Abstract
Bowel symptoms including diarrhoea can be produced when excess bile acids (BA) are present in the colon. This condition, known as bile acid or bile salt malabsorption, has been under recognized, as the best diagnostic method, the 75Se-homocholic acid taurine (SeHCAT) test, is not available in many countries and is not fully utilized in others. Reduced SeHCAT retention establishes that this is a complication of many other gastrointestinal diseases. Repeated studies show SeHCAT tests are abnormal in about 30% of patients otherwise diagnosed as diarrhoea-predominant irritable bowel syndrome or functional diarrhoea, with an estimated population prevalence of around 1%. Recent work suggests that the condition previously called idiopathic bile acid malabsorption (BAM) is not in fact due to a defect in absorption, but results from an overproduction of BA because of defective feedback inhibition of hepatic bile acid synthesis, a function of the ileal hormone fibroblast growth factor 19 (FGF19). The approach to treatment currently depends on binding excess BA, to reduce their secretory actions, using colestyramine, colestipol and, most recently, colesevelam. Colesevelam has a number of potential advantages that merit further investigation in trials directed at patients with bile acid diarrhoea.
Keywords: bile acids, colesevelam, colestipol, colestyramine, Crohn's disease, fibroblast growth factor 19, functional diarrhoea, irritable bowel syndrome, malabsorption, SeHCAT
Introduction
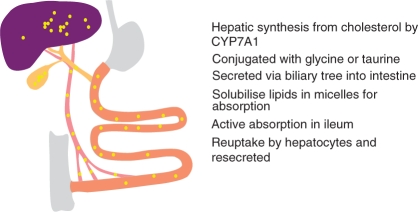
Bile acids (BA) are produced in the liver and have major roles in the absorption of lipids in the small intestine. They are synthesised from cholesterol by a number of steps, the first involving the enzyme CYP7A1, and are conjugated in the liver with _taurine and glycine, to give taurocholate, taurochenodeoxycholate, glycocholate and glycochenodeoxycholate, the primary BA. After their involvement in micelle formation in the small intestine, they are usually effectively reabsorbed in the terminal ileum, and taken up by the liver from the portal venous blood, and resecreted (Figure 1). This enterohepatic circulation is very efficient, with over 95% usually being absorbed and recycled. Estimates of the average kinetics of BA are shown in Table 1. Much of the history of the discovery of the roles of BA and their physiology has recently been reviewed by one of the major scientists in this area, Alan Hofmann [Hofmann, 2009a].
Figure 1.
The enterohepatic circulation of bile acids. The various steps in bile acid synthesis, secretion, absorption and resecretion are indicated.
Table 1.
Bile acid kinetics in a typical adult.
| Bile acid secretion | 12 g/day | 30 mmol/day |
| Bile acid pool size | 2–3 g | 5–7.5 mmol |
| Cycling frequency | 4–6 x/day | |
| Amount absorbed/cycle | ∼95% | |
| Faecal bile acid loss | <0.5 g/day | ∼1 mmol/day |
| Average half-life | ∼3 day |
Data taken from Hofmann [2009a] and Pattni and Walters [2009].
A small proportion of the secreted BA are not reabsorbed in the ileum and reach the colon. Here bacterial action results in deconjugation (removal of the taurine or glycine) and dehydroxylation (removal of the 7-hydroxy group), producing the secondary BA, deoxycholate and lithocholate. BA in the colon, in particular the dihydroxylated BA chenodeoxycholate and deoxycholate, stimulate electrolyte and water secretion. This will also increase motility, shortening the colonic transit time, and so producing diarrhoea with other gastrointestinal symptoms such as bloating, urgency and faecal incontinence. There have been several recent advances in the understanding of this condition of bile acid diarrhoea, which has also been called bile salt or bile acid malabsorption (BAM), and these are reviewed here.
Conditions associated with bile acid diarrhoea
In order to manage bile acid diarrhoea effectively, the diagnosis must be suspected: unfortunately this is a diagnosis that many gastroenterologists fail to consider [Khalid et al. 2010]. Many conditions give rise to BAM and diarrhoea [Pattni and Walters, 2009; Westergaard, 2007]. The usually effective enterohepatic circulation of bile salts is most obviously deranged in ileal disease. Following ileal resection, typically for Crohn's disease, BA are not absorbed efficiently, resulting in clear-cut BAM. This was called cholegenic diarrhoea or cholerheic enteropathy when first described by Alan Hofmann in 1967 [Hofmann, 1967]. The diarrhoea produced by increased faecal BA could be treated with bile acid sequestrants, as described in the following. Crohn's ileitis without resection will also affect bile acid absorption. It then became apparent that other conditions could mimic bile acid diarrhoea resulting from ileal resection or disease. The original type, secondary to ileal dysfunction, was then classified as type 1 BAM, with the idiopathic primary condition, first described by Thaysen and Pedersen [Thaysen and Pedersen, 1976, 1973], called type 2 BAM [Fromm and Malavolti, 1986].
