Abstract
Dyspepsia is a common clinical problem seen by both primary care physicians and gastroenterologists. Initial evaluation should focus on the identification and treatment of potential causes of symptoms such as gastroesophageal reflux disease (GERD), peptic ulcer disease, and medication side effects but also on recognizing those at risk for more serious conditions such as gastric cancer. This manuscript discusses the evaluation and management of dyspepsia including the role of proton-pump inhibitors, treatment of Helicobacter pylori, and endoscopy. Finally, treatment of refractory functional dyspepsia is addressed.
Keywords: dyspepsia, management, functional dyspepsia, evaluation, proton-pump inhibitor, Helicobacter pylori, endoscopy
Introduction
Of all adults 30–40% experience symptoms of upper abdominal pain or discomfort but an organic cause is found in only a minority who seek medical care [El-Serag and Talley, 2004; Talley et al. 1998b]. The remaining group is labeled as having functional dyspepsia. Individuals with functional dyspepsia suffer significant morbidity and expend significant resources through both direct and indirect costs. Despite periods of remission, patients will usually have continued intermittent symptoms long-term [Agreus et al. 1995; Talley et al. 1987]; with approximately 50% consulting a health care provider for their symptoms at some time in their life [Koloski et al. 2001].
Dyspepsia is defined as having one or more symptoms of epigastric pain, burning, postprandial fullness, or early satiation [Tack et al. 2006]. Bloating and nausea often coexist with dyspepsia but are nonspecific and are thus not included in its definition. Heartburn is also excluded from diagnostic symptom criteria for dyspepsia since it is thought to primarily arise from the esophagus and it is suggestive of gastroesophageal reflux disease (GERD) although it too may occur concomitantly [Talley et al. 1993; Klauser et al. 1990]. Similarly, retrosternal pain suggestive of esophageal origin such as that embraced by the term noncardiac chest pain is likewise distinguished from dyspepsia.
Differential diagnosis
Patients presenting with predominant epigastric pain or discomfort who have not undergone any investigations are defined as having uninvestigated dyspepsia. In patients with dyspepsia who are investigated, there are 5 major causes: gastroesophageal reflux (with or without esophagitis), medications, functional dyspepsia, chronic peptic ulcer disease (PUD), and malignancy [Talley et al. 2005b]. Less likely causes include pancreatic or hepatobiliary tract disease, motility disorders, infiltrative diseases of the stomach (e.g., eosinophilic gastritis, Crohn’s disease, sarcoidosis), celiac disease, intestinal angina, small intestine bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), metabolic disturbances (e.g., hypercalcemia, heavy metal), diabetic radiculopathy, hernia, and abdominal wall pain [Talley et al. 2005b; Heikkinen et al. 1995].
Gastroesophageal reflux disease
GERD, defined as symptoms or tissue damage that result from reflux of gastric contents into the esophagus [DeVault et al. 2005], can present with epigastric pain/discomfort although typically heartburn and regurgitation are more common symptoms. While approximately 40% of the US population has intermittent heartburn symptoms at least once monthly, the prevalence of GERD is 14% [Farup et al. 2001; Locke et al. 1997]. The prevalence of GERD in Europe ranges from 10 to 20%, while Asia has a much lower prevalence of 2–5% [Dent et al. 2005]. There are two patterns of acid reflux: upright (daytime) and supine (nocturnal) [Demeester et al. 1976]. Daytime or upright reflux commonly manifests as postprandial heartburn and may be associated with postprandial regurgitation. These symptoms are usually brief due to rapid clearance of gastric acid from the esophagus. Nocturnal GERD occurs when gastric contents reflux into the esophagus while a patient is recumbent. Approximately 80% of patients with GERD have nocturnal symptoms [Shaker et al. 2003; Farup et al. 2001]. The increased quantity, duration, and lack of clearance of gastric refluxate at night carry an increased risk of complications. GERD is usually a clinical diagnosis elicited by patient history and asking directed questions.
Medications
Medications are another frequent and often overlooked cause of dyspepsia. Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), including the cyclooxygenase-2-selective NSAIDs can cause ulcers and dyspepsia [Hawkey and Langman, 2003; Ofman et al. 2003; Bytzer and Hallas, 2000]. Many other medications (Table 1) can cause upper abdominal discomfort. Even herbal, OTC products, and home remedies have been implicated in causing symptoms [Holtmann et al. 2004].
Table 1.
Medications that cause dyspepsia.
| Some medications that commonly cause dyspepsia |
|---|
| NSAIDS |
| Cox-2 inhibitors |
| Bisphosphonates |
| Erythromycin |
| Tetracyclines |
| Iron |
| Potassium supplements |
| Acarbose |
| Digitalis |
| Theophylline Orlistat |
NSAIDS, nonsteroidal anti-inflammatory drugs.
Peptic ulcer disease
Ulcers are found in approximately 10% of patients undergoing evaluation for dyspepsia [Talley et al. 2005b; Shaib and El-Serag, 2004]. Until recently, chronic PUD was almost exclusively due to H. pylori infection with up to 90% of duodenal ulcers and 70% of gastric ulcers attributed to this bacterium [Talley et al. 1998b; Soll, 1996]. However, NSAIDs and aspirin are now responsible for most ulcer disease in developed countries [Liu et al. 2008; Ramsoekh et al. 2005]. This paradigm shift appears to be due to advances in public health and sanitation as well as effective treatment regimens for H. pylori [Ramsoekh et al. 2005]. Yet the combination of H. pylori infection and NSAID usage is synergistic with the risk of uncomplicated PUD estimated to be 17.5 times higher among H. pylori-positive NSAID users compared to H. pylori-negative non-users and a three- to four-fold increase in ulcer incidence with either risk factor alone [Papatheodoridis et al. 2006].
Functional dyspepsia
Functional dyspepsia is likely a heterogeneous disorder with subgroups identified based on different demographic, clinical, and pathophysiologic features [Sarnelli et al. 2003; Tack et al. 2002; Tack et al. 2001; Stanghellini et al. 1996]. The Rome III working group defined functional dyspepsia as the presence of symptoms thought to originate in the gastroduodenal region, in the absence of any organic, systemic, or metabolic disease that is likely to explain them [Tack et al. 2006]. Symptoms should be present for a minimum of 3 months; however, symptoms for greater than 6 months are typical. Rome III further characterized dyspepsia into two distinct categories: (1) Postprandial Distress Syndrome (PDS) and (2) Epigastric Pain Syndrome (EPS) [Tack et al. 2006] (Table 2).
