Abstract
Restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis is the operation of choice for patients with ulcerative colitis. Pouchitis is the most common cause of pouch dysfunction. Although the pathogenesis of this disease is not well understood, bacteria have been implicated in the disease process. Numerous bacterial studies have been reported over the last 25 years with few unifying findings. In addition, many different treatments for pouchitis have been reported with varying results. Antibiotic treatment remains the most studied and is the mainstay of treatment. In this article we review the aetiology of pouchitis and the evidenced-based treatment options.
Keywords: dysbiosis, familial adenomatous polyposis, ileal pouch-anal anastomosis, inflammatory bowel disease, pouchitis, restorative proctocolectomy, ulcerative colitis
Background
Restorative proctocolectomy (RPC) with ileal pouch-anal anastomosis is the operation of choice for patients with ulcerative colitis (UC) who require surgery and is also performed in selected patients with familial adenomatous polyposis (FAP). After surgery most patients experience a satisfactory functional outcome. Inflammation of the ileoanal reservoir (pouchitis) is the most common long-term complication. Pouchitis is diagnosed when histologically proven acute inflammation is associated with symptoms of frequency, urgency and liquid stool in the presence of endoscopic evidence of inflammation [McLaughlin et al. 2008b]. The incidence of pouchitis is markedly different between patients with UC and FAP. In UC the quoted incidence varies from 20% to 50% between studies [Hahnloser et al. 2007; Romanos et al. 1997; Stahlberg et al. 1996]. In FAP the incidence of pouchitis is much lower than in UC and varies from 0% to 11% [Barton et al. 2001; Nyam et al. 1997; Kartheuser et al. 1996; Fazio et al. 1995; Dozois et al. 1989]. This large interdisease variation in incidence is explained by the differences in definition and length of follow up. Some studies have based the diagnosis on symptoms alone whereas others correctly have required a triad of symptoms, endoscopic findings and compatible histology. Since the risk of pouchitis increases over time, studies of a longer duration are more likely to demonstrate a higher incidence of pouchitis.
About 39% of patients who develop pouchitis will have a single episode which is easily treated and does not recur. The remaining 61% will suffer at least one further episode [Lohmuller et al. 1990] and about 5–19% of patients will develop chronic relapsing pouchitis [Madiba and Bartolo, 2001; Mowschenson et al. 2000; Hurst et al. 1998]. In this article we review the aetiology of pouchitis and the evidenced-based treatment options.
The aetiology of pouchitis
Pathological similarities to ulcerative colitis and Crohn’s disease
Similarly to UC and Crohn’s disease (CD) the mucosal inflammation that occurs in pouchitis is localized to the areas with the highest concentration of bacteria. There is a tenfold increased risk of pouchitis in patients who undergo RPC for UC compared with FAP. Therefore, it has been suggested that pouchitis represents reactivation of UC in the colonized small bowel of the pouch.
Prepouch ileitis (PPI; small intestinal inflammation proximal to the pouch inlet) is known to occur in some RPC patients with UC [Slatter et al. 2008; Calabrese et al. 2007; Iwata et al. 2007; Kuisma et al. 2004]. The pathogenesis of PPI has not been studied. Ischaemia [Bell et al. 2006], CD and nonsteroidal anti-inflammatory drugs (NSAIDs) [Shen et al. 2006; Wolf et al. 2004] have been implicated in some cases, but where these factors have been excluded the pathogenesis is likely to be similar to that of pouchitis where bacteria are considered fundamental to the disease process. It is possible that reflux of pouch contents into the prepouch ileum analogous to backwash ileitis in UC, a higher bacterial load or more pathogenic bacteria may be responsible for the disease process. Evidence for this comes from knowledge that the antibiotic therapy is an effective treatment [McLaughlin et al. 2008a] and that PPI appears to be a risk factor for developing antibiotic resistance in patients treated with maintenance antibiotic therapy [McLaughlin et al. 2009b]. One group has suggested that inflammation proximal to the pouch is indicative of CD or associated with the use of NSAIDs [Shen et al. 2006; Wolf et al. 2004]. This study included 87 patients with available colectomy histology including 28 with UC, 32 with indeterminate colitis and 27 with CD. Only 5 UC patients had signs of PPI, all of whom were taking NSAIDs. Therefore, the study did not assess UC patients with PPI who were not taking NSAIDs. Recent work by our group has demonstrated that histologically PPI is a distinct entity which can be differentiated from CD [Bell et al. 2006] and it is therefore possible that PPI is similar to backwash ileitis seen in UC, providing evidence of similarity to UC.
Despite this there are several similarities between pouchitis and CD. Both are inflammatory conditions which can affect the small bowel, both respond to treatment with antibiotics and both improve with diversion of the faecal stream (importantly these treatments are not effective in UC). There is also evidence at an immunological level that pouchitis has a similar pathogenesis to CD. In UC inflammation is predominately Th2-cytokine driven, whereas in CD a Th1-mediated immune response dominates [Fiocchi, 1998; Gately et al. 1998; Sartor, 1991; Wolf et al. 1991; Kobayashi et al. 1989]. It might therefore be expected that pouchitis would be associated with a Th2-cytokine activation, but an increase in the Th1 cytokine interferon gamma (IFN-γ) has been described in acute and chronic pouchitis [Stallmach et al. 1998]. Our group has previously investigated the association between CD30 and pouchitis. CD30 was shown to be upregulated in UC but not in CD [Elewaut et al. 1998; Giacomelli et al. 1998]. In this study CD30 was elevated in acute pouchitis but not in those with chronic pouchitis [Thomas et al. 2001] and therefore it was postulated that this may be due to a more CD-type disease occurring in these individuals.
Evidence for pouchitis being a novel third form of inflammatory bowel disease
It has been suggested that pouchitis may represent a novel third form of inflammatory bowel disease (IBD) [Sandborn and Pardi, 2004]. The differences in treatment response seen in pouchitis patients compared with UC and CD could be considered to fit with this. Although antibiotics are of some clinical benefit in CD, they are much less so than in pouchitis where they are more effective than any other therapy. Interestingly there is no evidence that immunomodulators are effective in pouchitis (we return to this later) suggesting that the pathogenesis of the disease may differ from that of UC and CD where these agents are very effective.
The dysbiosis theory
An abnormal host–microbial interaction has long been implicated in all forms of IBD including pouchitis [Sartor, 2008]. There is evidence from clinical practice to implicate bacteria in pouchitis. In common with UC and CD, mucosal inflammation is localized to the area of gut with the highest concentration of bacteria [Sartor, 2008], antibiotics are effective treatment for both pouchitis and PPI [McLaughlin et al. 2008a; Shen et al. 2007a; Madden et al. 1994] and probiotics have been shown to reduce disease relapse [Mimura et al. 2004; Gionchetti et al. 2000], reduce the risk of disease onset [Gionchetti et al. 2005] and to be effective in mild to moderate pouchitis [Gionchetti et al. 2007a].
Based on the response of peripheral and local mononuclear cells our group has previously demonstrated that the inflammatory response in pouchitis appears to be at the local mucosal level and is not a general systemic reaction [Thomas et al. 2002] and that pouchitis-derived bacterial sonicates from metronidazole-sensitive bacterial species can stimulate healthy patients’ mononuclear cells significantly more than corresponding sonicates from nonpouchitis patients [Bell et al. 2004]. Both studies providing indirect evidence to implicate bacteria and justify studies of ileoanal pouch microbiota.
