Abstract
We performed a retrospective review of side effects and clinical outcomes in relapsing—remitting (RR) multiple sclerosis (MS) patients receiving long-term treatment with daclizumab. Twelve patients with RR MS were initially treated with daclizumab at 1 mg/kg IV, again 14 days later and then monthly treatments (average duration 42.1 months). Daclizumab dose (0.85 mg/kg to 1.5 mg/kg) was adjusted based on clinical response. Daclizumab was generally well tolerated. There was a significant reduction in relapse rate and improvement in Expanded Disability Status Scores (EDSSs) (p < 0.0001). Long-term treatment with daclizumab in RR MS patients has apparent benefit that will require formal confirmation.
Keywords: long-term daclizumab therapy, interleukin 2 receptor alpha, side effects, clinical outcomes
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) that results in demyelination and axonal injury [Sospedra and Martin, 2005; Noseworthy, 2000]. Current immunotherapies partially suppress disease activity in MS [Compston and Coles, 2008; Brashir and Whitacker, 2002]. A new immunotherapy would be advantageous for the population of patients that despite treatment with currently approved immunotherapies have persistent disease activity (as demonstrated by continuing relapses and contrast enhancing lesions [CELs] on magnetic resonance imaging [MRI] scans). A promising new line of therapies in MS includes immunotherapy utilizing monoclonal antibodies that target specific receptors. One potential target is the interleukin 2 receptor (IL2-R) that is involved in activation of T and B cells [Morris and Waldmann, 2000; Bielekova et al. 2004; Waldmann et al. 2000]. IL2-R directed immunotherapy can ameliorate the experimental autoimmune encephalomyelitis (EAE) model of MS [Rose et al. 1991; Engelhardt et al. 1989; Hayosh and Swanborg, 1987]. Daclizumab is a humanized monoclonal antibody (IgG1 subtype) that binds to the alpha chain (CD25) of the IL2-R, making it specific to the high affinity, but not the intermediate affinity, IL2-R receptor. Mechanisms of action by daclizumab may be more complex than blockage of the receptor. Recent studies have demonstrated that inhibition of CD40L expression by daclizumab contributes to clinical efficacy [Snyder et al. 2007]. In addition, daclizumab treatment increases the function and number of natural killer cells potentially contributing to immunoregulation of activated lymphocytes [Bielekova et al. 2006].
Daclizumab is approved by the Food and Drug Administration for prevention of renal allograft rejection [Vincenti et al. 1998]. Daclizumab has been examined as an investigational therapy for autoimmune diseases that include noninfectious uveitis [Nussenblatt et al. 2005, 1999] and multiple sclerosis [Bielekova et al. 2004; Rose et al. 2004]. Two small phase II clinical trials of daclizumab for MS and a previous retrospective study of MS patients on open-label daclizumab have demonstrated reduction of new lesions and relapse rate as well as improved clinical scores over duration of treatment up to 28.5 months [Rose et al. 2007]. In addition, a multicenter phase II trial demonstrated significant reduction in contrast-enhancing lesions with short term (44 weeks) daclizumab administration [Kaufman et al. 2008]. In the present report we examine the clinical outcomes and side-effect profiles in relapsing—remitting (RR) MS patients on long-term daclizumab therapy ranging from 24 to 60 months (average 42.1 months).
Methods
Patients
Twelve ambulatory patients treated in the MS clinic at the University of Utah (Salt Lake City, UT), with Expanded Disability Status Scores (EDSSs) of 2.5 to 6.5 were included in an IRB-approved retrospective study to evaluate side effects and clinical outcomes of daclizumab therapy. Side effects were monitored at clinical follow up visit every 3—6 months as well as by a formal survey. Follow-up phone contacts were done when necessary to evaluate specific side effects.
The patients included five women and seven men ranging between 30 and 61 years of age (Table 1). The duration of the disease ranged from 3 years to more than 21 years, averaging 11.1 years. All patients were failing multiple immunotherapies, defined as continuing relapses with new T2 lesions and/or contrast-enhancing lesions on MRI during an average of 20 months before treatment with daclizumab. The duration of treatment with daclizumab for all patients was from 24 months to 60 months with an average of 42.1 months.
