Abstract
Intrathecal injection of triamcinolone acetonide (TCA) has been shown to provide substantial benefit in a subset of progressive multiple sclerosis (MS) patients with predominant spinal symptoms. We examined whether atrophy of the upper spinal cord (USC) as measured by MRI can serve as a predictive marker for response to repetitive intrathecal TCA application. Repetitive administration of 40 mg TCA was performed in 31 chronic progressive MS patients up to six times within 3 weeks. Expanded Disability Status Scale (EDSS) and maximum walking distance (WD) were assessed before and after the treatment cycle. Cervical 3D T1-weighted images were acquired on a 1.5T scanner at baseline. Mean cross-sectional area of the USC was determined using a semi-automated volumetry method. Results were compared with a group of 29 healthy controls to group patients into those with and without atrophy. Results show a negative correlation between the degree of USC atrophy and treatment benefit. A higher treatment benefit in patients with little USC atrophy but short initial maximum WD was observed. Absence of USC atrophy as measured on MRI is a predictive marker for intrathecal TCA therapy outcome in progressive MS. Patients with initial poor walking abilities, but only little or no atrophy, benefited most from TCA therapy.
Keywords: atrophy, MRI, multiple sclerosis, spinal cord, triamcinolone acetonide
Introduction
In multiple sclerosis (MS), the spinal cord is a common site of involvement, affecting the cervical region more frequently than the thoracic and lumbar regions [Evangelou et al. 2005; Kidd et al. 1993]. Damage in this region can dramatically affect the functional outcome of patients. Acute symptoms are often caused by active lesions which are frequently associated with cord swelling [Thielen and Miller 1996]. While newer imaging modalities have improved the detection of MS lesions in the spinal cord, conventional magnetic resonance imaging (MRI) still lacks histopathological specificity [Kidd et al. 1993]. Techniques such as the measurement of spinal cord atrophy have become of significant importance. A number of cross-sectional and longitudinal studies have shown that spinal cord atrophy correlates with clinical disability [Rashid et al. 2006; Bakshi et al. 2005; Liu et al. 1999]. Amount and severity of MS pathology in the cervical cord are greater in the progressive forms of the disease [Filippi et al. 2000]. It is assumed that axonal loss in MS is an important factor for the occurrence of atrophy and that atrophy in general represents the endpoint of tissue destruction [Lin and Blumhardt 2001; Matthews 1999; Silber and Sharief 1999; Rovaris et al. 1997; Silver et al. 1997]. Since spinal cord atrophy already develops in the early phases of MS, the quantification of spinal cord volume by MRI is a potential marker to quantitatively monitor the course of the disease and furthermore to monitor treatment efficacy.
For patients with progressive multiple sclerosis available immunomodulatory and conventional steroid treatment regimens provide only a limited symptomatic benefit. Intrathecal application of a small dosage of a slow release steroid compound can be superior to i.v. injection since it facilitates higher drug concentrations at the origin of inflammation and the agent is released at long term without the typical adverse effects of systemic low or high dose steroid administration [Rohrbach et al. 1988]. Repeated intrathecal application of the sustained release steroid triamcinolone acetonide (TCA) can significantly improve the disability status and especially the walking capacities in progressive MS patients with predominant spinal symptoms [Hellwig et al. 2006a; Hoffmann et al. 2006, 2003]. Furthermore, a follow-up study demonstrated that in a majority of patients treatment benefit of TCA therapy remained stable during further intermittent TCA injections over one year [Hellwig et al. 2006a]. Although these studies demonstrated the safety of appropriate intrathecal TCA application, serious side effects, for example, sterile meningitis or adhesive arachnoiditis, may occur. Given the invasive character of this therapeutic approach, and the lack of class I evidence studies, a clinical or radiological predictor of treatment response would be useful in both aspects. The aim of this prospective study was to determine whether atrophy of the upper spinal cord (USC) as measured by MRI can serve as a predictive marker for response to repetitive intrathecal TCA application.