Studies of pouch microbiota
The interpretation of early work to establish the potential differences in bacterial microbiota between pouchitis and nonpouchitis patients is limited by the lack of universally accepted criteria for pouchitis prior to the Moskowitz score being described in 1986 [Moskowitz et al. 1986] and the pouch disease activity index (PDAI) in 1994 [Sandborn et al. 1994]. Many of these early studies were also limited by comparing well-functioning with poorly functioning pouches rather than nonpouchitis and pouchitis groups. In addition a further limitation of many studies was that many of the patients with a history of pouchitis were asymptomatic at the time of the study [Lim et al. 2006]. The majority of studies to establish the microbiota of the ileoanal pouch have been performed using culture methods, but it is now known that less than 50% of gut bacterial species can be cultured [Hayashi et al. 2002; Suau et al. 1999].
Estimates of the presence of 200–300 colonic species derived from culture-based studies have been revised to about 15, 000–36, 000 individual species following the introduction of 16s ribosomal RNA polymerase chain reaction (PCR) techniques [Frank et al. 2007]. There are several different PCR techniques each with a different sensitivity. Cloning and high-throughput sequencing of the 16 s gene is currently accepted as the ‘gold standard’ molecular method [Zoetendal et al. 2008; Lim et al. 2006] and therefore modern research should use molecular rather than culture-based techniques.
Studies using culture methods
Details of each of the culture studies are summarized in Tables 1 and 2.
Table 1.
Studies comparing the microbiota of UC pouchitis with nonpouchitis patient samples.
| Study | Patients | Faecal or mucosal study | Histological pouchitis? | Symptomatic pouchitis Y/N | Findings |
|---|---|---|---|---|---|
| Nicholls et al. [1981] | 10 UC, 4 FAP | Faecal | Yes | No | Increase in aerobes in pouchitis |
| Santavirta et al. [1991] | 30 UC-RPC (9 = Hx of pouchitis), 10 ileostomy | Faecal | No | Yes but only 3 were symptomatic | No difference |
| Ruseler-van Embden et al. [1994] | 12 UC-RPC, 2 FAP-RPC | Faecal | Yes | Yes but only 2 of 5 were symptomatic | Increase in number of aerobes and C. perfingens in pouchitis |
| Sandborn et al. [1995] | 10 UC-RPC nonpouchitis, 10 UC-RPC pouchitis, 5 FAP-RPC nonpouchitis, 5 ileostomy | Faecal | Yes | Yes (PDAI used) | No difference between pouchitis and nonpouchitis or FAP groups |
| Ohge et al. [2005] | 18 UC-RPC previous pouchitis, 8 UC non pouchitis, 8 UC-RPC antibiotic treated | Faecal | No | None | Increased counts of SRB in patients with active pouchitis |
| Kuisma et al. [2003] | 11 UC nonpouchitis, 21 with previous pouchitis | Faecal and mucosal | No | None | Increased aerobes and anaerobes in pouchitis faecal samples |
| Kmiot et al. [1993] | 6 RPC pouchitis, 6 RPC nonpouchitis | Faecal | Yes | None | No difference |
| O’Connell et al. [1986] | 14 nonpouchitis, 6 pouchitis | Faecal | No | Yes | No difference |
| Iwaya et al. [2006] | 9 pouchitis, 13 non-pouchitis | Faecal | Yes | Yes (PDAI used) | Reduction in Bifidobacteria, Bacteriodaceae and Lactobacillus in the pouchitis group. |
UC, ulcerative colitis; FAP, familial adenomatous polyposis; RPC, restorative proctocolectomy with ileal pouch-anal anastomosis; PDAI, pouch disease activity index.
Table 2.
Studies comparing the microbiota of UC with FAP patient samples (no pouchitis group).
| Study | Patient numbers | Faecal/ mucosal | Histological Px? | Symptomatic pouchitis Y/N | Findings |
|---|---|---|---|---|---|
| Duffy et al. [2002] | 10 UC-RPC, 7 FAP-RPC, 8 ileostomy | F | None | Increased SRB in UC patients | |
| Sandborn et al. [1995] | 10 UC-RPC nonpouchitis, 10 UC-RPC pouchitis, 5 FAP-RPC nonpouchitis, 5 ileostomy | Faecal | Yes | Yes (PDAI used) | No difference between pouchitis and nonpouchitis or FAP groups |
UC, ulcerative colitis; FAP, familial adenomatous polyposis; RPC, restorative proctocolectomy with ileal pouch-anal anastomosis; PDAI, pouch disease activity index; SRB, sulphate-reducing bacteria.
Nicholls and colleagues studied 14 patients who had undergone RPC at least 6 months earlier, seven patients had inflammation of the reservoir and seven did not [Nicholls et al. 1981]. Inflammation of the reservoir was associated with a higher count of aerobic but not anaerobic bacteria.
Santavirta and colleagues studied faecal samples from 30 UC patients after RPC and 10 with a conventional ileostomy [Santavirta et al. 1991]. Nine had a history of pouchitis defined as increased stool frequency, bleeding and abdominal cramping, however only three of these patients were symptomatic at the time of the study and only two with a history of pouchitis had faecal samples analysed. The authors found no difference in the numbers of anaerobes and aerobes in those with a history of pouchitis compared with those who had no history of pouchitis but did report an increase in the total bacterial counts, numbers of anaerobes and ratio of anaerobes to aerobes compared with patients with a conventional ileostomy.
Ruseler-van Embden and colleagues studied faecal samples of 14 RPC patients (12 UC; 2 FAP) [Ruseler-van Embden et al. 1994]. Pouchitis was defined by clinical, endoscopic and histological criteria. Five were classified as having pouchitis and nine nonpouchitis. Of the former two were symptomatic when studied. There was no difference in the total numbers of bacteria between pouchitis and nonpouchitis patients but an increase in the number of aerobes was found. Clostridium perfingens was present in over 75% of the pouchitis and in less than 25% of the nonpouchitis samples. Bifidobacterium and Lactobacillus were found in only one pouchitis sample but were present in 84% of nonpouchitis samples.
Duffy and colleagues studied 25 patients (10 UC RPC, 7 FAP RPC, 8 ileostomy) none of whom had a previous history of pouchitis [Duffy et al. 2002]. In the UC RPC patients 80% of the samples contained sulphate-reducing bacteria (SRB) in contrast to none of the FAP or ileostomy samples. The authors found no significant difference between UC and FAP in counts of Lactobacilli, C. perfingens, Bacteriodes and Bifidobacterium or enterococci and coliforms.
Sandborn and colleagues cultured the stool from 30 patients (10 UC RPC non-pouchitis, 10 UC RPC pouchitis, 5 FAP RPC non-pouchitis and 5 ileostomy) [Sandborn et al. 1995]. Pouchitis was defined as a PDAI ≥ 7. The authors found a higher ratio of anaerobic Gram-negative rods in RPC compared with ileostomy patients and there were no differences between pouchitis and non-pouchitis samples in FAP and UC RPC.
A study was carried out by Ohge and colleagues of 50 patients including 9 UC RPC with previous pouchitis but no active disease for 1 year, 9 UC RPC with pouchitis in the last year, but inactive for 6 weeks or more, 8 UC RPC more than 2 years ago and no previous pouchitis and 11 UC RPC pouchitis with ongoing antibiotic treatment [Ohge et al. 2005]. Stool samples were collected, and counts of SRB and output of hydrogen sulphide were measured. The authors found a fivefold greater release of hydrogen sulphide in all UC samples compared with the FAP samples except in the UC patients receiving antibiotic treatment, where there was no difference to the FAP group. Patients who had recently had pouchitis had a higher hydrogen sulphide production than those who had pouchitis more than 1 year previously which was higher than those who had never had pouchitis. SRB counts were higher in samples from active pouchitis patients than all other groups. Gosselink and colleagues cultured faecal samples from 13 patients with active pouchitis prior to antibiotic treatment, during treatment and following treatment. A comparison with nonpouchitis patients was not undertaken. They found that during an episode of pouchitis there was an increase in aerobes and decrease in anaerobes [Gosselink et al. 2004].
