Abstract
A large number of new therapies are in development for chronic hepatitis C including direct-acting antiviral drugs (DAA), which target specific hepatitis C virus enzymes. Two of these compounds have already advanced into phase 3 development in the USA and EU, and many more are in phase 2 trials and likely to advance. In this review, the results of recent studies on ribavirin analogues, nonstructural (NS) 3/4 serine protease inhibitors, NS5B polymerase inhibitors, cyclophilin inhibitors, silimarin components, and thiazolides have been updated. Each compound includes a brief summary of its proposed mechanism of action, results of early clinical trials, and more advanced trial data where available. These compounds are likely to be the first approved in the USA and EU and will initially be used in combination with the current standard of care. It is possible that future treatment paradigms with these agents will offer the potential of interferon-free regimens. It is most likely that patients for these new therapies will be selected carefully by identifying and treating first those who have excellent sustained virologic response rates with 24 weeks of pegylated interferon and ribavirin, the current standard of care. It is also likely that there will be a need to identify those patients who are not likely to have a sustained virologic response with the addition of a protease inhibitor to the current standard of care and delaying their therapy until combination viral suppression therapy becomes an option. The cost and side effects of the DAA will be important considerations for treating physicians. This review is current through 2009; however, data are rapidly changing.
Keywords: direct acting antivirals, chronic hepatitis C, NS3/4 protease inhibitors, NS5B polymerase inhibitors, ribavirin analogues
Introduction
A large number of new therapies are in development for chronic hepatitis C (CHC) including direct-acting antiviral (DAA) drugs, which target specific hepatitis C virus (HCV) enzymes. Two of these compounds have already advanced into phase 3 development in the USA and EU, and many more are in phase 2 trials and likely to advance. The results of recent studies on ribavirin analogues, nonstructural (NS) NS3/4 protease inhibitors, NS5B polymerase inhibitors, cyclophilin inhibitors, silimarin analogues, and thiazolides (see Table 1) have been updated in this review. A brief summary of proposed mechanism of action, results of early clinical trials, and more advanced trial data where available has been included for each compound. The goal of therapy for HCV remains viral eradication rather than viral suppression, a fundamental difference than that of antiviral therapy for chronic hepatitis B and human immunodeficiency virus [Pawlotsky, 2006].
Table 1.
Some direct-acting antivirals in development in 2009.
| Protease inhibitors |
Polymerase-nucleoside / nonnucleoside inhibitors |
|---|---|
| • Telaprevir (Vertex) Boceprevir (Schering Plough/Merck) | • PF00868554 (Pfizer) |
| • TMC435350 (Tibotec) | • Idenix 184/-IDX136/316 |
| • ITMN-191/R7227 (Roche) | • ANA598 (Anadys) |
| • MK7009 (Merck) | • BI 207127 NNPI (BI) |
| • B1201355 (BI) | • MK-3281 NNPI (Merck) |
| • SCH 900518 (Schering) | • ABT-072/333 NNPI (Abbott) |
| • BMS790052 (BMS NS5A) | • GS-9190 NNPI (Gilead) |
| Other novel agents | |
| • IV Silibin – silimarin (milk thistle) | |
| • Debio025, NIM811, SCY635 (cyclophilin inhibitors) | |
| • Nitazoxanide (unknown mode of action) | |
| • Taribavirin (ribavirin analogue) |
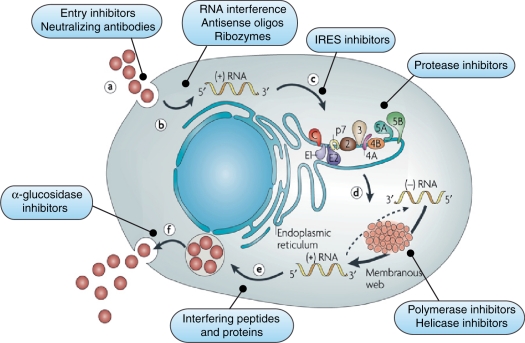
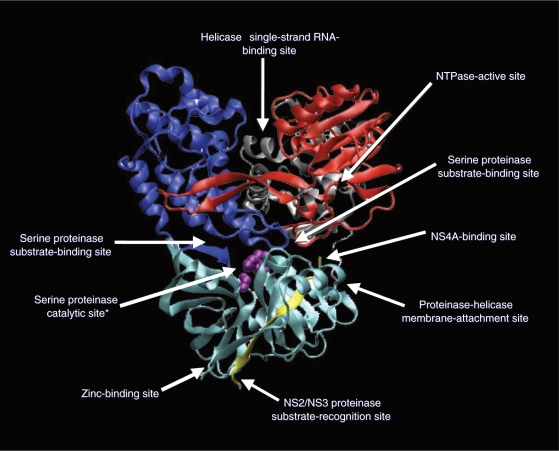
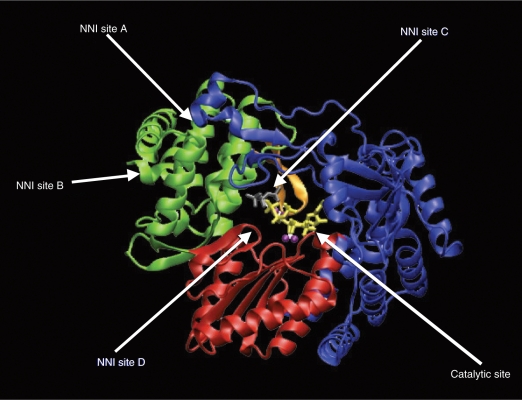
The HCV virus utilizes the NS3 protease and NS5B polymerase enzymes for posttranslational processing and replication. As demonstrated in Figure 1, these key steps in viral replication have become the prime targets for antiviral drug development. The HCV protease enzyme is a serine proteinase with a catalytic site, two substrate-binding sites, an NS4A-binding site and a NS2/NS3 proteinase substrate recognition site, a helicase single-strand RNA-binding site, and a zinc-binding site (see Figure 2). This offers multiple potential avenues to inhibit the enzyme and block HCV RNA posttranslational processing. The NS5B enzyme is an RNA-dependent RNA polymerase (RdRp), which is necessary for viral replication. As shown in Figure 3, the enzyme has a catalytic site for nucleoside binding and at least four other sites to which a nonnucleoside molecule could bind and cause allosteric alteration.
Figure 1.