Methods
Subjects
Thirty-one progressive MS-patients [22 with secondary progressive (SPMS), 9 with primary progressive (PPMS) disease course] with an Expanded Disability Status Scale (EDSS) score lower than 7.5 were enrolled in this study. Clinical characteristics are given in Table 1. All patients had to have experienced a distinct progression of MS symptoms, which corresponds to an EDSS change of at least one point in the last 2 years before study entry. Furthermore all patients had to be clinically stable for at least 4 weeks before inclusion and exhibit no enhancing USC lesions. EDSS before the start of the TCA therapy was in similar ranges in the SPMS and the PPMS group (range: 4.5–7.0). Subjects did not receive steroids for at least 4 weeks before study entry. Furthermore, in case of pretreatment patients had to be on a stable immunomodulatory or immunosuppresive drug treatment for at least 4 weeks before study entry. Patients with a history of seizure, subdural hematoma and/or severe postlumbar puncture syndrome were not included in the study. All patients were treated with repetitive administration of 40 mg triamcinolone acetonide of three to six times within 3 weeks. EDSS and maximum walking distance (WD) without support by walking aids were assessed at the same day prior and after the end of the treatment cycle (mean: 19 ± 1 days) (Table 2). To compare imaging findings a cohort of 29 age and gender-matched healthy controls was additionally enrolled in this study. The study was approved by the local ethics committee. All MS patients gave their written informed consent to intrathecal treatment and MRI examination as well as the further use of anonymized data.
Table 1.
Clinical data of MS patients and healthy controls.
|
All patientsz (n = 31) |
SPMS (n = 22) |
PPMS (n = 9) |
Healthy controls (n = 29) |
|
| Age/years | 43.3 ± 9.7 | 42.7 ± 7.4 | 46.6 ± 13.0 | 42.7 ± 15.7 |
| [24–9] | [27–3] | [26–9] | [23–3] | |
| Female: male | 21 : 10 | 13 : 9 | 8 : 1 | 16 : 13 |
| Disease duration/years | 9.5 ± 7.8 | 8.7 ± 8.8 | 11.7 ± 11.7 | |
| [1–42] | [1–22] | [1–42] | ||
| Years since conversion from RRMS to SPMS | 3.8±2.0 | |||
| [1–7] | ||||
| EDSS before therapy | 5.5 ± 0.9 | 5.5 ± 0.9 | 5.4 ± 1.0 | |
| [4.5–7.0] | [4.5–7.0] | [4.5–7.0] | ||
| Max. WD before therapy | 194 m | 168 m | 257m | |
| [10–800 m] | [10–400 m] | [40–800 m] | ||
| Pretreatment: (number of patients) | ||||
| None | 19 | 11 | 8 | |
| ImmunomoduLatory | 9 | 9 | 0 | |
| Immunosuppressive | 3 | 2 | 1 |
Data are quoted as mean (±SD] [range], n, number of subjects. Max. WD, maximum walking distance; SPMS, secondary progressive MS; PPMS, primary progressive MS; RRMS, relapsing—remitting MS. Immunomodulatory pretreament: interferon, glatiramer acetate, immuoglobulins. Immunosuppresive pretreatment: azathioprine, mitoxantrone.
Table 2.
Walking distance and Expanded Disability Symptom Scale during triamcinolone acetonide treatment.
