al. 2009].
Pathophysiology
A brief review of NAFLD pathogenesis will help inform the discussion of potential therapies. Although factors such as medications, toxins, parenteral nutrition, and certain surgical procedures can lead to hepatic steatosis (secondary NAFLD), this article focuses on primary NAFLD, which is associated with insulin resistance, obesity, and the metabolic syndrome.
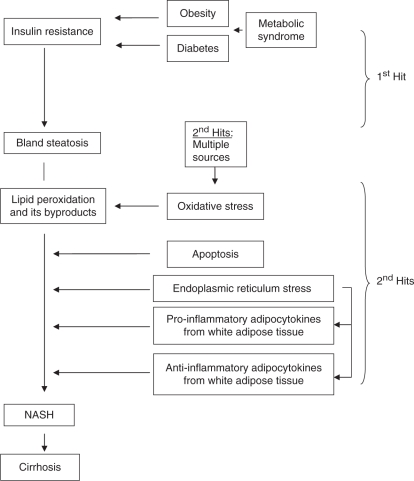
NAFLD pathogenesis is widely believed to result from a series of liver insults, commonly referred to as the ‘multi-hit’ hypothesis [Estep et al. 2009; Malhi and Gores, 2008; Miele et al. 2003; Charlton et al. 2002]. The first hit involves the development of hepatic steatosis due to insulin resistance. Insulin resistance leads to increased serum levels of nonesterified or free fatty acids (FFAs). Subsequently, increased FFA transport into hepatocytes and increased hepatic de novo lipogenesis exceed hepatic FFA β-oxidation and very low-density lipoprotein (VLDL) export, leading to increased hepatic steatosis [Malhi and Gores, 2008]. Several studies suggest that hepatic steatosis is largely due to the increase in lipogenesis and decreases in lipid export [Miele et al. 2003; Charlton et al. 2002].
The exact mechanisms which drive the progression from bland steatosis to steatohepatitis are still not fully elucidated. It is clear however that apoptosis can be the pathogenic marker of steatohepatitis in some NAFLD patients [Malhi and Gores, 2008]. For this reason, much attention has been focused on the mechanisms that lead to hepatocellular apoptosis. It has been proposed that accumulating FFAs in steatotic hepatocytes promote apoptosis through a number of pathways, including increased stress of membrane bound organelles such as mitochondria, endoplasmic reticula (ER), and lysosomes [Malhi and Gores, 2008]. Lipotoxicity occurs on a molecular level as circulating FFAs activate a number of complex intracellular pathways, including induction of Bim expression, activation of c-jun N-terminal kinase, the pro-apoptotic protein Bax and Toll-like receptor 4. Sufficient activation of these pathways may then lead to lysozomal and mitochondrial permeabilization, oxidative stress, inflammatory gene expression, ER stress, and hepatocyte apoptosis [Malhi and Gores, 2008].
Visceral obesity also seems to contribute to the development of NASH via the endocrine functions of white adipose tissue (WAT) as well as insulin resistance. Several adipokines secreted by WAT and implicated in the pathogenesis of NAFLD include adiponectin, leptin, resistin, tumor necrosis factor alpha (TNF-α), complement component 3, apelin, vaspin, visfatin, interleukins 1 beta, 6, 8, and 18, and plasminogen activator inhibitor type 1 [Estep et al. 2009]. Furthermore, angiotensinogen may also play a role in NASH pathogenesis [Estep et al. 2009]. These ‘second hits’ lead to increased fibrosis and progression to steatohepatitis and cirrhosis. Current treatment modalities target one or more of these components (Figure 1).
Figure 1.
Nonalcoholic steatohepatitis (NASH) pathogenesis.
Treatment
As the potential for liver disease progression occurs almost exclusively in patients with NASH, our efforts should focus mainly on prevention and treatment of this NAFLD subtype. However, given that NADFLD is associated with cardiovascular mortality, it is important to treat components of metabolic syndrome for all NAFLD patients to potentially improve cardiovascular outcomes.
As of 2009, no pharmacological agents have been approved for the treatment of NAFLD or NASH. Therefore, most clinical efforts have been directed at treating the components of metabolic syndrome, namely obesity, diabetes, hypertension, and dyslipidemia. Other interventions are directed at specific pathways potentially involved in the pathogenesis of NAFLD, such as insulin resistance, oxidative stress, pro-inflammatory cytokines, apoptosis, bacterial overgrowth, and the angiotensin pathway (Table 1).
Table 1.
Potential treatments and their targets.
| Target | Treatment |
|---|---|
| Obesity | Weight loss |
| - Diet with or without exercise | |
| - Pharmacologic | |
| • Orlistat | |
| • Sibutramine | |
| - Surgical treatment of obesity | |
| Insulin resistance | Insulin sensitizing agents |
| - Thiazolidinediones (TZDs) | |
| - Metformin | |
| - Meglitinides | |
| Dyslipidemia | Lipid lowering agents |
| - Statins | |
| - Fibrates | |
| - Omega-3 fatty acids | |
| Oxidative stress | Antioxidants |
| - Vitamin E | |
| - Other vitamins | |
| - Betaine | |
| - N-Acetyl-cysteine | |
| - Lecithin | |
| - Silymarin | |
| - Beta-carotene | |
| Pro-inflammatory cytokines | Anti-tumor necrosis factor agents |
| - Pentoxifylline | |
| Bacterial overgrowth | Probiotics |
| - VSL#3 | |
| Apoptosis | Cytoprotective agents |
| - Ursodeoxycholic acid (UDCA) | |
| Novel treatments | |
| - ACE inhibitors/ARBs | |
| - Oligofructose | |
| - Incretin analogs |
ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blockers
Obesity
The therapeutic measure with the best potential for treating NAFLD is sustained weight loss by dietary changes with or without exercise, or bariatric surgery. Several studies have investigated the effects of dietary changes upon NAFLD [Tendler et al. 2007; Baba et al. 2006; Thomas et al. 2006; Huang et al. 2005; Suzuki et al. 2005; Hickman et al. 2004; Kugelmas et al. 2003; Zhu et al. 2003; Okita et al. 2001; Knobler et al. 1999; Ueno et al. 1997; Andersen et al. 1991; Palmer and Schaffner, 1990]. Some include exercise because it may promote increased muscle mass in addition to promoting weight loss, thereby increasing peripheral insulin sensitivity [Stewart et al. 2005]. Of the studies that have focused on weight loss by dietary measures with or without exercise, all but six are limited by the absence of histologic evaluation. Owing to the invasive nature of liver biopsy, many studies use biochemical improvement as the primary measure of efficacy. One study using histological assessment by Huang and colleagues evaluated the effect of intense dietary counseling on 23 patients with biopsy-proven NASH [Huang et al. 2005]. After 1 year, 9 of 15 patients showed histologic improvement associated with greater weight loss. Most studies are also limited by small study size and short duration. The study with the largest cohort and longest duration to date reported that weight loss and exercise were significantly associated with ALT improvement in 136 patients over 36 months. However, this study did not include liver biopsies [Suzuki et al. 2005].