Many other intestinal conditions can interfere with the normal physiology of bile acid reabsorption; these have been called type 3 BAM and result from conditions such as cholecystectomy, small intestinal bacterial overgrowth (SIBO) or pancreatic insufficiency. One common situation where BAM is under recognized and causes significant ill health is in patients who have been treated for malignancy, who may have radiation ileitis, pancreatitis, resection and/or bacterial overgrowth [Andreyev, 2007; Danielsson et al. 1991].
Most patients with BAM have few histological changes, but BAM can be found in up to 40% of people who have microscopic, collagenous or lymphocytic colitis [Wildt et al. 2003; Fernandez-Banares et al. 2001; Ung et al. 2000a]. The majority of these patients will respond to bile acid sequestrants and budesonide, a steroid with high first-pass metabolism in the liver, can help both conditions [Bajor et al. 2006b; Ung et al. 2000a].
Diagnostic tests
An increase in faecal BA is the most definitive way to show that they are responsible for diarrhoea, but outside of a very few research centres, these are not measured. Faecal tests are unpopular and difficult to perform. Fortunately, the SeHCAT (75Se-homocholic acid taurine) test was developed and measures loss of BA in a simple and reliable manner.
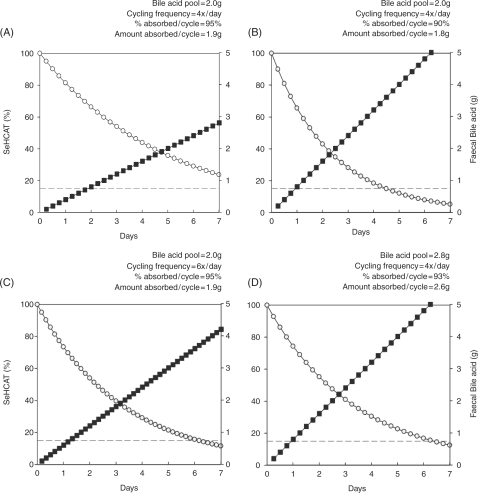
SeHCAT was first used in 1981 and can be measured in any nuclear medicine department with a gamma camera [Boyd et al. 1981]. This makes it much easier to use than the 14carbon-labelled glycocholate breath test which was used previously [Hofmann, 2009a; Roberts et al. 1977]. SeHCAT undergoes the same enterohepatic circulation as natural BA, and so tracks their retention and loss. Although faecal SeHCAT loss has been measured and it is possible to calculate the whole body retention half-life [van Tilburg et al. 1991], the usual measurement is the fraction retained at 7 days. This is simply calculated from the values obtained shortly after ingestion of the SeHCAT capsule and a repeat scan after a week. A normal 7-day value for SeHCAT retention is above 15% and retention below 10% is highly predictive of a successful response to therapy and so is used in many centres. In Figure 2, predicted SeHCAT retention and cumulative faecal loss of BA values are shown, calculated from a computer simulation model, in a normal situation with 95% absorption (Figure 2(A)) and with a somewhat impaired absorption of 90% (Figure 2(B)), where there is a doubling of faecal bile acid loss and a 7-day SeHCAT value of about 5%.
Figure 2.
Effects of specific changes affecting the enterohepatic circulation of bile acids on calculated 75Se-homocholic acid taurine (SeHCAT) retention and faecal bile acid loss. Predicted results from a computer simulation model of bile acid kinetics are presented. SeHCAT retention (open symbols, left axis) and cumulative faecal bile acid loss (closed symbols, right axis) are shown over 7 days. The dashed line indicates 10% SeHCAT retention. (A) Typical normal values. (B) Effects of reducing bile acid absorption on each cycle from 95% to 90%. (C) Effects of increasing the number of cycles to 6/day from 4/day. (D) Effects of increasing the total bile acid pool size from 2 g to 2.8 g. Absorption is increased from 1.9 g to 2.6 g; the fractional absorption decreases slightly from 95% to 93%.
Despite the utility, safety and relatively low cost of the SeHCAT test, many gastroenterologists fail to request this [Khalid et al. 2010]. It is not available in many countries and has never been licensed in the USA. This has hindered the recognition of BAM as a frequent finding in patients with chronic diarrhoea. Some physicians are content with a therapeutic trial of sequestrant therapy, but as this is often poorly tolerated (as described in the following), it is difficult to advocate continuing therapy in a situation of poor compliance when the diagnosis has not been unequivocally established with an independent test such as SeHCAT.