| All patients (n ¼ 31) | SPMS (n = 22) | PPMS (n = 9) | |
| WD before TCA | 194 m | 168 m | 257 m |
| (Mean [range]) | [10–800 m] | [10–400 m] | [40–800 m] |
| WD after TCA | 498 m | 488 m | 518 m |
| (Mean [range]) | [15–2000 m] | [90–2000 m] | [15–1200 m] |
| Significance p | p < 0.001 | p = 0.001 | p = 0.046 |
| EDSS before TCA (Mean±SD) | 5.5 ± 0.9 | 5.5±0.9 | 5.4 ±1.0 |
| EDSS after TCA (Mean±SD) | 4.9 ± 1.0 | 4.9 ±0.9 | 4.8± 1.1 |
| Significance p | p < 0.001 | p = 0.001 | p = 0.071 |
| Number of TCA injections | 3 inj.: 1 patient 6 inj.: 30 patients | 6 inj.: 22 patients | 3 inj.: 1 patient 6 inj.: 8 patients |
Data are quoted as mean (±SD) [range], n, number of subjects; WD, walking distance; SPMS, secondary progressive MS; PPMS, primary progressive MS; p, statistical significance of differences between WD (respectively EDSS) after therapy in comparison with therapy start by Wilcoxon signed rank testing.
TCA administration
In all patients an atraumatic (Sprotte®) needle was used for intrathecal treatment in order to reduce the risk for onset of postlumbar puncture syndrome. All patients were treated with repetitive administration of 40 mg TCA three to six times within 3 weeks. The individual decision on the number of injections depended on the improvement of the clinical parameters in each patient, which in earlier studies became evident after the third application and more significant after the sixth injection. The details of the procedure are given in Hoffmann et al. [2006].
Neuroimaging and data analysis
MRI scans were obtained from all the patients and control subjects using a standardized MRI protocol on a 1.5T scanner (Magnetom SymphonyTM, Siemens, Erlangen, Germany) at study entry. For assessing spinal cord cross-sectional area a sagittal high-resolution 3D T1-weighted MRI data set (MPRAGE; magnetization prepared rapid gradient echo) covering the whole brain and upper cervical spinal cord was acquired by using a Turbo FLASH 3D sequence (TE [echo time]: 3.93ms, TR [repetition time]: 1900 ms, TI [inversion time]: 1100 ms, flip angle: 15°, resolution: 1 mm × 1 mm, 128 sagittal slices, slice thickness: 1.5 mm). All subjects were positioned within the head coil using a standard procedure according to outer anatomical markers. In addition we performed sagittal T1 post-GdDTPA images of the cervical cord to rule out active enhancing lesions, which can be associated with cord swelling. Patients with acute lesions were excluded from this study under the assumption that acute cord swelling may alter spinal cord volumetry.
Postprocessing of MR images
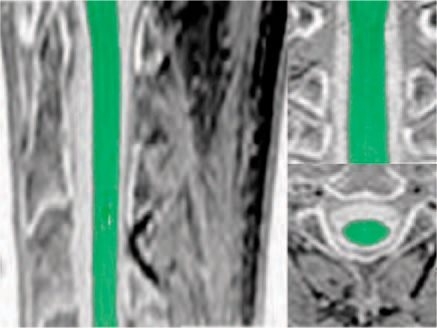
Images were postprocessed by an evaluator blinded to the subject's identity using a semi-automated volumetry method combining a marker-based segmentation and an automatic histogram analysis. Spinal cord volume was segmented and quantified within a section of 50mm thickness starting at upper border of C2 rostrally (Figure 1). The mean cross-sectional area within this volume was then calculated by dividing the volume by the defined section length. Details of the evaluation method can be found in Lukas et al. [2008]. To correct for intra-individual variations due to gender, natural aging and head size, all cross-sectional area results were normalized by multiplying by the ratio of the mean intracranial cavity volume (ICC) of the total population to the ICC for each individual. This is a common procedure in morphometric studies of the central nervous system (CNS) [Whitwell et al. 2001], which in our study reduced the interindividual variability of the mean USC area in the healthy control group from 9.1% to 6.8% (mean USC area in the healthy control group, not normalized: 89.4 ±8.2 mm2, normalized: 87.3 ±5.9 mm2). Furthermore, after normalization the mean USC area in the healthy control group showed no significant correlation with age.
Figure 1.
Spinal cord segmentation technique at the level C1 to C3.