(a) Receptor binding and cell entry can be blocked by entry inhibitors and neutralizing antibodies. Hepatitis C virus receptor complex contains several components including binding/attachment factors, which concentrate hepatitis C virus on the cell surface (low density lipoprotein receptor). (b) Uncoating and viral RNA release activates endogenous interferon pathways. (c) RNA to endoplasmic reticulum activates innate immunity. The internal ribosome entry site (IRES) acts as the GPS system to direct the virus to the endoplasmic reticulum. Antisense, ribozymes (heptazyme), siRNA, and IRES-eIF3-binding blocker are all potential methods for the inhibition of IRES. (d) Translation and polyprotein processing requires viral NS3/NS4a protease and can be blocked by protease inhibitors. (e) Transcription can be inhibited by translational arrest (protease inhibitors), inhibiting the binding of RNA and polymerase to the replicase complex (cyclophilin inhibitors), NS5A/B inhibition (polymerase inhibitors), and preventing the unwinding of template and progeny plus-strand RNA (helicase inhibitors). (f) Viral assembly and export can be blocked by impairing envelope glycosylation (UT-231B, celgosivir) or NS5A inhibition. Reprinted with permission from Macmillan Publishers Ltd: [Moradpour et al. 2007] copyright 2007.
Figure 2.
The hepatitis C virus protease enzyme is a serine proteinase with a catalytic site, two substrate-binding sites, an NS4A-binding site and NS2/NS3 proteinase substrate-recognition site, a helicase single-strand RNA-binding site, and a zinc-binding site. This offers multiple potential avenues to inhibit the enzyme and block HCV-RNA translation. Reprinted with permission from Elsevier [Pawlotsky et al. 2007].
Figure 3.
The NS5B enzyme is an RNA-dependent RNA polymerase, which is necessary for transcription. The enzyme has a catalytic site for nucleoside binding and at least four other sites to which a nonnucleoside molecule could bind and cause allosteric alteration. Reprinted with permission from Elsevier [Pawlotsky et al. 2007].
Protease inhibitors
Protease inhibition interrupts posttranslational processing by blocking the catalytic site (examples include telaprevir, boceprevir, and ITMN191) or blocking NS3/NS4A interaction (e.g. ACH-806). The first reported human study showing a profound and rapid reduction in viral load using a protease inhibitor was in 2003 [Lamarre et al. 2003]. Further development of this compound was discontinued after demonstration of cardiac toxicity in animal models. Since that time, protease inhibitor development has been rapid and two study medications, telaprevir and boceprevir, have moved to the forefront of novel HCV therapy, both of which are currently in phase 3 clinical trials.
Telaprevir (VX-950) is an oral inhibitor of the HCV protease. The results of two phase 2 clinical trials have been published [Hézode et al. 2009; McHutchison et al. 2009]. PROVE 1 was conducted in the USA in 250 patients with CHC, genotype 1, who were treatment naïve. These patients were randomized to four treatment arms: (a) 12 weeks of telaprevir 750 mg every 8 h plus pegylated interferon alpha-2a and ribavirin followed by 12 weeks of pegylated interferon and ribavirin alone; (b) 12 weeks of telaprevir 750 mg every 8 h plus pegylated interferon alpha-2a and ribavirin followed by pegylated interferon and ribavirin for 36 weeks; (c) 12 weeks of telaprevir 750 mg every h plus pegylated interferon alpha-2a and ribavirin; (d) a control arm with pegylated interferon alpha-2a and ribavirin for 48 weeks. In the telaprevir-treated arms, 81, 81, and 59% of patients in groups 1, 2 and 3, respectively, met criteria for a rapid virologic response (RVR) compared with 11% in the control arm. Sustained virologic response (SVR) rates in the arm receiving telaprevir plus pegylated interferon and ribavirin were 61, 67, and 35% for groups 1, 2, and 3, respectively, compared with 41% in the control arm. Adverse events leading to discontinuation of medication were more frequent in the telaprevir groups with a rash being the most frequent side effect [McHutchison et al. 2009].
PROVE 2 was a phase 2 trial with a different study design than PROVE 1. It was conducted in Europe with 323 patients with CHC, genotype 1, who were treatment naïve. This study had four treatment arms: (a) telaprevir 750 mg every 8 h with pegylated interferon and ribavirin for 12 weeks followed by pegylated interferon and ribavirin alone for 12 weeks; (b) telaprevir 750 mg every 8 h and pegylated interferon with ribavirin for 12 weeks; (c) telaprevir 750 mg every 8 h and pegylated interferon for 12 weeks; (d) a control arm receiving 48 weeks of pegylated interferon alpha-2a and ribavirin. The RVR rates for the four treatment arms were 69, 80, 50, and 13, respectively. The SVR rates were 69, 60, 36, and 46% with the first two telaprevir arms being significantly better than the control arm. A rash was the most frequently encountered adverse event in patients receiving 12 weeks of telaprevir and occurred in approximately 50% of patients resulting in premature discontinuation of therapy in 3–7% [Hézode et al. 2009]. This study demonstrated the importance of including ribavirin in the treatment regimen.
A third important phase 2b study, PROVE 3, had interim analysis results presented at the 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD 2008). This study is evaluating telaprevir with pegylated interferon alpha-2a and ribavirin in CHC, genotype 1 patients who are null or partial responders. This multicenter study involves 453 patients. It has four treatment arms: (a) a control arm receiving 48 weeks of pegylated interferon and ribavirin; (b) telaprevir 750 mg every 8 h with pegylated interferon and ribavirin for 12 weeks followed by 12 weeks of pegylated interferon and ribavirin; (c) telaprevir 750 mg every 8 h with pegylated interferon and ribavirin for 24 weeks followed by pegylated interferon and ribavirin for 24 weeks; (d) telaprevir 750 mg every 8 h with pegylated interferon for 24 weeks. SVR was attained in the control group in only 14% of patients while in the arms receiving telaprevir with pegylated interferon and ribavirin for 12 or 24 weeks followed by pegylated interferon and ribavirin alone for 12 or 36 weeks, SVR was achieved in 51–52% of patients [Manns et al. 2009a]. SVR was achieved in 69–76% of prior relapsers and 38–39% of prior nonresponders in this study, suggesting that there is an opportunity for the development of telapavir resistance in 60% of nonresponders if they are treated for an excessive period of time.