Orlistat and sibutramine are two US Food and Drug Administration (FDA)-approved medications for weight loss. Orlistat is an enteric lipase inhibitor. Sibutramine, a serotonin and norepinephrine reuptake inhibitor, promotes satiety and increases energy expenditure by stimulating thermogenesis. At least four studies have examined the effects of these medications on NAFLD [Hussein et al. 2007; Harrison et al. 2004, 2009; Sabuncu et al. 2003]. Sabuncu and colleagues treated patients with sibutramine or orlistat along with dietary restriction in an open-label study for 6 months [Sabuncu et al. 2003]. Improvements were noted in homeostatic model assessment (HOMA) scores, aminotransferases, and sonographic findings, but liver biopsies were not obtained. Three studies have evaluated the effect of treatment with orlistat over 6 months [Hussein et al. 2007; Harrison et al. 2004, 2009]. Paired biopsies revealed improvements in steatosis, inflammation, and fibrosis in most patients. A recently published study by Harrison and colleagues. randomized 50 overweight patients with biopsy-proven NASH to a 1400 kcal/day diet plus 800 IU vitamin E daily with or without orlistat for 36 weeks. They found that weight loss of 9% or more of body weight, not treatment with orlistat, resulted in significant improvements in insulin sensitivity, adiponectin, and steatosis, ballooning, inflammation and NAFLD score (NAS) on repeat biopsy [Harrison et al. 2009]. Thus, it is important to note that the improvements in NAFLD patients treated with these medications are largely attributed to weight loss. Given that medication-assisted weight loss is often difficult to sustain, these therapies may be most appropriate only in patients who are highly motivated.
On the other hand, several studies have shown that weight loss following bariatric surgery leads not only to biochemical improvement but also to histological improvement [Furuya et al. 2007; Liu et al. 2007; Sjostrom et al. 2007; Barker et al. 2006; Csendes et al. 2006; De Almeida et al. 2006; Jaskiewicz et al. 2006; Klein et al. 2006; Mathurin et al. 2006, 2009; O’Brien et al. 2006; Clark et al. 2005; Mattar et al. 2005; Mottin et al. 2005; Stratopoulos et al. 2005; Dixon et al. 2004, 2006; Kral et al. 2004; Silverman et al. 1995; Grimm et al. 1992; Ranlov and Hardt, 1990; Rucker et al. 1982]. While no randomized blinded studies can be conducted, an analysis of numerous paired biopsy studies reveals convincing evidence that significant improvement in steatosis and inflammation occurs in NAFLD patients after weight loss surgery. On the other hand, reports on the progression of fibrosis are mixed. Mild fibrosis progression is reported in some. However, a meta-analysis of bariatric surgery assessing 766 paired liver biopsies from 15 studies reports improvement in steatosis in 91.6% of patients, improvement in steatohepatitis in 81.3%, and improvement in fibrosis in 65.5%, with complete NASH resolution in 69.5% [Mummadi et al. 2008]. In most studies, these improvements occurred primarily in patients showing the greatest improvement in components of metabolic syndrome and insulin resistance.
Insulin resistance
Given that insulin resistance is a key contributor in the development of NAFLD and NASH, much attention has been placed on insulin sensitizers. Thiazolidinediones (TZDs) are peroxisomal proliferator activated receptor-γ (PPAR-γ) agonists that promote hepatic fatty acid oxidation, decrease hepatic lipogenesis, and increase peripheral and hepatic insulin sensitivity [Oh et al. 2008]. At least 11 studies have evaluated their potential as agents for NAFLD treatment [Argo et al. 2009; Chalasani et al. 2009; Juurlink et al. 2009; Aithal et al. 2008; Lutchman et al. 2007; Belfort et al. 2006; Ratziu et al. 2006, 2008; Promrat et al. 2004; Sanyal et al. 2004; Neuschwander-Tetri et al. 2003; Shadid and Jensen, 2003; Caldwell et al. 2001]. Troglitazone has been withdrawn from the market due to hepatotoxicity. Rosiglitazone and pioglitazone continue to be used and both improve insulin sensitivity and aminotransferases [Aithal et al. 2008; Ratziu et al. 2008; Neuschwander-Tetri et al. 2003]. Studies have generally shown improvements in steatosis. The effect of TZDs on inflammation, fibrosis, and other histological features of NASH are less uniform [Argo et al. 2009; Chalasani et al. 2009; Juurlink et al. 2009; Aithal et al. 2008; Lutchman et al. 2007; Belfort et al. 2006; Ratziu et al. 2006, 2008; Promrat et al. 2004; Sanyal et al. 2004; Neuschwander-Tetri et al. 2003; Shadid and Jensen, 2003; Caldwell et al. 2001].
For example, the Fatty Liver Improvement with Rosiglitazone Trial (FLIRT) by Ratziu and colleagues randomized 63 biopsy-proven NASH patients to receive rosiglitazone or placebo for 1 year [Ratziu et al. 2008]. They found that treatment with rosiglitazone improved steatosis, transaminase levels, adiponectin, and insulin sensitivity, despite significantly more weight gain than in the placebo cohort. No significant change was noted in fibrosis or NAS score. Adverse effects included painful swollen legs and anemia. No hepatic toxicity was noted.
Many have suggested that NASH often recurs after discontinuation of TZD therapy, suggesting that long-term therapy may be necessary [Argo et al. 2009; Lutchman et al. 2007]. This may be problematic given the concerns for cardiotoxicity, congestive heart failure, edema, osteoporosis, and weight gain with TZDs, especially with rosiglitazone [Juurlink et al. 2009; Aithal et al. 2008; Lutchman et al. 2007]. It has also been noted that TZD therapy in the absence of lifestyle modification is often not effective [Ratziu et al. 2008; Lutchman et al. 2007]. Carefully designed studies with larger populations and longer durations are needed to better assess the efficacy and safety of long-term TZD use in patients with NAFLD.
The results of the PIVENS (Pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis) trial by the NIDDK NASH CRN Research Group have recently become available [Chalasani et al. 2009]. This large randomized, multicenter, double-blind, placebo-controlled study randomized 247 nondiabetic NASH patients to receive 96 weeks of pioglitazone 30 mg qd, vitamin E 800 IU qd or placebo [Chalasani et al. 2009]. Ninety percent of the subjects had biopsies at the end of the study. In this cohort of nondiabetic NASH patients, treatment with pioglitazone did not meet the primary endpoint of the study, which was defined by a decrease in NAS by at least two points, with at least a one-point improvement in ballooning. Nevertheless, treatment with pioglitazone was associated with in significant improvements in steatosis, inflammation, serum ALT, but neither pioglitazone nor vitamin E improved fibrosis scores. Treatment with pioglitazone was also significantly associated with greater weight gain (mean 4.7 kg: p < 0.01 versus placebo) when compared to the vitamin E or placebo groups. While the primary endpoint was not met by treatment with pioglitazone in this cohort of nondiabetic adults, it would be beneficial to investigate the effect of pioglitazone in NASH patients with insulin resistance or type 2 diabetes.
Metformin improves insulin resistance by it reducing hepatic gluconeogenesis, lipogenesis and fatty acid oxidation, increasing peripheral and hepatic insulin sensitivity, decreasing intestinal glucose absorption, and lowering serum lipid concentration. Several small trials have shown that metformin is relatively well tolerated in NASH patients and effective in improving aminotransferase levels and insulin resistance [Loomba et al. 2008; Bugianesi et al. 2005; Schwimmer et al. 2005; Duseja et al. 2004; Haukeland et al. 2004; Nair et al. 2004; Uygun et al. 2004; Marchesini et al. 2001]. Notably, weight loss has been noted with metformin use, in contrast to the weight gain sometimes observed with TZD use. All but three of the trials were open label [Bugianesi et al. 2005; Haukeland et al. 2004; Uygun et al. 2004]. In the study by Uygun and colleagues, metformin treatment resulted in improvement in necroinflammatory activity, although the difference did not reach statistical significance [Uygun et al. 2004]. Bugianesi and coworkers reported significant improvement in steatosis, inflammation, and fibrosis [Bugianesi et al. 2005]. The recent randomized, double blind, placebo-controlled trial by Haukeland and colleagues. examined the effects of 6 months of metformin (N = 24) versus placebo (N = 24) in patients with biopsy-proven NAFLD [Haukeland et al. 2004]. Treatment with metformin did not significantly improve steatosis (by histological or CT evaluation), NAS score, transaminases, or markers of inflammation or insulin resistance. Significant improvements in body weight, total cholesterol, low-density lipoprotein (LDL), glucose, and HbA1c were noted in the metformin cohort. So while the results regarding histological improvement of NAFLD findings with metformin treatment are mixed at best, metformin may have utility due to its ability to address insulin resistance and the cardiometabolic risk factors which often accompany NAFLD.