As an alternative test, the bile acid precursor, 7α-OH-4-cholesten-3-one (C4), can be measured in blood by high-performance liquid chromatography (HPLC) [Sauter et al. 1999; Brydon et al. 1996; Sciarretta et al. 1987]. With increased faecal bile acid loss, hepatic bile acid synthesis increases to compensate. Fasting values have been shown to be increased in patients with BAM. Unfortunately this assay is relatively time consuming, requiring special expertise, and is not widely available. It is a test that will demonstrate increased bile acid production and can be used in countries such as the USA where SeHCAT is not available [Camilleri et al. 2009].
Prevalence of bile acid diarrhoea
The use of the SeHCAT test has allowed a clear estimate of the prevalence of bile acid diarrhoea to be obtained. Many groups have reported their experience in patients with chronic diarrhoea [Spiller and Thompson, 2010; Muller et al. 2004; Wildt et al. 2003; Fernandez-Banares et al. 2001; Ung et al. 2000b; Smith et al. 2000; Sinha et al. 1998; Niaz et al. 1997; Eusufzai, 1993; Ford et al. 1992; Williams et al. 1991; Sciarretta et al. 1987; Merrick et al. 1985]. For instance, in one of the largest series in a total of 304 patients, the Rotherham group reported SeHCAT retention of less than 10% in 97% of 37 Crohn's patients with resection, 54% of 44 Crohn's patients without resection, 58% of 26 patients with gastric surgery and/or cholecystectomy, and in 33% of 197 patients with otherwise unexplained, idiopathic chronic diarrhoea who would otherwise have been classified as having diarrhoea-predominant irritable bowel syndrome (IBS-D) [Smith et al. 2000]. This latter set of patients with so-called idiopathic BAM was by far the largest.
We have recently performed a systematic review of studies which have estimated the prevalence of idiopathic BAM using SeHCAT scanning in patients with chronic diarrhoea, who were usually labelled as having IBS-D [Wedlake et al. 2009a]. The 18 studies (15 prospective) comprising 1223 patients are summarized in Table 2. They variously reported SeHCAT retention values of less than 5%, 10% or 15%. At the value with the best confidence interval (SeHCAT retention less than 10%), almost one third of patients have abnormal faecal bile acid loss.
Table 2.
Summary of studies reporting abnormal SeHCAT values in patients with diarrhoea-predominant IBS [Wedlake et al. 2009a].
| Reported SeHCAT value | <5% | <10% | <15% | Total |
|---|---|---|---|---|
| Number of studies reporting | 5 | 17 | 7 | 18 |
| Total number of patients | 429 | 1073 | 618 | 1223 |
| Number abnormal | 43 | 339 | 163 | |
| Percentage abnormal [95% confidence intervals] | 10% [7–13] | 32% [29–35] | 26% [23–30] | |
| Percentage response to colestyramine | 96% | 80% | 70% |
Using these figures we have estimated the population prevalence of bile acid diarrhoea. There are up to 3.9 million adults in the UK being treated for IBS, approximately 10% of the population. With one third of these having IBS-D, and one third of those having idiopathic BAM, we estimate the prevalence in the general population of this condition to be over 1% overall. This means there are a possible 500,000 people in the UK, and many millions of people in the Western world, that have this condition, exceeding better known conditions such as Crohn's or ulcerative colitis, which have prevalence figures of about 0.2% and 0.3% respectively, and broadly similar to coeliac disease. It is clearly incorrect to think of this as a rare cause of IBS: a myth which unfortunately is still mistakenly held [Spiller and Thompson, 2010].
Mechanisms of primary bile acid diarrhoea
Primary bile acid diarrhoea is thus a surprisingly common disease which is under recognized. Until recently, as its mechanisms were unclear, it has been called idiopathic bile acid malabsorption (IBAM), but now recent studies have indicated a potential mechanism, the use of this term may not longer be accurate [Walters et al. 2009].
There is an absence of obvious histological changes in the ileum. In some patients, the onset is triggered by a gastrointestinal infection [Niaz et al. 1997], but there are no clear associations with infectious agents or changes in inflammatory cells. This is an area that will need further research.
In a few patients with BAM, abnormal bile acid transporters may be found in the ileum. A rare mutation has been described in the apical sodium-dependent bile transporter (ASBT, gene name SLC10A2) in one family [Oelkers et al. 1997] but this is not present in most patients [Montagnani et al. 2006, 2001]. We looked for mutations in the cytoplasmic ileal bile acid binding protein (IBABP, gene name FABP6) but found no significant differences in the frequency of IBABP polymorphisms in patients or controls [Balesaria et al. 2008]. Polymorphisms in the basolateral membrane transport complex (organic solute transporters, OSTα and OSTβ) have not been studied.