Table 1.
CLinicaL features, outcome and side effects of dacLizumab treatment.
| No | Age/sex | Years with MS | CLinicaL course | Previous Rx | Time Dac Rx (mo) | Comb Rx w/Dac | Pre RX EDSS | During Rx EDSS | EDSS Δ | Side effects |
| 1 | 31F | 12 | RR/A* | I,P,S | 43 | None | 3.5 | 1.5 | -2.0 | Rash |
| 2 | 44F | 12 | RR/A | I,P,S | 28b | None | 3.0 | 1.0 | -2.0 | None |
| 3 | 34F | 10 | RR/A | I,P,S | 41b | None | 4.0 | 1.5 | -2.5 | Fatigue |
| 7 | 40M | 8 | RR/A | I,P,S,G | 43a | None | 2.5 | 1.5 | -1.0 | Lymphadenopathy, rash, high fever |
| 12 | 61F | 21 | RR/A | I,P,S | 43 | None | 5.0 | 2.5 | -2.5 | Lymphadenopathy, EBV infection, headache, paresthesia |
| 14 | 46F | 12 | RR/A | I,P,S,G | 28b | (I) | 3.5 | 3.0 | -0.5 | None |
| 16 | 45M | 15 | RR/A | I,P,S | 45 | None | 4.0 | 1.5 | -2.5 | None |
| 17 | 30M | 8 | RR/A* | I,S,G,PP,M | 60 | (I) | 6.5 | 2.0 | -4.5 | Constipation, toe infection, fever, sore throat |
| 18 | 34M | 6 | RR/A | I,S,P,G | 51 | None | 3.5 | 1.0 | -2.5 | Transient eLevation LFT, seasonaL rash, chest discomfort |
| 19 | 39M | 12 | RR/A | I,S,P,G | 51 | None | 2.5 | 1.0 | -1.5 | None |
| 20 | 45M | 15 | RR/A | I,S,P | 49 | (I) | 3.5 | 2.0 | -1.5 | None |
| 22 | 33M | 3 | RR/A | S,P,I | 24 | None | 6.0 | 2.5 | -3.5 | Rash, chest discomfort |
Clinical characteristics: Five females and seven males ranging from 30 to 61 years of age. The average disease duration for aLL patients was 11.1 years.
DacLizumab treatment: The dose range from 0.85 to 1.5mg/kg IV. The average duration of treatment was 42.1 months. Nine patients received treatment for more that 3 years, two patients for more than two years and one patient for two years.
DacLizumab Rx status: Patients continuing Rx: 1,12,16,17,18,19,20 and 22.
aDiscontinued Rx due to side effects: (patient #7).
bDiscontinued Rx due to insurance issue: (patients #2, #3 and #14).
CLinicaL course: RR: reLapsing—remitting; RR/A: active (one ore two reLapses per year); RR/A*: active (three or more reLapses/year). Previous Rx: I, interferon; S, soLumedroL; P, prednisone; Mtx, methotrexate; G, gLatiramer acetate; PP, pLasmapheresis; Pac, pacLitaxeL. Combined therapy: (I): interferon.
Patients with RR MS were treated with daclizumab. RR MS includes those patients with active disease, one or two relapses per year (RR/A), and three or more relapses per year (RR/A*). The annual relapse rate was determined over a period of at least 15 and up to 27 months in each patient prior to daclizumab treatment (data not shown). The base line EDSS (before medication) was generally representative of the clinical status for at least 2 months before therapy. The disease course was (RR/A) in ten patients and (RR/A*) in two patients.
The 12 patients on long-term daclizumab therapy included in this study consisted of five patients that continued from the 2004 open-label study [Rose et al. 2004], one new open-label patient and six patients that transferred from phase II participation to open-label treatment [Rose et al. 2007]. Of these six patients, three were able to continue daclizumab in open-label treatment although three patients were not able to continue treatment due to lack of insurance funding; they were, however, observed in the subsequent year for development of any potential post-treatment side effects.