One small study evaluated the efficacy of nateglinide, a meglitinide that stimulates pancreatic insulin release and pancreatic beta cell growth [Morita et al. 2005]. This study showed that treatment with nateglinide in five biopsy-proven NASH patients resulted in statistically significant biochemical and histological improvement.
Dyslipidemia
Statins reduce cholesterol production by competitively inhibiting hepatic hydroxymethyl-glutaryl coenzyme A (HMG-CoA) reductase. While statin use in the setting of hepatic disease should be monitored due to possible hepatotoxicity and muscle toxicity, severe hepatotoxicity due to statins is exceedingly rare and statin use is widely believed to be safe in patients with liver disease [Lewis et al. 2007; Browning, 2006; Gómez-Domínguez et al. 2006]. Ekstedt et al.’s retrospective review of 17 NAFLD patients treated with statins for 10.3–16.3 years showed significant improvement in steatosis and no significant increase in fibrosis [Ekstedt et al. 2007]. Only a few small pilot studies have evaluated the efficacy of statins for NASH treatment [Antonopoulos et al. 2006; Hatzitolios et al. 2004; Rallidis et al. 2004; Kiyici et al. 2003]. These studies suggest some biochemical improvement, but long-term efficacy and safety remain to be established.
A few studies have also evaluated the therapeutic potential other lipid-lowering agents, such as fibrates and omega-3 fatty acids. Studies evaluating the role of fibrates indicate that fibrates may be efficacious in treating NASH since they activate peroxisome proliferator-activated receptors alpha (PPAR-α), leading to increased HDL-C and decreased triglycerides, LDL, and VLDL [Fernández-Miranda et al. 2008; Athyros et al. 2006; Nakamuta et al. 2005; Basaranoglu et al. 1999; Laurin et al. 1996]. More recently, Fernández-Miranda and coworkers studied the effects of 48 weeks of fenofibrate in 16 patients with biopsy-proven NAFLD [Fernández-Miranda et al. 2008]. They observed significant improvement in triglycerides, glucose, alkaline phosphatase gamma-glutamyl transpeptidase, aspartate transaminase (AST), ALT, apolipoprotein A1 levels, and the grade of hepatocellular ballooning degeneration. Unfortunately, no significant improvement was noted in insulin resistance, hepatic steatosis, necroinflammation, or fibrosis. In addition, an earlier open-label study by Laurin and colleagues that reported an absence of histologic improvement after 12 months of clofibrate therapy [Laurin et al. 1996].
Omega-3 fatty acid treatment results in some improvement in biochemical and radiological features of NAFLD, but no one has examined histological changes [Cussons et al. 2009; Zhu et al. 2008; Capanni et al. 2006; Hatzitolios et al. 2004]. While there may be a role for these agents in NASH treatment, larger placebo-controlled trials with histological assessment would be helpful.
Oxidative stress
The number of antioxidants with potential beneficial hepatic effects is increasing. On the other hand, only a few have been studied systematically. Antioxidants have therapeutic potential because fatty acid oxidation produces reactive oxygen species, which cause direct cellular damage and activate pro-inflammatory cytokines. Transforming growth factor beta1 is believed to promote fibrosis, and vitamin E has been shown to inhibit its activity. Of the antioxidants, vitamin E has been studied the most, with a few studies showing some positive preliminary results [Argo et al. 2009; Lutchman et al. 2007; Ersöz et al. 2005; Kawanaka et al. 2004; Vajro et al. 2004; Harrison et al. 2003; Kugelmas et al. 2003; Hasegawa et al. 2001; Lavine, 2000]. Four studies have evaluated histological improvement [Argo et al. 2009; Lutchman et al. 2007; Harrison et al. 2003; Hasegawa et al. 2001]. Hawegawa and coworkers report improvement in NASH features after 1 year of vitamin E [Hasegawa et al. 2001] treatment. Harrison and coworkers report improved fibrosis after 6 months of vitamin E, but inflammation did not improve [Harrison et al. 2003]. Sanyal et al. conducted a small pilot study in 2004 showing that vitamin E alone is not as effective as vitamin E and pioglitazone [Sanyal et al. 2004]. Significant improvement in steatosis, ballooning, and fibrosis were noted in the vitamin E and pioglitazone cohort.
As detailed above, the PIVENS trial by the NIDDK NASH Clinical Research Network found that 96 weeks of treatment with vitamin E in nondiabetic NASH patients resulted in significant improvement in the NAS score [Chalasani et al. 2009]. The primary endpoint of an improvement of at least two points in the NAS score was met, despite no improvement in fibrosis. These results provide encouraging support for further study of vitamin E therapy for NASH.
Several small studies have examined the potential therapeutic benefit of other antioxidants, such as betaine, S-adenosylmethionine (SAM), and N-acetyl-cysteine (NAC). SAM and betaine, which increases SAM levels, have anti-TNF-α, cytoprotective and anti-apoptotic activity [Purohit et al. 2007; Barak et al. 1993]. Studies with animal models show that SAM has anti-steatogenic properties [Kwon et al. 2009; Abdelmalek et al. 2001]. A 1-year open-label study of betaine by Abdelmalek and colleagues resulted in statistically significant aminotransferase improvement [Abdelmalek et al. 2001]. Histological improvement was not statistically significant, and betaine was not beneficial in a later randomized control trial [Patrick, 2002].
Finally, N-acetyl-cysteine has beneficial effects in animal models of NAFLD, largely as a result of increasing hepatic glutathione, which decreases oxidative stress and the resultant upregulation of the inflammatory cascade [Baumgardner et al. 2008]. Two small studies of short duration show significant improvement in liver-function tests (LFTs), but follow-up biopsies were not performed [Pamuk and Sonsuz, 2003; Gülbahar et al. 2000]. Findings regarding these agents and others, such as silymarin, lecithin, and beta-carotene are very preliminary. Although it is unlikely that these antioxidants will become the mainstay of NASH therapy, they may serve as adjunct therapy in combination with other more targeted therapies.
Pro-inflammatory cytokines
TNF-α is a pro-inflammatory cytokine that is activated by the reactive oxygen species created by lipid peroxidation. It promotes necroinflammation, fibrogenesis, hepatic insulin resistance, and apoptosis [Satapathy et al. 2004]. Pentoxifylline is a xanthine derivative that is currently approved for treatment of claudication due to its effects on blood viscosity. Pentoxifylline has been shown to inhibit TNF-α and improve histology in animal models of NASH [Yalniz et al. 2007] and to significantly decrease TNF-α levels in NASH patients [Duman et al. 2007]. Three prospective trials have examined pentoxifylline’s efficacy in NASH patients [Adams et al. 2004; Satapathy et al. 2004, 2007]. In 2007, Satapathy and colleagues treated nine patients with biopsy-proven NASH for 1 year with pentoxifylline 400 mg tid [Satapathy et al. 2007]. Significant reductions in AST and ALT were noted, as well as improvements in steatosis and lobular inflammation. Scores were significantly decreased by Brunt staging in six patients at the end of treatment, and four of six patients showed improved fibrosis. Again, larger studies are warranted.