We also looked for differences in expression levels of these transporter transcripts in ileal biopsies. These results were largely negative, with no differences overall in the mean expression of transcripts for the transporter molecules. Certain differences were found in the relationships of key transcription factors with some of these transporter transcripts [Balesaria et al. 2008].
The literature actually contains several studies showing no transport defect for BA in IBAM. Other groups failed to detect any differences in bile acid uptake into ileal mucosa or brush-border vesicles [Bajor et al. 2006a; van Tilburg et al. 1991]. There were even suggestions of a somewhat higher capacity for bile acid uptake in these patients. Alternative mechanisms that could produce this syndrome need consideration.
An increase in bile acid enterohepatic cycling frequency could be responsible. Figure 2(C) shows the computer model prediction that a change in bile acid cycling from four to six times a day will result in greater loss of BA into the faeces and a reduction in SeHCAT retention. It has also been suggested that malabsorption of BA could result from more rapid small intestinal transit, perhaps due to increased ileal secretion, and there is some evidence to support this [Bajor et al. 2009; Sadik et al. 2004].
However defective net ileal uptake of BA due to a shorter time for reabsorption would not be compatible with the study which showed an enlarged bile acid pool in typical patients with primary, idiopathic BAM [van Tilburg et al. 1992]. A depleted pool was found in patients with BAM secondary to resection. The enlarged bile acid pool was contrary to the expected finding in bile acid ‘malabsorption’, and as this was published in a supplement, it did not receive much attention, but is key to interpreting our findings. Figure 2(D) shows the computer simulation prediction that a larger pool, with unchanged cycling frequency, and an increased absolute ileal bile acid absorption (although slightly reduced fractional absorption) can produce reduced SeHCAT retention and increased faecal bile acid output.
We have proposed a new mechanism for primary bile acid diarrhoea, based on these findings and our recent results [Walters et al. 2009]. We suggest that this condition results from excess bile acid production due to impaired negative feedback by the ileal hormone, fibroblast growth factor 19 (FGF19). Fasting serum FGF19 is roughly half that of controls, with elevated levels of the bile acid precursor 7αOH-4-cholesten-3-one. Our theory proposes that bile acid absorption in the ileal enterocyte fails to generate the appropriate level of FGF19. Our key results in primary bile acid diarrhoea are presented in Table 3, together with the bile acid kinetics measured previously by van Tilburg and colleagues [van Tilburg et al. 1992].
Table 3.
Fibroblast growth factor 19 (FGF19)and bile acid kinetics compared in patients with primary bile acid diarrhoea and in controls.
| Study | Primary bile acid diarrhoea | Controls | |
|---|---|---|---|
| Walters et al. [2009] | Fasting serum values of: | (n = 13) | (n = 19) |
| FGF19 (pg/ml) | 103 ± 53** | 268 ± 145 | |
| 7αOH-4-cholesten-3-one (ng/ml) | 91 ± 74** | 17 ± 9 | |
| van Tilburg et al. [1992] | Bile acid kinetics: | (n = 8) | (n = 8) |
| Faecal bile acid loss (mmol/day) | 2.5 ± 1.0* | 1.0 ± 0.1 | |
| BA pool size (mmol) | 7.0 ± 4.4* | 3.7 ± 1.0 | |
| 75 SeHCAT retention (half-life in days) | 2.1 ± 1.1 | 2.6 ± 0.7 | |
| (calculated 7-day mean %) | 9% | 16% |
Means ± SD are shown. *p < 0.05; **p < 0.0005 (Mann–Whitney U-test).
The discovery of the involvement of FGF19, first in the enterohepatic circulation of bile acid, and now in the development of primary bile acid diarrhoea, appears to be a major advance [Hofmann, 2009b]. FGF19 in humans, and its orthologue FGF15 in the mouse, have recently been established as a negative regulator of bile acid synthesis in the liver, acting through the receptor FGFR4 and the coreceptor β-klotho. In ileal enterocytes, absorbed BA bind to the farnesoid X receptor (FXR) and transactivate the FGF15/FGF19 promoter. GW4064, a synthetic FXR agonist, increases FGF15 transcription more than any other ileal gene [Inagaki et al. 2006] and in Fxr-/- mice, FGF15 production is reduced [Inagaki et al. 2005]. Fgf15-/- mice have increased faecal bile acid concentrations, and FGF15 is effective in reducing watery diarrhoea in the Asbt -/- mouse [Jung et al. 2007].