Table 2.
Diagnostic criteria for Postprandial Distress Syndrome and Epigastric Pain Syndrome (Reprinted from Gastroenterology 130, Tack et al., Functional gastroduodenal disorders, 1466–1479, Copyright (2006), with permission from Elsevier).
| Postprandial Distress Syndrome | Epigastric Pain Syndrome |
|---|---|
| 1. Bothersome postprandial fullness, occurring after ordinary sized meals, at least several times per week AND/OR | 1. Pain or burning localized to the epigastrium of at least moderate severity at least once per week AND |
| 2. Early satiation that prevents finishing a regular meal, at least several times per week | 2. The pain is intermittent AND |
| 3. Not generalized or localized to other abdominal or chest regions AND | |
| 4. Not relieved by defecation or passage of flatus AND | |
| 5. Not fulfilling criteria for gallbladder and sphincter of Oddi disorders | |
| Supportive criteria | Supportive criteria |
| 1. Upper abdominal bloating, postprandial nausea or excessive belching can be present | 1. The pain may be of a burning quality but without a retrosternal component |
| 2. Epigastric Pain Syndrome may coexist | 2. The pain is commonly induced or relieved by ingestion of a meal but may occur while fasting |
| 3. Postprandial distress syndrome may coexist |
Several pathophysiologic mechanisms can underlie functional dyspeptic symptoms including: delayed gastric emptying, impaired gastric accommodation to a meal, hypersensitivity to gastric distention, H. pylori infection, altered duodenal response to lipids or acid, abnormal duodenojejunal motility, or central nervous system dysfunction [Tack et al. 2004]. Research is needed to better characterize these heterogeneous abnormalities, allowing for mechanism specific diagnostic studies and directed treatment.
Other
While gastric or esophageal cancer is an unusual finding in patients with dyspepsia, excluding malignancy is a common reason given for performing endoscopy [Lieberman et al. 2004]. Biliary or pancreatic pain can usually be distinguished by history since it is more severe, unpredictable, often localized to the right upper quadrant (RUQ) or radiates to the shoulder or back, and may last from hours to days [Ceyhan et al. 2008; Behar et al. 2006]. Bacterial overgrowth is an under-recognized cause of abdominal pain, but is usually associated with diarrhea, diffuse cramping pain and bloating in combination with some predisposing factor (prior abdominal surgery, diabetes, etc). Celiac disease and lactose intolerance are other less likely causes of dyspepsia but should be considered in the appropriate clinical setting [Giangreco et al. 2008; Locke et al. 2004; Sahai et al. 2000].
Clinical evaluation and management
As with any subacute or chronic illness, the initial evaluation of a patient with dyspepsia begins with a thorough history and physical examination. Directed questioning for the presence of alarm symptoms (e.g., unexplained weight loss, recurrent vomiting, progressive dysphagia, odynophagia, gastrointestinal blood loss, and family history of upper gastrointestinal cancer) is important; however, the presence of alarm symptoms may indicate advanced disease and thus limited treatment options [Bowrey et al. 2006; Vakil et al. 2006; Hammer et al. 2004; Blackshaw et al. 2003]. Regardless, an upper endoscopy is recommended for all patients with alarm symptoms [Tack et al. 2006; Talley et al. 2005a; Talley et al. 2005b; Veldhuyzen van Zanten et al. 2005; National Institute for Clinical Excellence, 2004; Scottish Intercollegiate Guidelines Network, 2003; Talley et al. 1998a]. Experts also recommend endoscopic evaluation for new onset dyspepsia in people over age 50 [Tack et al. 2006] although the diagnostic yield is low [Lieberman et al. 2004]. Age specific thresholds to trigger endoscopic evaluation may differ by sex and locality given gender and regional disease specific risks [Marmo et al. 2005; Talley et al. 2005a; Talley et al. 2005b; Lieberman et al. 2004; National Institute for Clinical Excellence, 2004; Scottish Intercollegiate Guidelines Network, 2003; Talley et al. 1998a].
Special attention should be given to a thorough medication review since patients often do not inform their physicians of over-the-counter medication or herbal medicine use. Directly questioning patients about the use of NSAIDs may be facilitated by listing common available medications or questioning what they take for their headaches, backaches, arthritis, etc. If a potential offending medication is identified, it should be stopped if clinically feasible or an alternate medication substituted. If NSAIDs cannot be stopped, the addition of a proton pump inhibitor (PPI) or changing the NSAID to a selective COX-2 inhibitor may minimize symptoms [Targownik et al. 2008].
Typical reflux symptoms suggest a diagnosis of GERD and should prompt empiric PPI treatment. The Rome II criteria for dyspepsia specifically excluded patients with GERD symptoms; however, the Rome III guidelines acknowledged that symptoms of GERD and dyspepsia often overlap [Tack et al. 2006]. According to the revised criteria, the presence of heartburn does not exclude a diagnosis of functional dyspepsia if symptoms persist despite a trial of adequate acid suppression therapy. In fact, overlap of GERD with functional dyspepsia is probably frequent and needs to be carefully considered in clinical practice.
Helicobacter pylori
Once alarm features, typical GERD symptoms and possible offending medications have been excluded in a younger dyspeptic patient, either evaluation for H. pylori or a trial of empiric antisecretory therapy is generally warranted. Noninvasive testing for H. pylori infection, followed by eradication (‘test and treat’) has been shown to be an approach that decreases the number of endoscopies [Arents et al. 2003; Manes et al. 2003; McColl et al. 2002; Lassen et al. 2000]. The American Gastroenterology Association (AGA) and Scottish Intercollegiate Guidelines Network (SIGN) recommends ‘test and treat’ for patients less than 50 years old without alarm features [Talley et al. 2005b; Scottish Intercollegiate Guidelines Network, 2003]. A test and treat strategy can cure underlying PUD and prevent future ulcer recurrence. However, false positive serology results are common in areas where the prevalence of infection is low and many patients remain symptomatic despite eliminating their infection [Moayyedi et al. 2006; Moayyedi et al. 2000]. The effect of eradication of H. pylori on symptoms of functional dyspepsia has also been questioned. The updated Cochrane review of 13 trials including 3180 patients with functional dyspepsia indicated that eradication therapy was superior to placebo, but the number needed to treat (NNT) was 17 [Moayyedi et al. 2004]. This strategy also leads to increased antibiotic use and risks increasing the incidence of antibiotic resistance both to H. pylori and other bacteria. The success of the ‘test and treat’ approach is highest in places with a high prevalence of H. pylori and related PUD [Moayyedi et al. 2005]. H. pylori is also an important cause of gastric cancer, so proponents of a test and treat approach to dyspepsia argue that it eliminates a potential carcinogen despite not eliminating symptoms in everyone. Yet the question remains as to whether H. pylori eradication reduces gastric cancer risk and if so at what stage of infection it can do so.
