Abstract
Although zonisamide was previously only used to treat epilepsy, recently more applications have been forthcoming. Due to a good side effect profile, a lower frequency of interactions and a more comfortable posology, there are several studies regarding its uses in other pathologies such as migraine, neuropathic pain, essential tremor and various psychiatric diseases. A multicentered, randomized, double-blind, placebo-controlled study conducted in Japan suggested that zonisamide, as an add-on treatment, has efficacy in treating motor symptoms in patients with Parkinson's disease. In addition, other studies support the utility of zonisamide in other symptoms of this disease. The therapeutic doses of zonisamide for the treatment of Parkinson's disease are considerably lower than those for the treatment of epilepsy. This antiepileptic drug has been used in Japan for more than 15 years and so it is expected that it will be safe and well tolerated in patients with Parkinson's disease. However, the pharmacological mechanisms of the antiparkinsonian actions of zonisamide remain unclear and more basic investigation is warranted. The aim of this paper is to review the structure, mechanisms of action, pharmacokinetics and antiparkinsonian action of zonisamide.
Keywords: antiepileptic, neuromodulator, Parkinson's disease, zonisamide
Introduction
Zonisamide (ZNS) is a sulphonamide anticonvulsant drug originally synthesized in Japan used to treat seizures worldwide [Arzimanoglou and Rahbani, 2006]. In addition to its effects on patients with epilepsy, ZNS has been suggested to have beneficial efficacy in various neurological and psychiatric diseases. Migraine [Bermejo and Dorado, in press], neuropathic pain [Guay, 2003], essential tremor [Bermejo et al. 2008], impulse control disorders [Bermejo and Velasco, 2008] and Parkinson's disease (PD) are possible uses of this drug. Similar to ZNS, other antiepileptic drugs have been proposed to treat different pathologies and the name ‘neuromodulators’ has been proposed for them [Bazil, 2004].
Until now, neuromodulators have not had an important role in of PD and the treatment is based on different combinations of L-DOPA with peripheral inhibitors of dopamine decarboxylase, such as carbidopa and benserazide, catechol-O-methyltransferase (COMT) inhibitors such as entacapone and tolcapone, monoamine oxidase-B (MAO-B) inhibitors such as selegiline and rasagiline, several dopamine agonists and deep brain stimulation [Jankovic, 2006].
Recent studies have provided data suggesting that ZNS has an efficacy in treating motor and nonmotor symptoms in patients with PD. The aim of this article is to review the structure, mechanism of action, pharmacokinetics and antiparkinsonian action of ZNS.
Structure and mechanisms of action of ZNS
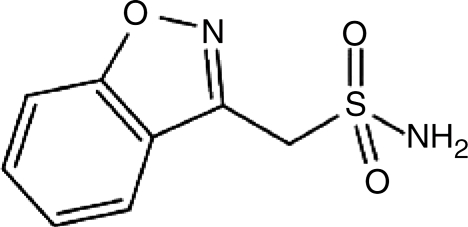
ZNS has multiple mechanisms of action, including blockage of sodium and T-type calcium channels, inhibition of carbonic anhydrase, inhibition of glutamate release and modulation of the GABAA receptor. In the dopaminergic system, therapeutic doses of ZNS increase intracellular and extracellular dopamine in the rat striatum. In contrast, supratherapeutic doses reduce intra-cellular intracellular dopamine. Thus, ZNS has a biphasic effect on the dopaminergic system [Biton, 2007]. In these different mechanisms of action may contribute to its clinical efficacy in different disorders. The structure ofZNS is shown in Figure 1.
Figure 1.
Structure of zonisamide.
Antiparkinsonian mechanisms of action of ZNS
The pharmacological mechanisms underlying the beneficial effects of ZNS in PD are unclear and both dopaminergic and nondopaminergic mechanisms seem to be involved. These mechanisms could be different from others used by ZNS to treat other diseases such as epilepsy or migraine since therapeutic doses of ZNS are 50—100mg/day, considerably lower than those for the treatment of epilepsy (200—400 mg/day) [Murata et al. 2007]. We will now review the potential mechanisms.