Human FGF19 normally varies during the day with meals, consistent with a feedback role in bile acid synthesis [Lundasen et al. 2006]. Interestingly, some of our patients with bile acid diarrhoea, as well as having low fasting values of FGF19, fail to respond with any increase in FGF19 levels following meals [Walters et al. 2009]. Possibly different mechanisms may be responsible for basal and stimulated FGF19 secretion. A further possibility that may produce bile acid overproduction in some patients could come from abnormal FGF19 signalling in the liver through changes in FGFR4, β-klotho or other downstream molecules.
Treatment options
The mainstay of treatment for bile acid diarrhoea has been the use of anion exchange resins to bind BA with high affinity. Cholestyramine (now spelt colestyramine) has been given for many years for diarrhoea in patients with ileal resection [Hofmann and Poley, 1969]. Until the advent of statins, colestyramine and colestipol were commonly used in the treatment of hypercholesterolaemia, as sequestering bile in the intestine led to increased conversion of cholesterol into BA in the liver. The principle for their use in diarrhoea has been to prevent free BA from stimulating secretion in the colon. They are effective and, as described above, most patients with abnormal SeHCAT values will respond [Wedlake et al. 2009a]. Subjects with lower SeHCAT values (less than 5%) are more likely to respond than those with higher values (below 15%).
The high response rates to bile acid sequestrants has resulted in their use as a therapeutic trial in patients suspected as having bile acid diarrhoea [Westergaard, 2007; Fromm and Malavolti, 1986]. Although this may be a pragmatic approach in countries such as the USA where SeHCAT testing is not available, this can mean that patients do not have a clear diagnosis established, which can prevent satisfactory treatment. Many patients find that colestyramine and colestipol are poorly tolerated, often because of the poor texture and taste of the resin powder [Ford et al. 1992]. Over half of patients given bile acid sequestrants for hyperlipidaemia discontinued them within a year [Hiatt et al. 1999] and similar findings were described in a follow up of treated patients with IBAM [Rössel et al. 1999]. Patients may find that the treatment is too effective, particularly if they have been prescribed large doses previously given for lowering cholesterol. Although the stools may be more solid and become less frequent, they may complain of constipation, bloating, abdominal cramps or nausea. Thus, it is important to titrate the dosage to find that which is best tolerated. For drugs that have been used for over 40 years and for a condition that has a prevalence of 1%, it is surprising that the best dosing regimen is still unclear, with uncertainties regarding whether they are more effective if taken on an empty stomach, before bedtime or with food.
Formulation of colestyramine into enterocoated tablets designed to disintegrate in the colon was shown to be more acceptable in a small study reported in 1985 [Jacobsen et al. 1985] but this preparation was not marketed. Fortunately another bile acid sequestrant, colesevelam, is available in tablet form, being used initially for hyperlipidaemia [Davidson et al. 1999]. There is now experience in the use of colesevelam in BAM and IBS-D [Odunsi-Shiyanbade et al. 2010; Wedlake et al. 2009b; Puleston et al. 2005]. It has a greater affinity for binding BA, which may be an advantage. Colesevelam was shown to be effective at doses between 1.25 and 3.75 g/day, and was well tolerated [Wedlake et al. 2009b]. Patients who had failed to tolerate colestyramine were able to accept colesevelam, with the majority of them continuing with colesevelam long term. However, there have been no large-scale, double-blind or randomized studies of colesevelam in bile acid diarrhoea, and hence the use of this drug remains unlicensed. Such studies are clearly warranted.
All bile acid sequestrants are capable of binding other compounds and it is advised that other drugs are taken 1 h before or 4–6 h afterwards. It is also possible that deficiencies of fat-soluble vitamins (A, D, E and K) may occur and it is reasonable to check these periodically. Patients with BAM following extensive ileal resection may also have a more generalized fat malabsorption as part of a short-bowel syndrome. In these patients, steatorrhoea and diarrhoea can best be prevented with a low-fat diet supplemented with medium-chain triglycerides, and perhaps with cholylsarcosine [Westergaard, 2007]. Patients with bile acid diarrhoea secondary to Crohn's ileitis will be helped with glucocorticoid treatment and it has been shown that these will induce expression of the apical membrane bile acid transporter ASBT [Jung et al. 2004]. Microscopic colitis is also helped by steroids, perhaps through this mechanism. Budesonide which is highly metabolized in the liver and so has few systemic effects, is highly effective [Bajor et al. 2006b; Ung et al. 2000a]. Patients with BAM secondary to SIBO require antibiotic therapy [Roberts et al. 1977].