The intracranial volume was assessed on the basis of the volumetric T1-weighted 3D data by using a semi-automated volumetry method for brain volumetry which is identical to the method used in Lukas et al. [2004]. The histogram based volume computation approach employed therein is described in more detail in Hahn et al. [2004].
Statistical analysis
Statistical analyses were performed using the SPSS14 (SPSS Inc., Chicago, IL) software. Wilcoxon signed rank test for repeated measurements was used to examine changes of EDSS and maximum walking distance after treatment. Correlation between relative WD gain and the mean normalized cross-sectional spinal cord area in subjects were evaluated using Spearman's rank correlation. To examine the effects of mean USC area and initial maximum walking distance on the relative WD gain a multiple linear regression analysis was performed. Possible influences of the pretreatment at the beginning of the TCA therapy on the relative WD gain were assessed by an analysis of variance with the mean USC area as covariable and pretreatment (present/absent) as a fixed factor. Analysis between subgroups according to the rate of atrophy was done by Mann—Whitney U-testing.
Results
Mean walking distance for the whole group increased from 255 m at baseline to 566 m after TCA therapy. The mean EDSS of the whole patient group showed a slight decrease by 0.6. The differences between the mean walking distance and EDSS before and after TCA therapy were statistically highly significant with p ≤ 0.001 for the whole patient group and SPMS subgroup and significant for the mean walking distance in the PPMS subgroup (p = 0.046; see Table 2).
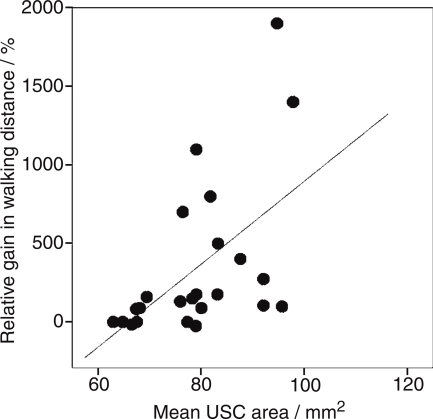
Treatment benefit was evaluated according to the relative gain in maximum walking distance: rel. WDgain=(WDafter TCA- WDbefore TCA)/ WDbefore TCA; range: 67% to +1900%. Correlation between the relative WD gain and the mean cross-sectional spinal cord area revealed a significant correlation with r = 0.442 and p = 0.019, indicating that treatment benefit tends to be higher for patients with less USC atrophy than for patients with marked atrophy of the USC. In the scatter plot (Figure 2) the distribution of the relative WD gain values in dependence on the mean USC area is presented for clarification. Presence or absence of pretreatment showed no significant impact on the TCA benefit in this study group (p = 0.937).
Figure 2.
Scatter plot of relative walking distance improvement (treatment benefit) dependent of the mean upper spinal cord area.
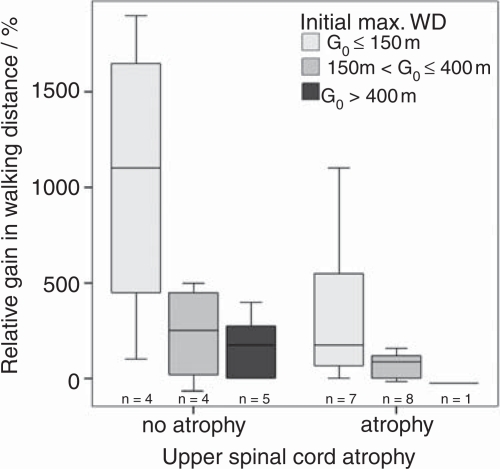
Relative WD gain in subjects was significantly dependent on mean USC area and initial maximum walking distance indicating that higher treatment benefit prevails in patients with little USC atrophy but short maximum walking distance at the start of the TCA therapy (Table 3). To illustrate this effect further (Figure 3) the patients were grouped into those with no atrophy (>80mm2, n=14), or atrophy (≤80mm2, n= 17) on the basis of the mean USC area of the control group (mean: 87.1 ±5.8 mm2). A further subdivision of the patients according to the initial maximum walking distance G0 into groups: G0 ≤ 150 m, G0: 150–400 m, G0 > 400 m was performed. Although statistical testing between these subgroups did not reach significance, the box plots demonstrate that patients with an initial short maximum walking distance (≤150m) and no atrophy exhibited greatest benefit in improved maximum walking distance (median > 1000%) (Figure 3).