Bacterial overgrowth
One possible source of increased oxidative stress may be bacterial overgrowth in the gastrointestinal tract. Bacterial overgrowth leads to increased hepatic exposure to ethanol and bacterial lipopolysaccharide, which are hepatotoxic and upregulate TNF-α and other pro-inflammatory mediators [Velayudham et al. 2008; Lirussi et al. 2007]. Two small studies have evaluated the effect of the probiotic VSL#3 in humans. Loguercio and colleagues reported improvement in LFTs, malondialdehyde, 4-hydroxynonenal, and S-nitrosothiol levels after 120 days of treatment [Loguercio et al. 2005]. Solga and colleagues reported that steatosis measured by proton magnetic resonance spectroscopy actually increased in three out of four patients treated with VSL#3 for 4 months [Solga et al. 2008]. While animal studies have suggested beneficial effects of probiotics, convincing evidence has yet to be demonstrated in humans, and further studies will be necessary before conclusions can be drawn.
Apoptosis
As detailed above, increased hepatocyte apoptosis may play a key role in progression from NAFLD to NASH [Malhi and Gores, 2008]. With the number of complex intracellular pathways in the apoptotic cascade, a large number of possible therapeutic targets exist. Although several studies of caspase inhibitors are underway, the long-term efficacy and safety of these agents are unknown.
On the other hand, several researchers have evaluated the potential efficacy of ursodeoxycholic acid (UDCA) because of its purported cytoprotective and immunomodulatory effects. It is postulated that UDCA may also be anti-apoptotic [Lazaridis et al. 2001]. UDCA is a naturally occurring bile acid that is approved for gallstone prevention and treatment of primary sclerosing cholangitis and primary biliary cirrhosis. Initial open-label pilot studies report improvements in aminotransferases and steatosis [Ersöz et al. 2005; Kiyici et al. 2003; Laurin et al. 1996; Guma and Viola, 1992]. However, a randomized placebo-controlled study of NASH patients did not confirm these findings [Dufour et al. 2006; Lindor et al. 2004; Mendez-Sanchez et al. 2004; Vajro et al. 2000]. In this study, 166 patients with biopsy-proven NASH were randomized to receive UDCA at 13–15 mg/kg/day or placebo for 2 years [Lindor et al. 2004]. Of the cohort enrolled, 126 patients completed the study and 107 had paired liver biopsies. When compared with placebo, UDCA did not result in statistically significant improvements in aminotransferases, steatosis, inflammation, or fibrosis. UDCA was well tolerated. Nonetheless, these findings may warrant further investigation of UDCA as an adjunct with antioxidants or TZDs or in higher doses.
Other novel treatments
As stated earlier, many factors may contribute to NASH development and progression, and so a number of preliminary studies have been conducted on various other agents. As noted, the renin–angiotensin system (RAS) promotes fibrosis when hepatic stellate cells are activated by angiotensin II (ATII). Animal studies show that inhibiting the RAS, specifically at the ATII type 1a receptor, decreases stellate cell activity and fibrosis [Fujita et al. 2007; Ibanez et al. 2007; Yoshiji et al. 2001]. Angiotensin-receptor blockers are thought to decrease insulin resistance by their activation of PPAR-γ. A small pilot study of seven patients with hypertension and NASH reported improved aminotransferases and significant improvement in serum markers of fibrosis [Yokohama et al. 2004]. Necroinflammation improved in five patients and fibrosis improved in four patients.
Oligofructose, which has also been studied in the treatment of NASH, is an oligosaccharide derived from fruits and vegetables such as bananas, onions, chicory root, garlic, and others. It is indigestible and considered a prebiotic, as it may stimulate the growth of beneficial gut bacteria. Animal studies show that oligofructose decreases serum and hepatic triglycerides by decreasing hepatic triglyceride uptake and lipogenesis [Daubioul et al. 2000; Kok et al. 1996]. A small 8-week crossover study by Daubioul and colleagues randomized seven patients with biopsy-proven NASH to receive oligofructose or maltodextrine, which served as placebo [Daubioul et al. 2005]. Improvements in insulin and aminotransferase levels were reported.
Finally, some animal studies and a few case reports in humans suggest that incretin analogs may have therapeutic potential for NASH. This class promotes glucose-dependent insulin secretion, decreases excess glucagon secretion, and promotes satiety by delaying gastric emptying [Ding et al. 2006; Tushuizen et al. 2006]. There is obviously much room for future research into pathogenic mechanisms and possible therapeutic agents for NAFLD.
Conclusion
While much progress has been made in the epidemiology, natural history, and pathogenesis of NAFLD, as yet no single treatment modality has established efficacy in the treatment for NASH. To date, no pharmacological agent has been approved for treatment of NASH or could be recommended for routine use in clinical practice. While a number of agents targeting insulin resistance and pro-inflammatory factors demonstrate some potential, additional research is warranted to establish their safety and efficacy.
Even so, it is important to attempt to reverse conditions associated with NAFLD. If insulin resistance or type 2 diabetes is present, an insulin sensitizer may be helpful. For patients with dyslipidemia, lipid-lowering agents including statins should be considered. Furthermore, ACE inhibitors may be helpful, especially in hypertensive or diabetic patients. Finally, a multi-disciplinary team of experts may be needed to effectively treat patients with visceral obesity. For patients who are appropriate candidates, bariatric surgery may be helpful. Despite the absence of an established treatment for NAFLD, future investigations of NAFLD will continue to be very active and exciting.
Conflict of interest statement
None declared.