Treatment protocol
The treatment protocol in this investigation was based on the design by Dr Roland Martin and colleagues in the Neuroimmunology Branch at the National Institutes of Health [NIH] [Snyder et al. 2007; Bielekova et al. 2006]. This design was also used in our earlier reports of open-label use and a small phase II trial [Rose et al. 2007, 2004]. Patients initially were treated with 1 mg/kg IV daclizumab; the same dose was repeated 14 days later, followed by subsequent treatments every 28 days. As therapy continued, the dosage was adjusted based on end of dose accentuation of previous neurologic symptoms or continued disease activity. The dose of daclizumab ranged between 0.85 and 1.5 mg/kg. When the daclizumab dose needed to be adjusted to optimize response, changes were made in one or two increments of an additional 0.25 mg/kg. In one patient (#1) the dosage was decreased to 0.85 mg/kg due to a medication related rash (granuloma annulare) [Mehta and Rose, 2009]. Liver function tests and blood cell counts including absolute lymphocyte counts were monitored every month during therapy.
The duration of therapy for all patients ranged between 24 months and 60 months with an average of 42.1 months. Nine patients received therapy for more than 3 years, two patients received therapy for more than 2 years and one patient received therapy for 2 years. Nine of these patients received monotherapy with daclizumab and three patients continued in combined therapy with interferon; one patient discontinued therapy due to side effects including lymphadenopathy, fever and interface dermatitis and three patients due to insurance issues. Eight patients continued to receive treatment according to the treatment protocol (The clinical features and outcomes of daclizumab treatment are outlined in Table 1).
Statistical analysis
Comparisons of EDSS scores between pretreatment and after sustained long-term treatment were performed with a nonparametric two-tailed Mann—Whitney test for pairwise comparison and analysis of variance Kruskal—Wallis Test (nonparametric ANOVA) for multiple comparisons. Comparison of annual relapse rate between pretreatment and during sustained long term treatment was performed with two-tailed unpaired i-test. Statistical analyses were performed using the Graphpad (San Diego, CA) Instat statistical program.
Results
Side effect profile
Overall, the treatment was well tolerated in all patients. In one patient (#7) the therapy was discontinued after 43 months of treatment because of side effects including lymphadenopathy, fever and interface dermatitis.
During treatment with daclizumab, five patients reported no side effects and the remaining seven patients reported one or more side effects, as summarized in Tables 1 and 2. The most common side effects were rash, lymphadenopathy, chest discomfort and fever. Other possible side effects with single occurrences were headache, paresthesia, fatigue, constipation, Epstein-Barr virus (EBV) infection, toe infection, sore throat and transient mild liver function test elevation of alanine amonitransferase (ALT: 80) which was World Health Organization WHO grade I (Table 2).
Table 2.
Side-effect profile with daclizumab treatment — the numbers of patients exhibiting each side effect is shown.
| Side effects | Number of patients |
| None | 5 |
| Rash | 4 |
| Chest discomfort | 2 |
| Lymphadenopathy | 2 |
| Fever | 2 |
| EBV infection | 1 |
| Headache | 1 |
| Paresthesia | 1 |
| Constipation | 1 |
| Toe infection | 1 |
| Sore throat | 1 |
| Transient mild LFT elevation | 1 |
| Fatigue | 1 |
Rash has been frequently observed in patients on daclizumab therapy [Rose et al. 2007, 2004]. The rashes that we observed in our patients included eczema, granuloma annulare and interface dermatitis. Two patients [#18 and #22] had small patches of eczema that responded to topical steroids; daclizumab therapy was continued in these patients. One patient (#1) developed granuloma annulare, which resolved with oral prednisone treatment and cessation of daclizumab. Once the rash cleared, daclizumab was successfully reinitiated.
Patient #7 on daclizumab monotherapy developed a truncal and axillary rash associated with daily fever (as high as 40° C) as well as lymphadenopathy; skin biopsy revealed interface dermatitis and daclizumab was discontinued. This illness resolved following intravenous and subsequent oral corticosteroid therapy.