Enhancement of dopamine release
Therapeutic doses of ZNS increase intracellular and extracellular dopamine in the rat striatum while supratherapeutic doses reduce intracellular dopamine. This effect is not observed in rats with 6-hydroxydopamine-induced denervation of dopaminergic fibers except when ZNS is administered with L-DOPA and a dopa decarboxylase inhibitor [Gluck et al. 2004]. The dual effect of ZNS (because of the biphasic effect on dopaminergic system described above) has been proposed to be the cause of the several cases of restless legs syndrome described in the literature [Bermejo et al. 2007; Chen et al. 2003].
Blockade of T-type calcium channels
The pattern of neuronal activity in basal nuclei neurons in PD patients and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) monkeys changes to a bursting discharge pattern [Wichmann and DeLong, 2006]. This activity could be reduced by the blockage of T-type calcium channels, one of the mechanisms of action of ZNS. However, the effects of modulating T-type calcium channels on PD symptoms are unknown and more studies are warranted.
Inhibition of MAO-B
MAO-B inhibitors such as rasagiline and selegiline are well known treatments in PD and ZNS does inhibit MAO-B. However the potency of this activity is unknown. In the study conducted by Murata et al. [2007], ZNS was effective even in the group of patients who were on a sufficient dose of selegiline, suggesting that the inhibition of MAO-B is not the principal mechanism of action of ZNS to improve parkinsonian symptoms.
Kubo et al. [2008], in recent experiments using marmosets, suggested that ZNS does not inhibit the effects of MPTP, which is inhibited by MAO-B inhibitors. However, the administration of ZNS on MPTP-treated marmosets was effective at increasing dopamine metabolism in the striatum, therefore the authors suggest that ZNS may not a MAO-B inhibitor in vivo, but it activates impaired dopamine neurons.
Neuroprotection
There has been a growing interest in the use of antiepileptic drugs for neuroprotection. Established antiepileptic drugs such as phenytoin, phenobarbital and carbamazepine, have shown neuroprotective activity in an ischemic/hypoxic model of neuronal injury. Animal model studies also have suggested that newer antiepileptic drugs such as levetiracetam, topiramate and ZNS, may have not only antiepileptigenic but also neuroprotective properties [Willmore, 2005]. Since neurodegeneration seems to be present in PD, ZNS neuroprotective properties could have a role in the progression of the disease.
Pharmacokinetics
ZNS is completely absorbed from the gastrointestinal tract and its bioavailability is not affected by food. In the blood, 40% is bound to plasma albumin and penetrates the blood—brain barrier via lipid-mediated transport. Its elimination halflife is 49.7—62.5 hours and plasma steady state is achieved in 10—12 days of dosing. Serum concentration is similar on dosing once or twice daily [Miwa, 2007]. The P450 enzyme CYP3 A4 is the principal responsible for the metabolism of ZNS although CYP2 C19 and CYP3 A5 may also contribute [Morita et al. 2005]. All ZNS derivates are excreted in the urine. Possible interactions of ZNS with antiparkinsonian drugs or other drugs that may be used in PD patients should also be taken into account.
Efficacy of ZNS on motor symptoms of PD
A multicenter, randomized, double-blind, placebo-controlled study conducted by Murata et al. [2007] in Japan provided data suggesting that ZNS, as an add-on treatment, has efficacy in treating motor symptoms in patients with PD. In this study, 279 patients with PD who had problems receiving L-DOPA therapy were enrolled. ZNS (25, 50 or 100 mg/day) or placebo was administered for 12 weeks and symptoms were evaluated using the Unified Parkinson's Disease
Rating Scale (UPDRS) Part III and the total daily ‘off’ time. There was a significant improvement in the change from baseline in the total score of the UPDRS Part III in the 25 mg and 50 mg groups versus placebo. The duration of ‘off’ time was also significantly reduced in the 50 mg and 100 mg groups versus placebo. Additionally, ZNS was demonstrated to have a good safety profile in PD patients. The incidence of adverse effects was similar between the 25 mg, 50 mg and placebo groups although was higher in the 100 mg group. The principal adverse events were somnolence (10.9%), apathy (8.5%), body weight loss (6.9%) and constipation (6.5%). Additionally, dyskinesia was not increased in ZNS groups. This author performed a small open trial some years before with similar results [Murata etal. 2001].
Although the Murata study is the most important so far, others support the antiparkinsonian effect of this drug. Kajimoto et al. [2008] examined the preservation of the efficacy and safety of ZNS for parkinsonism one year after starting ZNS administration as an adjunct to ordinary antiparkinsonian drug therapy. According to this study this drug seems to be safe and efficacious during the 1-year follow-up period.