Kuisma and colleagues studied faecal microbiology and mucosal microbiology in 32 patients (11 nonpouchitis and 21 with a previous history of pouchitis) [Kuisma et al. 2003]. No patient had active pouchitis. The authors found a significantly increased total number of faecal anaerobic and aerobic bacteria in patients with a history of pouchitis as compared with nonpouchitis patients, but no difference in the bacteria from mucosal samples.
Kmiot and colleagues studied 46 UC patients (10 end ileostomy, 12 RPC pouchitis, 12 nonpouchitis good function and 12 nonpouchitis poor function) [Kmiot et al. 1993]. Pouchitis was defined as endoscopic and histological inflammation and patients did not need to be symptomatic. Bacterial counts of stool samples were performed on 6 non-pouchitis and 6 pouchitis patients before and following metronidazole. The authors found no significant difference in the total aerobic or anaerobic bacterial counts between the non-pouchitis and pouchitis groups, there were also no differences between the numbers of Escherichia coli, Streptococcus faecalis or Bacteriodes in each group. Bacteriodes were not present in any samples following metronidazole. There was no significant difference in counts of E. coli or S. faecalis between the pouchitis and nonpouchitis groups following metronidazole.
O’Connell and colleagues studied 20 UC patients (8 nonpouchitis with good function, defined as <6 stools per 24 h, 6 nonpouchitis poor function, defined as >6 stools per 24 h, and 6 pouchitis, defined as episodic bloody diarrhoea and malaise) [O’Connell et al. 1986]. Three patients had active pouchitis and three did not. Jejunal aspirates and stool samples were analysed. There was no difference in the numbers of aerobic and anaerobic bacteria between the pouchitis and nonpouchitis groups in the jejunal aspirates and the values were within the normal range for healthy controls. Stool culture showed overgrowth of both aerobic and anaerobic bacteria in all samples compared with values for ileal chyme in health. There were no differences between the pouchitis and nonpouchitis groups. Iwaya and colleagues cultured stool samples from 22 UC RPC patients (9 pouchitis, 13 nonpouchitis) [Iwaya et al. 2006]. Pouchitis was diagnosed using the PDAI. The authors found a significant reduction in the Bifidobacteria, Bacteriodaceae and Lactobacillus in the pouchitis group.
Studies using molecular methods
Falk and colleagues studied two UC RPC patients from the time of stoma closure for 1 year with repeated mucosal biopsies using terminal restriction fragment length polymorphism (T-RFLP), cloning and sequencing [Falk et al. 2007]. Neither patient developed pouchitis during this time. They found that the microbiota evolved during this time and differed between the two patients. The microbiotal composition was similar to normal colon except for the presence of Clostridium perfringens and Turicibacter. Kuhbacher and colleagues studied the differences in microbiota between patients with chronic pouchitis who were treated with the probiotic VSL#3 and those treated with a placebo following a successful remission induced by antibiotic treatment with ciprofloxacin and metronidazole [Kuhbacher et al. 2006]. This study did not compare nonpouchitis and pouchitis microbiota and did not study the microbiota of pouchitis patients before antibiotic therapy. The authors used real-time PCR, denaturing gel gradient electrophoresis (DGGE) and fluorescent in-situ hybridization (FISH) to study the microbiota. Patients who relapsed on placebo had a lower bacterial diversity and increased fungal diversity than those who maintained remission with VSL#3, with an increase in the diversity of Lactobacilli and Bifidobacteria species. However, the authors stated that the methods used did not reliably identify all bacteria present.
Casadesus and colleagues studied the possible role of Cytomegalovirus (CMV) in pouchitis; 34 UC RPC patients were studied [Casadesus et al. 2007]. Pouchitis was diagnosed using the modified PDAI [Shen et al. 2003] and Japanese classification of pouchitis (JCP) [Fukushima et al. 2007]. The JCP defines pouchitis as a condition with severe endoscopic findings or with two or more clinical symptoms and moderate endoscopic findings. Endoscopic biopsies from the pouch mucosa were taken on multiple occasions in each patient resulting in a total of 473 specimens (103 pouchitis patients, 370 nonpouchitis patients). CMV was detected in both pouchitis and nonpouchitis biopsies using PCR and sequencing and no significant difference was found in the incidence of CMV in each group when classified using the modified PDAI. CMV was, however, present in significantly more samples from pouchitis than from nonpouchitis patients when classified using the JCP. All pouchitis biopsies with CMV detected were obtained during the first episode of pouchitis, and in earlier samples from the same patients suggesting that CMV infection causes symptoms similar to pouchitis but is not the cause of pouchitis.
Four studies have used a molecular biological method to establish the differences between the microbiota in pouchitis and nonpouchitis patients. In the first Komanduri and colleagues studied ileal pouch biopsies and faecal samples from 33 patients (13 non-IBD patients, 5 UC pouchitis, 15 non-pouchitis) [Komanduri et al. 2007]. Pouchitis was classified using the PDAI. Ileal biopsies were taken from non-IBD patients who had an intact colon. Length heterogeneity PCR (LH-PCR) was performed and output data were expressed as electropherograms with the peaks representing different populations of microbiota with amplicons of different length in base pairs. Because this technique provides limited information about the bacterial differences at a species/phylotype level, the LH-PCR products from three non-IBD controls, five UC nonpouchitis and three UC pouchitis patients were pooled and cloned. Data were then filtered so that only phylotypes representing more than 5% of the total clone library were analysed. The authors reported an increase in the bacterial diversity in pouchitis samples compared with non-IBD controls, an increase in the proportion of Enterobacters and Fusobacter (proteobacteria), reduction in Streptococci (firmicutes) and a difference in the Rumicoccus species associated with pouchitis (R. obeum) and nonpouchitis (R. gnavus).
The use of pooled data for each of the disease groups was a significant source of error in this study. Since it is known that there is a wide variation in gut microbiota between individuals [Zoetendal et al. 2008; Komanduri et al. 2007], comparisons can only be made between groups when the data are derived from different individuals. In addition. according to the authors the technique used identified at most 73% of the microbial population.
The second molecular study (from our group) by Johnson and colleagues investigated the mucosal adherent microbiota in 32 RPC patients (7 UC pouchitis, 15 UC non-pouchitis, 9 FAP nonpouchitis, 1 FAP pouchitis) [Johnson et al. 2009]. Mucosal biopsies were first cultured on agar, the bacterial DNA was then extracted and amplified using PCR and profiles were generated using TRFLP [Johnson et al. 2009]. No differences between pouchitis and nonpouchitis groups or between FAP and UC groups were found but this study also had significant limitations. The use of an enrichment culture technique potentially favoured identification of bacteria supported by the culture conditions and TRFLP can only identify dominant species groups and is not able to identify individual bacterial species.
A further study by Lim and colleagues investigated the luminal microbiota in 20 RPC patients (5 UC pouchitis and 15 UC non-pouchitis) using TRFLP. They reported no differences in diversity or TRFLP profile between pouchitis and nonpouchitis samples. Again this study was limited by the use of TRFLP [Lim et al. 2009].