Lymphadenopathy occurred in two of the 12 patients who were treated with open-label daclizumab. These patients were on treatment for more than 2 years and the dosage was 1.5 mg/kg (two patients). EBV infection was associated in one lymphadenopathy (patient #12). Patient #7 initially developed a truncal rash with cervical lymphadenopathy, which was treated empirically with antibiotics. Three weeks later the patient presented with tender inguinal lymphadenopathy. The rash spread to the abdomen, axillas and extremities. At this point, daclizumab infusion was suspended for 7 weeks then restarted at a lower dose. Within 10 days postinfusion, the patient developed pronounced cervical, axillary and inguinal lymphadenopathy accompanied by progressively severe night sweats, fever, rigors, arthralgia, anorexia and weight loss. Liver function studies, sedimentation rate and C-reactive protein were significantly elevated. Computed tomography (CT) scans revealed diffuse pelvic, abdominal, and thoracic lymphadenopathy. Skin and lymph node biopsies were obtained. Changes on skin biopsy demonstrated ‘interface dermatitis’ felt to be compatible with either drug rash or possibly an autoimmune disease. Lymph node pathology revealed one necrotic node and another with hyperplasia with preservation of the architecture. Immunohistochemistry of the hyperplasic node revealed abundant populations of CD3, CD4, CD20 and CD68. The CD4: CD8 ratio was normal. CD10 staining was observed only in the germinal center B cells. Natural killer (NK) cell stains did not reveal a population of significance in the node and EBV stain demonstrated rare positive cells not thought to be significant. Other infectious etiologies were ruled out following extensive work up. Also, there was no laboratory evidence of a lupus-like autoimmune illness. The patient was hospitalized and treated with solumedrol, 1000 mg IV per day for 3 days, followed by a 2-month tapered course of prednisone and mycophenolate, 1000 mg per day, which resulted in resolution of fever, night sweats and lymphadenopathy. Because the patient required hospitalization for treatment of this complex of side effects the patient qualified as a serious adverse event (SAE). At present the patient is being worked up for persistent bilateral wrist synovitis that developed during treatment.
Patient #12 was on therapy for over 2 years when she developed night sweats associated with lymphadenopathy. A biopsy was performed and lymphoid hyperplasia was observed. In addition, EBV positive cells were detected by immunohistochemical analysis (data not shown). The patient was withdrawn from daclizumab therapy for 7 weeks during which the night sweats and lymphadenopathy resolved. Treatment was then successfully restarted without recurrence of symptoms or lymphadenopathy. The patient has also been monitored by PCR for EBV recurrence with no detectable viral DNA over the subsequent year.
Clinical outcomes
EDSS
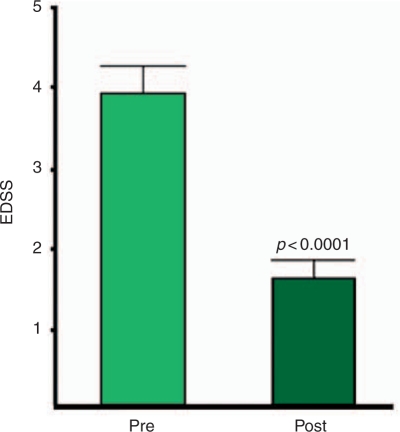
Improvement in EDSS was observed after sustained long-term treatment, with an average reduction of 2.2 points (p < 0.0001) (Figure 1). Patients had sustained improvement in EDSS over their entire course, defined as a change in EDSS of 1.0 or more points over the total course of follow-up for each patient.
Figure 1.
Clinical response to daclizumab treatment based on Expanded Disability Status Scores (EDSS). EDSS scores for pretreatment and post-sustained long-term treatment were compared for all relapsing—remitting patients (n = 12). An average reduction in EDSS of 2.2 points was observed after sustained long-term treatment (p < 0.0001; by Mann—Whitney test paired t-test).
Patient #17 had severe disability and was refractory to solumedrol therapy and had an EDSS of 8.0, 5 months before daclizumab therapy and improved after plasmapheresis, but the EDDS score regressed to 6.5 points 1 month prior initiation of daclizumab therapy. The average duration of therapy was 42.1 months for all relapsing remitting patients with variable treatment duration from 24 to 60 months (Table 1). A similar magnitude of improvement in EDSS was observed between patients on combined therapy or monotherapy, although the number of combined therapy patients (n = 3) was too small to assess any significant differences between groups.