Table 3.
Multiple linear regression of relative walking distance (WD) gain (treatment benefit) in dependency on mean upper spinal cord (USC) area and initial walking distance in MS patients.
| R2 | Independent variable | Regression coefficient | Standard error | p value |
| 0.308 | Mean USC area | 18.97 | 6.97 | 0.011 |
| Initial WD | —0.51 | 0.21 | 0.021 |
Figure 3.
Relative gain in max. walking distance (WD) is highest for patients with short WD at therapy start and no USC atrophy (boxes depict median values and 25%/75% quartiles, error bars show ranges).
Discussion
Efficiency and safety of TCA therapy in progressive MS have been reported in a few studies so far [Hellwig et al. 2006a; Hoffmann et al. 2006, 2003]. Previous selection of patients has been based on clinical symptoms; for example, predominant spinal cord disability. Given the invasive character of this therapy, additional paraclinical findings such as the presence of cervical cord atrophy may be useful to preselect patients with good prospects to benefit from the treatment. We observed that patients with no or little atrophy in the upper spinal cord achieved the strongest treatment benefit especially when their initial walking abilities were impaired. Our findings are not surprising, but rather corroborate current pathophysiological models. The spinal cord is a clinically eloquent region and damage in this area has the potential to affect dramatically the functional outcome of MS patients. Damage can be due to acute lesions, which are often accompanied by cord swelling or result from degenerative processes, which lead to spinal cord atrophy. The latter seems to be the end state of tissue destruction resulting in an irreversible damage in which therapeutic treatment effects may be ineffective. Furthermore there is evidence that tissue damage in the cord is not restricted to MS lesions itself. By using magnetization-transfer histogram analysis subtle tissue damage can also be assessed outside MS cord lesions in the normal appearing white matter (NAWM) [Rovaris et al. 2001, 2000; Bozzali et al. 1999]. Besides the accumulation of macroscopic lesions and the severity of tissue destruction within those lesions, NAWM changes also contribute to the clinical status in MS patients [Rovaris et al. 2000]. In patients with no or little spinal cord atrophy subtle inflammation and patchy edema may play a relevant role in functional disability. In the absence of Gd enhancement these processes may be beyond the resolution of conventional MRI techniques as shown by DTI and MTR studies [Agosta et al. 2005; Rovaris et al. 2001; Filippi et al. 2000]. It can be hypothesized that in this cohort anti-inflammatory drug treatment when given intrathecally may lead to a reduction of subacute inflammation and/or edema inducing an improvement of disability. In contrast to i.v. injection, intrathecal application of a small dosage of a slow-release steroid compound leads to higher concentrations at the origin of inflammation which has been demonstrated by Rohrbach et al. [1988]. This effect has also been hypothesized in a clinical TCA study in relapsing remitting MS patients with painful dysasthesia due to acute spinal cord lesions in which prior treatment with i.v. steroids failed [Hellwig et al. 2006b].
In contrast, in patients with moderate to distinct atrophy subacute inflammation may play only a minor role and disability in these patients may result from pronounced irreversible tissue loss, which will not be affected by anti-inflammatory treatment approaches. However, even in the subgroup of patients with severe atrophy in the spinal cord, we observed cases of increase of the walking distance. One possible explanation for this contradictory finding can be that to some degree locomotor disability is a result of MS pathology in the brain. Intrathecal TCA therapy also acts upon inflammatory processes in the brain. Since we have not explicitly excluded acute or subacute brain inflammation by MRI, which is a major shortcoming of this study, in some patients the observed treatment profit can result from brain involvement. On the other hand, patients in this study have been selected on the basis of slow disease progression rather than on acute relapse. Thus, acute disease activity within the brain seems to be unlikely. Finally, it has to be mentioned that we cannot fully exclude prior effects on the development of spinal cord atrophy due to immunosupressive or immunomodulatory treatment which might confound the results of this study.