We recently performed a further study of the mucosal adherent microbiota in 24 RPC patients (8 UC pouchitis, 8 nonpouchitis, 5 FAP nonpouchitis and 3 FAP pouchitis). PCR amplification of bacterial 16S rRNA genes was performed and samples were individually cloned and sequenced to the species/phylotype level [McLaughlin et al. 2010]. There was a significant increase in Proteobacteria and a significant reduction in Bacteroidetes and Faecalibacterium prausnitzii in the total UC compared with the total FAP cohort with only small differences between the UC nonpouchitis and UC pouchitis groups and the FAP pouchitis and FAP nonpouchitis groups. Bacterial diversity was significantly greater in FAP nonpouchitis compared with UC nonpouchitis samples and significantly greater in UC nonpouchitis samples compared with UC pouchitis samples. No individual species or phylotype was specifically associated with either UC or FAP pouchitis.
Summary
The results from culture-based studies of pouch microbiota using culture methods are varied and inconclusive. Advances in our understanding of gut microbiota following the introduction of molecular techniques have led to the realization that culture-based techniques are inadequate for the study of gut microbiota. The differences in findings between earlier and the most recent molecular studies are likely to be explained by the different techniques employed and the limitations of the earlier studies. The findings of the most recent study [McLaughlin et al. 2010] suggest that a reduction in bacterial diversity but not dysbiosis occurs in pouchitis and that UC RPC patients have a different, less-diverse microbiota with increased Proteobacteria and reduced Bacteroidetes and F. prausnitzii.
Treatment of pouchitis
Before drug treatment is initiated a clinical diagnosis of pouchitis should be confirmed by flexible pouchoscopy and histological biopsy demonstrating acute inflammation since other causes of pouch dysfunction may present with similar symptoms [McLaughlin et al. 2008b]. In addition other precipitants should be considered; NSAIDs are associated with pouchitis and withdrawal may induce remission [Shen et al. 2007b]. Infectious agents such as Clostridium difficile and CMV should be excluded in those with pouchitis that fails to respond to antibiotics [Pfau and Lichtenstein, 2000].
Antibiotics
Ciprofloxacin or metronidazole for 14 days has been effective in several studies and should be considered as first-line treatment [Shen et al. 2001; Madden et al. 1994]. Topical metronidazole, given per anum, has also been shown to be effective and should be considered in patients intolerant to oral metronidazole who fail treatment with ciprofloxacin [Johnson et al. 2001]. Rifaxamin when used as a single agent for the treatment of pouchitis was found to be no more effective than placebo in a randomized placebo-controlled study of 18 patients [Isaacs et al. 2007].
Treatment options for patients who fail to respond to combination antibiotics or who relapse on maintenance treatment are very limited and some patients will have chosen indefinite diversion with an end ileostomy (with or without pouch excision). Using faecal coliform sensitivity testing our group recently demonstrated that ineffective combination antibiotic treatment may be due to the development of bacterial resistance. In a study of 15 patients who had failed to respond to empirical antibiotic treatment, ciprofloxacin resistance was identified in all stool samples and selection of another antibiotic based on bacterial sensitivity analysis resulted in remission in 80% of patients [McLaughlin et al. 2009a].
Other agents
Budesonide has been shown to be effective in those with refractory pouchitis who do not respond to a single antibiotic in an open-label study [Gionchetti et al. 2007b] but a randomized double-blind study found it to be no more effective than metronidazole [Sambuelli et al. 2002]. Its efficacy in those who fail to respond to ciprofloxacin and metronidazole or other combination regimes has not been demonstrated.
Bismuth-citrate carbomer enemas were shown to be effective in an open-label study using with 83% of patients entering remission [Gionchetti et al. 1997], however a subsequent double-blind randomised trial found no difference when compared with placebo [Tremaine et al. 1997].
There are no controlled trials of oral or topical mesalamine for pouchitis. A study which compared a combination of ciprofloxacin and tinidazole in pouchitis with a historical cohort treated with mesalamine found that 50% achieved clinical remission and 50% had a clinical response. Overall there was a significant reduction in the PDAI but no improvement in the Cleveland global quality of life (CGQoL) score, whereas of the patients treated with ciprofloxacin and tinidazole, 87.5% achieved a clinical remission and 87.5% achieved a clinical response with a significant reduction in PDAI and improvement in CGQoL score [Shen et al. 2007a]. Sulphasalazine was recently found to be effective for the treatment of pouchitis. In an open-label study of 11 patients 63% of patients were in remission after 8 weeks treatment [Belluzzi et al. 2010].
Allopurinol was found to be ineffective in preventing pouchitis in a randomised placebo controlled study [Joelsson et al. 2001]. Treatment with short chain fatty acids (SCFAs) was also ineffective in uncontrolled studies [de Silva et al. 1989].
Infliximab was beneficial in one study of patients with pouchitis and extensive PPI; however, they were found to have almost total small bowel inflammation (demonstrated by video-capsule endoscopy). It is possible therefore that this patient group had CD rather than UC [Calabrese et al. 2008]. In a recent retrospective uncontrolled study of 10 patients, seven maintained a clinical response at a median follow up of 8.5 months and endoscopy demonstrated four to have continuing mucosal inflammation [Ferrante et al. 2010].
Other therapies that have been tried include oral prednisolone and azathioprine. There is, however, no good evidence that these are effective in pouchitis [Pardi and Sandborn, 2006]. There are two reports of the development of pouchitis in patients taking azathioprine for primary sclerosing cholangitis [Rowley et al. 1995; Zins et al. 1995] suggesting that immunomodulators may not be effective in pouchitis.
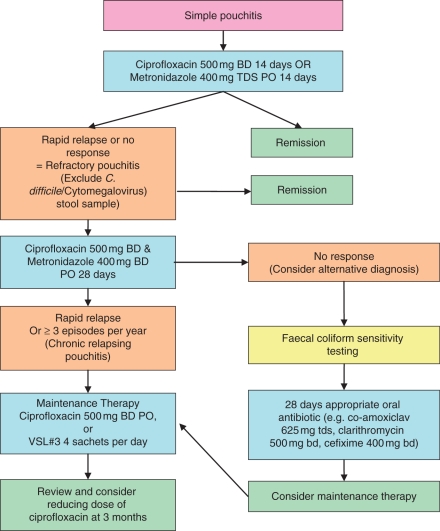
An algorithm for the treatment of pouchitis is given in Figure 1.
Figure 1.
An algorithm for the treatment of pouchitis. Reproduced with permission from Blackwell Publishing Ltd (McLaughlin et al. [2008b]).
Treatment of prepouch ileitis
Two studies have reported the results of treatment for PPI. In the first, seven patients with chronic relapsing pouchitis and total small bowel inflammation identified at video-capsule endoscopy were treated with a standard infliximab induction regime of three infusions. Symptomatic and endoscopic resolution was reported in six [Calabrese et al. 2008]. Although CD had been excluded on histological grounds the presence of extensive small bowel inflammation raises the possibility that these patients were suffering from CD and not UC.
In the second study 14 patients with symptomatic PPI were treated with ciprofloxacin and metronidazole for 28 days. Twelve (86%) patients experienced symptomatic remission with a reduction in stool frequency from a median of 12 to 6 [McLaughlin et al. 2008a]. Given the cost and potentially significant complications associated with infliximab it is generally recommended that combination antibiotic therapy should be first-line treatment in patients with symptomatic PPI.
Maintenance therapy
It has been recommended that patients with chronic pouchitis who achieve remission following antibiotic therapy but relapse more than three times per year should be treated with maintenance therapy [Pardi and Sandborn, 2006]. Treatment with ciprofloxacin or the probiotic VSL#3 are recommended in the British Society of Gastroenterology IBD guidelines [Carter et al. 2004].