The results of another ongoing open-label, randomized phase 2 study of telaprevir administered every 8 h or every 12 h in combination with pegylated interferon alpha-2a or pegylated interferon alpha-2b and ribavirin in treatment-naïve genotype 1 HCV were presented at the 2008 AASLD meeting [Forns et al. 2008]. In this trial, 161 subjects were randomized in four arms: (a) pegylated interferon alpha-2a (180 µg/week), telaprevir 750 mg every 8 h, ribavirin 1000–1200 mg/day (n = 40); (b) pegylated interferon alpha-2b (1.5 µg/kg/week), telaprevir 750 mg every 8 h, ribavirin 800–1200 mg/day (n = 42); (c) pegylated interferon alpha-2a (180 µg/week), telaprevir 1125 mg every 12 h, ribavirin 1000–1200 mg/day (n = 40); (d) pegylated interferon alpha-2b (1.5 µg/kg/week), telaprevir 1125 mg every 12 h, ribavirin 800–1200 mg/day (n = 39). All subjects received telaprevir with pegylated interferon and ribavirin for 12 weeks, and received either 12 or 36 weeks additional pegylated interferon and ribavirin based on RVR. Week 4 HCV RNA clearance rates were similar in the pegylated interferon alpha-2a arms regardless of the telaprevir dosing regimen (a = 82%, c = 85%). A similar finding was seen with pegylated interferon alpha-2b (b = 71% and d = 68%). The differences between the pegylated interferon alpha-2a and pegylated interferon alpha-2b arms did not reach statistical significance due to the small numbers in each group. The final SVR rates were presented at the 2009 AASLD meeting and were roughly equal in all four groups (a: 85%; b: 81%; c: 83%; d: 82%) suggesting that the every 12 h dosing regimen will be equivalent to an every 8 h regimen [Marcellin et al. 2009].
An early study carried out with monotherapy of telaprevir demonstrated the rapid emergence of telaprevir-resistant variants within 14 days [Sarrazin et al. 2007]. Long-term follow up over 3–7 months postdosing showed re-emergence of the wild-type virus, however some telaprevir-resistant variants persisted as dominant species. For this reason, subsequent studies with telaprevir and all other DAAs studied in the USA have been limited to 3 days of monotherapy. The combination of pegylated interferon and ribavirin with telaprevir prevents the emergence of drug-resistant variants and has thus become the standard regimen for drug development of all DAAs [Kieffer et al. 2007].
Boceprevir (SCH-503034) is the second oral protease inhibitor that is furthest along in development. The results of an interim analysis of a phase 2 trial were presented at AASLD 2008. SPRINT 1 is a study involving 595 patients with CHC, genotype 1, who are treatment naïve. This study evaluated boceprevir 800 mg three times a day in three treatment regimens of pegylated interferon alpha-2b and ribavirin versus a control arm of standard of care (SOC) with pegylated interferon alpha-2b and ribavirin. SVR-12 rates of 38% were reported in the control arm compared with 74% in an arm that had a 4-week lead-in phase of pegylated interferon and ribavirin followed by 44 weeks of boceprevir with pegylated interferon and ribavirin. These results suggest that a 48-week regimen may be more effective when using this drug. Adverse events have been similar across all treatment arms with the most common being fatigue, nausea, and headache [Kwo et al. 2008]. Anemia was more common in the boceprevir-treated arms suggesting the drug causes bone marrow suppression or potentiates ribavirin-associated hemolysis. SVR-24 results from the SPRINT 1 trials were reported at AASLD 2009 and showed that patients who achieved an RVR had high SVR rates (82 versus 94%, p = NS), whether treated for 24 or 44 weeks of bocepravir with pegylated interferon alpha-2b and ribavirin, whereas those who did not achieve an RVR clearly needed the longer duration therapy (21 versus 79%, respectively, p < 0.004) [Kwo et al. 2009].
TMC435350 is a novel and potent reversible NS3/4A protease inhibitor (noncovalent), which has demonstrated synergy with interferon-alpha and polymerase inhibitors in vitro and is additive with ribavirin. Data indicating that the drug achieved a rapid decline in HCV viral load was observed in HCV patients treated with monotherapy (TMC43550 200 mg daily) in genotype 1 nonresponders/relapsers [Manns et al. 2008]. As TMC435350 is dosed once daily, it has a distinctive advantage as telaprevir and boceprevir require every 8 h dosing schedules. A recent interim analysis of an ongoing combination trial showed the drug in combination with pegylated interferon and ribavirin was very effective over 4 weeks of treatment at 75 and 200 mg doses (all patients achieved HCV-RNA levels <25 IU/ml) [Manns et al. 2009b]. In addition, TMC43550 in combination with SOC for 28 days generally was safe and well tolerated.
At the 2009 AASLD meeting results of phase 1b or phase 2 trials of four other protease inhibitors were shown to be effective and appear safe in early stage development: narlaprevir (Shering-Plough), BI201335 (Boehringer Ingelheim), ITMN-191 (Intermune/Roche), and MK-7009 (Merck) [Forestier et al. 2008; Manns et al. 2009c; Sulkowski et al. 2009; Vierling et al. 2009]. A number of other protease inhibitors in development at this time will not be reviewed here.
To summarize, protease inhibitors are extremely potent inhibitors of HCV RNA, they may enhance host innate immunity, may improve sensitivity to interferons, and are oral drugs. Despite these advantages, they may be genotype specific to a degree, result in the rapid emergence of resistance when used as monotherapies, all have some side effects, and will likely be expensive.
Polymerase inhibitors
The NS5B RdRp enzyme is a highly conserved structure across all hepatitis C genotypes. It is, therefore, an ideal target for drug therapy. There are two classes of polymerase inhibitors: nucleoside/nucleotide analogues and nonnucleoside RdRp inhibitors. Nucleoside inhibitors target the catalytic sites of the enzyme and act as chain terminators (e.g. NM283, R1626, and R7128). This mode of action makes variants less fit and unable to replicate, thus providing a higher barrier to antiviral resistance. Nonnucleoside inhibitors are allosteric inhibitors (e.g. HCV-796, ABT-072/333, and GS-9190) making them more susceptible to developing resistance.
R1626 was the NS5B RNA polymerase inhibitor furthest along in development prior to being discontinued due to unexpected drug toxicities. In a phase 2a study, 104 patients with CHC, genotype 1 who were treatment naïve were randomized to four arms in combination with pegylated interferon or pegylated interferon and ribavirin. This was the first study to demonstrate a potent antiviral effect with a polymerase inhibitor, attaining RVR rates >80% in combination with pegylated interferon and ribavirin. The end-of-treatment response rates were 52, 66, 81, and 60%, respectively. The corresponding SVR rates were 24, 53, 58, and 50% [Pockros et al. 2008b]. A large phase 2b study has revealed two serious toxicities: severe lymphopenia resulting in the death of one patient and visual impairment, which affected eight patients. These toxicities have resulted in the discontinuation of R1626 for CHC treatment [Pockros et al. 2008c].