References
- Abdelmalek M.F., Angulo P., Jorgensen R.A., Sylvestre P.B., Lindor K.D. (2001) Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol 96: 2711–2717 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Angulo P. (2007) Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis 11: 25–35 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Lymp J.F., St. Sauver J., Sanderson S.O., Lindor K.D., Feldstein A., et al. (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Waters O.R., Knuiman M.W., Elliott R.R., Olynyk J.K. (2009) NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 104: 861–867 [DOI] [PubMed] [Google Scholar]
- Adams L.A., Zein C.O., Angulo P., Lindor K.D. (2004) A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol 99: 2365–2368 [DOI] [PubMed] [Google Scholar]
- Afendy A., Kallman J.B., Stepanova M., Younoszai Z., Aquino R.D., Bianchi G., et al. (2009) Predictors of health-related quality of life in patients with chronic liver disease. Ailment Pharmacol Ther 30: 469–476 [DOI] [PubMed] [Google Scholar]
- Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I., et al. (2008) Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 135: 1176–1184 [DOI] [PubMed] [Google Scholar]
- Almeda-Valdés P., Cuevas-Ramos D., Aguilar-Salinas C.A. (2009) Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol 8(Suppl 1): S18–S24 [PubMed] [Google Scholar]
- Amarapurkar D., Kamani P., Patel N., Gupte P., Kumar P., Agal S., et al. (2007) Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol 6: 161–163 [PubMed] [Google Scholar]
- Andersen T., Gluud C., Franzmann M.B., Christoffersen P. (1991) Hepatic effects of dietary weight loss in morbidly obese subjects. J Hepatol 12: 224–229 [DOI] [PubMed] [Google Scholar]
- Antonopoulos S., Mikros S., Mylonopoulou M. (2006) Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis 184: 233–234 [DOI] [PubMed] [Google Scholar]
- Argo C.K., Northup P.G., Al-Osaimi A.M., Caldwell S.H. (2009) Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 51: 371–379 [DOI] [PubMed] [Google Scholar]
- Athyros V.G., Mikhailidis D.P., Didangelos T.P., Giouleme O.I., Liberopoulos E.N., Karagiannis A., et al. (2006) Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin 22: 873–883 [DOI] [PubMed] [Google Scholar]
- Baba C.S., Alexander G.A., Kalyani B., Pandy R., Rastogi S., Pandey A., et al. (2006) Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol 21: 191–198 [DOI] [PubMed] [Google Scholar]
- Bacon B.R., Farahvash M.J., Janney C.G., Neuschwander Tetri B.A. (1994) Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 107: 1103–1109 [DOI] [PubMed] [Google Scholar]
- Barak A.J., Beckenhauer H.C., Junnila M., Tuma D.J. (1993) Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res 17: 552–555 [DOI] [PubMed] [Google Scholar]
- Barker K.B., Palekar N.A., Bowers S.P. (2006) Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol 101: 368–373 [DOI] [PubMed] [Google Scholar]
- Basaranoglu M., Acbay O., Sonsuz A. (1999) A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol 31: 384–384 [DOI] [PubMed] [Google Scholar]
- Baumgardner J.N., Shankar K., Hennings L., Albano E., Badger T.M., Ronis M.J. (2008) N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr 138: 1872–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J., et al. (2006) A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307 [DOI] [PubMed] [Google Scholar]
- Browning J.D. (2006) Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology 44: 466–471 [DOI] [PubMed] [Google Scholar]
- Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., et al. (2004) Prevalence of hepatic steatosis in an urban population in the united states: impact of ethnicity. Hepatology 40: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Gentilcore E., Manini R., Natale S., Vanni E., Villanova N., et al. (2005) A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 100: 1082–1090 [DOI] [PubMed] [Google Scholar]
- Caldwell S.H., Harris D.M., Patrie J.T., Hespenheide E.E. (2002) Is NASH underdiagnosed among African Americans? Am J Gastroenterol 97: 1496–1500 [DOI] [PubMed] [Google Scholar]
- Caldwell S.H., Hespenheide E.E., Redick J.A., Iezzoni J.C., Battle E.H., Sheppard B.L. (2001) A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol 96: 519–525 [DOI] [PubMed] [Google Scholar]
- Capanni M., Calella F., Biagini M.R., Genise S., Raimondi L., Bedogni G., et al. (2006) Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Ailment Pharmacol Ther 23: 1143–1151 [DOI] [PubMed] [Google Scholar]
- Chalasani N.P., Sanyal A.J., Kowdley K.V., Robuck P.R., Hoofnagle J., Kleiner D.E., et al. for the NASH CRN Research Group (2009) Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp Clin Trials 30: 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charatcharoenwitthaya P., Lindor K.D. (2007) Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 11: 37–54, viii [DOI] [PubMed] [Google Scholar]
- Charlton M., Sreekumar R., Rasmussen D., Lindor K., Nair K.S. (2002) Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology 35: 898–904 [DOI] [PubMed] [Google Scholar]
- Chen C.H., Juang M.H., Yang J., Nien C.K., Yang C.C., Yeh Y.H., et al. (2006) Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of Taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol 40: 745–752 [DOI] [PubMed] [Google Scholar]
- Clark J., Brancati F.L., Diehl A.M. (2002) Nonalcoholic fatty liver disease. Gastroenterology 122: 1649–1657 [DOI] [PubMed] [Google Scholar]
- Clark J.M., Alkhuraishi A.R., Solga S.F., Alli P., Diehl A.M., Magnuson T.H. (2005) Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res 13: 1180–1186 [DOI] [PubMed] [Google Scholar]
- Csendes A., Smok G., Burgos A.M. (2006) Histological findings in the liver before and after gastric bypass. Obes Surg 16: 607–611 [DOI] [PubMed] [Google Scholar]
- Cussons A.J., Watts G.F., Mori T.A., Stuckey B.G. (2009) Omega-3 fatty acid supplementation decreases liver fat content in Polycystic Ovarian Syndrome: a randomised controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab 94: 3842–3848 [DOI] [PubMed] [Google Scholar]
- Daubioul C.A., Horsmans Y., Lambert P., Danse E., Delzenne N.M. (2005) Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: results of a pilot study. Eur J Clin Nutr 59: 723–726 [DOI] [PubMed] [Google Scholar]
- Daubioul C.A., Taper H.S., De Wispelaere L.D., Delzenne N.M. (2000) Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J Nutr 130: 1314–1319 [DOI] [PubMed] [Google Scholar]
- De Almeida S.R., Rocha P.R., Sanches M.D. (2006) Roux-en-Y gastric bypass improves the nonalcoholic steatohepatitis (NASH) of morbid obesity. Obes Surg 16: 270–278 [DOI] [PubMed] [Google Scholar]
- Ding X., Saxena N.K., Lin S. (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.B., Bhathal P.S., Hughes N.R., O’Brien P.E. (2004) Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 39: 1647–1654 [DOI] [PubMed] [Google Scholar]
- Dixon J.B., Bhathal P.S., O’Brien P.E. (2001) Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 121: 91–100 [DOI] [PubMed] [Google Scholar]
- Dixon J.B., Bhathal P.S., O’Brien P.E. (2006) Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg 16: 1278–1286 [DOI] [PubMed] [Google Scholar]
- Dufour J.F., Oneta C.M., Gonvers J.J., Bihl F., Cerny A., Cereda J.M., et al. for the Swiss Association for the Study of the Liver (2006) Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 4: 1537–1543 [DOI] [PubMed] [Google Scholar]
- Duman D.G., Ozdemir F., Birben E., Keskin O., Ekşioğlu-Demiralp E., Celikel C., et al. (2007) Effects of pentoxifylline on TNF-alpha production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci 52: 2520–2524 [DOI] [PubMed] [Google Scholar]
