Abstract
Irritable bowel syndrome (IBS) is a highly prevalent functional gastrointestinal disorder that causes a range of symptoms. Currently, alosetron hydrochloride (Lotronex®), a selective serotonin type 3 receptor antagonist, is the only medication approved for the treatment of severe diarrhea-predominant irritable bowel syndrome (IBS-D) in women who have inadequately responded to conventional therapy. Alosetron has demonstrated efficacy compared with placebo in clinical trials and has been shown to improve overall health-related quality of life (HRQoL). However, rare instances of ischemic colitis and severe complications of constipation have been reported. As a result, in 2000 alosetron was voluntarily withdrawn from the market but was reintroduced in 2002 with a more restricted indication and a requirement that clinicians and patients follow a prescribing program. Although the efficacy and benefit of alosetron has been clearly demonstrated, it has been used sparingly since its reintroduction. This brief review describes the history of alosetron, efficacy of alosetron in the treatment of IBS, the impact of severe IBS on HRQoL, safety considerations, the risk evaluation and mitigation strategy program under which alosetron is now prescribed, and an update on postmarketing surveillance data.
Keywords: Irritable bowel syndrome, diarrhea, 5-HT3 antagonist, alosetron
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by recurrent abdominal pain or discomfort, disturbed bowel function, abdominal distension or bloating, and the passage of mucus. IBS is diagnosed more often in women than in men and typically in patients under 50 years of age [Drossman et al. 2002]. Subtypes of the disorder include diarrhea-predominant IBS (IBS-D), constipation-predominant IBS, mixed IBS, and unsubtyped IBS [Longstreth et al. 2006]. While the true prevalence of IBS subtypes remains unknown, IBS-D and mixed IBS were the most frequently reported subtypes in two published surveys [Andrews et al. 2005; Hungin et al. 2005]. Conventional treatments, including antidiarrheals, antispasmodics, and antidepressants, are commonly used for IBS-D [American College of Gastroenterology Task Force on Irritable Bowel Syndrome, 2009; Drossman et al. 2002]; however, no medication in these classes is specifically approved for use in IBS-D. Currently, in the United States, only alosetron is approved for IBS-D, indicated in women with severe IBS-D who have had an inadequate response to conventional therapy.
History of alosetron
Alosetron was introduced in early 2000 for the treatment of women with IBS-D but was voluntarily withdrawn from the market later that year owing to reports of infrequent but serious adverse events associated with its use, specifically ischemic colitis (IC) and complications of constipation (CoC), which included fecal impaction, intestinal obstruction, toxic megacolon, and intestinal perforation [Chang et al. 2006]. These adverse events resulted in hospitalizations and rare instances of blood transfusion, surgery, and death [Chang et al. 2006; Horton, 2001]. Two deaths were related to CoC; no deaths were related to IC [Chang et al. 2006]. After market withdrawal, the US Food and Drug Administration (FDA) received numerous requests from both patients and physicians to bring alosetron back to the market [Horton, 2001]. An advisory committee was convened by the FDA to discuss the risks and benefits of alosetron, which resulted in a recommendation for the reapproval of alosetron in June 2002 [Andresen and Hollerbach, 2004; McCarthy, 2002]. Alosetron was subsequently reintroduced for patient use in November 2002 with a lower recommended starting dose (0.5 mg twice daily, instead of 1 mg twice daily) and a more restricted indication specifying it be used in women with ‘severe’ IBS-D who have had an inadequate response to conventional therapy. For purposes of the more restrictive indication, ‘severe’ IBS was defined as the presence of one or more of the following: (1) frequent and severe abdominal pain or discomfort; (2) frequent bowel urgency or fecal incontinence; or (3) disability or restriction of daily activities due to IBS. It is important to note that only one of these parameters need be present for IBS to be considered severe [Lewis, 2010]. In addition, alosetron use is now governed by a risk evaluation and mitigation strategy (REMS).