R7128 is a polymerase inhibitor, which is a prodrug of PSI-6130, an oral cytidine nucleoside analogue [Reddy et al. 2007]. The interim analysis results of an ongoing phase 2a clinical trial were presented at the 43rd Annual Meeting of the European Association for the Study of the Liver in 2008 (EASL 2008). In this study, patients with CHC who were genotype 1 were randomized to R7128 500 mg twice a day, 1500 mg twice a day, or placebo for 4 weeks. All patients received pegylated interferon alpha-2a and ribavirin during the 4-week lead-in phase followed by open-label SOC. Eighty-five percent of patients achieved an RVR in the 1500 mg twice a day arm of R7128 compared with 10% in the placebo plus SOC arm. Safety and tolerability during the 4-week period were similar between placebo and R7128 arms [Lalezari et al. 2008]. The follow-on data of this trial were presented at the AASLD 2008 meeting [Rodriguez-Torres et al. 2008] as well as the data for two new polymerase inhibitors in earlier stages of development [Hammond et al. 2008; Steffy et al. 2008].
Recently a trial of an NS3 protease inhibitor (R7227/ITMN-191) and NS5B polymerase inhibitor (R7128) given together to genotype 1 treatment-naïve patients without pegylated interferon and ribavirin was presented at the EASL 2009 meeting (INFORM-1 trial) [Gane et al. 2009a]. Over a 14-day treatment duration, the orally administered combination of R7128 and R7227 demonstrated significant antiviral potency, sustained viral suppression, and acceptable safety and tolerability. All patients were subsequently placed on pegylated interferon and ribavirin therapy for 48 weeks. Higher doses and twice-daily regimens are being evaluated in additional INFORM-1 cohorts currently. These results demonstrate for the first time that two DAAs can be safely combined in HCV patients and may represent a future treatment regimen either with or without pegylated interferon plus ribavirin. Furthermore, this study demonstrated that prior nonresponders and even null-responders can have a dramatic reduction in HCV RNA over 14 days (–4.0 and –4.9 log IU/ml, respectively), when treated with a noninterferon antiviral regimen [Gane et al. 2009b].
HCV-796 is an orally bioavailable compound, which is a noncompetitive allosteric inhibitor (nonnucleoside) of the HCV NS5B RdRp and achieves NS5B inhibition by binding to one of at least five allosteric enzyme sites resulting in conformational changes in the protein. This compound was the first to be studied in combination with pegylated interferon and ribavirin in genotype 1, treatment-naïve individuals [Pockros et al. 2009]. Although the drug demonstrated antiviral efficacy with increased RVR rates in the triple combination-treated arm, there was significant hepatotoxicity, which was seen in three patients causing the trial to stop. There are at least four other nonnucleoside polymerase inhibitors currently in development, which remain promising at the time of writing: GS 9190 (Gilead), PF-868554 (Filibuvir; Pfizer), ANA-598 (Anadys), and VHC-916 (ViroPharma). None of these have suffered from adverse events to cause early discontinuation of development and all have demonstrated efficacy through to at least week 4 thus far [Jacobson et al. 2009; Lawitz et al. 2008a; Thompson et al. 2008a].
Ribavirin analogues
Conventional treatment of CHC involves pegylated interferon alpha in combination with weight-based ribavirin. The most common significant adverse effect of ribavirin is hemolytic anemia. Taribavirin is a prodrug of ribavirin that is preferentially liver targeted. Once inside the hepatocyte, it is converted to ribavirin by adenosine deaminase. The results of a large, phase 3 clinical trial have been published. VISER 1 compared taribavirin plus pegylated interferon alpha-2b versus ribavirin plus pegylated interferon alpha-2b in patients with CHC, all genotypes. Overall, SVR rates were lower in those receiving taribavirin (38%) versus those receiving ribavirin (52%). Taribavirin did not meet the noninferiority to ribavirin efficacy endpoint on intent-to-treat basis. It did demonstrate a superior safety profile with anemia rates (Hb < 100 g/L) being significantly less (5 versus 24%) [Benhamou et al. 2009]. VISER 2 compared taribavirin plus pegylated interferon alpha-2a versus ribavirin plus pegylated interferon alpha-2a and produced similar results as VISER 1 with SVR rates of 40% in taribavirin-treated patients and 55% in ribavirin-treated patients. Again, anemia rates were significantly less with taribavirin versus ribavirin (6 versus 22%) [Marcellin et al. 2007]. A post-hoc subgroup analysis suggested improved SVR rates with higher weight-based dosing of taribavirin [Pockros et al. 2008a].
This data was presented as a poster at the 2008 AASLD meeting and showed that SVR rates would be equivalent to those with ribavirin if taribavirin was used at 20 mg/kg/day, but anemia rates would be less than half that of ribavirin. Therefore, a phase 2b study was initiated in treatment naïve patients with CHC, genotype 1, comparing taribavirin at doses of 20, 25, or 30 mg/kg/day versus weight-based dosing of ribavirin. All patients received pegylated interferon alpha-2b. Treatment week 24 results of this study were presented at AASLD 2008 [Lawitz et al. 2008b]. RVR rates for the three arms of taribavirin were 16.4, 14.3, and 16.2% versus 11.4% for ribavirin. Undetectable viral RNA rates at week 12 were 41.8, 41.4 and 25% versus 31.4% for ribavirin. Rates of anemia (Hb < 100 g/L) were significantly less in the taribavirin arms with rates of 13.4, 11.4, and 19.1% versus 30% for the ribavirin arm. The most common adverse events fatigue, headache and nausea were similar across all study arms with the exception of diarrhea, which occurs more frequently with taribavirin [Lawitz et al. 2008b]. The final results of this trial confirmed the SVR rates in the taribavirin arms were identical to ribavirin (28, 27, 28%, and 27%, respectively), but the rate of anemia in the 20–25 mg/kg taribavirin arms was half that of the ribavirin arm (13 and 16% taribavirin 20 mg/kg and 25 mg/kg versus 33% for ribavirin; p < 0.05) [Poordad et al. 2009].
Other direct-acting antiviral drugs
Debio 025 is a synthetic, nonimmunosuppressive cyclosporine analogue that inhibits viral replication through binding and inhibition of the host cell protein, cyclophilin A. The results of a phase 2a study have been published. This was a double-blind, placebo-controlled trial of 90 patients with CHC, genotypes 1 and 4. Patients were randomized to five treatment arms: (a) pegylated interferon alpha-2a plus placebo; (b) pegylated interferon plus Debio 025 200 mg/day; (c) pegylated interferon plus Debio 600 mg/day; (d) pegylated interferon plus Debio 025 1000 mg/day; (d) Debio 1000 mg/day alone. At day 29, viral RNA levels had decreased by –4.6 log10 IU/ml in the pegylated interferon/Debio 025 600 mg/day arm and by –4.8 log10 IU/ml in the pegylated interferon/Debio 025 1000 mg/day arm. This was significant compared with the other three treatment arms. In fact, 60% of patients in the pegylated interferon/Debio 1000 mg arm had undetectable viral levels at 29 days [Flisiak et al. 2008].