- Duseja A. (2006) Nonalcoholic fatty liver disease in India—is it different? Trop Gastroenterol 27: 142–146 [PubMed] [Google Scholar]
- Duseja A., Das A., Das R., Dhiman R.K., Chawla Y., Bhansali A., et al. (2007) The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci 52: 2368–2374 [DOI] [PubMed] [Google Scholar]
- Duseja A., Murlidharan R., Bhansali A., Sharma S., Das A., Das R., et al. (2004) Assessment of insulin resistance and effect of metformin in nonalcoholic steatohepatitis—a preliminary report. Indian J Gastroenterol 23: 12–15 [PubMed] [Google Scholar]
- Ekstedt M., Franzen L.E., Mathiesen U.L. (2007) Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol 47: 135–141 [DOI] [PubMed] [Google Scholar]
- Ekstedt M., Franzén L.E., Mathiesen U.L., Thorelius L., Holmqvist M., Bodemar G., et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873 [DOI] [PubMed] [Google Scholar]
- Ersöz G., Günşar F., Karasu Z., Akay S., Batur Y., Akarca U.S. (2005) Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol 16: 124–128 [PubMed] [Google Scholar]
- Estep J.M., Baranova A., Hossain N., Elariny H., Ankrah K., Afendy A., et al. (2009) Expression of cytokine signaling genes in morbidly obese patients with non-alcoholic steatohepatitis and hepatic fibrosis. Obes Surg 19: 617–624 [DOI] [PubMed] [Google Scholar]
- Fan J.G., Zhu J., Li X.J., Chen L., Lu Y.S., Li L., et al. (2005) Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol 20: 1825–1832 [DOI] [PubMed] [Google Scholar]
- Fernández-Miranda C., Pérez-Carreras M., Colina F., López-Alonso G., Vargas C., Solís-Herruzo J.A. (2008) A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis 40: 200–205 [DOI] [PubMed] [Google Scholar]
- Fujita K., Yoneda M., Wada K., Mawatari H., Takahashi H., Kirikoshi H. (2007) Telmisartan, an angiotensin II type 1 receptor blocker, controls progress of nonalcoholic steatohepatitis in rats. Dig Dis Sci 52: 3455–64 [DOI] [PubMed] [Google Scholar]
- Furuya C.K., Jr,, de Oliveira C.P., de Mello E.S. (2007) Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol 22: 510–514 [DOI] [PubMed] [Google Scholar]
- Gómez-Domínguez E., Gisbert J.P., Moreno-Monteagudo J.A., García-Buey L., Moreno-Otero R. (2006) A pilot study of atorvastatin treatment in dyslipemidic, non-alcoholic fatty liver patients. Ailment Pharmacol Ther 23: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Grimm I.S., Schindler W., Haluszka O. (1992) Steatohepatitis and fatal hepatic failure after biliopancreatic diversion. Am J Gastroenterol 87: 775–779 [PubMed] [Google Scholar]
- Gülbahar O., Karasu Z.A., Ersöz G. (2000) Treatment non-alcoholic steatohepatitis with N-acetylcysteine. Gastroenterology 118: A1444–A1444 [Google Scholar]
- Guma C., Viola L. (1992) [Ursotherapy in hepatobiliary diseases]. Acta Gastroenterol Latinoam 22: 205–206 [PubMed] [Google Scholar]
- Harrison S.A., Fecht W., Brunt E.M., Neuschwander-Tetri B.A. (2009) Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 49: 80–86 [DOI] [PubMed] [Google Scholar]
- Harrison S.A., Fincke C., Helinski D., Torgerson S., Hayashi P. (2004) A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Ailment Pharmacol Ther 20: 623–628 [DOI] [PubMed] [Google Scholar]
- Harrison S.A., Torgerson S., Hayashi P., Ward J., Schenker S. (2003) Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 98: 2485–2490 [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Yoneda M., Nakamura K., Makino I., Terano A. (2001) Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Ailment Pharmacol Ther 15: 1667–1672 [DOI] [PubMed] [Google Scholar]
- Hatzitolios A., Savopoulos C., Lazaraki G., Sidiropoulos I., Haritanti P., Lefkopoulos A. (2004) Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 23: 131–134 [PubMed] [Google Scholar]
- Haukeland J.W., Konopski Z., Eggesbø H.B., von Volkmann H.L., Raschpichler G., Bjøro K. (2004) Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 44: 853–860 [DOI] [PubMed] [Google Scholar]
- Hickman I.J., Jonsson J.R., Prins J.B., Ash S., Purdie D.M., Clouston A.D., et al. (2004) Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 53: 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain N., Afendy A., Stepanova M., Nader F., Srishord M., Goodman Z., et al. (2009) Independent predictors of fibrosis in patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol 7(11): 1224–1229 [DOI] [PubMed] [Google Scholar]
- Huang M.A., Greenson J.K., Chao C., Anderson L., Peterman D., Jacobson J., et al. (2005) One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 100: 1072–1081 [DOI] [PubMed] [Google Scholar]
- Hussein O., Grosovski M., Schlesinger S., Szvalb S., Assy N. (2007) Orlistat reverse fatty infiltration and improves hepatic fibrosis in obese patients with nonalcoholic steatohepatitis (NASH). Dig Dis Sci 52: 2512–2519 [DOI] [PubMed] [Google Scholar]
- Ibanez P., Solis N., Pizarro M., Aguayo G., Duarte I., Miquel J.F., et al. (2007) Effect of losartan on early liver fibrosis development in a rat model of nonalcoholic steatohepatitis. J Gastroenterol Hepatol 22: 846–846 [DOI] [PubMed] [Google Scholar]
- Jaskiewicz K., Raczynska S., Rzepko R., Sledziński Z. (2006) Nonalcoholic fatty liver disease treated by gastroplasty. Dig Dis Sci 51: 21–26 [DOI] [PubMed] [Google Scholar]
- Juurlink D.N., Gomes T., Lipscombe L.L., Austin P.C., Hux J.E., Mamdani M.M. (2009) Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 18(339): b2942–b2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanaka M., Mahmood S., Niiyama G., Izumi A., Kamei A., Ikeda H., et al. (2004) Control of oxidative stress and reduction in biochemical markers by vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatol Res 29: 39–41 [DOI] [PubMed] [Google Scholar]
- Kiyici M., Gulten M., Gurel S., Nak S.G., Dolar E., Savci G., et al. (2003) Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 17: 713–718 [DOI] [PubMed] [Google Scholar]
- Klein S., Mittendorfer B., Eagon J.C. (2006) Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology 130: 1564–1572 [DOI] [PubMed] [Google Scholar]
- Knobler H., Schattner A., Zhornicki T., Malnick S.D., Keter D., Sokolovskaya N., et al. (1999) Fatty liver—an additional and treatable feature of the insulin resistance syndrome. QJM 92: 73–79 [DOI] [PubMed] [Google Scholar]
- Kok N., Roberfroid M., Robert A., Delzenne N.M. (1996) Involvement of lipogenesis in the lower VLDL secretion induced by oligofructose in rats. Br J Nutr 76: 881–890 [DOI] [PubMed] [Google Scholar]
- Kral J.G., Thung S.N., Biron S. (2004) Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery 135: 48–58 [DOI] [PubMed] [Google Scholar]
- Kugelmas M., Hill D.B., Vivian B., Marsano L., McClain C.J. (2003) Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 38: 413–419 [DOI] [PubMed] [Google Scholar]
- Kwon do Y., Jung Y.S., Kim S.J., Park H.K., Park J.H., Kim Y.C. (2009) Impaired sulfur-amino acid metabolism and oxidative stress in nonalcoholic fatty liver are alleviated by betaine supplementation in rats. J Nutr 139: 63–68 [DOI] [PubMed] [Google Scholar]
- Laurin J., Lindor K.D., Crippin J.S., Gossard A., Gores G.J., Ludwig J., et al. (1996) Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology 23: 1464–1467 [DOI] [PubMed] [Google Scholar]
- Lavine J.E. (2000) Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr 136: 734–738 [PubMed] [Google Scholar]
- Lazaridis K.N., Gores G.J., Lindor K.D. (2001) Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol 35: 134–146 [DOI] [PubMed] [Google Scholar]