Risk evaluation and mitigation strategy
The REMS includes the Prescribing Program for Lotronex® (PPL), which outlines the responsibilities of the physician and the patient before initiation of alosetron therapy [Prometheus Laboratories, 2008a]. Physicians who enroll in the PPL are required to be qualified to diagnose IBS, to counsel patients on the benefits and risks of alosetron therapy, to sign a physician–patient agreement form, to affix program stickers to alosetron prescriptions, to monitor patients, and to report any serious adverse events to the manufacturer or to the FDA [Prometheus Laboratories, 2008a]. Patients receiving alosetron must sign the physician–patient agreement form, report adverse events, and have the opportunity to participate in a voluntary survey. Finally, pharmacists must confirm that a program sticker is affixed to an alosetron prescription before they dispense the medication and provide an alosetron medication guide [Ameen et al. 2008]. These requirements were put in place with the goal of maximizing therapeutic benefit through proper patient selection and reducing the risk of complications or consequences of serious adverse events.
Efficacy of alosetron
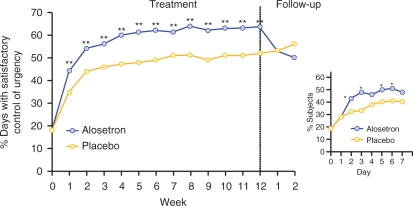
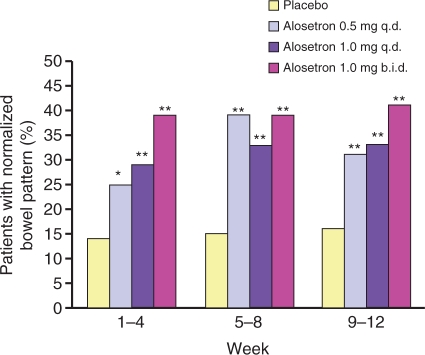
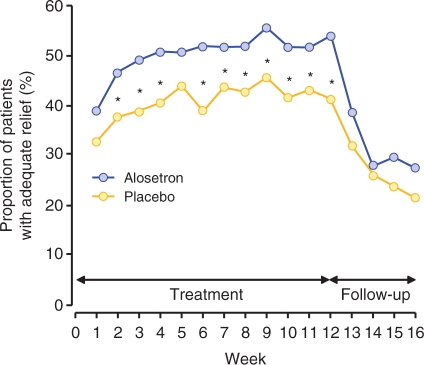
Alosetron hydrochloride is a potent, selective serotonin 3 (5-HT3) receptor antagonist indicated for the treatment of women with chronic (lasting >6 months), severe IBS-D and no anatomic or biochemical abnormalities of the GI tract who have not responded adequately to conventional therapy. Compared with placebo, alosetron demonstrated effectiveness in decreasing fecal urgency and improving stool consistency and frequency in women with IBS-D (Figures 1 and 2) [Krause et al. 2007; Lembo et al. 2004, 2001]. Alosetron has been shown to significantly improve IBS-related abdominal pain and discomfort in randomized, double-blind clinical trials (Figure 3) [Krause et al. 2007; Camilleri et al. 2001, 2000].
Figure 1.
Percentage of days with satisfactory control of urgency throughout a 12-week clinical trial (**p < 0.003). Inset shows the percentage of patients with satisfactory urgency control during the first week of treatment (*p < 0.05). [Reprinted with permission from Lembo et al. 2004].
Figure 2.
Proportion of patients with normalized bowel pattern (defined as having a stool consistency ≤3 and stool frequency of ≤2 per day (*p < 0.004; **p < 0.001). [Reprinted with permission from Krause et al. 2007].
Figure 3.
Proportion of patients with adequate relief of abdominal pain and discomfort by week (*p < 0.05). [Reprinted with permission from Camilleri et al. 2000].
Impact of irritable bowel syndrome
Numerous studies have indicated that IBS is associated with substantial compromise in health-related quality of life (HRQoL) [American College of Gastroenterology Task Force on Irritable Bowel Syndrome, 2009]. A survey using the Short Form 36 question general health questionnaire (SF-36) to assess HRQoL revealed scores significantly lower than country-specific normative values for IBS patients from the US (n = 287) and the UK (n = 343) across each of eight measured domains: physical functioning, role limitations due to physical/emotional components, bodily pain, general health, emotional well-being, energy/fatigue, and social functioning [Hahn et al. 1997]. Using the same instrument, Gralnek and colleagues compared HRQoL scores from patients with IBS with scores collected previously from patients with moderate to severe gastroesophageal reflux disease (GERD), diabetes mellitus (DM), dialysis-dependent end-stage renal disease (ESRD), and depression [Gralnek et al. 2000]. IBS patients scored significantly lower than GERD patients in seven of the eight SF-36 domains (excluding physical functioning) and significantly lower than DM patients in six of the eight domains (excluding physical functioning and general health perception). In another comparison [Gralnek et al. 2000], IBS patients scored significantly lower than ESRD patients in emotional well-being but had similar scores for bodily pain, role limitations due to emotional components, energy/fatigue, and social functioning. Lastly, IBS patients reported significantly worse bodily pain than patients with depression but comparable limitations due to physical components and general health perception [Gralnek et al. 2000].