In a second phase 2 study in genotype 1 null-responders, DEBIO 025 at the 400 mg and 800 mg doses was well tolerated in combination with pegylated interferon and ribavirin but associated with minor and reversible hyperbilirubinemia [Nelson et al. 2009]. At 400 mg daily the drug showed no antiviral activity in previous null responders, but after a very slow build-up of plasma levels without a loading dose, DEBIO 025 in combination with pegylated interferon and ribavirin can produce significant antiviral activity in previous null responders. The data suggest a loading dose of DEBIO 025 would enhance the pharmacokinetic profile.
Data from a phase 1b trial of a second nonimmunosuppressive analogue of cyclosporine, SCY-635, were presented at EASL 2009 [Hopkins et al. 2009]. This compound was able to suppress HCV RNA titers by approximately 2 logs when given to genotype 1 patients as monotherapy for 2 weeks. Combination trials with pegylated interferon and ribavirin are planned.
BMS-790052 is a first-in-class potent HCV NS5A inhibitor for patients with chronic HCV infection. The results from a proof-of-concept study using this compound as a single dose monotherapy in HCV-infected patients was presented at the meetings this year [Nettles et al. 2008]. The drug was safe and well tolerated in single doses up to 100 mg, has a pharmacokinetic profile that potentially supports once-daily dosing and produced a robust decline in HCV RNA (–3.6 logs after 48 h from a single 100 mg). Furthermore, the drug is additive/synergistic with protease and polymerase inhibitors in the replicon model, suggesting it is a new class of enzyme inhibitor, which may eventually be used in combination with protease and/or polymerase inhibitors.
Other novel compounds
Nitazoxanide is a thiazolide approved in the USA for the treatment of diarrhea caused by Cryptosporidium and Giardia. The drug appeared to have an antiviral effect on HCV patients who were treated for parasitic disease, although the mechanism of action was poorly understood. The results of a phase 2 study, STEALTH C-1, were presented at EASL 2008. In this randomized, controlled trial of 96 treatment-naïve and 24 previously treated patients with CHC genotype 4, 28 patients received nitazoxanide 500 mg twice a day as a 12-week lead-in phase followed by 36 weeks of nitazoxanide plus pegylated interferon alpha-2a and ribavirin and achieved an SVR of 79% compared with the SOC arm of 40 patients who received pegylated interferon and ribavirin for 48 weeks and achieved an SVR of 50%. Interestingly, 28 patients received a 12-week lead-in of nitazoxanide followed by 36 weeks of nitazoxanide and pegylated interferon (without ribavirin) and achieved an SVR of 61%, which was better than the SOC arm [Rossignol et al. 2008b]. The results of a second study evaluating a 4-week lead-in phase of nitazoxanide were presented at AASLD 2008. In this study, 44 treatment-naïve patients with CHC (40 genotype 4, 3 genotype 3, and 1 genotype 1) were treated with nitazoxanide 500 mg twice a day for 4 weeks followed by pegylated interferon alpha-2a and nitazoxanide for 36 weeks. An SVR was achieved in 80% of patients. Unfortunately, there was no active control group in this trial [Rossignol et al. 2008a]. Two other studies are ongoing and are evaluating nitazoxanide in patients with CHC genotype 1 who are treatment naïve as well as prior nonresponders [US National Institutes of Health, 2008]: STEALTH C-2 to investigate the role of nitazoxanide (plus pegylated interferon and ribavirin) in 64 genotype 1 prior nonresponders compared with pegylated interferon/ribavirin therapy, and STEALTH C-3 to investigate in 112 HCV genotype 1 treatment-naïve patients. An interim analysis of the treatment-naïve genotype 1 trial has shown no effect of nitazoxanide on RVR but possibly better complete early virologic response (cEVR) and EVR rates in the nitazoxanide arm compared with pegylated interferon/ribavirin therapy (cEVR: 60 versus 49%, EVR: 80 versus 68%, respectively; no p values were reported) [Bacon et al. 2009].
Silibinin is a component of silimarin, which inhibits HCV in replicons, but appears to be ineffective in man when given orally. Intravenous silibinin was given to nonresponders with hepatitis C in a pilot trial combined with SOC. In this study the investigators gave silibinin 5, 10, 15, or 20 mg/kg intravenous on days 1–14, followed by silimarin 280 mg p.o. three times a day. pegylated interferon alpha-2a plus ribavirin 1–1.2 g/d was started on day 7 [Ferenci et al. 2008]. No additional adverse events were seen in the patients receiving silibinin. The remarkable antiviral effects seen in this difficult-to-treat population are quite surprising with four out of nine patients receiving the 20 mg/kg dose HCV RNA undetectable after 14 days therapy. The data are compelling enough to pursue this approach further.
Treatment guidelines
Owing to the side-effect cost of DAAs and the risk of development of drug-resistant viral strains, it will be necessary to guide treating physicians through a growing maze of confounding factors. These will likely not only include new drugs but also important predictive tests and relevant mutation analysis. One such predictive test is the IL28B genetic polymorphism, which has recently been reported to be associated with SVR by three separate groups [Ge et al. 2009; Suppiah et al. 2009; Tanaka et al. 2009]. The IL28B genotype will become a commercially available polymerase chain reaction assay in 2010 and may be helpful for decisions using SOC treatment. It is unknown if the C/C, C/T, and T/T alleles of IL28B will be associated with response rates to DAAs and this information will need to be carefully collected in ongoing trials.
In Tables 2 and 3, a simple classification system is proposed that might permit physicians to solidify decisions regarding therapy. In this proposed system, Class I patients would be those with a very high chance of SVR with SOC therapy for 24 weeks, that is, treatment naïve, interferon-tolerant patients with genotype 2/3, or genotype 1 infection with low viral loads or the C/C IL28B allele [Thompson et al. 2009]. As these patients would have an 80–90% probability of cure without the risk of additional side effects or the additional cost of DAAs, they might be well served to be treated with current SOC alone. These patients represent approximately 20–25% of the US population with HCV [Ghany et al. 2009].
Table 2.