- Lewis J.H., Mortensen M.E., Zweig S. (2007) Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 46: 1453–1463 [DOI] [PubMed] [Google Scholar]
- Lindor K.D., Kowdley K.V., Heathcote E.J., Harrison M.E., Jorgensen R., Angulo P., et al. (2004) Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39: 770–778 [DOI] [PubMed] [Google Scholar]
- Lirussi F., Mastropasqua E., Orando S., Orlando R. (2007) Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev 24: CD005165–CD005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lazenby A.J., Clements R.H., Jhala N., Abrams G.A. (2007) Resolution of nonalcoholic steatohepatitis after gastric bypass surgery. Obes Surg 17: 486–492 [DOI] [PubMed] [Google Scholar]
- Loguercio C., Federico A., Tuccillo C., Terracciano F., D’Auria M.V., De Simone C., et al. (2005) Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 39: 540–543 [DOI] [PubMed] [Google Scholar]
- Loomba R., Lutchman G., Kleiner D., Ricks M., Feld J.J., Borg B.B., et al. (2008) Clinical trial: Pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Ailment Pharmacol Ther. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. (1980) Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 55: 434–438 [PubMed] [Google Scholar]
- Lutchman G., Modi A., Kleiner D.E., Promrat K., Heller T., Ghany M., et al. (2007) The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology 46: 424–429 [DOI] [PubMed] [Google Scholar]
- Madan K., Batra Y., Gupta S.D., Chander B., Rajan K.D., Tewatia M.S., et al. (2006) Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol 12: 3400–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H., Gores G.J. (2008) Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 28: 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Cheah P.L., Hilmi I.N., Chan S.P., Goh K.L. (2007) Non-alcoholic fatty liver disease in Malaysia: a demographic, anthropometric, metabolic and histological study. J Dig Dis 8: 58–64 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Brizi M., Bianchi G., Tomassetti S., Zoli M., Melchionda N. (2001) Metformin in non-alcoholic steatohepatitis. Lancet 358(9285): 893–894 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R. (2003) Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917–923 [DOI] [PubMed] [Google Scholar]
- Mathurin P., Gonzalez F., Kerdraon O. (2006) The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology 130: 1617–1624 [DOI] [PubMed] [Google Scholar]
- Mathurin P., Hollebecque A., Arnalsteen L., Buob D., Leteurtre E., Caiazzo R., et al. (2009) Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 137: 532–540 [DOI] [PubMed] [Google Scholar]
- Mattar S.G., Velcu L.M., Rabinovitz M. (2005) Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg 242: 610–617, discussion 618-620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. (1999) Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 116: 1413–1419 [DOI] [PubMed] [Google Scholar]
- McCullough A.J. ((2005)) The epidemiology and risk factors of NASH.. In: Farrell G.C., George J., Hall P., McCullough A.J. (eds), Fatty Liver Disease: NASH and Related Disorders, Blackwell: Oxford [Google Scholar]
- Mendez-Sanchez N., Gonzalez V., Chavez-Tapia N., Ramos M.H., Uribe M. (2004) Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol 3: 108–112 [PubMed] [Google Scholar]
- Miele L., Grieco A., Armuzzi A., Candelli M., Forgione A., Gasbarrini A., et al. (2003) Hepatic mitochondrial-beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol 98: 2335–2336 [DOI] [PubMed] [Google Scholar]
- Misra A., Chowbey P., Makkar B.M., Vikram N.K., Wasir J.S., Chadha D., et al. for the Consensus Group (2009) Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 57: 163–170, Review. PMID: 19582986 [PubMed] [Google Scholar]
- Misra A., Vikram N.K. (2004) Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition 20: 482–491 [DOI] [PubMed] [Google Scholar]
- Morita Y., Ueno T., Sasaki N., Tateishi Y., Nagata E., Kage M., et al. (2005) Nateglinide is useful for nonalcoholic steatohepatitis (NASH) patients with type 2 diabetes. Hepatogastroenterology 52: 1338–1343 [PubMed] [Google Scholar]
- Mottin C.C., Moretto M., Padoin A.V. (2005) Histological behavior of hepatic steatosis in morbidly obese patients after weight loss induced by bariatric surgery. Obes Surg 15: 788–793 [DOI] [PubMed] [Google Scholar]
- Mummadi R.R., Kasturi K.S., Chennareddygari S., Sood G.K. (2008) Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 6: 1396–1402 [DOI] [PubMed] [Google Scholar]
- Nair S., Diehl A.M., Wiseman M., Farr G.H., Jr,, Perrillo R.P. (2004) Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Ailment Pharmacol Ther 20: 23–28 [DOI] [PubMed] [Google Scholar]
- Nakamuta M., Morizono S., Soejima Y., Yoshizumi T., Aishima S., Takasugi S., et al. (2005) Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation 80: 608–612 [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421 [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A., Brunt E.M., Wehmeier K.R., Wehmeier K.R., Oliver D., Bacon B.R. (2003) Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017 [DOI] [PubMed] [Google Scholar]
- O’Brien P.E., Dixon J.B., Laurie C., Skinner S., Proietto J., McNeil J., et al. (2006) Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 144: 625–633 [DOI] [PubMed] [Google Scholar]
- Oh M.K., Winn J., Poordad F. (2008) Review article: diagnosis and treatment of non-alcoholic fatty liver disease. Ailment Pharmacol Ther 28: 503–522 [DOI] [PubMed] [Google Scholar]
- Okita M., Hayashi M., Sasagawa T., Takagi K., Suzuki K., Kinoyama S., et al. (2001) Effect of moderately energy-restricted diet on obese patients with fatty liver. Nutrition 17: 542–547 [DOI] [PubMed] [Google Scholar]
- Ong J.P., Elariny H., Collantes R., Younoszai A., Chandhoke V., Reines H.D., et al. (2005) Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg 15: 310–315 [DOI] [PubMed] [Google Scholar]
- Ong J.P., Pitts A., Younossi Z.M. (2008) Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 49: 608–612 [DOI] [PubMed] [Google Scholar]
- Ong J.P., Younossi Z.M. (2007) Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 11: 1–16, vii [DOI] [PubMed] [Google Scholar]
- Palmer M., Schaffner F. (1990) Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology 99: 1408–1413 [DOI] [PubMed] [Google Scholar]
- Pamuk G.E., Sonsuz A. (2003) N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterol Hepatol 18: 1220–1221 [DOI] [PubMed] [Google Scholar]
- Park S.H., Jeon W.K., Kim S.H., Kim H.J., Park D.I., Cho Y.K., et al. (2006) Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 21: 138–143 [DOI] [PubMed] [Google Scholar]
- Patrick L. (2002) Nonalcoholic fatty liver disease: relationship to insulin sensitivity and oxidative stress. Treatment approaches using vitamin E, magnesium, and betaine. Altern Med Rev 7: 276–291 [PubMed] [Google Scholar]
- Powell E.E., Cooksley W.G., Hanson R., Searle J., Halliday J.W., Powell L.W. (1990) The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 11: 74–80 [DOI] [PubMed] [Google Scholar]
- Promrat K., Lutchman G., Uwaifo G.I., Freedman R.J., Soza A., Heller T., et al. (2004) A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 39: 188–196 [DOI] [PubMed] [Google Scholar]
- Purohit V., Abdelmalek M.F., Barve S., Benevenga N.J., Halsted C.H., Kaplowitz N., et al. (2007) Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr 86: 14–24 [DOI] [PubMed] [Google Scholar]
- Rafiq N., Bai C., Fang Y., Srishord M., McCullough A., Gramlich T., et al. (2009) Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 7: 234–238 [DOI] [PubMed] [Google Scholar]