The level of IBS severity also correlates with impairments in HRQoL [American College of Gastroenterology Task Force on Irritable Bowel Syndrome, 2009; Naliboff et al. 1998]. Using an IBS-specific QoL instrument (IBSQoL), Hahn and colleagues found that patients with severe IBS had significantly poorer scores in physical functioning, social functioning, energy/fatigue, mental health, and role limitations due to emotional and physical components than those with moderately severe disease [Hahn et al. 1997]. A recent international Internet survey of 1966 IBS patients [Drossman et al. 2009] confirmed these earlier findings, showing that patients with severe IBS scored lower than those with less severe disease in all domains of the IBSQoL and had more days of restricted usual/social activities. Patients with severe IBS (n = 400) in this survey reported a willingness to risk at least a 1 in 1000 chance of death (24.9%), serious or permanent side effects (18.8%), or mild side effects (65.1%) to be able to take a medication that provided total IBS symptom relief [Drossman et al. 2009].
Although traditionally IBS has not been considered a life-threatening disorder, recent data reveal that the negative impact of IBS on patients’ lives may be associated with increased suicidal ideation and suicidal behavior [Spiegel et al. 2007; Miller et al. 2004]. Miller and colleagues assessed suicidal ideation or suicide attempts specifically linked to bowel problems in IBS and inflammatory bowel disease (IBD) patients followed in the UK National Health Service clinic system [Miller et al. 2004]. Investigators questioned patients at all levels of clinical care: primary care (pIBS; 100 patients managed in general practice), secondary care (sIBS; 100 patients referred from primary care), and tertiary care (tIBS; 100 patients referred by another specialist). The control group comprised 100 IBD patients with quiescent, minimally active, or remitted disease. Results showed that patients in the tIBS group were significantly more likely to consider suicide because of their symptoms (38%) than those in the sIBS (16%), pIBS (4%), or IBD (15%) group (tIBS versus sIBS [p = 0.002] versus pIBS [p < 0.001] versus IBD [p < 0.001]). Multiple regression analysis revealed that symptom severity, level of clinical care (i.e. tertiary versus secondary versus primary), anxiety, and depression were all independent predictors of suicidal ideation [Miller et al. 2004]. The potential of IBS, especially in its most severe form, to induce suicidal ideation underscores the seriousness of IBS and the need to optimize treatment outcomes [Miller et al. 2004]. To date, studies have not specifically examined the impact of IBS subtypes on HRQoL or suicidal ideation and suicidal behavior; further investigations in this area are needed.
Alosetron and potential for drug–drug interactions
In vivo data suggest that alosetron is primarily metabolized by cytochrome P450 (CYP) 1A2, with minor contributions from CYP3A4 and CYP2C9. Therefore, agents that induce or inhibit these enzymes can alter the clearance of alosetron. For example, concomitant administration of alosetron and fluvoxamine (a selective serotonin reuptake inhibitor) is contraindicated because fluvoxamine strongly inhibits CYP1A2. Fluvoxamine produces approximately six-fold increases in mean alosetron plasma concentrations (AUC) and an approximately three-fold prolongation of its half-life [Lewis, 2010]. Use of alosetron with more moderate CYP1A2 inhibitors, such as quinolone antibiotics or cimetidine, has not been assessed but should be avoided unless clinically necessary given the drug interaction potential. In addition, caution is recommended during concomitant use of alosetron and the strong CYP3A4 inhibitor ketoconazole, which can increase alosetron plasma concentrations by 29% [Lewis, 2010]. Alosetron has not been evaluated in drug-interaction studies with more modest CYP3A4 inhibitors (e.g. clarithromycin, telithromycin, protease inhibitors, voriconazole, itraconazole).