Studies using VSL#3
The probiotic VSL#3 was shown to maintain remission in 85% of patients with refractory pouchitis who had entered clinical and endoscopic remission following 4 weeks treatment with ciprofloxacin and rifaxamin in a randomized placebo-controlled study of 40 patients [Gionchetti et al. 2000], whilst a later two-centre randomised placebo-controlled study of 36 patients found that 85% of patients could maintain remission following 4 weeks treatment with ciprofloxacin and metronidazole [Mimura et al. 2004]. In contrast, however, a more recent 8-month open-label clinical study reported that less than 20% of patients were able to maintain remission [Shen et al. 2005].
The reason for the difference in these results is not clear but differences in the design and protocol of the two studies may have contributed. The most important and modifiable factors were as follows.
Studies by Gionchetti and colleagues [Gionchetti et al. 2000] and Mimura and colleagues [Mimura et al. 2004] excluded patients who did not achieve complete or near-complete endoscopic remission with a score of ≤3 in the clinical component of the PDAI, whereas Shen et al. [2005] did not repeat the pouchoscopy after clinical remission. It is known that some patients do not achieve endoscopic remission despite clinical remission following antibiotic treatment [Madden et al. 1994] and it is possible that this subset of patients have a more difficult to treat disease which may not respond to probiotics.
Gionchetti’s and Mimura’s groups used a combination of ciprofloxacin and rifaxamin or metronidazole for 4 weeks whereas Shen and colleagues used a 2-week course of ciprofloxacin only to induce remission. It is possible that probiotic therapy is more effective following a combination of two different antibiotic agents for a prolonged period. Gionchetti and colleagues’ and Mimura and colleagues’ studies recruited patients with refractory pouchitis, defined as three or more episodes of pouchitis per year, whereas Shen and colleagues only recruited patients with chronic antibiotic-dependent pouchitis, defined as four or more episodes of pouchitis per year. Therefore, many of the patients included in the studies of Gionchetti’s and Mimura’s groups had less-aggressive disease in which maintenance of remission may have been easier to achieve.
Figure 2.
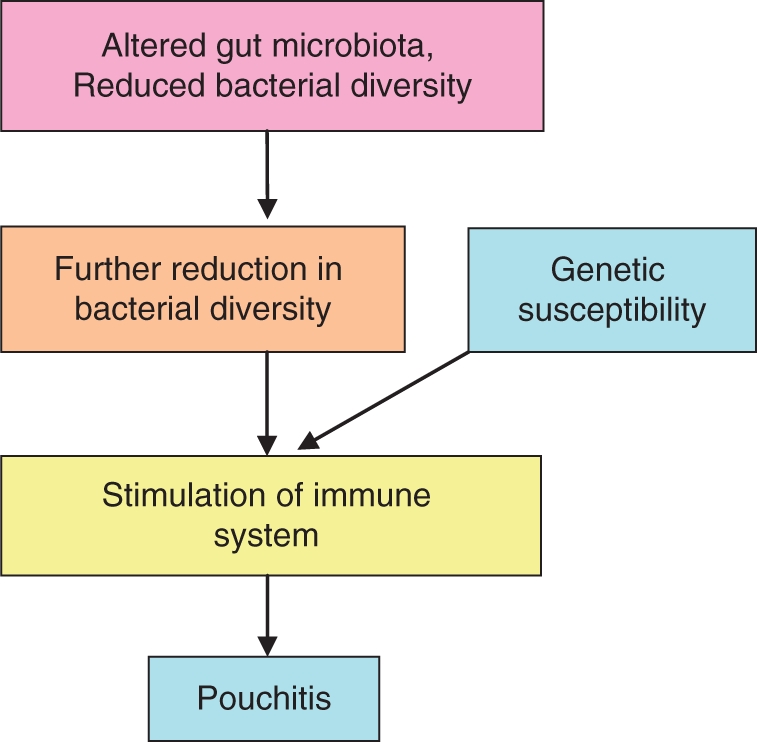
Potential mechanism of dysbiosis in pouchitis.
Studies of antibiotic maintenance therapy
Two studies have reported the outcome of maintenance antibiotic treatment. In the first, 51 patients were treated with rifaxamin maintenance therapy after induction of remission with single or combination antibiotic therapy [Shen et al. 2008]. At 3, 12 and 24 months 65%, 58% and 3% of patients were in remission. Importantly those who failed to achieve endoscopic as well as clinical remission were excluded from the study. In the second study (from our group) [McLaughlin et al. 2009b] which included both patients who had and who had not achieved endoscopic as well as clinical remission, retrospective data on 25 patients were reported. Several antibiotics had been used including ciprofloxacin, trimethoprim, coamoxiclav, colistin, nitrofurantoin, cefixime and trimethoprim. During a median follow up of 15.8 months no patient developed C. difficile colonization but 24% relapsed. In these, faecal coliform sensitivity testing [McLaughlin et al. 2009a] demonstrated bacterial resistance in all cases and identified an appropriate change of antibiotic which resulted in the induction and maintenance of remission in all of these patients. Those who developed antibiotic resistance were successfully treated with two-weekly alternating antibiotics. In this study the presence of PPI but not failure of mucosal healing was associated with an increased risk of relapse.
The recent comprehensive molecular study comparing the microbiota in UC and FAP RPC patients [McLaughlin et al. 2010] identified similar differences to those reported previously in the colonic mucosa of UC and CD patients compared with patients without IBD. Frank and colleagues reported a reduction in Bacteroidetes and Lachnospiraceae and a reciprocal increase in Proteobacteria in a subset of IBD patients [Frank et al. 2007]. Other studies too have reported increases in Proteobacteria in IBD patients [Gophna et al. 2006; Seksik et al. 2003]. F. prausnitzii which was reduced in the UC RPC group has previously been postulated to have anti-inflammatory properties and found to be reduced in IBD patients [Sokol et al. 2009, 2008].
Conclusion
Bacterial diversity has been demonstrated in UC and CD and appears to be important in maintaining normal gut homeostasis [Andoh et al. 2007; Sokol et al. 2006; Ott et al. 2004]. In RPC there is a reduction in diversity in UC patients compared with FAP patients and a further reduction in diversity in patients with pouchitis [McLaughlin et al. 2010]. Overall these findings suggest that the pathogenesis of pouchitis is similar to that of UC and CD and that either a reduction in bacterial diversity leads to stimulation of the immune system resulting in mucosal inflammation or that dysbiosis predisposes UC patients to pouchitis by increasing the likelihood of immune system stimulation.
Antibiotics remain the mainstay of treatment for pouchitis and PPI. The majority of patients will enter clinical remission following single-agent or combination antibiotic therapy. Failure to respond to this treatment is likely to be due to the presence of resistant organisms. Faecal coliform sensitivity testing to identify an appropriate alternative antibiotic should be performed. About 5–19% of patients will develop chronic relapsing pouchitis [Madiba and Bartolo, 2001; Mowschenson et al. 2000; Hurst et al. 1998]. These should be treated with maintenance therapy to maintain symptomatic remission. In those patients where maintenance therapy fails to control symptoms indefinite diversion with an ileostomy with or without pouch excision should be considered.
Conflict of interest statement
The Authors declare that there are no conflicts of interest.
Financial support
S.D. McLaughlin is supported by the Broad medical research programme and the St Mark’s Hospital foundation.