On the other hand there is increasing evidence of an antitremor effect of ZNS. A preliminary open study conducted by Nakanishi et al. [2003] suggested that ZNS has effects on residual parkinsonian tremor in PD patients whose motor symptoms were treated with dopamine replacement therapy. Additionally several studies demonstrate the effectiveness of ZNS in suppressing essential tremor [Bermejo et al. 2008; Ondo, 2007; Zesiewicz et al. 2007].
Another study performed by our group [Bermejo, 2007] suggested a possible beneficial role of ZNS in those patients with PD and essential tremor (ET), which is called ‘ET-PD syndrome'. This entity shares characteristics with both diseases and no pharmacological approach has demonstrated to be useful for ET and PD so far. In fact, drugs used to treat either ET or PD are not effective in controlling symptoms of the other disorder except ZNS [Ondo, 2007]. This study enrolled six patients with both tremor (including acting, postural and resting) and other parkinsonian features, such as rigidity and bradykinesia, improved in a high percentage of patients. ZNS doses (200mg/day average) were slightly higher than previous studies (50—600 mg/day). Since AMPA [2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid] receptor blockage seems to improve levodopa-induced dyskinesias [Konitsiotis et al. 2000] and ZNS also blocks these receptors [Huang et al. 2005], this drug could have a potential beneficial role in these patients.
Efficacy of ZNS on nonmotor symptoms of PD
Current studies about the role of neuromodulators in PD are focused on specific aspects of the disease including tremor, control of dyskinesias, neuroprotection, psychiatric complications and associated pathologies such as essential tremor-PD syndrome or restless legs syndrome [Bermejo et al. 2008]. According to recent studies, ZNS may be useful in several symptoms other than motor complications of PD:
Impulse control disorders. They are a set of behaviors which are now recognized to occur in a subgroup of patients with PD. Their pathophysiology remains unclear although a dopaminergic mechanism seems to be implicated. ZNS has been suggested to improve several impulse control disorders such as binge eating disorder and alcohol intake. Our group [Bermejo and Velasco, 2008] evaluated the safety and efficacy of ZNS in a small group of patients with impulse control disorders and PD who did not improve following either a reduction of or switch to L-DOPA or dopamine agonists. Results suggested that this drug might also play a role when these disorders are associated with PD. Similar results have been obtained with topiramate, another neuromodulator with several similar mechanisms of action to ZNS [Bermejo, 2008].
Anxiety. According to a recent study, anxiety and depression are frequent disorders in PD [Carod-Artal et al. 2008]. In this respect, ZNS may be useful in the treatment of this problem, especially when it is associated to PD [Kinrys et al. 2007].
Tolerability and safety
ZNS is widely used to treat epilepsy and has been used in Japan for more than 15 years. Therefore, ZNS may be considered as a well-tolerated drug with a good safety profile. In addition, doses used to treat PD are significantly lower than those used to treat epilepsy. In the study conducted by Murata et al. [2007] ZNS was well tolerated and the principal side effects were somnolence, apathy, body weight loss and constipation. Although they are not severe, it is important to take into account that PD may produce several gastrointestinal symptoms including body weight loss and constipation, symptoms that may be worsened by ZNS. There is little information concerning interactions between ZNS and other parkinsonian drugs. Other situations that have to be taken into account in ZNS-treated PD patients are the following:
Psychosis. This complication is common in patients with PD and is related to an increase in morbidity. There are several articles reporting mental side effects such as psychosis during the treatment with some neuromodulators, including ZNS [Michael and Starr, 2007]. Although there is no case of worsening of psychosis in advanced PD due to ZNS, this possibility should be taken into account in PD patients treated with this drug.
Restless legs syndrome. This syndrome is a phenomenon characterized by an intense and irresistible urge to move lower limbs and is associated with sensitive complaints and motor restlessness. Symptoms usually occur at night provoking insomnia and daily fatigue. The pathophysiology of this disorder is still unknown and dopaminergic mechanisms have been implicated. It has been associated to PD in up to 20% of the patients [Gomez-Esteban et al. 2007]. Several ZNS-induced restless legs syndrome cases have been reported in the literature [Bermejo et al. 2007; Chen et al. 2003] but the effect of this drug on patients with PD and restless legs syndrome is unknown.