Data from in vitro and in vivo studies suggest that alosetron is unlikely to inhibit CYP enzymes, including CYP1A2, 2C9, 2C19, 2D6, 2E1, or 3A4 [Lewis, 2010; Koch et al. 2004b], nor is it likely to induce the CYP2C19, 2E1, or 3A4 enzymes. No adjustments in alosetron dosing are needed during coadministration of oral contraceptives such as ethinyl estradiol or levonorgestrel or coadministration of fluoxetine, alprazolam, or theophylline [Koch et al. 2004a, 2001; D’Souza et al. 2001a, 2001b]. It is unknown whether alosetron induces other enzymes.
Alosetron use in pregnancy
Given that alosetron is indicated only for women with IBS-D, its potential influence on pregnancy should be considered. Alosetron belongs to Pregnancy Category B. Preclinical reproduction studies in rats and rabbits (at ∼160 and ∼240 times the recommended human dose based on body surface area, respectively) have revealed no evidence of impaired fertility or fetal harm from alosetron. Despite this, no randomized, placebo-controlled studies of alosetron have been performed in pregnant women; therefore, it is recommended that alosetron be used during pregnancy only if there is a clear indication of need [Prometheus Laboratories, 2008b].
Safety: association of ischemic colitis and complications of constipation with alosetron
A systematic blinded review performed by Chang and colleagues examined the incidence of IC and CoC in patients treated with alosetron in clinical trials and during postmarketing surveillance of the initial marketing period [Chang et al. 2006]. Notably, the randomized, controlled trials examined in the Chang et al. analysis included patients with the full spectrum of IBS illness, not just women with severe IBS-D, for whom the drug is now indicated [Chang et al. 2006]. Table 1 outlines the screening criteria used to identify IC and CoC that were probably related to alosetron use in this analysis.
Table 1.
Screening criteria to identify potential cases of ischemic colitis and serious complications of constipation in patients receiving alosetron (identified from pooled clinical trial data). [Adapted with permission from Chang et al. 2006].
| Probable ischemic colitis (IC) |
Probable serious complications of constipation (CoC) |
|---|---|
| • Medical history consistent with IC (e.g. abdominal discomfort, hematochezia, diarrhea) and … • Supported by the results of colonoscopy or other imaging tests and /or histological evaluation of a relevant tissue biopsy and … • No evidence for any more likely diagnosis | • Medical history consistent with serious CoC (e.g. patient complained of constipation) and met regulatory definition of a serious adverse event* • Medical history is supported by hospital or medical records |
| • Colonoscopy results (or other imaging test) do not identify a more likely diagnosis for the patient’s symptoms |
Serious adverse event is defined as ‘death, a life-threatening adverse drug experience, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, a congenital anomaly/birth defect.’ Serious adverse events also include: ‘important medical events that may not result in death, be life-threatening, or require hospitalization … [but] based upon appropriate medical judgment, they may jeopardize the patients and may require medical or surgical intervention to prevent one of the outcomes listed in the definition.’
Clinical trial program
In their pooled analysis of clinical trials, Chang et al. [2006] found an increased risk of IC among patients receiving alosetron (0.15%) compared with those receiving placebo (0%, p = 0.03; 6.4 cases per 1000 patient years [pt-yr] of alosetron use versus 0.0 cases per 1000 pt-yr of placebo use). All patients developing IC associated with alosetron use (n = 19) had transient symptoms with no long-term sequelae; the majority of cases (63.1%) occurred within the first 30 days of treatment [Chang et al. 2006].
Rates of serious CoC in clinical trials did not differ significantly between alosetron- and placebo-treated patients (3.3 cases per 1000 pt-yr of alosetron use versus 1.0 case per 1000 pt-yr of placebo use) [Chang et al. 2006]. Of 10 cases of serious CoC that occurred in the program, 90% occurred within the first 90 days of treatment, highlighting the importance of vigilance during the first 3 months of therapy [Chang et al. 2006]. All patients were hospitalized, one patient underwent abdominal surgery, and there were no deaths.
Postmarketing surveillance before June 2002
In postmarketing surveillance data collected before June 2002, post-adjudication rates of IC and CoC were 0.96 cases per 1000 pt-yr and 0.59 cases per 1000 pt-yr of use, respectively [Chang et al. 2006].
Postmarketing surveillance after reintroduction (November 2002–December 2007)
Postmarketing surveillance data were obtained from REMS reports submitted to the FDA between November 2002 and December 2007 (including spontaneous reports from patients and health care professionals, responses from a voluntary patient survey, and serious adverse event reports from physicians as required by the prescribing program), applying the same adjudication criteria for probable relation to alosetron used by Chang et al. (Table 1). Based on these data, 15 cases of IC were confirmed, revealing an IC incidence rate of 1.14 cases per 1000 pt-yr [Ameen et al. 2008]. Over that same period, six confirmed cases of CoC were reported, corresponding to an incidence rate of 0.46 per 1000 pt-yr of treatment [Ameen et al. 2008].