References
- Andoh A., Sakata S., Koizumi Y., Mitsuyama K., Fujiyama Y., Benno Y. (2007) Terminal restriction fragment length polymorphism analysis of the diversity of fecal microbiota in patients with ulcerative coliti? Inflamm Bowel Dis 13: 955–962 [DOI] [PubMed] [Google Scholar]
- Barton J.G., Paden M.A., Lane M., Postier R.G. (2001) Comparison of postoperative outcomes in ulcerative colitis and familial polyposis patients after ileoanal pouch operation? Am J Surg 182: 616–620 [DOI] [PubMed] [Google Scholar]
- Bell A.J., Nicholls R.J., Forbes A., Ellis H.J., Ciclitira P.J. (2004) Human lymphocyte stimulation with pouchitis flora is greater than with flora from a healthy pouch but is suppressed by metronidazol? Gut 53: 1801–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.J., Price A.B., Forbes A., Ciclitira P.J., Groves C., Nicholls R.J. (2006) Pre-pouch ileitis: a disease of the ileum in ulcerative colitis after restorative proctocolectom? Colorectal Dis 8: 402–410 [DOI] [PubMed] [Google Scholar]
- Belluzzi A., Serrani M., Roda G., Bianchi M.L., Castellani L., Grazia M., et al. (2010) Pilot study: the use of sulfasalazine for the treatment of acute pouchiti? Aliment Pharmacol Ther 31(2): 228–232 [DOI] [PubMed] [Google Scholar]
- Calabrese C., Fabbri A., Gionchetti P., Rizzello F., Morselli C., Liguori G., et al. (2007) Controlled study using wireless capsule endoscopy for the evaluation of the small intestine in chronic refractory pouchiti? Aliment Pharmacol Ther 25: 1311–1316 [DOI] [PubMed] [Google Scholar]
- Calabrese C., Gionchetti P., Rizzello F., Liguori G., Gabusi V., Tambasco R., et al. Short-term treatment with infliximab in chronic refractory pouchitis and ileitis. (2008) Aliment Pharmacol Ther 27: 759–764 [DOI] [PubMed] [Google Scholar]
- Carter M.J., Lobo A.J., Travis S.P. (2004) Guidelines for the management of inflammatory bowel disease in adult? Gut 53(Suppl 5): V1–V16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus D., Tani T., Wakai T., Maruyama S., Iiai T., Okamoto H., et al. (2007) Possible role of human cytomegalovirus in pouchitis after proctocolectomy with ileal pouch-anal anastomosis in patients with ulcerative coliti? World J Gastroenterol 13: 1085–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva H.J., Ireland A., Kettlewell M., Mortensen N., Jewell D.P. (1989) Short-chain fatty acid irrigation in severe pouchiti? N Engl J Med 321: 1416–1417 [DOI] [PubMed] [Google Scholar]
- Dozois R.R., Kelly K.A., Welling D.R., Gordon H., Beart R.W., Jr, Wolff B.G., et al. (1989) Ileal pouch-anal anastomosis: comparison of results in familial adenomatous polyposis and chronic ulcerative coliti? Ann Surg 210: 268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M., O’Mahony L., Coffey J.C., Collins J.K., Shanahan F., Redmond H.P., et al. (2002) Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposi? Dis Colon Rectum 45: 384–388 [DOI] [PubMed] [Google Scholar]
- Elewaut D., De Keyser F., Cuvelier C., Lazarovits A.I., Mielants H., Verbruggen G., et al. (1998) Distinctive activated cellular subsets in colon from patients with Crohn’s disease and ulcerative coliti? Scand J Gastroenterol 33: 743–748 [DOI] [PubMed] [Google Scholar]
- Falk A., Olsson C., Ahrne S., Molin G., Adawi D., Jeppsson B. (2007) Ileal pelvic pouch microbiota from two former ulcerative colitis patients, analysed by DNA-based methods, were unstable over time and showed the presence of Clostridium perfringen? Scand J Gastroenterol 42: 973–985 [DOI] [PubMed] [Google Scholar]
- Fazio V.W., Ziv Y., Church J.M., Oakley J.R., Lavery I.C., Milsom J.W., et al. (1995) Ileal pouch-anal anastomoses complications and function in 1005 patient? Ann Surg 222: 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante M., D’Haens G., Dewit O., Baert F., Holvoet J., Geboes K., et al. (2010) Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case serie? Inflamm Bowel Dis 16(2): 243–249 [DOI] [PubMed] [Google Scholar]
- Fiocchi C. (1998) Inflammatory bowel disease: etiology and pathogenesi? Gastroenterology 115: 182–205 [DOI] [PubMed] [Google Scholar]
- Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel disease? Proc Natl Acad Sci U S A 104: 13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Fujii H., Yamamura T., Sugita A., Kameoka S., Nagawa H., et al. (2007) Pouchitis atlas for objective endoscopic diagnosi? J Gastroenterol 42: 799–806 [DOI] [PubMed] [Google Scholar]
- Gately M.K., Renzetti L.M., Magram J., Stern A.S., Adorini L., Gubler U., et al. (1998) The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune response? Annu Rev Immunol 16: 495–521 [DOI] [PubMed] [Google Scholar]
- Giacomelli R., Passacantando A., Parzanese I., Vernia P., Klidara N., Cucinelli F., et al. (1998) Serum levels of soluble CD30 are increased in ulcerative colitis (UC) but not in Crohn’s disease (CD? Clin Exp Immunol 111: 532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Morselli C., Poggioli G., Tambasco R., Calabrese C., et al. (2007a) High-dose probiotics for the treatment of active pouchiti? Dis Colon Rectum 50: 2075–2082 [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Poggioli G., Morselli C., Lammers K.M., Campieri M. (2005) Probiotic therapy to prevent pouchitis onse? Dis Colon Rectum 48: 1493–1494 [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Poggioli G., Pierangeli F., Laureti S., Morselli C., et al. (2007b) Oral budesonide in the treatment of chronic refractory pouchiti? Aliment Pharmacol Ther 25: 1231–1236 [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Venturi A., Brigidi P., Matteuzzi D., Bazzocchi G., et al. (2000) Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled tria? Gastroenterology 119: 305–309 [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Venturi A., Ferretti M., Brignola C., Peruzzo S., et al. (1997) Long-term efficacy of bismuth carbomer enemas in patients with treatment-resistant chronic pouchiti? Aliment Pharmacol Ther 11: 673–678 [DOI] [PubMed] [Google Scholar]
- Gophna U, Sommerfeld K, Gophna S, Doolittle W.F, Veldhuyzen van Zanten S.J.2006Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative coliti? J Clin Microbiol 44: 4136–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselink M.P., Schouten W.R., van Lieshout L.M., Hop W.C., Laman J.D., Ruseler-van Embden J.G. (2004) Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchiti? Dis Colon Rectum 47: 1519–1525 [DOI] [PubMed] [Google Scholar]
- Hahnloser D., Pemberton J.H., Wolff B.G., Larson D.R., Crownhart B.S., Dozois R.R. (2007) Results at up to 20 years after ileal pouch-anal anastomosis for chronic ulcerative coliti? Br J Surg 94: 333–340 [DOI] [PubMed] [Google Scholar]
- Hayashi H., Sakamoto M., Benno Y. (2002) Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based method? Microbiol Immunol 46: 535–548 [DOI] [PubMed] [Google Scholar]
- Hurst R.D., Chung T.P., Rubin M., Michelassi F. (1998) The implications of acute pouchitis on the long-term functional results after restorative proctocolectom? Inflamm Bowel Dis 4: 280–284 [DOI] [PubMed] [Google Scholar]