Others. Weight loss [Kashihara, 2006] and fatigue [Friedman et al. 2007] may be associated with PD and ZNS may potentiate them, therefore they should be taken into account when treating PD with ZNS.
Conclusions
There is increasing evidence for a role of ZNS in the treatment of both motor and nonmotor symptoms in PD. The therapeutic doses of ZNS for the treatment of PD are 50—100mg/day, considerably lower than those for the treatment of epilepsy (200—400 mg/day). It is expected that ZNS will have a good safety profile and that it will be well tolerated in patients with PD, as it has been used as an antiepileptic for more than 15 years; however, further studies are required to evaluate its safety and tolerability in the treatment of PD due to specific characteristics of these patients.
There are several limitations for the use of ZNS in PD. One of the most important is that there is only one randomized, double-blind study demonstrating this effect and other similar studies are warranted. In addition, most observations of a beneficial effect of ZNS have been in Japanese people, and the antiparkinsonian mechanism of action is unclear. In our opinion, ZNS is a promising but still investigational drug to treat PD and more studies are warranted.
Conflict of interest statement
None declared.
Contributor Information
Pedro Emilio Bermejo, Sanatorio Nuestra Señora del Rosario — Hospital Sanitas La Zarzuela, Madrid, Spain pedro_bermejo@hotmail.com.
Buenaventura Anciones, Sanatorio Nuestra Señora del Rosario — Hospital Sanitas La Zarzuela, Madrid, Spain.
References
- Arzimanoglou A., Rahbani A. (2006) Zonisamide for the treatment of epilepsy. Expert Rev Neurother 6: 1283–1292 [DOI] [PubMed] [Google Scholar]
- Bazil C.W. (2004) Anticonvulsant drug or neuromodulator? The growing case for anticonvulsant uses beyond epilepsy. Curr Neurol Neurosci Rep 4: 305–307 [DOI] [PubMed] [Google Scholar]
- Bermejo P.E. (2008) Topiramate in managing impulse control disorders in Parkinson's disease. Parkinsonism Relat Disord 14: 448–449 [DOI] [PubMed] [Google Scholar]
- Bermejo P.E. (2007) Zonisamide in patients with essential tremor and Parkinson's disease. Mov Disord 22: 2137–2138 [DOI] [PubMed] [Google Scholar]
- Bermejo P.E., Dorado R.Zonisamide for migraine prophylaxis in refractory patients to topira-mate. Clin Neuropharmacol, in press [DOI] [PubMed] [Google Scholar]
- Bermejo P.E., Gomez-Argüelles J.M., Sepiilveda J.M. (2008) Role of antiepileptic drugs in Parkinson's disease. Med Clin (Barc) 131: 466–471 [DOI] [PubMed] [Google Scholar]
- Bermejo P.E., Ruiz-Huete C., Dorado R., Anciones B. (2008) Zonisamide in refractory essential tremor. Rev Neurol 46: 139–142 [PubMed] [Google Scholar]
- Bermejo P.E., Velasco R. (2008) Zonisamide in managing impulse control disorders in Parkinson's disease. J Neurol 255(Suppl 2): 167 [Abstract] [DOI] [PubMed] [Google Scholar]
- Bermejo P.E., Zabala J.A., Saez R. (2007) Restless legs syndrome induced by zonisamide. Mov Disord 22: 1517–1518 [DOI] [PubMed] [Google Scholar]
- Biton V. (2007) Clinical pharmacology and mechanism of action of zonisamide. Clin Neuropharmacol 30: 230–240 [DOI] [PubMed] [Google Scholar]
- Carod-Artal F.J., Ziomkowski S., Mourao Mesquita H., Martínez-Martín P. (2008) Anxiety and depression: Main determinants of health-related quality of life in Brazilian patients with Parkinson's disease. Parkinsonism Relat Disord 14: 102–108 [DOI] [PubMed] [Google Scholar]
- Chen J.T., Garcia P.A., Alldredge B.K. (2003) Zonisamide-induced restless legs syndrome. Neurology 60: 147. [DOI] [PubMed] [Google Scholar]