Whether to look for H. pylori depends to some extent on the likelihood of finding it. So in regions with a high prevalence of infection (>60%) testing is appropriate. Cost-effectiveness studies suggest that the choice of a noninvasive test should also be based on the prevalence of infection in the community. In low- and intermediate-prevalence situations, the stool antigen test or the urea breath test are favored [Vakil et al. 2000] but to ensure accuracy, these tests should only be done when the patient has been off PPIs for at least 2 weeks [Gatta et al. 2004; Malfertheiner et al. 2002]. The accuracy of H. pylori serology has been questioned, especially in low prevalence areas such as many parts of North America. Negative serology likely excludes infection but a positive result is often erroneous so confirmation with a breath or stool test is appropriate before starting treatment. Table 3 lists common treatment regimens for H. pylori.
Table 3.
Common treatment regimens for Helicobacter pylori.
| Regimen | Comment |
|---|---|
| Triple therapy PPI; amoxicillin 1 g BID; clarithromycin 500 mg BID for 10–14 days | First line treatment |
| Sequential therapy PPI and amoxicillin 1 g BID for 5 days followed by PPI, clarithromycin 500 mg BID, tinidazole 500 mg BID for 5 days | May be first line where macrolide resistance is common |
| Quadruple therapy PPI; bismuth 525 mg QID; metronidazole 500 mg QID; and tetracycline 500 mg QID for 14 days | Treatment for failure |
PPI, proton-pump inhibitor; BD, twice daily; QID, four times a day.
Empiric proton-pump inhibitors
In regions with low (less than 20–30%) prevalence of H. pylori, patients less than 50 years old without alarm symptoms should receive an empiric trial of PPIs [Chey et al. 2007; Peura et al. 2007; Ladabaum et al. 2002]. In fact, Peura and associates found that the majority of patients in an American population with uninvestigated dyspepsia had an acid mediated condition that should respond to empiric acid suppression [Peura et al. 2007].
In uninvestigated dyspepsia, an empiric PPI trial will treat the most frequent causes of dyspepsia including GERD, medication-induced gastritis, and peptic ulcers, thus minimizing the need for costly and invasive testing with an NNT of 5 [Peura et al. 2007; Talley et al. 2005b]. Two independent cost-effectiveness analyses found that empiric PPI trial was superior to ‘test and treat’ when treating a population having a low prevalence of H. pylori infection [Ladabaum et al. 2002; Spiegel et al. 2002]. In populations with intermediate H. pylori prevalence (30–60%) cost-effectiveness analyses are equivalent between the 2 strategies; however, the empiric PPI strategy avoids use of antibiotics and the associated risk of antibiotic resistance [Ladabaum et al. 2002]. PPIs are also effective in treating patients with investigated functional dyspepsia with a NNT of 14.6 [Delaney et al. 2000; Wang et al. 2007]. Similarly, a meta-analysis of randomized controlled trials comparing ‘test and treat’ and empiric PPI strategies found no difference in symptoms or treatment costs at 12 months [Ford et al. 2008]. This differs from the recommendations from the most recent AGA guidelines, which lists empiric PPI as an alternative to ‘test and treat’; however, these guidelines were published prior to randomized controlled trials comparing empiric PPI to ‘test and treat’ [Tack et al. 2006]. The British National Institute for Clinical Excellence (NICE) guidelines recommend initiation of a 4 week trial of full dose PPI therapy in patients with uninvestigated dyspepsia [National Institute for Clinical Excellence, 2004]. Thus, the prevalence of H. pylori as well as patient and physician preference should determine the initial approach to the management of uncomplicated dyspepsia.
Endoscopy
Prompt endoscopy is recommended in patients with alarm symptoms or patients over a threshold age (35–55 years, depending on the incidence of malignant disease in the population and health care access) and in the presence of alarm symptoms [Tack et al. 2006; Delaney et al. 2005; Talley et al. 2005a; Talley et al. 2005b; Veldhuyzen van Zanten et al. 2005; National Institute for Clinical Excellence, 2004; Scottish Intercollegiate Guidelines Network, 2003; Delaney et al. 2000; Talley et al. 1998a]. Men have an increased prevalence and younger age at diagnosis of upper GI malignancy, thus a lower threshold for endoscopic evaluation may be warranted [Marmo et al. 2005; Lieberman et al. 2004]. The value of alarm symptoms has been questioned, as their presence often indicate advanced disease [Blackshaw et al. 2003]. Recent data has shown that early endoscopic evaluation performed in an open access endoscopy unit resulted in diagnosis of earlier stage cancers and a better 5-year survival; however, the diagnostic yield is low [Bowrey et al. 2006; Vakil et al. 2006; Lieberman et al. 2004; Blackshaw et al. 2003].
Once a patient has failed a 4–8 week trial of PPI therapy (in an area of low prevalence of H. pylori) or failed to respond to eradication of H. pylori (in an H. pylori endemic region) upper endoscopy is indicated. Dyspepsia, with or without reflux symptoms, accounts for 43% of upper endoscopies performed in the USA. Among endoscopies performed to evaluate dyspeptic patients, greater than 35% are done in patients younger than 50 years of age without alarm symptoms [Lieberman et al. 2004]. In a US study, the number of endoscopies needed to diagnose one cancer in a 50-year-old patient without alarm symptoms is approximately 375 in men and 750 in women; however, this varies depending on race and comorbidities [Lieberman et al. 2004]. Asians and Native Americans may be at significantly increased risk, thus early endoscopy may facilitate a cancer diagnosis [Lieberman et al. 2004].