- Isaacs K.L., Sandler R.S., Abreu M., Picco M.F., Hanauer S.B., Bickston S.J., et al. (2007) Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot stud? Inflamm Bowel Dis 13: 1250–1255 [DOI] [PubMed] [Google Scholar]
- Iwata T., Yamamoto T., Umegae S., Matsumoto K. (2007) Pouchitis and pre-pouch ileitis developed after restorative proctocolectomy for ulcerative colitis: a case repor? World J Gastroenterol 13: 643–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya A., Iiai T., Okamoto H., Ajioka Y., Yamamoto T., Asahara T., et al. (2006) Change in the bacterial flora of pouchiti? Hepatogastroenterology 53: 55–59 [PubMed] [Google Scholar]
- Joelsson M., Andersson M., Bark T., Gullberg K., Hallgren T., Jiborn H., et al. (2001) Allopurinol as prophylaxis against pouchitis following ileal pouch-anal anastomosis for ulcerative colitis. A randomized placebo-controlled double-blind stud? Scand J Gastroenterol 36: 1179–1184 [DOI] [PubMed] [Google Scholar]
- Johnson E., Carlsen E., Nazir M., Nygaard K. (2001) Morbidity and functional outcome after restorative proctocolectomy for ulcerative coliti? Eur J Surg 167: 40–45 [DOI] [PubMed] [Google Scholar]
- Johnson M.W., Rogers G.B., Bruce K.D., Lilley A.K., von Herbay A., Forbes A., et al. (2009) Bacterial community diversity in cultures derived from healthy and inflamed ileal pouches after restorative proctocolectom? Inflamm Bowel Dis 15(12): 1803–1811 [DOI] [PubMed] [Google Scholar]
- Kartheuser A.H., Parc R., Penna C.P., Tiret E., Frileux P., Hannoun L., et al. (1996) Ileal pouch-anal anastomosis as the first choice operation in patients with familial adenomatous polyposis: a ten-year experienc? Surgery 119: 615–623 [DOI] [PubMed] [Google Scholar]
- Kmiot W.A., Youngs D., Tudor R., Thompson H., Keighley M.R. (1993) Mucosal morphology, cell proliferation and faecal bacteriology in acute pouchiti? Br J Surg 80: 1445–1449 [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R.M., Clark S.C., Chan S., Loudon R., et al. (1989) Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocyte? J Exp Med 170: 827–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komanduri S., Gillevet P.M., Sikaroodi M., Mutlu E., Keshavarzian A. (2007) Dysbiosis in pouchitis: evidence of unique microfloral patterns in pouch inflammatio? Clin Gastroenterol Hepatol 5: 352–360 [DOI] [PubMed] [Google Scholar]
- Kuhbacher T., Ott S.J., Helwig U., Mimura T., Rizzello F., Kleessen B., et al. (2006) Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchiti? Gut 55: 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuisma J., Jarvinen H., Kahri A., Farkkila M. (2004) Factors associated with disease activity of pouchitis after surgery for ulcerative coliti? Scand J Gastroenterol 39: 544–548 [DOI] [PubMed] [Google Scholar]
- Kuisma J., Mentula S., Luukkonen P., Jarvinen H., Kahri A., Farkkila M. (2003) Factors associated with ileal mucosal morphology and inflammation in patients with ileal pouch-anal anastomosis for ulcerative coliti? Dis Colon Rectum 46: 1476–1483 [DOI] [PubMed] [Google Scholar]
- Lim M., Adams J.D., Wilcox M., Finan P., Sagar P., Burke D. (2009) An assessment of bacterial dysbiosis in pouchitis using terminal restriction fragment length polymorphisms of 16S ribosomal DNA from pouch effluent microbiot? Dis Colon Rectum 52: 1492–1500 [DOI] [PubMed] [Google Scholar]
- Lim M., Sagar P., Finan P., Burke D., Schuster H. (2006) Dysbiosis and pouchiti? Br J Surg 93: 1325–1334 [DOI] [PubMed] [Google Scholar]
- Lohmuller J.L., Pemberton J.H., Dozois R.R., Ilstrup D., van H.J. (1990) Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosi? Ann Surg 211: 622–627 [PMC free article] [PubMed] [Google Scholar]
- Madden M.V., McIntyre A.S., Nicholls R.J. (1994) Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchiti? Dig Dis Sci 39: 1193–1196 [DOI] [PubMed] [Google Scholar]
- Madiba T.E., Bartolo D.C. (2001) Pouchitis following restorative proctocolectomy for ulcerative colitis: incidence and therapeutic outcom? J R Coll Surg Edinb 46: 334–337 [PubMed] [Google Scholar]
- McLaughlin S.D., Clark S.K., Bell A.J., Tekkis P.P., Ciclitira P.J., Nicholls R.J. (2008a) An open study of antibiotics for the treatment of pre-pouch ileitis following restorative proctocolectomy with ileal pouch-anal anastomosi? Aliment Pharmacol Ther 27(10): 895–909 [Epub 2008 Feb 9. Review]. [DOI] [PubMed] [Google Scholar]
- McLaughlin S.D., Clark S.K., Shafi S., Petrovksa L., Tekkis P.P., Ciclitira P.J., et al. (2009a) Fecal coliform testing to identify effective antibiotic therapies for patients with antibiotic-resistant pouchiti? Clin Gastroenterol Hepatol 7: 545–548 [DOI] [PubMed] [Google Scholar]
- McLaughlin S.D., Clark S.K., Tekkis P.P., Ciclitira P.J., Nicholls R.J. (2008b) Review article: restorative proctocolectomy, indications, management of complications and follow-up–a guide for gastroenterologist? Aliment Pharmacol Ther 27: 895–909 [DOI] [PubMed] [Google Scholar]
- McLaughlin S.D., Clark S.K., Tekkis P.P., Ciclitira P.J., Nicholls R.J. (2009b) An open study of maintenance antibiotic therapy for chronic antibiotic dependent pouchitis; efficacy, complications and outcom? Clin Gastroenterol Hepatol 7(5): 545–548 [DOI] [PubMed] [Google Scholar]
- McLaughlin S.D., Walker A.W., Churcher C., Clark S.K., Tekkis P.P., Johnson M.W., et al. (2010) The bacteriology of pouchitis: a molecular phylogenetic analysis using 16srRNA gene cloning and sequencin? Ann Surg, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T., Rizzello F., Helwig U., Poggioli G., Schreiber S., Talbot I.C., et al. (2004) Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchiti? Gut 53: 108–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz R.L., Shepherd N.A., Nicholls R.J. (1986) An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoi? Int J Colorectal Dis 1: 167–174 [DOI] [PubMed] [Google Scholar]
- Mowschenson P.M., Critchlow J.F., Peppercorn M.A. (2000) Ileoanal pouch operation: long-term outcome with or without diverting ileostom? Arch Surg 135: 463–465 [DOI] [PubMed] [Google Scholar]
- Nicholls R.J., Belliveau P., Neill M., Wilks M., Tabaqchali S. (1981) Restorative proctocolectomy with ileal reservoir: a pathophysiological assessmen? Gut 22: 462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyam D.C., Brillant P.T., Dozois R.R., Kelly K.A., Pemberton J.H., Wolff B.G. (1997) Ileal pouch-anal canal anastomosis for familial adenomatous polyposis: early and late result? Ann Surg 226: 514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P.R., Rankin D.R., Weiland L.H., Kelly K.A. (1986) Enteric bacteriology, absorption, morphology and emptying after ileal pouch-anal anastomosi? Br J Surg 73: 909–914 [DOI] [PubMed] [Google Scholar]
- Ohge H., Furne J.K., Springfield J., Rothenberger D.A., Madoff R.D., Levitt M.D. (2005) Association between fecal hydrogen sulfide production and pouchiti? Dis Colon Rectum 48: 469–475 [DOI] [PubMed] [Google Scholar]
- Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Folsch U.R., et al. (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel diseas? Gut 53: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi D.S., Sandborn W.J. (2006) Systematic review: the management of pouchiti? Aliment Pharmacol Ther 23: 1087–1096 [DOI] [PubMed] [Google Scholar]