Annual relapse rate
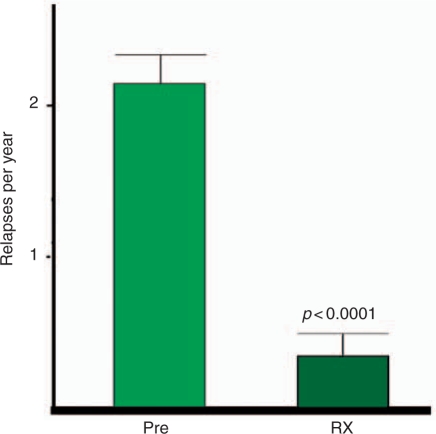
Treatment with daclizumab significantly decreased the number of relapses. The combined relapse rate for all RR MS patients decreased from 2.15 relapses per year the in pretreatment period to 0.33 relapses per year after sustained long-term therapy (p < 0.0001) (Figure 2). The pretreatment relapse rate was evaluated over a range of 15–27 months before daclizumab treatment. All patients had a substantial reduction in the annual relapse rate that was sustained during therapy.
Figure 2.
Clinical response to daclizumab treatment based on annual relapse rate (ARR). Pretreatment and during treatment ARR was compared for all relapsing—remitting patients (n = 12). The ARR decreased from 2.15 relapses per year in pretreatment observations to 0.33 relapses per year during sustained therapy (p < 0.0001 by unpaired t-test; p < 0.0001 by unpaired f-test).
Discussion
In this retrospective study, 12 RR MS patients refractory to interferon therapy were treated with off label daclizumab for an average of 42.1 months. The medication was well tolerated during therapy and the side-effect profile was acceptable. However, monitoring for rash and lymphadenopathy is advisable, as these were the most common side effects. In two patients, minor rashes required either no treatment or occasional topical steroid administration. However, one patient required oral steroid treatment and a second patient with rash and lymphadenopathy developed recurrent fevers and required discontinuation of monoclonal antibody and intravenous steroid therapy to ameliorate this syndrome.
Monitoring blood cell counts including absolute lymphocyte counts and liver function test are recommended during daclizumab treatment. Clinical results in our series of patients indicate that patients with RR disease respond to daclizumab therapy. The majority of these patients (n = 11) had clinical improvements that were sustained during the course of treatment. A regression to the mean phenomena could be a factor, but seems less likely in view of ongoing relapses and CELs while on immu-notherapy over a substantial period of time (20 months average duration) prior to daclizumab treatment.
Daclizumab represents an effective alternative to aggressive immunosuppresion in patients who do not tolerate or do not respond to conventional MS therapies. An initial study revealed that daclizumab paired with interferon could result in significant reduction in CELs [Bielekova et al. 2004]. In a previous review of patients on open-label daclizumab from 5 to 25 months, significant improvements in clinical course and MRIs were observed [Rose et al. 2004]. In addition, we have shown in a small phase II study a substantial benefit in reduction of CELs as well as improvement on standard clinical rating scales during daclizumab therapy over 27.5 months [Rose et al. 2007]. In the present study, the duration of therapy is much longer and demonstrates consistent benefit from daclizumab therapy in active RR MS patients [Rose et al. 2007, 2004].
In our initial evaluation of open-label therapy we found that monotherapy with daclizumab was effective in the majority of patients [Rose et al. 2004]. In our small phase II trial, six of nine patients treated with daclizumab therapy were controlled on monotherapy [Rose et al. 2007]. In the present study, nine of 12 patients were maintained on monotherapy but, as we have previously observed, some patients (three in this study) required addition of interferon for optimal disease control with respect to CELs or clinical relapse rate.
Patient #17 was maintained on combined therapy with interferon during the whole duration of the study due to the aggressive clinical course. Patient #14 developed three new CELs 3 months after discontinuation of interferon treatment. Patient #20 experienced one relapse 1 month after discontinuation of interferon and developed one new CEL on MRI 2 months after discontinuation of interferon. Reinstitution of interferon therapy in patients 14 and 20 resulted in no further relapses or CELs.
Long-term daclizumab treatment appears to be associated with sustained clinical improvement in RR MS patients; but this open-label retrospective study of a small number of patients will require formal confirmation. The results of ongoing long-term, controlled clinical trials with daclizumab will be of considerable interest.