Sequelae of ischemic colitis and complications of constipation cases in association with alosetron
In the time period prior to its reintroduction, alosetron use was not associated with fatal outcomes resulting from the development of IC but two fatalities did occur as a result of CoC. Since the market reintroduction of alosetron, confirmed cases of IC (n = 15) and CoC (n = 6) have not resulted in the need for surgical procedures or transfusions, nor have they been associated with fatal outcomes. Moreover, all confirmed events of IC and CoC resolved upon discontinuation of alosetron [Ameen et al. 2008]. Thus, IC and CoC events observed over this 5-year period have remained rare, idiosyncratic, unrelated to alosetron dose, and with incidence rates similar to those noted in the postmarketing surveillance prior to its reintroduction [Ameen et al. 2008].
Discussion
Since the reapproval and reintroduction of alosetron in 2002, the efficacy, safety, and tolerability of this medication have been well characterized. The alosetron REMS program provides health care professionals and patients with information about alosetron (including new dosing guidelines). Since the introduction of the REMS, no new, clinically relevant safety concerns have been observed. Likewise, reports of IC and CoC since reintroduction have remained rare and stable [Ameen et al. 2008; Chang et al. 2006] and no confirmed serious outcomes have been reported (e.g. surgeries, transfusions, deaths) to result from these events [Ameen et al. 2008; Krause et al. 2007; Chang et al. 2006]. The risk of developing IC and the pathologic mechanism by which alosetron might cause this adverse event remains unclear [Camilleri, 2007]. Effects of alosetron on colonic mucosal blood flow are unknown; however, submucosal vasomotor reflexes appear to be modulated by 5-HT4 or 5-HT1p receptors, not 5-HT3 receptors [Camilleri, 2007]. In experimental settings, 5-HT3 antagonists do not inhibit normal dilatory responses in preconstricted arterioles during balloon distension [Reed and Vanner, 2003]. Likewise, there is no evidence that 5-HT3 antagonists cause changes in coagulation factors or platelet or endothelial function [Camilleri, 2007]. Moreover, medical claims data from a large managed care database (United Healthcare) showed that IBS patients have a 3.4 times higher incidence of IC than the general population [Cole et al. 2004]. One remaining point of controversy is whether alosetron predisposes patients to developing IC, exacerbates other risk factors for IC, or causes IC.
One unintentional outcome of the REMS is that it has led to a much more restricted usage of alosetron than when it initially came to market. A survey of patients enrolled in the RiskMAP found that 76% of alosetron-treated women who responded to the questionnaire met all three severity criteria for the diagnosis of severe IBS-D, although only one criterion is necessary to receive the diagnosis of ‘severe’ IBS-D [Miller et al. 2006]. This finding indicates that there are likely many more women suffering from less severe IBS-D who may benefit from a trial of alosetron.
Selection of therapy for the IBS patient should be based not only on the severity of GI symptoms (e.g. abdominal pain, fecal urgency) but should also address the impact of the symptoms on the patient’s functional status and HRQoL. Routine screening of HRQoL in IBS patients is recommended by the American College of Gastroenterology (ACG) Task Force on IBS, and treatment should be initiated when symptoms reduce functional status and diminish overall HRQoL [American College of Gastroenterology Task Force on Irritable Bowel Syndrome, 2009]. Indeed, in women with IBS-D, disability or restriction of daily activities due to IBS is an HRQoL parameter that supports the characterization of IBS as severe. Furthermore, recent evidence suggesting increased suicidal ideation and suicidal behavior in patients with IBS, independent of depression or other psychiatric comorbid conditions, underscores the importance of timely treatment of severe IBS [Spiegel et al. 2007; Miller et al. 2004]. Appropriate use of alosetron across a broader population of patients with severe IBS—not just in those with the severest disease—would likely optimize outcomes in the population of women who suffer from this serious disorder.