- Pfau P.R., Lichtenstein G.R. (2000) Cytomegalovirus infection as a cause of ileoanal pouchiti? Dis Colon Rectum 43: 113–114 [DOI] [PubMed] [Google Scholar]
- Romanos J., Samarasekera D.N., Stebbing J.F., Jewell D.P., Kettlewell M.G., Mortensen N.J. (1997) Outcome of 200 restorative proctocolectomy operations: the John Radcliffe Hospital experienc? Br J Surg 84: 814–818 [PubMed] [Google Scholar]
- Rowley S., Candinas D., Mayer A.D., Buckels J.A., McMaster P., Keighley M.R. (1995) Restorative proctocolectomy and pouch anal anastomosis for ulcerative colitis following orthotopic liver transplantatio? Gut 37: 845–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruseler-van Embden J.G., Schouten W.R., van Lieshout L.M. (1994) Pouchitis: result of microbial imbalance? Gut 35: 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambuelli A., Boerr L., Negreira S., Gil A., Camartino G., Huernos S., et al. (2002) Budesonide enema in pouchitis—a double-blind, double-dummy, controlled tria? Aliment Pharmacol Ther 16: 27–34 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Pardi D.S. (2004) Clinical management of pouchiti? Gastroenterology 127: 1809–1814 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Tremaine W.J., Batts K.P., Pemberton J.H., Phillips S.F. (1994) Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Inde? Mayo Clin Proc 69: 409–415 [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Tremaine W.J., Batts K.P., Pemberton J.H., Rossi S.S., Hofmann A.F., et al. (1995) Fecal bile acids, short-chain fatty acids, and bacteria after ileal pouch-anal anastomosis do not differ in patients with pouchiti? Dig Dis Sci 40: 1474–1483 [DOI] [PubMed] [Google Scholar]
- Santavirta J., Mattila J., Kokki M., Matikainen M. (1991) Mucosal morphology and faecal bacteriology after ileoanal anastomosi? Int J Colorectal Dis 6: 38–41 [DOI] [PubMed] [Google Scholar]
- Sartor R.B. (1991) Pathogenetic and clinical relevance of cytokines in inflammatory bowel diseas? Immunol Res 10: 465–471 [DOI] [PubMed] [Google Scholar]
- Sartor R.B. (2008) Microbial influences in inflammatory bowel disease? Gastroenterology 134: 577–594 [DOI] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, et al. (2003) Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52: 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Achkar J.P., Connor J.T., Ormsby A.H., Remzi F.H., Bevins C.L., et al. (2003) Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchiti? Dis Colon Rectum 46: 748–753 [DOI] [PubMed] [Google Scholar]
- Shen B., Achkar J.P., Lashner B.A., Ormsby A.H., Remzi F.H., Brzezinski A., et al. (2001) A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchiti? Inflamm Bowel Dis 7: 301–305 [DOI] [PubMed] [Google Scholar]
- Shen B., Brzezinski A., Fazio V.W., Remzi F.H., Achkar J.P., Bennett A.E., et al. (2005) Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practic? Aliment Pharmacol Ther 22: 721–728 [DOI] [PubMed] [Google Scholar]
- Shen B., Fazio V.W., Remzi F.H., Bennett A.E., Brzezinski A., Lopez R., et al. (2006) Risk factors for clinical phenotypes of Crohn’s disease of the ileal pouc? Am J Gastroenterol 101: 2760–2768 [DOI] [PubMed] [Google Scholar]
- Shen B., Fazio V.W., Remzi F.H., Bennett A.E., Lopez R., Brzezinski A., et al. (2007a) Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchiti? Dis Colon Rectum 50(4): 498–508 [DOI] [PubMed] [Google Scholar]
- Shen B., Fazio V.W., Remzi F.H., Bennett A.E., Lopez R., Lavery I.C., et al. (2007b) Effect of withdrawal of nonsteroidal anti-inflammatory drug use on ileal pouch disorder? Dig Dis Sci 52(12): 3321–3328 [Epub 2007 Apr 5]. [DOI] [PubMed] [Google Scholar]
- Shen B., Remzi F.H., Lopez A.R., Queener E. (2008) Rifaximin for maintenance therapy in antibiotic-dependent pouchiti? BMC Gastroenterol 8: 26–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatter C., Girgis S., Huynh H., El Matary W. (2008) Pre-pouch ileitis after colectomy in paediatric ulcerative coliti? Acta Paediatr 97: 381–383 [DOI] [PubMed] [Google Scholar]
- Sokol H., Lepage P., Seksik P., Dore J., Marteau P. (2006) Temperature gradient gel electrophoresis of fecal 16S rRNA reveals active Escherichia coli in the microbiota of patients with ulcerative coliti? J Clin Microbiol 44: 3172–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermudez-Humaran L.G., Gratadoux J.J., et al. (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patient? Proc Natl Acad Sci U S A 105: 16731–16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Seksik P., Furet J.P., Firmesse O., Nion-Larmurier I., Beaugerie L., et al. (2009) Low counts of Faecalibacterium prausnitzii in colitis microbiot? Inflamm Bowel Dis 15: 1183–1189 [DOI] [PubMed] [Google Scholar]
- Stahlberg D., Gullberg K., Liljeqvist L., Hellers G., Lofberg R. (1996) Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factor? Dis Colon Rectum 39: 1012–1018 [DOI] [PubMed] [Google Scholar]
- Stallmach A., Schafer F., Hoffmann S., Weber S., Muller-Molaian I., Schneider T., et al. (1998) Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchiti? Gut 43: 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suau A., Bonnet R., Sutren M., Godon J.J., Gibson G.R., Collins M.D., et al. (1999) Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gu? Appl Environ Microbiol 65: 4799–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.D., Forbes A., Nicholls R.J., Ciclitira P.J. (2001) Altered expression of the lymphocyte activation markers CD30 and CD27 in patients with pouchiti? Scand J Gastroenterol 36: 258–264 [DOI] [PubMed] [Google Scholar]
- Thomas P.D., Forbes A., Price A.B., Nicholls R.J., Ciclitira P.J. (2002) Differential expression of cell adhesion molecules within inflamed ileal pouch mucosa: relationship to recruited cell subtype? Eur J Gastroenterol Hepatol 14: 137–144 [DOI] [PubMed] [Google Scholar]
- Tremaine W.J., Sandborn W.J., Wolff B.G., Carpenter H.A., Zinsmeister A.R., et al. (1997) Bismuth carbomer foam enemas for active chronic pouchitis: a randomized, double-blind, placebo-controlled tria? Aliment Pharmacol Ther 11: 1041–1046 [DOI] [PubMed] [Google Scholar]
- Wolf J.M., Achkar J.P., Lashner B.A., Delaney C.P., Petras R.E., Goldblum J.R., et al. (2004) Afferent limb ulcers predict Crohn’s disease in patients with ileal pouch-anal anastomosi? Gastroenterology 126: 1686–1691 [DOI] [PubMed] [Google Scholar]
- Wolf S.F., Temple P.A., Kobayashi M., Young D., Dicig M., Lowe L., et al. (1991) Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cell? J Immunol 146: 3074–3081 [PubMed] [Google Scholar]
- Zins B.J., Sandborn W.J., Penna C.R., Landers C.J., Targan S.R., Tremaine W.J., et al. (1995) Pouchitis disease course after orthotopic liver transplantation in patients with primary sclerosing cholangitis and an ileal pouch-anal anastomosi? Am J Gastroenterol 90: 2177–2181 [PubMed] [Google Scholar]
- Zoetendal E.G., Rajilic-Stojanovic M., De Vos W.M. (2008) High-throughput diversity and functionality analysis of the gastrointestinal tract microbiot? Gut 57: 1605–1615 [DOI] [PubMed] [Google Scholar]