When subdividing MS patients according to their disease subtype, results showed no significant EDSS changes after treatment in the PPMS group. However, these results must be kept with caution due to the small number of patients in this subgroup. Further MRI studies monitoring the long-term benefit of TCA and its impact on the evolution of spinal cord atrophy in a larger cohort of patients are currently ongoing and will also include brain MRI. In conclusion, absence of USC atrophy as measured by a volumetric MR-based strategy is a predictive marker for intrathecal TCA therapy outcome in progressive MS with predominant spinal symptoms.
Conflict of interest statement
None declared.
Contributor Information
Carsten Lukas, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany Carsten.Lukas@ruhr-uni-bochum.de.
Barbara Bellenberg, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany.
Horst K. Hahn, Fraunhofer MEVIS, Institute for Medical Image Computing, Bremen, Germany
Jan Rexilius, Fraunhofer MEVIS, Institute for Medical Image Computing, Bremen, Germany.
Robert Drescher, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany.
Kerstin Hellwig, Department of Neurology, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany.
Odo Köster, Department of Diagnostic and Interventional Radiology and Nuclear Medicine, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany.
Sebastian Schimrigk, Department of Neurology, St Josef Hospital, Ruhr-University of Bochum, Bochum, Germany.
References
- Agosta F., Benedetti B., Rocca M.A., Valsasina P., Rovaris M., Comi G.et al. (2005) Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology 64:631–635 [DOI] [PubMed] [Google Scholar]
- Bakshi R., Dandamudi V.S., Neema M., De C., Bermel R.A. (2005) Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 15 (4 Suppl): 30S–45S [DOI] [PubMed] [Google Scholar]
- Bozzali M., Rocca M.A., Iannucci G., Pereira C, Comi G., Filippi M. (1999) Magnetization-transfer histogram analysis of the cervical cord in patients with multiple sclerosis. AJNR Am J Neuroradiol 20:1803–1808 [PMC free article] [PubMed] [Google Scholar]
- Evangelou N., DeLuca G.C., Owens T., Esiri M.M. (2005) Pathological study of spinal cord atrophy in multiple sclerosis suggests limited role of local lesions. Brain 128 (Pt 1): 29–34 [DOI] [PubMed] [Google Scholar]
- Filippi M., Bozzali M., Horsfield M.A., Rocca M.A., Sormani M.P., Iannucci G.et al. (2000) A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology 54:207–213 [DOI] [PubMed] [Google Scholar]
- Hahn H.K., Millar W.S., Klinghammer O., Durkin M.S., Tulipano P.K., Peitgen H.O. (2004) A reliable and efficient method for cerebral ventricular volumetry in pediatric neuroimaging. Methods InfMed 43:376–382 [PubMed] [Google Scholar]
- Hellwig K., Schimrigk S., Lukas C, Hoffmann V., Brune N., Przuntek H.et al. (2006a) Efficacy of mitoxantrone and intrathecal triamcinolone acetonide treatment in chronic progressive multiple sclerosis patients. Clin Neuropharmacol 29:286–291 [DOI] [PubMed] [Google Scholar]
- Hellwig K., Lukas C, Brune N., Schimrigk S., Przuntek H., Muller T. (2006b) Repeat intrathecal triamcinolone acetonide application reduces acute occurring painful dysesthesia in patients with relapsing remitting multiple sclerosis. Scientific World Journal 6:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann V., Kuhn W., Schimrigk S., Islamova S., Hellwig K, Lukas C.et al. (2006) Repeat intrathecal triamcinolone acetonide application is beneficial in progressive MS patients. Eur J Neurol 13:72–76 [DOI] [PubMed] [Google Scholar]