Conclusions
Alosetron is currently the only 5-HT3 receptor antagonist approved for the management of severe IBS-D in women [American College of Gastroenterology Task Force on Irritable Bowel Syndrome, 2009; Prometheus Laboratories, 2008b]. Several clinical trials have shown that alosetron effectively treats the multiplicity of GI symptoms, including fecal urgency, stool consistency and frequency, and abdominal pain and discomfort, as well as improves HRQoL in patients with IBS-D. Whereas the use of alosetron has been associated with serious but rare adverse events, the incidence of these events has essentially remained rare and stable and they have not resulted in any deaths since alosetron was reintroduced in November 2002. Despite these findings, it appears that only the most severe IBS cases are being treated with alosetron. Outcomes in patients with IBS-D might be optimized through the accurate assessment and diagnosis of IBS severity as it relates to both GI symptomatology and impact on HRQoL. With such an assessment, an appropriate and effective treatment plan can be initiated to improve symptoms and ease the suffering related to IBS.
Acknowledgments
The author would like to thank John Simmons, MD, for his assistance in the preparation of this manuscript. Writing support was provided with funding from Prometheus Laboratories, Inc.
Conflict of interest statement
The author declares that she is a speaker and consultant for Prometheus Labs, Inc., Salix, and Takeda pharmaceutical companies; she is also a consultant for Forest Labs/Ironwood. The author serves as a legal expert for Novartis and was a legal expert for GlaxoSmithKline.
References
- Ameen, V.Z., Tong, K. and Pan, H. (2008) The Risk Management Program (RiskMAP) is effective in mitigating serious outcomes of ischemic colitis and complications of constipation with marketed use of alosetron since reintroduction [abstract P693]. Presented at the 2008 Annual Meeting of the American College of Gastroenterology, 6 October 2008, Orlando, FL, USA. [Google Scholar]
- American College of Gastroenterology Task Force on Irritable Bowel Syndrome (2009) An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 104(Suppl 1): S1–S35 [DOI] [PubMed] [Google Scholar]
- Andresen V., Hollerbach S. (2004) Reassessing the benefits and risks of alosetron: what is its place in the treatment of irritable bowel syndrome? Drug Saf 27: 283–292 [DOI] [PubMed] [Google Scholar]
- Andrews E.B., Eaton S.C., Hollis K.A., Hopkins J.S., Ameen V., Hamm L.R., et al. (2005) Prevalence and demographics of irritable bowel syndrome: results from a large Web-based survey. Aliment Pharmacol Ther 22: 935–942 [DOI] [PubMed] [Google Scholar]
- Camilleri M. (2007) Is there an experimental basis for the development of ischaemic colitis as a result of 5-HT3 antagonist treatment? Neurogastroenterol Motil 19: 77–84 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Chey W.Y., Mayer E.A., Northcutt A.R., Heath A., Dukes G.E., et al. (2001) A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med 161: 1733–1740 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Northcutt A.R., Kong S., Dukes G.E., McSorley D., Mangel A.W. (2000) Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet 355: 1035–1040 [DOI] [PubMed] [Google Scholar]
- Chang L., Chey W.D., Harris L., Olden K., Surawicz C., Schoenfeld P. (2006) Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 101: 1069–1079 [DOI] [PubMed] [Google Scholar]
- Cole J.A., Cook S.F., Sands B.E., Ajene A.N., Miller D.P., Walker A.M. (2004) Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol 99: 486–491 [DOI] [PubMed] [Google Scholar]
- D’Souza D.L., Dimmitt D.C., Robbins D.K., Nezamis J., Simms L., Koch K.M. (2001a) Effect of alosetron on the pharmacokinetics of fluoxetine. J Clin Pharmacol 41: 455–458 [DOI] [PubMed] [Google Scholar]
- D’Souza D.L., Levasseur L.M., Nezamis J., Robbins D.K., Simms L., Koch K.M. (2001b) Effect of alosetron on the pharmacokinetics of alprazolam. J Clin Pharmacol 41: 452–454 [DOI] [PubMed] [Google Scholar]
- Drossman D.A., Camilleri M., Mayer E.A., Whitehead W.E. (2002) AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131 [DOI] [PubMed] [Google Scholar]