Unmet needs and future possibilities
Perhaps the greatest need is for increased recognition of the role of BA in the generation of the symptoms of chronic diarrhoea, urgency, incontinence, bloating and discomfort in what would have been labelled as IBS-D or as functional diarrhoea. Diagnostic tests need to be made more available. SeHCAT testing seems unlikely to ever become available in the USA unless legislation changes, as it involves the administration of a synthetic radiolabelled compound. However, in many parts of the world, it is available and needs to be employed to allow the size of the population with this problem to be fully appreciated.
Other tests such as 7α-OH-4-cholesten-3-one have promise as alternatives and should be rolled out as centres adopt the methodology. Gastroenterologists need to be prepared to attempt a therapeutic trial of bile acid sequestrants in all patients with chronic diarrhoea if no other diagnostic test is available. More work is required to document the extent of the ill health resulting from BAM and how an early correct diagnosis will produce savings in avoiding and repeating unnecessary other investigations to pursue alternative causes.
The use of the therapeutic agents currently available will need refinement in properly conducted trials. Patient acceptability of bile acid sequestrants needs to be improved and the availability of colesevelam in tablet form is clearly an advance. It should be borne in mind when the next generation of treatment is developed that it is in the colon where the unabsorbed, deconjugated, dihydroxylated BA produce their adverse effects; formulations that deliver sequestrants here may be preferable.
Identification of the role of FGF19 and an enlarged bile acid pool due to an impaired negative feedback opens up a large number of possibilities in so-called idiopathic BAM. Potentially it may become possible to directly modulate this pathway, with specific FXR agonists, or other agents. Addressing what appears to be the primary defect in primary bile acid diarrhoea seems a logical aim but will require a long-term time frame. Optimal bile acid sequestrant therapy is a more achievable immediate therapeutic goal which should greatly benefit a large number of patients.
Acknowledgments
We acknowledge the contribution of many fruitful discussions with Dr Jervoise Andreyev, Dr Basumani Pandurangan and Professor K. D. Bardhan.
Funding
S.P. is supported by the Bardhan Research and Education Trust.
Conflict of interest statement
J.W. has been a member of scientific advisory boards for GE Healthcare and Genzyme.
References
- Andreyev J. (2007) Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patient? Lancet Oncol 8: 1007–1017 [DOI] [PubMed] [Google Scholar]
- Bajor A., Kilander A., Fae A., Galman C., Jonsson O., Ohman L., et al. (2006a) Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorptio? Eur J Gastroenterol Hepatol 18: 397–403 [DOI] [PubMed] [Google Scholar]
- Bajor A., Kilander A., Galman C., Rudling M., Ung K.A. (2006b) Budesonide treatment is associated with increased bile acid absorption in collagenous coliti? Ailment Pharmacol Ther 24: 1643–1649 [DOI] [PubMed] [Google Scholar]
- Bajor A., Ung K.A., Ohman L., Simren M., Thomas E.A., Bornstein J.C., et al. (2009) Indirect evidence for increased mechanosensitivity of jejunal secretomotor neurones in patients with idiopathic bile acid malabsorptio? Acta Physiol (Oxf) 197: 128–137 [DOI] [PubMed] [Google Scholar]
- Balesaria S., Pell R.J., Abbott L.J., Tasleem A., Chavele K.M., Barley N.F., et al. (2008) Exploring possible mechanisms for primary bile acid malabsorption: evidence for different regulation of ileal bile acid transporter transcripts in chronic diarrhoe? Eur J Gastroenterol Hepatol 20: 413–422 [DOI] [PubMed] [Google Scholar]
- Boyd G.S., Merrick M.V., Monks R., Thomas I.L. (1981) Se-75-labeled bile acid analogs, new radiopharmaceuticals for investigating the enterohepatic circulatio? J Nucl Med 22: 720–725 [PubMed] [Google Scholar]
- Brydon W.G., Nyhlin H., Eastwood M.A., Merrick M.V. (1996) Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoe? Eur J Gastroenterol Hepatol 8: 117–123 [DOI] [PubMed] [Google Scholar]
- Camilleri M., Nadeau A., Tremaine W.J., Lamsam J., Burton D., Odunsi S., et al. (2009) Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometr? Neurogastroenterol Motil 21: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A., Nyhlin H., Persson H., Stendahl U., Stenling R., Suhr O. (1991) Chronic diarrhoea after radiotherapy for gynaecological cancer: occurrence and aetiolog? Gut 32: 1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M.H., Dillon M.A., Gordon B., Jones P., Samuels J., Weiss S., et al. (1999) Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effect? Arch Intern Med 159: 1893–1900 [DOI] [PubMed] [Google Scholar]