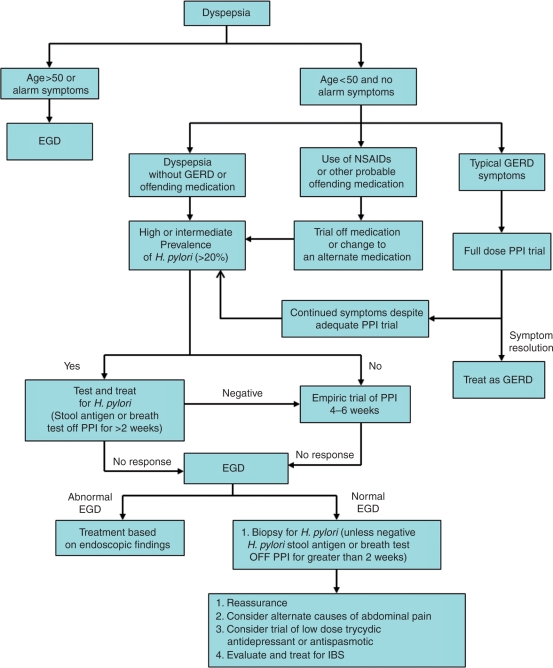
Performing upper endoscopy during a symptomatic period especially while the patient is off acid-suppressant therapy is important to making a diagnosis of functional dyspepsia by excluding other potential causes of symptoms. Biopsies of the stomach (and small bowel if celiac disease is suspected) can be obtained at the time of endoscopy to detect H. pylori infection and, in view of the association of H. pylori with PUD and dyspepsia, eradication is recommended in all positive cases [Moayyedi et al. 2005; Malfertheiner et al. 2002; Moayyedi et al. 2000]. H. pylori culture and sensitivity may also be useful in the setting of treatment failure for H. pylori, but are generally reserved for infection that has proven refractory to several courses of treatment. Figure 1 is a schematic diagram of the evaluation and management of dyspepsia. Note: diagnostic algorithm may differ based on regional cancer risk, gender, and age of patient at presentation.
Figure 1.
Schematic diagram of the management of dyspepsia. EGD, esophagogastroduodenoscopy; NSAIDS, nonsteroidal anti-inflammatory drugs; GERD, gastroesophogeal reflux disease; PPI, proton pump inhibitor; IBS, irritable bowel syndrome. Note: diagnostic algorithm may differ based on regional cancer risk, gender, and age of patient at presentation.
Gastric emptying studies
Dysfunctional gastric motility has traditionally been considered a major pathophysiologic mechanism underlying symptoms in functional dyspepsia [Sarnelli et al. 2003; Talley et al. 2001; Stanghellini et al. 1996]. In a meta-analysis of 17 studies involving 868 dyspeptic patients and 397 controls significant delay of solid gastric emptying was present in 30–40% of patients with functional dyspepsia [Quartero et al. 1998]; however, these findings did not correlate with patient symptoms [Jian et al. 1989; Talley et al. 1989; Wegener et al. 1989]. Results of solid-phase gastric emptying are mixed; however, a large-scale study suggested an association between delayed emptying for liquids and symptoms of postprandial fullness [Sarnelli et al. 2003]. Conversely, Delgado-Aros and associates found that low fasting gastric volumes and faster gastric emptying were associated with functional dyspepsia [Delgado-Aros et al. 2004]. Thus a liquid gastric emptying study may allow for identification of subgroups of dysmotility induced functional dyspepsia; however, this has not been validated. Recently, 2 subgroups of PDS-related dyspepsia were identified as having either accelerated gastric emptying in the early postprandial period or impaired inhibitory gastric emptying in the mid postprandial period supporting the observation that functional dyspepsia is a heterogeneous disorder [Zai and Kusano, 2009].
Other studies
Other investigations that might be considered in the evaluation of the dyspeptic patient may include ultrasound of the liver and biliary system; small bowel radiography; cross-sectional imaging of the abdomen, including the pancreas, with either a CT or MRI; vascular enterography; and hydrogen breath testing. These investigations should not be performed in all patients but instead specialized testing should be based on the clinical features, severity, and refractoriness of the symptoms. Upper GI barium radiography is inferior to upper endoscopy and is generally not recommended as part of the work up for dyspepsia [Talley et al. 2005b; Dooley et al. 1984]. Barium x-ray study of the small bowel is only useful in cases of suspected mechanical obstruction or to look for potential causes of stasis that may contribute to overgrowth of bacteria of the small bowel. Glucose or lactulose hydrogen breath testing may be used to detect SIBO; however, test characteristics are less than optimal. Hydrogen breath testing should be reserved for patients with structural abnormality or other predisposing clinical conditions.
Treatment for refractory functional dyspepsia
Patients who do not respond to empiric PPI therapy, have normal upper endoscopy, and who either are negative for H. pylori or have cleared infection following treatment yet continue to have dyspepsia represent a challenging group. First, the diagnosis should be re-evaluated, considering other disorders that may be mistaken for dyspepsia. In the absence of an alternate disease, reassurance and education of the patient with functional dyspepsia becomes important. Although not validated in the functional dyspepsia population, a positive physician–patient interaction including reassurance can reduce health care seeking behavior [Owens et al. 1995]. Patients are often also educated to eat smaller, more frequent meals to avoid gastric distention and to avoid food that aggravates symptoms.
Alternatively, pharmacotherapy will be considered for some patients; however, the benefits may be limited. The lack of effective therapeutics highlights our incomplete understanding of functional dyspepsia and that it may in fact represent a very heterogeneous disorder of visceral hypersensitivity and altered motility. Recently identified subgroups within functional dyspepsia may allow for cause-specific therapeutic interventions [Zai and Kusano, 2009]; however, more studies are needed to further characterize these differences.
Antidepressants
Antidepressants are frequently used to treat refractory functional dyspepsia, with tricyclic antidepressants often utilized as first-line agents. This practice is based in part on studies of functional abdominal pain consisting of predominantly IBS [Jackson et al. 2000]. More recently, a meta-analysis of antidepressants (mostly tricyclic antidepressants) in functional dyspepsia confirmed the efficacy of antidepressants with a relative risk reduction of symptoms of 0.55 [Hojo et al. 2005]. Whether benefit was related to control of underlying depression is unclear, since many studies did not exclude concomitant depression.