A proposed classification system for the treatment of hepatitis C virus in the direct-acting antiviral era.
| Class I: treatment naïve, IT, G2/3, G1 low viral load, and/or CC allele (20–25% of US patients) |
| Class II: treatment naïve, IT, G1 high viral load, or CT/TT allele (40–50% of US patients) |
| Class III: G1/4 and G2/3 relapsers and nonresponders to current standard of care who become RNA undetectable by week 12 of treatment (20% of US patients) |
| Class IV: Class IIIs who do not become undetectable by week 12, interferon-intolerant patients and all treatment failures from treated Classes I–III (30% US patients assuming 5% from Classes I and III, and 10% from Class II) |
CC, IL28B allele genotype C/C; CT, IL28B allele genotype C/T; IT, interferon tolerant; TT, IL28B allele genotype T/T.
Table 3.
Likely treatment durations, sustained virologic response rates, and expected costs.
| Class I |
Class II |
Class III |
Class IV |
|---|---|---|---|
| SOC × 24 weeks | PI + SOC × 24 weeks | PI + SOC × 48 weeks | PI + NS5B pol + other agents |
| SVR = 80–90% | SVR = 80% | SVR = 70–75% | SVR = nil (lifetime suppression) |
| Cost = US$25,000 (AWP) | Cost < US$50,000* | Cost > US$50,000 but <US$100,000* | Cost > US$100,000* |
Costs assume US$25,000/year for standard of care, likely use of growth factors for extended duration therapy, and costs of direct-acting antivirals <US$25,000/regimen.
AWP, average wholesale cost; NS5B pol, NS5B HCV polymerase inhibitor; PI, NS3/4 hepatitis C virus protease inhibitor; SOC, standard of care; SVR, sustained virologic response.
Class II patients might be those who are treatment naïve, interferon-tolerant with genotype 1 who have high viral loads and/or have C/T or T/T IL28B alleles. These patients would be less likely to have an SVR with current SOC but would have approximately a 75–80% chance of an SVR with the addition of an NS3/4 protease inhibitor followed by consolidation therapy for a total of 24 weeks (if an RVR is attained). The added cost of DAA therapy and the risk of side effects would need to be considered in these patients, who represent the majority of the US HCV population (40–50%) [Ghany et al. 2009]. Class III patients would be those requiring 48 weeks of total therapy in order to attain an SVR with SOC and protease inhibitor therapy, such as relapsers and nonresponders of all genotypes, and those in Class I who do not attain an RVR. It would be important to establish a limit on exposure to protease inhibitor therapy in this group as many who are interferon refractory would not clear virus permitting the emergence of resistance. Although this period will require refinement in the future it appears to be 12 weeks based on the data we have presented herein. These patients are prevalent in practices but actually do not represent a majority of the infected population, certainly no more than 20%. It would be anticipated that half of these patients would not attain an SVR based on the data seen in treatment-failure patients.
Class IV patients would include Class IIIs who do not become undetectable by week 12, interferon-intolerant patients and all treatment failures from treated Classes I and II. This could be up to 30% of the US population infected with HCV based on the SVR rates proposed above. These patients would probably be best served by waiting for the availability of combination therapy with an NS3/4 protease and either an NS5B polymerase, an NS5A inhibitor, a cyclophilin inhibitor, or another interferon-free combination regimen. The goal of therapy in these patients would be viral eradication but this is not likely to be an attainable outcome for most. Instead, viral suppression with cessation of histological injury will likely be the new goal in these patients. As such, the risks for resistance and the cost of long-term therapies will need to be major considerations in this population.
Conclusions
In conclusion, multiple promising new drugs are currently in development for the treatment of CHC. The current SOC medications, pegylated interferon and ribavirin, have uncertain mechanisms of action but appear to enhance the host’s immune response to the virus. These two medications are likely to be used in combination regimens for the foreseeable future. Understanding the HCV life cycle has led to a number of options for novel treatment. Two of these compounds, telaprevir and boceprevir, have entered phase 3 trials. The earliest date that these drugs would be approved by the US Food and Drug Administration is 2011. We are hopeful that numerous other compounds will prove effective and become available over the ensuing 5–10 years. The ultimate goal of therapy will remain improving efficacy and patient tolerability through lessening side effects and shortening duration of treatment.
Conflict of interest statement
Research or unrestricted CME grants from Roche, Gilead, BMS, Tibotec, Vertex, Conatus, HGS, Pfizer, Wyeth, Globeimmune, Debio, and Sciclone. Consulting or speaking honorariums from Roche, Gilead, BMS, Tibotec, Vertex, Conatus, Novartis, Three Rivers, Merck and Pharmacet.
References
- Bacon B.R., Shiffman M.L., Lim J.K., Berman A., Rustgi V.K., Keeffe E.B., et al. (2009) Phase 2 randomized, double-blind, placebo-controlled study of nitazoxanide plus peginterferon and ribavirin in HCV genotype 1 naïve patients: interim analysis shows increase in EVR. J Hepatol 50(Suppl 1): S381–S381 [Google Scholar]
- Benhamou Y., Afdhal N.H., Nelson D.R., Shiffman M.L., Halliman D.G., Heise J., et al. (2009) A phase III study of the safety and efficacy of viramidine vs ribavirin in treatment-naїve patients with chronic hepatitis C: VISER 1 results. Hepatology 50: 717–726 [DOI] [PubMed] [Google Scholar]
- Ferenci P., Scherzer T.-M., Kerchner H., Rutter K., Beinhardt S., Hofer H., et al. (2008) Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology 135: 1561–1567 [DOI] [PubMed] [Google Scholar]