- Eusufzai S. (1993) Bile acid malabsorption in patients with chronic diarrhoe? Scand J Gastroenterol 28: 865–868 [DOI] [PubMed] [Google Scholar]
- Fernandez-Banares F., Esteve M., Salas A., Forne T.M., Espinos J.C., Martin-Comin J., et al. (2001) Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhe? Dig Dis Sci 46: 2231–2238 [DOI] [PubMed] [Google Scholar]
- Ford G.A., Preece J.D., Davies I.H., Wilkinson S.P. (1992) Use of the SeHCAT test in the investigation of diarrhoe? Postgrad Med J 68: 272–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H., Malavolti M. (1986) Bile acid-induced diarrhoe? Clin Gastroenterol 15: 567–582 [PubMed] [Google Scholar]
- Hiatt J.G., Shamsie S.G., Schectman G. (1999) Discontinuation rates of cholesterol-lowering medications: implications for primary car? Am J Manag Care 5: 437–444 [PubMed] [Google Scholar]
- Hofmann A.F. (1967) The syndrome of ileal disease and the broken enterohepatic circulation: cholerheic enteropath? Gastroenterology 52: 752–757 [PubMed] [Google Scholar]
- Hofmann A.F. (2009a) Bile acids: trying to understand their chemistry and biology with the hope of helping patient? Hepatology 49: 1403–1418 [DOI] [PubMed] [Google Scholar]
- Hofmann A.F. (2009b) Chronic diarrhea caused by idiopathic bile acid malabsorption: an explanation at las? Expert Rev Gastroenterol Hepatol 3: 461–464 [DOI] [PubMed] [Google Scholar]
- Hofmann A.F., Poley J.R. (1969) Cholestyramine treatment of diarrhea associated with ileal resectio? N Engl J Med 281: 397–402 [DOI] [PubMed] [Google Scholar]
- Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., et al. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasi? Cell Metab 2: 217–225 [DOI] [PubMed] [Google Scholar]
- Inagaki T., Moschetta A., Lee Y.K., Peng L., Zhao G., Downes M., et al. (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid recepto? Proc Natl Acad Sci U S A 103: 3920–3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen O., Hojgaard L., Hylander M.E., Wielandt T.O., Thale M., Jarnum S., et al. (1985) Effect of enterocoated cholestyramine on bowel habit after ileal resection: a double blind crossover stud? Br Med J (Clin Res Ed) 290: 1315–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D., Fantin A.C., Scheurer U., Fried M., Kullak-Ublick G.A. (2004) Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid recepto? Gut 53: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D., Inagaki T., Gerard R.D., Dawson P.A., Kliewer S.A., Mangelsdorf D.J., et al. (2007) FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorptio? J Lipid Res 48: 2693–2700 [DOI] [PubMed] [Google Scholar]
- Khalid U., Lalji A., Stafferton R., Andreyev J. (2010) Bile acid malabsorption: a forgotten diagnosis? Clin Medicine 10: 124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundasen T., Galman C., Angelin B., Rudling M. (2006) Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in ma? J Intern Med 260: 530–536 [DOI] [PubMed] [Google Scholar]
- Merrick M.V., Eastwood M.A., Ford M.J. (1985) Is bile acid malabsorption underdiagnosed? An evaluation of accuracy of diagnosis by measurement of SeHCAT retentio? Br Med J (Clin Res Ed) 290: 665–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M., Abrahamsson A., Galman C., Eggertsen G., Marschall H.U., Ravaioli E., et al. (2006) Analysis of ileal sodium/bile acid cotransporter and related nuclear receptor genes in a family with multiple cases of idiopathic bile acid malabsorptio? World J Gastroenterol 12: 7710–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M., Love M.W., Rössel P., Dawson P.A., Qvist P. (2001) Absence of dysfunctional ileal sodium-bile acid cotransporter gene mutations in patients with adult-onset idiopathic bile acid malabsorptio? Scand J Gastroenterol 36: 1077–1080 [DOI] [PubMed] [Google Scholar]
- Muller M., Willen R., Stotzer P.O. (2004) Colonoscopy and SeHCAT for investigation of chronic diarrhe? Digestion 69: 211–218 [DOI] [PubMed] [Google Scholar]
- Niaz S.K., Sandrasegaran K., Renny F.H., Jones B.J.M. (1997) Postinfective diarrhoea and bile acid malabsorptio? J Roy Coll Phys Lond 31: 53–56 [PMC free article] [PubMed] [Google Scholar]