Other antidepressant agents have also been studied. Hashash and associates in a double-blind crossover study in 25 patients found that a combination of flupenthixol (antipsychotic) and melitracen (tricyclic antidepressant) significantly improved symptoms of functional dyspepsia (symptom relief in 71% of the treatment group compared to 24% of control) [Hashash et al. 2008]. This study excluded patients with a history of depression or use of antidepressants, thus benefits were due to improvement of the dyspepsia. A larger trial of venlafaxine showed no symptom benefit; however, results were confounded by a high dropout rate in the venlafaxine group due to medication intolerance [Van Oudenhove and Tack, 2009]. Theoretically, selective serotonin reuptake inhibitors (SSRIs) could be an effective treatment for functional dyspepsia through relaxation of the fundus (increasing serotonin in the gastric wall) and potentially modulating pain pathways. However, there have been no adequate randomized, controlled trials with any of the SSRIs. Therefore, it is the current practice of the authors to initiate a trial of low-dose tricyclic antidepressants for treatment of refractory functional dyspepsia.
Prokinetics
Functional dyspepsia is often treated with prokinetic agents; however, the efficacy of prokinetic agents has been questioned. The most recent Cochrane review of 19 heterogeneous studies implied a benefit of prokinetics with a relative symptom reduction of 33% [Moayyedi et al. 2006]. However, the authors noted that results were difficult to interpret due to the degree of heterogeneity between studies and likely publication bias [Moayyedi et al. 2006]. Also studies indicating potential benefit of prokinetics were short-term studies, and long-term efficacy is unproven. Prokinetic agents that have been studied include metoclopramide, domperidone, cisapride, erythromycin, and tegaserod.
Metoclopramide is a dopamine receptor antagonist that readily crosses the blood–brain barrier and therefore has a high incidence of dose-related side effects including restlessness, tremor, fatigue, Parkinsonism, tardive dyskinesia, and hyperprolactinemia which limit its utility. Domperidone works similarly to metoclopramide, but has a more favorable side effect profile as it does not cross the blood–brain barrier. Domperidone is available throughout Europe and Canada but is not approved in the USA, making its use in US populations problematic. Cisapride has been withdrawn from most world markets because of rare fatal arrhythmias.
The macrolide antibiotic erythromycin acts on the motilin receptor to increase gastric emptying in patients with gastroparesis, but its side effects and tachyphylaxis limit its clinical utility [Richards et al. 1993; Janssens et al. 1990]. Several motilin receptor agonists without antibiotic properties have been evaluated for the treatment of functional dyspepsia, but none have shown benefit over placebo [Karamanolis and Tack, 2006; Moayyedi et al. 2006].
Tegaserod is a partial 5-HT4 agonist that been used in the treatment of constipation predominant IBS and idiopathic constipation. Tegaserod has been shown to enhance gastric accommodation and normalize delayed gastric emptying [Thumshirn et al. 2007; Beattie et al. 2004]. The results of 2 large clinical trials yielded mixed results, but a meta-analysis demonstrated an improvement with tegaserod (NNT of 16) [Vakil et al. 2008]. Thus, tegaserod may not be an appropriate treatment for all patients, but may have favorable response in selected patients. Adverse effects may limit its use including diarrhea and risk of myocardial infarction and stroke. Tegaserod has been withdrawn from the US and Canadian markets due to the increased risks of heart attack and stroke. Alosteron is a 5-HT3 receptor antagonist with mixed results in functional dyspepsia. Alosteron also has significant adverse effects including constipation and ischemic colitis limiting its utility.
Dysfunctional gastric motility is considered a major pathophysiologic mechanism underlying functional dyspepsia [Sarnelli et al. 2003; Talley et al. 2001; Stanghellini et al. 1996]. The symptom profile and relationship to gastric and duodenal distention imply that functional dyspepsia is a heterogeneous disorder. Recent studies confirm this by finding the presence of subgroups of PDS-related dyspepsia [Zai and Kusano, 2009]. Further studies are needed to characterize the contributions of motility and visceral sensitivity which may lead to cause-specific therapeutic interventions. With our current limitations, clinicians must often use trial and error in treatment for their patients. In the absence of a contraindication the authors propose using a tricyclic antidepressant as first line in most patients with functional dyspepsia. Additionally, patients with PDS may be more likely to benefit from prokinetic agents than those with EPS.
Antibiotics
Bacterial overgrowth may cause abdominal pain but is usually associated with diarrhea and bloating [Rana and Bhardwaj, 2008; Singh and Toskes, 2003]. It is often associated with some predisposing factor including prior abdominal surgery (especially small bowel resection), diabetes, or duodenal diverticulosis that induces stasis of small bowel contents [Rana and Bhardwaj, 2008; Singh and Toskes, 2003]. If a diagnosis of SIBO is confirmed on breath testing or small bowel aspiration, antibiotics may be used to suppress bacterial growth. While an empiric trial of an antibiotic (for example: rifaximin, tetracycline, ciprofloxacin, amoxicillin/clavulanate, etc.) may be used to both diagnose and treat SIBO, this is not generally recommended given the epidemic of increasing antibiotic resistance worldwide.
Psychological treatments
Psychotherapy and behavioral therapy may also provide benefit in selected patients. Hypnotherapy has been shown to be superior to supportive therapy or an H2-receptor antagonist. Applied relaxation therapy, psychodynamic psychotherapy and cognitive therapy have also shown potential benefits, but studies are limited and poorly designed [Soo et al. 2004].
Conclusion
Dyspepsia is a common clinical problem seen both by primary care physicians and gastroenterologists. Initial evaluation should focus on the identification and treatment of potential causes of symptoms such as GERD, PUD, and medication side effects but also on recognizing those at risk of more serious conditions such as gastric cancer. An empiric PPI trial or ‘test and treat’ strategy for H. pylori are the initial approaches to a patient with dyspepsia, followed by endoscopy if initial management fails. Once an organic cause for symptoms is excluded, a diagnosis of functional dyspepsia is made. Unfortunately, effective treatments for functional dyspepsia are limited although acid suppression, antidepressants, antispasmodics and prokinetics may provide some symptom relief in selected patients. Providing reassurance of the absence of significant pathology is important to subsequent management and often precludes the need for further testing. Severe or ‘refractory’ functional dyspepsia should alert clinicians to the possibility of misdiagnosis and may warrant further evaluation. More research is needed to better understand the pathogenesis of functional dyspepsia, thus allowing development of better and more specific treatment.
Conflict of interest statement
CS has nothing to declare. DP declares to have received speaker and consulting fees from Takeda Pharmaceuticals North America.