The CHOICE study is a phase II, randomized, double-blind, placebo-controlled clinical study that evaluated the efficacy and safety of daclizumab versus placebo when added to interferon beta therapy. Two-hundred-and-thirty patients with active MS with ongoing interferon beta therapy enrolled at study centers in the US and Europe were randomized to received daclizumab 1 mg/kg or 2 mg/kg subcutaneously every 2 weeks or placebo. The primary efficacy analysis at 24 weeks demonstrated a significant reduction in new or enlarged gadolinium enhancing lesions in the 2 mg/kg daclizumab group. A multicenter phase II monotherapy investigation of subcutaneous daclizumab (DacHyp) called the SELECT trial is ongoing [Rose et al. 2009; Lutterotti and Martin, 2008]
Recently, IL2-R alpha chain was identified by genetic analysis as a factor contributing to MS susceptibility [Hafler et al. 2007]. Daclizumab specifically binds to the alpha chain of the IL2- R. This chain is present on activated T and B cell but not in natural killer (NK) cells [Bielekova et al. 2006]. The mechanisms of action for daclizumab are complex and include inhibition of IL2 binding to the receptor, as well as the inhibition of CD4+CD25+ effector cells resulting from blockade of CD40L [Snyder et al. 2007]. Daclizumab also has the potential to increase function and number of NK cells designated by a high density of CD56. These CD56 bright cells may have an important regulatory function on T cells which may contribute to the efficacy of daclizumab [Bielekova et al. 2006]. Further study of the mechanism of action of daclizumab may help to delineate new pathways of interest for development of new immunotherapies.
We conclude that intravenous daclizumab was relatively well tolerated over the average 3.5 years course of therapy. Importantly, in this retrospective study we document the side-effect profile of daclizumab during long-term treatment for RR MS patients. Depending on the outcomes of controlled clinical trials, daclizumab may become an important therapy for patients with active RR MS [Buttman and Rieckmann, 2008; Linker et al. 2008; Schippling and Martin, 2008].
Acknowledgements
Therapy for one patient was supported by the Cumming Foundation. The investigators wish to thank the staff of the University of Utah Infusion Center and Dr Roland Martin for providing the protocol adapted for treatment of these patients. Dr Rojas is a fellow supported by the Cumming Foundation.
Conflict of interest statement
None declared.
Contributor Information
Monica A. Rojas, Neurovirology Research Laboratory, VASLCHCS and Department of Neurology, University of Utah, Salt Lake City, UT, USA
Noel G. Carlson, Neurovirology Research Laboratory, Geriatric Research Education Clinical Center (GRECC), VASLCHCS, Departments of Neurology, Neurobiology and Anatomy, Brain Institute and Center on Aging, University of Utah, Salt Lake City, UT, USA
Thomas L. Miller, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA
John W. Rose, Neurovirology Research Laboratory, Veterans Affairs Salt Lake City Health Care System, Salt Lake City, UT, USA jrose@genetics.utah.edu
References
- Bielekova B., Catalfamo M., Reichert-Scrivner S., Packer A., Cerna M., Walmann T.et al. (2006) Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2 R alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl AcadSci 103: 5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B., Richert N., Howard T., Blevins G., Markovic-Plese S., McCartin J.et al. (2004) Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl AcadSci 101: 8705–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashir K., Whitacker J. (2002) Curent immunotherapy for demyelinating diseases. Arch Neurol 59: 726–731 [DOI] [PubMed] [Google Scholar]
- Buttman M., Rieckmann P. (2008) Treating multiple sclerosis with monoclonal antibodies. Expert Rev Neurother 8: 433–455 [DOI] [PubMed] [Google Scholar]