- Odunsi-Shiyanbade S.T., Camilleri M., McKinzie S., Burton D., Carlson P., Busciglio I.A., et al. (2010) Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel functio? Clin Gastroenterol Hepatol 8: 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers P., Kirby L.C., Heubi J.E., Dawson P.A. (1997) Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2? J Clin Invest 99: 1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni S.S., Walters J.R.F. (2009) Recent advances in the understanding of bile acid malabsorptio? Br Med Bull 92: 79–93 [DOI] [PubMed] [Google Scholar]
- Puleston J., Morgan H., Andreyev J. (2005) New treatment for bile salt malabsorptio? Gut 54: 441–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.H., James O., Jarvis E.H. (1977) Bacterial overgrowth syndrome without “blind loop”: A cause for malnutrition in the elderl? Lancet 2: 1193–1195 [DOI] [PubMed] [Google Scholar]
- Rössel P., Jensen H.S., Qvist P., Arveschoug A. (1999) Prognosis of adult-onset idiopathic bile acid malabsorptio? Scand J Gastroenterol 34: 587–590 [DOI] [PubMed] [Google Scholar]
- Sadik R., Abrahamsson H., Ung K.-A., Stotzer P.-O. (2004) Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorptio? Am J Gastroenterol 99: 711–718 [DOI] [PubMed] [Google Scholar]
- Sauter G.H., Munzing W., von Ritter C., Paumgartner G. (1999) Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in seru? Dig Dis Sci 44: 14–19 [DOI] [PubMed] [Google Scholar]
- Sciarretta G., Fagioli G., Furno A., Vicini G., Cecchetti L., Grigolo B., et al. (1987) 75SeHCAT test in the detection of bile acid malabsorption in functional diarrhoea and its correlation with small bowel transi? Gut 28: 970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha L., Liston R., Testa H.J., Moriarty K.J. (1998) Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramin? Ailment Pharmacol Ther 12: 839–844 [DOI] [PubMed] [Google Scholar]
- Smith M.J., Cherian P., Raju G.S., Dawson B.F., Mahon S., Bardhan K.D. (2000) Bile acid malabsorption in persistent diarrhoe? J R Coll Physicians Lond 34: 448–451 [PMC free article] [PubMed] [Google Scholar]
- Spiller R.C., Thompson W.G. (2010) Bowel disorder? Am J Gastroenterol 105: 775–785 [DOI] [PubMed] [Google Scholar]
- Thaysen E.H., Pedersen L. (1973) Diarrhoea associated with idiopathic bile acid malabsorption. Fact or fantasy? Dan Med Bull 20: 174–177 [PubMed] [Google Scholar]
- Thaysen E.H., Pedersen L. (1976) Idiopathic bile acid catharsi? Gut 17: 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K.A., Gillberg R., Kilander A., Abrahamsson H. (2000a) Role of bile acids and bile acid binding agents in patients with collagenous coliti? Gut 46: 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K.A., Kilander A.F., Lindgren A., Abrahamsson H. (2000b) Impact of bile acid malabsorption on steatorrhoea and symptoms in patients with chronic diarrhoe? Eur J Gastroenterol Hepatol 12: 541–547 [DOI] [PubMed] [Google Scholar]
- van Tilburg A.J., de Rooij F.W., van den Berg J.W., van Blankenstein M. (1991) Primary bile acid diarrhoea without an ileal carrier defect: quantification of active bile acid transport across the ileal brush border membran? Gut 32: 500–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilburg A.J., de Rooij F.W., van den Berg J.W., van Blankenstein M. (1992) Primary bile acid malabsorption: a pathophysiologic and clinical entity? Scand J Gastroenterol Suppl 194: 66–70 [DOI] [PubMed] [Google Scholar]
- Walters J.R.F., Tasleem A.M., Omer O.S., Brydon W.G., Dew T., Le Roux C.W. (2009) A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesi? Clin Gastro Hepatol 7: 1189–1194 [DOI] [PubMed] [Google Scholar]
- Wedlake L., A'Hern R., Thomas K., Walters J.R.F., Andreyev H.J.N. (2009a) Systematic review: the prevalence of idiopathic bile acid malabsorption (I‐BAM) as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome (IBS? Aliment Pharmacol Ther 30: 707–717 [DOI] [PubMed] [Google Scholar]
- Wedlake L., Thomas K., Lalji A., Anagnostopoulos C., Andreyev J. (2009b) A 4-year experience with colesevelam hydrochloride: a new treatment for bile acid malabsorption? Gut 58: A117–A117 [DOI] [PubMed] [Google Scholar]
- Westergaard H. (2007) Bile Acid malabsorptio? Curr Treat Options Gastroenterol 10: 28–33 [DOI] [PubMed] [Google Scholar]
- Wildt S., Rasmussen S.N., Madsen J.L., Rumessen J.J. (2003) Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT tes? Scand J Gastroenterol 38: 826–830 [DOI] [PubMed] [Google Scholar]
- Williams A.J.K., Merrick M.V., Eastwood M.A. (1991) Idiopathic bile acid malabsorption—a review of clinical presentation, diagnosis, and response to treatmen? Gut 32: 1004–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]