References
- Agreus L., Svardsudd K., Nyren O., Tibblin G. (1995) Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology 109: 671–680 [DOI] [PubMed] [Google Scholar]
- Arents N.L., Thijs J.C., van Zwet A.A., Oudkerk Pool M., Gotz J.M., van de Werf G.T., et al. (2003) Approach to treatment of dyspepsia in primary care: a randomized trial comparing ‘test-and-treat’ with prompt endoscopy. Arch Intern Med 163: 1606–1612 [DOI] [PubMed] [Google Scholar]
- Beattie D.T., Smith J.A., Marquess D., Vickery R.G., Armstrong S.R., Pulido-Rios T., et al. (2004) The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol 143: 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar J., Corazziari E., Guelrud M., Hogan W., Sherman S., Toouli J. (2006) Functional gallbladder and sphincter of oddi disorders. Gastroenterology 130: 1498–1509 [DOI] [PubMed] [Google Scholar]
- Blackshaw G.R., Barry J.D., Edwards P., Allison M.C., Lewis W.G. (2003) Open-access gastroscopy is associated with improved outcomes in gastric cancer. Eur J Gastroenterol Hepatol 15: 1333–1337 [DOI] [PubMed] [Google Scholar]
- Bowrey D.J., Griffin S.M., Wayman J., Karat D., Hayes N., Raimes S.A. (2006) Use of alarm symptoms to select dyspeptics for endoscopy causes patients with curable esophagogastric cancer to be overlooked. Surg Endosc 20: 1725–1728 [DOI] [PubMed] [Google Scholar]
- Bytzer P., Hallas J. (2000) Drug-induced symptoms of functional dyspepsia and nausea. A symmetry analysis of one million prescriptions. Aliment Pharmacol Ther 14: 1479–1484 [DOI] [PubMed] [Google Scholar]
- Ceyhan G.O., Michalski C.W., Demir I.E., Muller M.W., Friess H. (2008) Pancreatic pain. Best Pract Res Clin Gastroenterol 22: 31–44 [DOI] [PubMed] [Google Scholar]
- Chey W.D., Wong B.C.the Practice Parameters Committee of the American College of Gastroenterology (2007) American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102: 1808–1825 [DOI] [PubMed] [Google Scholar]
- Delaney B., Ford A.C., Forman D., Moayyedi P., Qume M. (2005) Initial management strategies for dyspepsia. Cochrane Database Syst Rev )(4): CD001961–CD001961 [DOI] [PubMed] [Google Scholar]
- Delaney B.C., Wilson S., Roalfe A., Roberts L., Redman V., Wearn A., et al. (2000) Cost effectiveness of initial endoscopy for dyspepsia in patients over age 50 years: a randomised controlled trial in primary care. Lancet 356: 1965–1969 [DOI] [PubMed] [Google Scholar]
- Delgado-Aros S., Camilleri M., Cremonini F., Ferber I., Stephens D., Burton D.D. (2004) Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology 127: 1685–1694 [DOI] [PubMed] [Google Scholar]
- Demeester T.R., Johnson L.F., Joseph G.J., Toscano M.S., Hall A.W., Skinner D.B. (1976) Patterns of gastroesophageal reflux in health and disease. Ann Surg 184: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J., El-Serag H.B., Wallander M.A., Johansson S. (2005) Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54: 710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault K.R., Castell D.O., the American College of Gastroenterology (2005) Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 100: 190–200 [DOI] [PubMed] [Google Scholar]
- Dooley C.P., Larson A.W., Stace N.H., Renner I.G., Valenzuela J.E., Eliasoph J., et al. (1984) Double-contrast barium meal and upper gastrointestinal endoscopy. A comparative study. Ann Intern Med 101: 538–545 [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Talley N.J. (2004) Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther 19: 643–654 [DOI] [PubMed] [Google Scholar]
- Farup C., Kleinman L., Sloan S., Ganoczy D., Chee E., Lee C., et al. (2001) The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med 161: 45–52 [DOI] [PubMed] [Google Scholar]
- Ford A.C., Moayyedi P., Jarbol D.E., Logan R.F., Delaney B.C. (2008) Meta-analysis: Helicobacter pylori ‘test and treat’ compared with empirical acid suppression for managing dyspepsia. Aliment Pharmacol Ther 28: 534–544 [DOI] [PubMed] [Google Scholar]
- Gatta L., Vakil N., Ricci C., Osborn J.F., Tampieri A., Perna F., et al. (2004) Effect of proton pump inhibitors and antacid therapy on 13C urea breath tests and stool test for Helicobacter pylori infection. Am J Gastroenterol 99: 823–829 [DOI] [PubMed] [Google Scholar]
- Giangreco E., D’agate C., Barbera C., Puzzo L., Aprile G., Naso P., et al. (2008) Prevalence of celiac disease in adult patients with refractory functional dyspepsia: value of routine duodenal biopsy. World J Gastroenterol 14: 6948–6953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J., Eslick G.D., Howell S.C., Altiparmak E., Talley N.J. (2004) Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut 53: 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashash J.G., Abdul-Baki H., Azar C., Elhajj I.I., El Zahabi L., Chaar H.F., et al. (2008) Clinical trial: a randomized controlled cross-over study of flupenthixol+melitracen in functional dyspepsia. Aliment Pharmacol Ther 27: 1148–1155 [DOI] [PubMed] [Google Scholar]
- Hawkey C.J., Langman M.J. (2003) Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut 52: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen M., Pikkarainen P., Takala J., Rasanen H., Julkunen R. (1995) Etiology of dyspepsia: four hundred unselected consecutive patients in general practice. Scand J Gastroenterol 30: 519–523 [DOI] [PubMed] [Google Scholar]
- Hojo M., Miwa H., Yokoyama T., Ohkusa T., Nagahara A., Kawabe M., et al. (2005) Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol 40: 1036–1042 [DOI] [PubMed] [Google Scholar]
- Holtmann G., Siffert W., Haag S., Mueller N., Langkafel M., Senf W., et al. (2004) G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology 126: 971–979 [DOI] [PubMed] [Google Scholar]
- Jackson J.L., O’Malley P.G., Tomkins G., Balden E., Santoro J., Kroenke K. (2000) Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 108: 65–72 [DOI] [PubMed] [Google Scholar]
- Janssens J., Peeters T.L., Vantrappen G., Tack J., Urbain J.L., De Roo M., et al. (1990) Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 322: 1028–1031 [DOI] [PubMed] [Google Scholar]