- Friedman J.H., Brown R.G., Comella C., Garber C.E., Krupp L.B., Lou J.S.et al. (2007) Fatigue in Parkinson's disease: a review. Mov Disord 22: 297–308 [DOI] [PubMed] [Google Scholar]
- Gluck M.R., Santana L.A., Granson H., Yahr M.D. (2004) Novel dopamine releasing response of an anticonvulsant agent with possible anti-Parkinson's activity. J Neural Transm 111: 713–724 [DOI] [PubMed] [Google Scholar]
- Gomez-Esteban J.C., Zarranz J.J., Tijero B., Velasco F., Barcena J., Rouco I.et al. (2007) Restless legs syndrome in Parkinson's disease. Mov Disord 22: 1912–1916 [DOI] [PubMed] [Google Scholar]
- Guay D.R. (2003) Oxcarbazepine, topiramate, zonisamide, and levetiracetam: potential use in neuropathic pain. Am J Geriatr Pharmacother 1: 18–37 [DOI] [PubMed] [Google Scholar]
- Huang C.W., Ueno S., Okada M., Kaneko S. (2005) Zonisamide at clinically relevant concentrations inhibits field EPSP but not presynaptic fiber volley in rat frontal cortex. Epilepsy Res 67: 51–60 [DOI] [PubMed] [Google Scholar]
- Jankovic J. (2006) An update on the treatment of Parkinson's disease. Mt Sinai J Med 73: 682–689 [PubMed] [Google Scholar]
- Kajimoto Y., Nakanishi I., Kondo Wakayama T. (2008) One year follow-up study of the zonisamide (ZNS) efficacy on parkinsonism. Mov Disord 23(Suppl 1): 214 [Abstract] [Google Scholar]
- Kashihara K. (2006) Weight loss in Parkinson's disease. J Neurol 253: 38–41 [DOI] [PubMed] [Google Scholar]
- Kinrys G., Vasconcelos e Sa D., Nery F. (2007) Adjunctive zonisamide for treatment refractory anxiety. IntJClin Pract 61: 1050–1053 [DOI] [PubMed] [Google Scholar]
- Konitsiotis S., Blanchet P.J., Verhagen L., Lamers E., Chase T.N. (2000) AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology 54: 1589–1595 [DOI] [PubMed] [Google Scholar]
- Kubo M., Nishikawa N., Yabe H., Nagai M., Moritoyo H., Moritoyo T.et al. (2008) Zonisamide increased metabolism of dopamine neurons in MPTP-treated C57BL/6 and common marmosets. Mov Disord 23(Suppl 1): 311 [Abstract]18044768 [Google Scholar]
- Michael C.T., Starr J.L. (2007) Psychosis following initiation of zonisamide. Am J Psychiatry 164: 682. [DOI] [PubMed] [Google Scholar]
- Miwa H. (2007) Zonisamide for the treatment of Parkinson's disease. Expert Rev Neurother 7: 1077–1083 [DOI] [PubMed] [Google Scholar]
- Morita S., Miwa H., Kondo T. (2005) Effect of zonisamide on essential tremor: a pilot crossover study in comparison with arotinolol. Parkinsonism Relat Disord 11: 101–103 [DOI] [PubMed] [Google Scholar]
- Murata M., Hasegawa K., Kanazawa I. (2007) Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68: 45–50 [DOI] [PubMed] [Google Scholar]
- Murata M., Horiuchi E., Kanazawa I. (2001) Zonisamide has beneficial effects on Parkinson's disease patients. Neurosci Res 41: 397–399 [DOI] [PubMed] [Google Scholar]
- Nakanishi I., Kohmoto J., Miwa H., Kondo T. (2003) Effect of zonisamide on resting tremor resistant to antiparkinsonian medication. No To Shinkei 55: 685–689 [PubMed] [Google Scholar]
- Ondo W.G. (2007) Zonisamide for essential tremor. Clin Neuropharmacol 30: 345–349 [DOI] [PubMed] [Google Scholar]
- Wichmann T., DeLong M.R. (2006) Basal ganglia discharge abnormalities in Parkinson's disease. J Neural Transm 70: 21–25 [DOI] [PubMed] [Google Scholar]
- Willmore L.J. (2005) Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav 7(Suppl. 3): 25–28 [DOI] [PubMed] [Google Scholar]
- Zesiewicz T.A., Ward C.L., Hauser R.A., Sanchez-Ramos J., Staffetti J.F., Sullivan K.L. (2007) A double-blind placebo-controlled trial of zonisamide (zonegran) in the treatment of essential tremor. Mov Disord 22: 279–282 [DOI] [PubMed] [Google Scholar]