- Flisiak R., Horban A., Gallay P., Bobardt M., Selvarajah S., Wiercinska-Drapalo A., et al. (2008) The cyclophilin inhibitor Debio 025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology 47: 817–826 [DOI] [PubMed] [Google Scholar]
- Forestier N., Larrey D., Marcellin P., Benhamou Y., Guyader D., Bradford W., et al. (2008) Antiviral activity and safety of ITMN-191 in combination with peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C virus (HCV). J Hepatol 48(Suppl 2): S35–S35 [Google Scholar]
- Forns X., Marcellin P., Goeser T., Feremco P., Nevens F., Carosi G., et al. (2008) Phase 2 study of telaprevir administered Q8H or Q12H with peginterferon-alfa-2A or -alfa-2B and ribavirin in treatment-naïve subjects with genotype 1 hepatitic C: week 4 interim results. Hepatology 48(Suppl): 1136A–1136A [Google Scholar]
- Gane E.J., Roberts S.K., Stedman C., Angus P.W., Ritchie B., Elston E., et al. (2009a) Potent antiviral activity with a nucleoside polymerase (R7128) and protease (R7227/ITMN-191) inhibitor combination in HCV genotype 1: initial safety, pharmacokinetics, and virologic results from INFORM-1. J Hepatol 50(50Suppl): S380–S380 [Google Scholar]
- Gane E.J., Roberts S.K., Stedman C.A., Angus P.W., Ritchie B., Elston E., et al. (2009b) Combination therapy with a nucleoside polymerase (R7128) and protease (R7227/ITMN-191) inhibitor in HCV: safety, pharmacokinetics, and virologic results from INFORM-1. Hepatology 50(Suppl): 394A–395A [Google Scholar]
- Ge D., Fellay J., Thompson A.J., Simon J.S., Shianna K.V., Urban T.J., et al. (2009) Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461: 399–401 [DOI] [PubMed] [Google Scholar]
- Ghany M.G., Strader D.B., Thomas D.L., Seeff L.B. (2009) Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49: 1335–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J.L., Purohit V.S., Fang J., DeBruin M.F. (2008) Safety, tolerability and pharmacokinetics of the HCV polymerase inhibitor PF-00868554 following multiple dose administration in healthy volunteers. Hepatology 48(Suppl): 1159A–1159A [Google Scholar]
- Hézode C., Forestier N., Dusheiko G., Ferenci P., Pol S., Goeser T., et al. (2009) Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 360: 1839–1850 [DOI] [PubMed] [Google Scholar]
- Hopkins S., Heuman D., Gavis E., Lalezari J., Glutzer E., DiMassimo B., et al. (2009) Safety, plasma pharmacokinetics and anti-viral activity of SCY-635 in adult patients with chronic hepatitis C virus infection. J Hepatol 50(Suppl 1): S36–S36 [Google Scholar]
- Jacobson I., Pockros P., Lalezari J., Lawitz E., Rodriguez-Torres M., DeJesus E., et al. (2009) Antiviral activity of filibuvir in combination with pegylated interferon alfa-2a and ribavirin for 28 days in treatment naïve patients chronically infected with HCV genotype 1. J Hepatol 50(Suppl 1): S382–S382 [Google Scholar]
- Kieffer T.L., Sarrazin C., Miller J.S., Welker M.W., Forestier N., Reesink H.W., et al. (2007) Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology 46: 631–639 [DOI] [PubMed] [Google Scholar]
- Kwo P., Lawitz E.J., McCone J., Schiff E.R., Vierling J.M., Pound D., et al. (2008) HCV SPRINT-1: boceprevir plus peginterferon alfa-2b/ribavirin for treatment of genotype 1 chronic hepatitis C in previously untreated patients. Hepatology 48(Suppl): 1027A–1027A18821594 [Google Scholar]
- Kwo P.Y., Lawitz E., McCone J., Schiff E.R., Vierling J.M., Pound D., et al. (2009) Response-guided therapy (RGT) for bocepravir (BOC) combination treatment-results from HCV SPRINT-1. Hepatology 50(Suppl): 1035A–1035A [Google Scholar]
- Lalezari J., Gane E., Rodriguez-Torres M., De Jesus E., Nelson D., Everson G., et al. (2008) Potent antiviral activity of the HCV nucleoside polymerase inhibitor R7128 with PEG IFN and ribavirin: interim results of R7128 500 mg BID for 28 days. J Hepatol 48(Suppl 2): S29–S29 [Google Scholar]
- Lamarre D., Anderson P.C., Bailey M., Beaulieu P., Bolger G., Bonneau P., et al. (2003) An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426: 186–189 [DOI] [PubMed] [Google Scholar]
- Lawitz E., Cooper C., Rodriguez-Torres M., Ghalib R., Lalonde R., Sheikh A., et al. (2008a) Safety, tolerability and antiviral activity of VCH-916, a novel non-nucleoside polymerase inhibitor in patients with chronic HCV genotype-1 infection. J Hepatol 48(Suppl 2): S37–S37 [Google Scholar]
- Lawitz E., Muir A., Poordad F., Chun E., Hammond J. (2008b) Treatment week 24 results of weight-based taribavirin versus weight-based ribavirin, both with peginterferon alfa-2b, in naïve chronic hepatitis C, genotype 1 patients. Hepatology 48(Suppl): 433A–433A [DOI] [PubMed] [Google Scholar]
- Manns M., Muir A., Adda N., Jacobson I., Afdhal N., Heathcote J., et al. (2009a) Telaprevir in hepatitis C genotype-1-infected patients with prior non-response, viral breakthrough or relapse to peginterferon-alfa-2A/B and ribavirin therapy: SVR results of the PROVE 3 study. J Hepatol 50(Suppl): S379–S379 [Google Scholar]
- Manns M., Reesink H., Moreno C., Berg T., Benhamou Y., Horsmans Y., et al. (2009b) OPERA-1 trial: interim analysis of safety and antiviral activity of TMC435 in treatment-naïve genotype 1 HCV patients. J Hepatol 50(Suppl 1): S7–S7 [Google Scholar]
- Manns M.P., Gane E., Rodriguez-Torres M., Stoehr A., Yeh C.-T., Wiedmann R., et al. (2009c) MK-7009 significantly improves rapid viral response (RVR) in combination with pegylated interferon alfa-2a and ribavirin in patients with chronic hepatitis C (CHC) genotype 1 infection. J Hepatol 50(Suppl 1): S384–S384 [Google Scholar]
- Manns M.P., Reesink H.W., Moreno C., Berg T., Behamou Y., Horsmans Y.J., et al. (2008) Safety and antiviral activity of TMC435350 in treatment-naïve genotype 1 HCV-infected patients. Hepatology 48(Suppl): LB8–LB8 [Google Scholar]
- Marcellin P., Forns X., Goeser T., Ferenci P., Nevens F., Giampiero C., et al. (2009) Virologic analysis of patients receiving telapravir administered Q8H or Q12H with peginterferon-alfa-2a or –alfa-2b and ribavirin in treatment-naïve patients with genotype 1 hepatitis C: study C208. Hepatology 50(Suppl): 395A–395A [Google Scholar]