- Jian R., Ducrot F., Ruskone A., Chaussade S., Rambaud J.C., Modigliani R., et al. (1989) Symptomatic, radionuclide and therapeutic assessment of chronic idiopathic dyspepsia. A double-blind placebo-controlled evaluation of cisapride. Dig Dis Sci 34: 657–664 [DOI] [PubMed] [Google Scholar]
- Karamanolis G., Tack J. (2006) Promotility medications – now and in the future. Dig Dis 24: 297–307 [DOI] [PubMed] [Google Scholar]
- Klauser A.G., Schindlbeck N.E., Muller-Lissner S.A. (1990) Symptoms in gastro-oesophageal reflux disease. Lancet 335: 205–208 [DOI] [PubMed] [Google Scholar]
- Koloski N.A., Talley N.J., Boyce P.M. (2001) Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol 96: 1340–1349 [DOI] [PubMed] [Google Scholar]
- Ladabaum U., Chey W.D., Scheiman J.M., Fendrick A.M. (2002) Reappraisal of non-invasive management strategies for uninvestigated dyspepsia: a cost-minimization analysis. Aliment Pharmacol Ther 16: 1491–1501 [DOI] [PubMed] [Google Scholar]
- Lassen A.T., Pedersen F.M., Bytzer P., Schaffalitzky de Muckadell O.B. (2000) Helicobacter pylori test-and-eradicate versus prompt endoscopy for management of dyspeptic patients: a randomised trial. Lancet 356: 455–460 [DOI] [PubMed] [Google Scholar]
- Lieberman D., Fennerty M.B., Morris C.D., Holub J., Eisen G., Sonnenberg A. (2004) Endoscopic evaluation of patients with dyspepsia: results from the national endoscopic data repository. Gastroenterology 127: 1067–1075 [DOI] [PubMed] [Google Scholar]
- Liu N.J., Lee C.S., Tang J.H., Cheng H.T., Chu Y.Y., Sung K.F., et al. (2008) Outcomes of bleeding peptic ulcers: a prospective study. J Gastroenterol Hepatol 23: e340–7 [DOI] [PubMed] [Google Scholar]
- Locke G.R., 3rd, Murray J.A., Zinsmeister A.R., Melton L.J., 3rd, Talley N.J. (2004) Celiac disease serology in irritable bowel syndrome and dyspepsia: a population-based case–control study. Mayo Clin Proc 79: 476–482 [DOI] [PubMed] [Google Scholar]
- Locke G.R., 3rd, Talley N.J., Fett S.L., Zinsmeister A.R., Melton L.J., 3rd (1997) Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112: 1448–1456 [DOI] [PubMed] [Google Scholar]
- McColl K.E., Murray L.S., Gillen D., Walker A., Wirz A., Fletcher J., et al. (2002) Randomised trial of endoscopy with testing for Helicobacter pylori compared with non-invasive H pylori testing alone in the management of dyspepsia. BMJ 324: 999–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfertheiner P., Megraud F., O’Morain C., Hungin A.P., Jones R., Axon A., et al. (2002) Current concepts in the management of Helicobacter pylori infection – the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther 16: 167–180 [DOI] [PubMed] [Google Scholar]
- Manes G., Menchise A., de Nucci C., Balzano A. (2003) Empirical prescribing for dyspepsia: randomised controlled trial of test and treat versus omeprazole treatment. BMJ 326: 1118–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmo R., Rotondano G., Piscopo R., Bianco M.A., Russo P., Capobianco P., et al. (2005) Combination of age and sex improves the ability to predict upper gastrointestinal malignancy in patients with uncomplicated dyspepsia: a prospective multicentre database study. Am J Gastroenterol 100: 784–791 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Feltbower R., Brown J., Mason S., Mason J., Nathan J., et al. (2000) Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Leeds HELP Study Group. Lancet 355: 1665–1669 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Soo S., Deeks J., Delaney B., Harris A., Innes M., et al. (2005) Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev (1): CD002096–CD002096 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Soo S., Deeks J., Delaney B., Harris A., Innes M., et al. (2006) Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev (2): CD002096–CD002096 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Soo S., Deeks J., Delaney B., Innes M., Forman D. (2004) Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev (4): CD001960–CD001960 [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (2004) Dyspepsia: managing dyspepsia in adults in primary care. [Google Scholar]
- Ofman J.J., Maclean C.H., Straus W.L., Morton S.C., Berger M.L., Roth E.A., et al. (2003) Meta-analysis of dyspepsia and nonsteroidal antiinflammatory drugs. Arthritis Rheum 49: 508–518 [DOI] [PubMed] [Google Scholar]
- Owens D.M., Nelson D.K., Talley N.J. (1995) The irritable bowel syndrome: long-term prognosis and the physician–patient interaction. Ann Intern Med 122: 107–112 [DOI] [PubMed] [Google Scholar]
- Papatheodoridis G.V., Sougioultzis S., Archimandritis A.J. (2006) Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol 4: 130–142 [DOI] [PubMed] [Google Scholar]
- Peura D.A., Gudmundson J., Siepman N., Pilmer B.L., Freston J. (2007) Proton pump inhibitors: effective first-line treatment for management of dyspepsia. Dig Dis Sci 52: 983–987 [DOI] [PubMed] [Google Scholar]
- Quartero A.O., de Wit N.J., Lodder A.C., Numans M.E., Smout A.J., Hoes A.W. (1998) Disturbed solid-phase gastric emptying in functional dyspepsia: a meta-analysis. Dig Dis Sci 43: 2028–2033 [DOI] [PubMed] [Google Scholar]
- Ramsoekh D., van Leerdam M.E., Rauws E.A., Tytgat G.N. (2005) Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol 3: 859–864 [DOI] [PubMed] [Google Scholar]
- Rana S.V., Bhardwaj S.B. (2008) Small intestinal bacterial overgrowth. Scand J Gastroenterol 43: 1030–1037 [DOI] [PubMed] [Google Scholar]
- Richards R.D., Davenport K., McCallum R.W. (1993) The treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycin. Am J Gastroenterol 88: 203–207 [PubMed] [Google Scholar]
- Sahai A.V., Mishra G., Penman I.D., Williams D., Wallace M.B., Hadzijahic N., et al. (2000) EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia. Gastrointest Endosc 52: 153–159 [DOI] [PubMed] [Google Scholar]
- Sarnelli G., Caenepeel P., Geypens B., Janssens J., Tack J. (2003) Symptoms associated with impaired gastric emptying of solids and liquids in functional dyspepsia. Am J Gastroenterol 98: 783–788 [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (2003) Dyspepsia: a national clinical guideline. [Google Scholar]