- Drossman D.A., Morris C.B., Schneck S., Hu Y.J., Norton N.J., Norton W.F., et al. (2009) International survey of patients with IBS: symptom features and their severity, health status treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 43: 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnek I.M., Hays R.D., Kilbourne A., Naliboff B., Mayer E.A. (2000) The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 119: 654–660 [DOI] [PubMed] [Google Scholar]
- Hahn B.A., Kirchdoerfer L.J., Fullerton S., Mayer E. (1997) Patient-perceived severity of irritable bowel syndrome in relation to symptoms, health resource utilization and quality of life. Aliment Pharmacol Ther 11: 553–559 [DOI] [PubMed] [Google Scholar]
- Horton R. (2001) Lotronex and the FDA: a fatal erosion of integrity. Lancet 357: 1544–1545 [DOI] [PubMed] [Google Scholar]
- Hungin A.P., Chang L., Locke G.R., Dennis E.H., Barghout V. (2005) Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 21: 1365–1375 [DOI] [PubMed] [Google Scholar]
- Koch K., Campanella C., Baidoo C.A., Manzo J.A., Ameen V.Z., Kersey K.E. (2004a) Pharmacodynamics and pharmacokinetics of oral contraceptives co-administered with alosetron (Lotronex). Dig Dis Sci 49: 1244–1249 [DOI] [PubMed] [Google Scholar]
- Koch K.M., Corrigan B.W., Manzo J., James C.D., Scott R.J., Stead A.G., et al. (2004b) Alosetron repeat dose pharmacokinetics, effects on enzyme activities, and influence of demographic factors. Aliment Pharmacol Ther 20: 223–230 [DOI] [PubMed] [Google Scholar]
- Koch K.M., Ricci B.M., Hedayetullah N.S., Jewell D., Kersey K.E. (2001) Effect of alosetron on theophylline pharmacokinetics. Br J Clin Pharmacol 52: 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause R., Ameen V., Gordon S.H., West M., Heath A.T., Perschy T., et al. (2007) A randomized, double-blind, placebo-controlled study to assess efficacy and safety of 0.5 mg and 1 mg alosetron in women with severe diarrhea-predominant IBS. Am J Gastroenterol 102: 1709–1719 [DOI] [PubMed] [Google Scholar]
- Lembo A.J., Olden K.W., Ameen V.Z., Gordon S.L., Heath A.T., Carter E.G. (2004) Effect of alosetron on bowel urgency and global symptoms in women with severe, diarrhea-predominant irritable bowel syndrome: analysis of two controlled trials. Clin Gastroenterol Hepatol 2: 675–682 [DOI] [PubMed] [Google Scholar]
- Lembo T., Wright R.A., Bagby B., Decker C., Gordon S., Jhingran P., et al. (2001) Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 96: 2662–2670 [DOI] [PubMed] [Google Scholar]
- Lewis, J.H. (2010) Alosetron for severe diarrhea-predominant irritable bowel syndrome; efficacy and safety in perspective. Expert Rev Gastroenterol Hepatol In press. [DOI] [PubMed] [Google Scholar]
- Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. (2006) Functional bowel disorders. Gastroenterology 130: 1480–1491 [DOI] [PubMed] [Google Scholar]
- McCarthy M. (2002) FDA recommends bringing controversial IBS drug back. Lancet 359: 1491–1492 [DOI] [PubMed] [Google Scholar]
- Miller D., Bennett L., Hollis K., Tennis P., Cook S., Andrews E. (2006) A patient follow-up survey programme for alosetron: assessing compliance to and effectiveness of the risk management programme. Aliment Pharmacol Ther 24: 869–878 [DOI] [PubMed] [Google Scholar]
- Miller V., Hopkins L., Whorwell P.J. (2004) Suicidal ideation in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Naliboff B.D., Balice G., Mayer E.A. (1998) Psychosocial moderators of quality of life in irritable bowel syndrome. Eur J Surg Suppl 57–59 [DOI] [PubMed] [Google Scholar]
- Prometheus Laboratories (2008a) Prescribing program for Lotronex physician enrollment form. Available at: http://www.lotronex.com/download/physician_enrollment.pdf (accessed 10 July 2009). [Google Scholar]
- Prometheus Laboratories (2008b) Lotronex (alosetron hydrochloride) tablets [package insert], San Diego, CA: Prometheus Laboratories Inc [Google Scholar]
- Reed D.E., Vanner S.J. (2003) Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol (Lond) 553: 911–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel B., Schoenfeld P., Naliboff B. (2007) Systematic review: the prevalence of suicidal behaviour in patients with chronic abdominal pain and irritable bowel syndrome. Aliment Pharmacol Ther 26: 183–193 [DOI] [PubMed] [Google Scholar]