- Marcellin P., Lurie Y., Rodrigues-Torres M., Chasen R., Xu Y., Murphy B. (2007) The safety and efficacy of taribavirin plus pegylated interferon alfa 2a versus ribavirin plus pegylated interferon alfa 2a in therapy-naïve patients infected with HCV: phase 3 results. J Hepatol 46: 7A–7A [Google Scholar]
- McHutchison J.G., Everson G.T., Gordon S.C., Jacobson I.M., Sulkowski M., Kauffman R., et al. (2009) Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 360: 1827–1838 [DOI] [PubMed] [Google Scholar]
- Moradpour D., Penin F., Rice C.M. (2007) Replication of hepatitis C virus. Nat Rev Microbiol 5: 453–463 [DOI] [PubMed] [Google Scholar]
- Nelson D.R., Ghalib R.H., Sulkowski M., Schiff E., Rustigi V., Pockros P.J., et al. (2009) Efficacy and safety of the cyclophilin inhibitor DEBIO 025 in combination with pegylated interferon alpha-2a and ribavirin in previously null-responder genotype 1 HCV patients. J Hepatol 50(Suppl 1): S40–S40 [Google Scholar]
- Nettles R., Chien C., Chung E., Persson A., Gao M., Belema M., et al. (2008) BMS-790052 is a first-in-class potent hepatitis C virus (HCV) NS5A inhibitor for patients with chronic HCV infection: Results from a proof-of-concept study. Hepatology 48(Suppl): LB12–LB12 [Google Scholar]
- Pawlotsky J.M. (2006) Virology of hepatitis B and C viruses and antiviral targets. J Hepatol 44: S10–S13 [DOI] [PubMed] [Google Scholar]
- Pawlotsky J.M., Chevaliez S., McHutchison J.G. (2007) The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology 132: 1979–1998 [DOI] [PubMed] [Google Scholar]
- Pockros P.J., Jacobson I.M., Bacon B.R., Afdhal N.H., Poordad F., Chun E., et al. (2008a) Taribavirin exposure analysis from a previous phase 3 trial correlates with phase 2B weight based dosing interim results. Hepatology 48(Suppl): 1138A–1138A [Google Scholar]
- Pockros P.J., Nelson D., Godofsky E., Rodriguez-Torres M., Everson G.T., Fried M.W., et al. (2008b) R1626 plus peginterferon alfa-2a provides potent suppression of hepatitis C RNA and significant anti-viral synergy in combination with ribavirin. Hepatology 48: 385–397 [DOI] [PubMed] [Google Scholar]
- Pockros P.J., Nelson D., Godofsky E., Rodriguez-Torres M., Everson G.T., Fried M.W., et al. (2008c) High relapse rate seen at week 72 for patients treated with R1626 combination therapy. Hepatology 48: 1349–1350 [DOI] [PubMed] [Google Scholar]
- Pockros P., Rodriguez-Torres M., Villano S., Maller E., Chojkier M. (2009) A phase 2, randomized study of HCV-796 in combination with pegylated-interferon (PEG) plus ribavirin (RBV) versus PEG plus RBV in hepatitis C virus genotype-1 infection. J Hepatology 50(Suppl 1): S7–S7 [Google Scholar]
- Poordad F., Lawitz E., Hassanein T., Schiffman M.L., Bacon B.R., Muir A., et al. (2009) Sustained virologic response (SVR) results for weight-based-taribavirin versus weight-based-ribavirin, in naïve chronic-hepatitis C, genotype 1 patients. Hepatology 50(Suppl): 334A–334A [DOI] [PubMed] [Google Scholar]
- Reddy R., Rodriguez-Torres M., Gane E., Robson R., Lalezari J., Everson G.T., et al. (2007) Antiviral activity, pharmacokinetics, safely and tolerability of R7128, a novel nucleoside HCV RNA polymerase inhibitor, following multiple, ascending, oral doses in patients with HCV genotype 1 infection who have failed prior interferon therapy. Hepatology 46(Suppl. 1): 862A–862A [Google Scholar]
- Rodriguez-Torres M., Lalezari J., Gane E., De Jesus E., Nelson D.R., Everson G.T., et al. (2008) Potent anti-viral response to the HCV nucleoside polymerase inhibitor R7128 for 28 days with PEG-IFN and ribavirin: subanalysis by race/ethnicity, weight and HCV genotype. Hepatology 48(Suppl): 1160A–1160A [Google Scholar]
- Rossignol J., Elfert A., Keeffe E.B. (2008a) Evaluation of a 4 week lead-in phase with nitazoxanide (NTZ) prior to peginterferon (PEGIFN) plus NTZ for treatment of chronic hepatitis C: final report. Hepatology 48(Suppl): 1132A–1132A [Google Scholar]
- Rossignol J.F., Elfert A., El-Gohary Y., Keeffe E.B. (2008b) Randomized controlled trial of nitazoxanide-peginterferon-ribavirin, nitazoxanide-peginterferon and peginterferon-ribavirin in the treatment of patients with chronic hepatitis C genotype 4. J Hepatol 48(Suppl 2): S30–S30 [Google Scholar]
- Sarrazin C., Kieffer T.L., Bartels D., Hanzelka B., Müh U., Welker M., et al. (2007) Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132: 1767–1777 [DOI] [PubMed] [Google Scholar]
- Steffy K., Kirkovsky L., Lanford R.E., Showalter R.E., Sergeeva M., Zhao J., et al. (2008) Antiviral efficacy of the HCV RNA polymerase inhibitor ANA 598 in the chimpanzee model of HCV infection. Hepatology 48(Suppl): 1163A–1163A [Google Scholar]
- Sulkowski M.S., Ferenci P., Emanoil C., Asselah T., Caruntu F., Lalezari J., et al. (2009) SILEN-C1: early antiviral activity and safety of BI 201335 combined with peginterferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV infection. Hepatology 50(Suppl): LB3–LB3 [Google Scholar]
- Suppiah V., Moldovan M., Ahlenstiel G., Berg T., Weltman M., Abate M.L., et al. (2009) IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat Genet. doi:10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Nishida N., Sugiyama M., Kurosaki M., Matsuura K., Sakamoto N., et al. (2009) Genome-wide association of chronic hepatitis C. Nat Genet. doi:10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- Thompson R., Patel R., Steffy J., Appleman J. (2009) Preclinical studies of ANA 598 combined with other anti-viral agents demonstrate potential of combination treatment. J Hepatol 48(Suppl 2): S37–S37 [Google Scholar]
- US National Institutes of Health. (2008) http://www.clinicaltrials.gov/ct2/results?term=nitazoxanide+and+hepatitis+c (accessed November 2008). [Google Scholar]
- Vierling J.M., Poordad F., Lawitz E., Ghalib R.H., Lee W.L., Ravendhran N., et al. (2009) Once daily narlaprevir (SCH 900518) in combination with pegintron (peginterferon alfa-2B)/ribavirin for treatment-naïve subjects with genotype-1 CHC: interim results from NEXT-1, a phase 2a study. Hepatology 50(Suppl): LB3–LB3 [Google Scholar]