- Shaib Y., El-Serag H.B. (2004) The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol 99: 2210–2216 [DOI] [PubMed] [Google Scholar]
- Shaker R., Castell D.O., Schoenfeld P.S., Spechler S.J. (2003) Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol 98: 1487–1493 [DOI] [PubMed] [Google Scholar]
- Singh V.V., Toskes P.P. (2003) Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep 5: 365–372 [DOI] [PubMed] [Google Scholar]
- Soll A.H. (1996) Consensus conference. Medical treatment of peptic ulcer disease. Practice guidelines. Practice Parameters Committee of the American College of Gastroenterology. JAMA 275: 622–629 [DOI] [PubMed] [Google Scholar]
- Soo S., Forman D., Delaney B.C., Moayyedi P. (2004) A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol 99: 1817–1822 [DOI] [PubMed] [Google Scholar]
- Spiegel B.M., Vakil N.B., Ofman J.J. (2002) Dyspepsia management in primary care: a decision analysis of competing strategies. Gastroenterology 122: 1270–1285 [DOI] [PubMed] [Google Scholar]
- Stanghellini V., Tosetti C., Paternico A., Barbara G., Morselli-Labate A.M., Monetti N., et al. (1996) Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 110: 1036–1042 [DOI] [PubMed] [Google Scholar]
- Tack J., Bisschops R., Sarnelli G. (2004) Pathophysiology and treatment of functional dyspepsia. Gastroenterology 127: 1239–1255 [DOI] [PubMed] [Google Scholar]
- Tack J., Caenepeel P., Fischler B., Piessevaux H., Janssens J. (2001) Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology 121: 526–535 [DOI] [PubMed] [Google Scholar]
- Tack J., Demedts I., Dehondt G., Caenepeel P., Fischler B., Zandecki M., et al. (2002) Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology 122: 1738–1747 [DOI] [PubMed] [Google Scholar]
- Tack J., Talley N.J., Camilleri M., Holtmann G., Hu P., Malagelada J.R., et al. (2006) Functional gastroduodenal disorders. Gastroenterology 130: 1466–1479 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Lam S.K., Goh K.L., Fock K.M. (1998a) Management guidelines for uninvestigated and functional dyspepsia in the Asia-Pacific region: First Asian Pacific Working Party on Functional Dyspepsia. J Gastroenterol Hepatol 13: 335–353 [DOI] [PubMed] [Google Scholar]
- Talley N.J., McNeil D., Hayden A., Colreavy C., Piper D.W. (1987) Prognosis of chronic unexplained dyspepsia. A prospective study of potential predictor variables in patients with endoscopically diagnosed nonulcer dyspepsia. Gastroenterology 92: 1060–1066 [PubMed] [Google Scholar]
- Talley N.J., Shuter B., McCrudden G., Jones M., Hoschl R., Piper D.W. (1989) Lack of association between gastric emptying of solids and symptoms in nonulcer dyspepsia. J Clin Gastroenterol 11: 625–630 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Silverstein M.D., Agreus L., Nyren O., Sonnenberg A., Holtmann G. (1998b) AGA technical review: evaluation of dyspepsia. American Gastroenterological Association. Gastroenterology 114: 582–595 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Vakil N., the Practice Parameters Committee of the American College of Gastroenterology (2005a) Guidelines for the management of dyspepsia. Am J Gastroenterol 100: 2324–2337 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Vakil N.B., Moayyedi P. (2005b) American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 129: 1756–1780 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Verlinden M., Jones M. (2001) Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol 96: 1422–1428 [DOI] [PubMed] [Google Scholar]
- Talley N.J., Weaver A.L., Tesmer D.L., Zinsmeister A.R. (1993) Lack of discriminant value of dyspepsia subgroups in patients referred for upper endoscopy. Gastroenterology 105: 1378–1386 [DOI] [PubMed] [Google Scholar]
- Targownik L.E., Metge C.J., Leung S., Chateau D.G. (2008) The relative efficacies of gastroprotective strategies in chronic users of nonsteroidal anti-inflammatory drugs. Gastroenterology 134: 937–944 [DOI] [PubMed] [Google Scholar]
- Thumshirn M., Fruehauf H., Stutz B., Tougas G., Salter J., Fried M. (2007) Clinical trial: effects of tegaserod on gastric motor and sensory function in patients with functional dyspepsia. Aliment Pharmacol Ther 26: 1399–1407 [DOI] [PubMed] [Google Scholar]
- Vakil N., Laine L., Talley N.J., Zakko S.F., Tack J., Chey W.D., et al. (2008) Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am J Gastroenterol 103: 1906–1919 [DOI] [PubMed] [Google Scholar]
- Vakil N., Moayyedi P., Fennerty M.B., Talley N.J. (2006) Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology 131: 390–401, quiz 659–660 [DOI] [PubMed] [Google Scholar]
- Vakil N., Rhew D., Soll A., Ofman J.J. (2000) The cost-effectiveness of diagnostic testing strategies for Helicobacter pylori. Am J Gastroenterol 95: 1691–1698 [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L., Tack J. (2009) Is the antidepressant venlafaxine effective for the treatment of functional dyspepsia? Nat Clin Pract Gastroenterol Hepatol 6: 74–75 [DOI] [PubMed] [Google Scholar]
- Veldhuyzen van Zanten S.J., Bradette M., Chiba N., Armstrong D., Barkun A., Flook N., et al. (2005) Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: an update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Can J Gastroenterol 19: 285–303 [DOI] [PubMed] [Google Scholar]
- Wang W.H., Huang J.Q., Zheng G.F., Xia H.H., Wong W.M., Liu X.G., et al. (2007) Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol 5: 178–85;, quiz 140 [DOI] [PubMed] [Google Scholar]
- Wegener M., Borsch G., Schaffstein J., Reuter C., Leverkus F. (1989) Frequency of idiopathic gastric stasis and intestinal transit disorders in essential dyspepsia. J Clin Gastroenterol 11: 163–168 [DOI] [PubMed] [Google Scholar]
- Zai H., Kusano M. (2009) Investigation of gastric emptying disorders in patients with functional dyspepsia reveals impaired inhibitory gastric emptying regulation in the early postcibal period. Digestion 79(Suppl. 1): 13–18 [DOI] [PubMed] [Google Scholar]