- Hoffmann V., Schimrigk S., Islamova S., Hellwig K, Lukas C, Brune N.et al. (2003) Efficacy and safety of repeated intrathecal triamcinolone acetonide application in progressive multiple sclerosis patients. J Neurol Sci 211:81–84 [DOI] [PubMed] [Google Scholar]
- Kidd D., Thorpe J.W., Thompson A.J., Kendall B.E., Moseley I.F., MacManus D.G.et al. (1993) Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 43:2632–2637 [DOI] [PubMed] [Google Scholar]
- Lin X., Blumhardt L.D. (2001) Inflammation and atrophy in multiple sclerosis: MRI associations with disease course. J Neurol Sci 189:99–104 [DOI] [PubMed] [Google Scholar]
- Liu C, Edwards S., Gong Q., Roberts N., Blumhardt L.D. (1999) Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 66:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C, Hahn H.K, Bellenberg B., Hellwig K, Globas C, Schimrigk S.Ket al. (2008) Spinal cord atrophy in spinocerebellar ataxia type 3 and 6: Impact on clinical disability. J Neurol 255:1244–1249 [DOI] [PubMed] [Google Scholar]
- Lukas C, Hahn H.K, Bellenberg B., Rexilius J., Schmid G., Schimrigk S.K.et al. (2004) Sensitivity and reproducibility of a new fast 3D segmentation technique for clinical MR-based brain volumetry in multiple sclerosis. Neuroradiology 46:906–915 [DOI] [PubMed] [Google Scholar]
- Matthews P.M. (1999) Axonal loss and demyelation in multiple sclerosis. J Neurol Neurosurg Psychiatry 67:708–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid W., Davies G.R., Chard D.T., Griffin CM., Altmann D.R., Gordon R.et al. (2006) Increasing cord atrophy in early relapsing-remitting multiple sclerosis: a 3 year study. J Neurol Neurosurg Psychiatry 77:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach E., Kappos L., Stadt D. (1988) Intrathecal versus oral corticosteroid therapy of spinal symptoms in multiple sclerosis: a double-blind controlled trial. Neurology 38:256 [Google Scholar]
- Rovaris M., Bozzali M., Santuccio G., Ghezzi A., Caputo D., Montanari E.et al. (2001) In vivo assessment of the brain and cervical cord pathology of patients with primary progressive multiple sclerosis. Brain 124:2540–2549 [DOI] [PubMed] [Google Scholar]
- Rovaris M., Bozzali M., Santuccio G., Iannucci G., Sormani M.P., Colombo B.et al. (2000) Relative contributions of brain and cervical cord pathology to multiple sclerosis disability: a study with magnetisation transfer ratio histogram analysis. J Neurol Neurosurg Psychiatry 69:723–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris M., Filippi M., Calori G., Rodegher M., Campi A., Colombo B.et al. (1997) Intra-observer reproducibility in measuring new putative MR markers of demyelination and axonal loss in multiple sclerosis: a comparison with conventional T2-weighted images. J Neurol 244:266–270 [DOI] [PubMed] [Google Scholar]
- Silber E., Sharief M.K. (1999) Axonal degeneration in the pathogenesis of multiple sclerosis. J Neurol Sci 170:11–18 [DOI] [PubMed] [Google Scholar]
- Silver N.C., Barker G.J., Losseff N.A., Gawne-Cain M.L., MacManus D.G., Thompson A.J.et al. (1997) Magnetisation transfer ratio measurement in the cervical spinal cord: a preliminary study in multiple sclerosis. Neuroradiology 39:441–445 [DOI] [PubMed] [Google Scholar]
- Thielen K.R., Miller G.M. (1996) Multiple sclerosis of the spinal cord: magnetic resonance appearance. J Comput Assist Tomogr 20:434–438 [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Crum W.R., Watt C., Fox N.C. (2001) Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol 22:1483–1489 [PMC free article] [PubMed] [Google Scholar]