- Composton A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517 [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Diamanstein T., Wekerle H. (1989) Immunotherapy of experimental autoimmune encephalomyelitis (EAE): differential effect of anti-IL-2 receptor antibody therapy on actively induced and T-line mediated EAE of the Lewis Rat. J Autoimmun 2: 61–73 [DOI] [PubMed] [Google Scholar]
- Hafler D., Comston A., Sawcer S., Lander S., Daly M., De Jager P.et al. (2007) Risk alleles for multiple sclerosis identified by a genomic study. N Engl J Med 357: 851–862 [DOI] [PubMed] [Google Scholar]
- Hayosh N., Swanborg R. (1987) Autoimmune effector cells: IX Inhibtion of adoptive transfer of autoimmune encephalomyelitis with a monoclonal antibody specific for interleukin 2 receptors. J Immunol 138: 3771–3775 [PubMed] [Google Scholar]
- Kaufman M.D., Wynn D.R., Montalban X., Wang M., Fong A. (2008) A phase 2 randomized, double-blinded, placebo-controlled, multicenter study of subcutaneous daclizumab, a humanized anti-CD-25 monoclonal antibody, in patients with active, relapsing forms of multiple sclerosis - week 44 results. Neurology 70: PL01.003 [Google Scholar]
- Linker R.A., Kieseier B.C., Gold R. (2008) Identification and development of new therapeutics for multiple sclerosis. Trends Pharmacol Sci 29: 558–565 [DOI] [PubMed] [Google Scholar]
- Lutterotti A., Martin R. (2008) Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol 7: 538–547 [DOI] [PubMed] [Google Scholar]
- Mehta L., Rose J.W. (2009) Recurrent granuloma annulare during treatment with daclizumab. Mult Scler 15: 527–528 [DOI] [PubMed] [Google Scholar]
- Morris J.C., Waldmann T. (2000) Advances in interleukin 2 receptor targeted treatment. Ann Rheum Dis 59(Suppl. 1): i109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy J. (2000) Multiple sclerosis. N Engl J Med 343: 938–952 [DOI] [PubMed] [Google Scholar]
- Nussenblatt R.B., Fortin E., Schiffman R., Rizzo L., Smith J., Van Veldhuisen P.et al. (1999) Treatment of noninfectious intermediate and posterior uveitis with humanized anti-TAC mAb: A phase I/II clinical trial. Proc Natl Acad Sci 96: 7462–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R.B., Peterson J.S., Foster C.S., Rao N.A., See R.F., Letko E.et al. (2005) Initial evaluation of subcutaneous daclizumab treatment for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology 112: 764–770 [DOI] [PubMed] [Google Scholar]
- Rose J., Lorberboum-Galski H., Fitzgerald D., Hill K., Townsend J., Pastan I. (1991) Chimeric cytotoxin IL2-PE40 inhibits EAE in the adoptive transfer model. J Neuroimmunology 32: 209–217 [DOI] [PubMed] [Google Scholar]
- Rose J.W., Burns J.B., Bjorklund J., Klein J., Watt H.E., Carlson N.G. (2007) Daclizumab phase II trial in relapsing remitting multiple sclerosis: MRI and clinical results. Neurology 69: 785–789 [DOI] [PubMed] [Google Scholar]
- Rose J.W., Foley J.F., Carlson N.G. (2009) Monoclonal antibody treatment for multiple sclerosis. Curr Treatment Options Neurol 11: 211–220 [DOI] [PubMed] [Google Scholar]
- Rose J.W., Watt H.E., White A.T., Carlson N.G. (2004) Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol 56: 864–867 [DOI] [PubMed] [Google Scholar]
- Schippling D.S., Martin R. (2008) Spotlight on anti-CD25: daclizumab in MS. Int Mult Scler J 15: 94–98 [PubMed] [Google Scholar]
- Snyder J.T., Shen J., Azmi H., Hou J., Fowler D.H., Ragheb J.A. (2007) Direct inhibition of CD40L expression contributes to clinical efficacy of daclizumab independently of its effects on cell division and Th1/Th2 cytokine production. Blood 109: 5399–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M., Martin R. (2005) Immunology of multiple sclerosis. Ann Rev Immunol 23: 683–747 [DOI] [PubMed] [Google Scholar]
- Vincenti F., Kirkman R., Light S., Bumgardner G., Pescovitz M., Halloran P.et al. (1998) Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med 338: 161–165 [DOI] [PubMed] [Google Scholar]
- Waldmann T., Levy R., Coller B. (2000) Emerging therapies: spectrum of applications of monoclonal antibody therapy. Hematology 84: 394–406 [DOI] [PubMed] [Google Scholar]