- Rafiq N., Younossi Z. (2009) Evaluation and management of non-alcoholic fatty liver disease. Clin Liver Dis 13: 249–266 [DOI] [PubMed] [Google Scholar]
- Rallidis L.S., Drakoulis C.K., Parasi A.S. (2004) Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 174: 193–196 [DOI] [PubMed] [Google Scholar]
- Ranlov I., Hardt F. (1990) Regression of liver steatosis following gastroplasty or gastric bypass for morbid obesity. Digestion 47: 208–214 [DOI] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Jacqueminet S. (2006) One year randomized placebo-controlled double-blind trial of rosiglitazone in nonalcoholic steatohepatitis: results of the Pilot trial. Hepatol 44(Suppl 1): 201A–201A [Google Scholar]
- Ratziu V., Giral P., Jacqueminet S., Charlotte F., Hartemann-Heurtier A., Serfaty L., et al. for the LIDO Study Group (2008) Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 135: 100–110 [DOI] [PubMed] [Google Scholar]
- Rucker R.D., Jr,, Horstmann J., Schneider P.D., Varco R.L., Buchwald H. (1982) Comparisons between jejunoileal and gastric bypass operations for morbid obesity. Surgery 92: 241–249 [PubMed] [Google Scholar]
- Ruhl C.E., Everhart J.E. (2003) Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 124: 71–79 [DOI] [PubMed] [Google Scholar]
- Sabuncu T., Nazligul Y., Karaoglanoglu M., Ucar E., Kilic F.B. (2003) The effects of sibutramine and orlistat on the ultrasonographic findings, insulin resistance and liver enzyme levels in obese patients with non-alcoholic steatohepatitis. Rom J Gastroenterol 12: 189–192 [PubMed] [Google Scholar]
- Sanyal A.J., Mofrad P.S., Contos M.J., Sargeant C., Luketic V.A., Sterling R.K., et al. (2004) A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Satapathy S.K., Garg S., Chauhan R., Sakhuja P., Malhotra V., Sharma B.C., et al. (2004) Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99: 1946–1952 [DOI] [PubMed] [Google Scholar]
- Satapathy S.K., Sakhuja P., Malhotra V., Sharma B.C., Sarin S.K. (2007) Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 22: 634–638 [DOI] [PubMed] [Google Scholar]
- Schwimmer J.B., Middleton M.S., Deutsch R., Lavine J.E. (2005) A phase 2 clinical trial of metformin as a treatment for nondiabetic paediatric nonalcoholic steatohepatitis. Ailment Pharmacol Ther 21: 871–879 [DOI] [PubMed] [Google Scholar]
- Shadid S., Jensen M.D. (2003) Effect of pioglitazone on biochemical indices of non-alcoholic fatty liver disease in upper body obesity. Clin Gastroenterol Hepatol 1: 384–387 [DOI] [PubMed] [Google Scholar]
- Shifflet A., Wu G.Y. (2009) Non-alcoholic steatohepatitis: an overview. J Formos Med Assoc 108: 4–12 [DOI] [PubMed] [Google Scholar]
- Silverman E.M., Sapala J.A., Appelman H.D. (1995) Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Pathol 104: 23–31 [DOI] [PubMed] [Google Scholar]
- Singh S.P., Nayak S., Swain M., Rout N., Mallik R.N., Agrawal O., et al. (2004) Prevalence of nonalcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol 25: 76–79 [PubMed] [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C.D., Karason K., Larsson B., Wedel H., et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–751 [DOI] [PubMed] [Google Scholar]
- Solga S.F., Buckley G., Clark J.M., Horska A., Diehl A.M. (2008) The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol 42: 1117–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K.J., Bacher A.C., Turner K., Lim J.G., Hees P.S., Shapiro E.P., et al. (2005) Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med 28: 9–18 [DOI] [PubMed] [Google Scholar]
- Stratopoulos C., Papakonstantinou A., Terzis I. (2005) Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes Surg 15: 1154–1160 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Lindor K., St. Saver J., Lymp J., Mendes F., Muto A., et al. (2005) Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol 43: 1060–1066 [DOI] [PubMed] [Google Scholar]
- Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S., et al. (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468 [DOI] [PubMed] [Google Scholar]
- Targher G., Arcaro G. (2007) Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 191: 235–240 [DOI] [PubMed] [Google Scholar]
- Tendler D., Lin S., Yancy W.S. (2007) The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci 52: 589–593 [DOI] [PubMed] [Google Scholar]
- Thomas E.L., Brynes A.E., Hamilton G., Patel N., Spong A., Goldin R.D., et al. (2006) Effect of nutritional counseling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol 12: 5813–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushuizen M.E., Brunck M.C., Pouwels P.J. (2006) Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver Int 26: 1015–1017 [DOI] [PubMed] [Google Scholar]
- Ueno T., Sugawara H., Sujaku K., Hashimoto O., Tsuji R., Tamaki S., et al. (1997) Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107 [DOI] [PubMed] [Google Scholar]
- Uslusoy H.S., Nak S.G., Gülten M., Biyikli Z. (2009) Liver histology according to the presence of metabolic syndrome in nonalcoholic fatty liver disease cases. World J Gastroenterol 15: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun A., Kadayifci A., Isik A.T., Ozgurtas T., Deveci S., Tuzun A., et al. (2004) Metformin in the treatment of patients with non-alcoholic steatohepatitis. Ailment Pharmacol Ther 19: 537–544 [DOI] [PubMed] [Google Scholar]
- Vajro P., Franzese A., Valerio G., Iannucci M.P., Aragione N. (2000) Lack of efficacy of ursodeoxycholic acid for the treatment of liver abnormalities in obese children. J Pediatr 136: 739–743 [PubMed] [Google Scholar]
- Vajro P., Mandato C., Franzese A., Ciccimarra E., Lucariello S., Savoia M., et al. (2004) Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr 38: 48–55 [DOI] [PubMed] [Google Scholar]
- Velayudham A., Dolganiuc A., Ellis M., Petrasek J., Kodys K., Mandrekar P., et al. (2008) VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 49: 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless I.R., Lentz J.S. (1990) Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 12: 1106–1110 [DOI] [PubMed] [Google Scholar]
- Weston S.R., Leyden W., Murphy R., Bass N.M., Bell B.P., Manos M.M., et al. (2005) Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology 41: 372–379 [DOI] [PubMed] [Google Scholar]
- Wieckowska A., Feldstein A.E. (2008) Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis 28: 386–395 [DOI] [PubMed] [Google Scholar]
- Yalniz M., Bahçecioğlu I.H., Kuzu N., Celebi S., Ataseven H., Ustündağ B., et al. (2007) Amelioration of steatohepatitis with pentoxifylline in a novel nonalcoholic steatohepatitis model induced by high-fat diet. Dig Dis Sci 52: 2380–2386 [DOI] [PubMed] [Google Scholar]
- Yiu D.C., Leung N.W. (2004) Epidemiological study: nonalcoholic fatty liver disease in Hong Kong Chinese. Hepatology 40(Suppl 1): 582A–582A [Google Scholar]
- Yokohama S., Yoneda M., Haneda M., Okamoto S., Okada M., Aso K., et al. (2004) Therapeutic efficacy of an angiotensin II receptor antagonist in patients with non alcoholic steatohepatitis. Hepatology 40: 1222–1225 [DOI] [PubMed] [Google Scholar]
- Yoshiji H., Kuriyama S., Yoshii J., Ikenaka Y., Noguchi R., Nakatani T., et al. (2001) Angiotensin II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 34: 745–750 [DOI] [PubMed] [Google Scholar]
- Zhu F.S., Liu S., Chen X.M., Huang Z.G., Zhang D.W. (2008) Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol 14: 6395–6400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.J., Shi Y.F., Hu M.M., Jiang Y.X., Tan L., Liu Y.P. (2003) The effects of weight reduction in reversing fatty liver changes in overweight and obese patients. Zhonghua Nei Ke Za Zhi 42: 98–102 [PubMed] [Google Scholar]