Abstract
Bacterial meningitis is a medical emergency requiring immediate diagnosis and immediate treatment. Streptococcus pneumoniae and Neisseria meningitidis are the most common and most aggressive pathogens of meningitis. Emerging antibiotic resistance is an upcoming challenge. Clinical and experimental studies have established a more detailed understanding of the mechanisms resulting in brain damage, sequelae and neuropsychological deficits. We summarize the current pathophysiological concept of acute bacterial meningitis and present current treatment strategies.
Keywords: bacterial meningitis, meningoencephalitis, pneumococci, meningococci, dexamethasone
Introduction
Despite modern antibiotics and improved critical care, bacterial meningitis (BM) is still an unresolved problem in clinical medicine. Although highly effective antibiotics kill bacteria efficiently, mortality rates are still up to 34% [van de Beek et al. 2006]. Up to 50% of the survivors suffer from long-term sequelae [Weisfelt et al. 2006; de Gans and van de Beek, 2002; Schuchat et al. 1997; Bohr et al. 1984]. This review focuses mostly on the typical and most common causes of community acquired bacterial meningitis such as meningococci and pneumococci.
Two landmark studies suggested an approach to improve the outcome of acute BM by decreasing inflammation using dexamethasone as an adjunctive treatment to antibiotics [de Gans and van de Beek, 2002; Odio et al. 1991]. In contrast to these clinical data collected in the USA or Western Europe, several studies performed in resource poor countries did not detect any positive effect. Potential reasons for this difference may include other underlying diseases, in particular AIDS [Scarborough et al. 2007; Molyneux et al. 2002], tuberculosis [Nguyen et al. 2007], malnutrition and the fact that patients in these studies presented to emergency rooms at more advanced stages of the disease [Scarborough and Thwaites, 2008].
Consequently, the widening gap between wealthier societies and countries with limited resources presents an equally important issue beside the scientific and medical challenges in unraveling the molecular basis of bacterial meningitis, developing new treatments and meeting new upcoming challenges such as increasing resistance of pathogens to currently used antibiotics; for example, pneumococci up to 35% [Richter et al. 2002; Doern et al. 2001; Whitney et al. 2000]. It is important to state that the proportion of resistant isolates is extremely dependent on geographical and other factors.
Definition of bacterial meningitis
Bacterial meningitis is an inflammation of the meninges, in particular the arachnoid and the pia mater, associated with the invasion of bacteria into the subarachnoid space, principles known for more than 100 years [Flexner, 1907]. The pathogens take advantage of the specific features of the immune system in the CNS, replicate and induce inflammation [Simberkoff et al. 1980]. A hallmark of bacterial meningitis is the recruitment of highly activated leukocytes into the CSF. Beside bacteria, viruses, fungi and non-infectious causes as in systemic and neoplastic disease as well as certain drugs can induce meningeal inflammation. Usually the inflammatory process is not limited to the meninges surrounding the brain but also affects the brain parenchyma (meningoencephalitis) [Swartz, 1984], the ventricles (ventriculitis) and spreads along the spinal cord [Kastenbauer et al. 2001]. In recent years the damage of neurons, particularly in hippocampal structures, has been identified as a potential cause of persistent neuropsychological deficits in survivors [Zysk et al. 1996; Nau et al. 1999b]. Bacterial meningitis is a medical emergency requiring immediate diagnosis and subsequent treatment.
Epidemiology
During the last 20 years, the epidemiology of bacterial meningitis has dramatically changed. Haemophilus influenzae, formerly a major cause of meningitis, has disappeared in developed countries and serves as a remarkable example of a successful vaccination campaign. Nowadays, pneumococci are the most important cause of bacterial meningitis in children and adults in the US as well as in Europe. The incidence of the disease varies from 1.1 to 2 in the US [Schuchat et al. 1997; Wenger et al. 1990] and in Western Europe [Berg et al. 1996] up to 12 in 100 000 per year in Africa [O'Dempsey et al. 1996]. The risk of disease is highest in individuals younger than 5 years and older than 60 years. Some predisposing factors such as a former splenectomy, malnutrition or sickle cell disease are known [Kastenbauer and Pfister, 2003; Fraser et al. 1973]. The use of conjugate pneumococcal vaccines has led to a significant decline in invasive pneumococcal disease, including meningitis, in those regions promoting this approach [Hsu et al. 2009; Whitney et al. 2003]. An emerging problem is the growing prevalence of pneumococci resistant to beta-lactam antibiotics [Stanek and Mufson, 1999]. Prolonged persistence of pneumococci in the cerebrospinal fluid (CSF) may result in higher mortality as well as in pronounced neurological damage in survivors [Fiore et al. 2000; McCullers et al. 2000]. These effects of living bacteria urge us to understand in detail the effects of bacterial toxins and released cell wall and surface components and their contribution to neuronal damage.
With Haemophilus on the decline, Neisseria meningitides has become the leading meningitis pathogen in developing countries, but it continues to pose a major health problem in the US and Europe. In addition to classical meningitis, meningococci frequently cause systemic disease including fulminant gram-negative sepsis and disseminated intravascular coagulopathy. WHO estimates at least 500 000 newly symptomatic infections per year worldwide, leading to at least 50 000 deaths [Stephens et al. 2007]. The highest incidence is observed in the sub-Sahara meningitis belt where cyclic epidemics occur at least once per decade.
Pathogenesis
Bacterial invasion
The current assumption is that high-grade bacteremia precedes meningitis and that bacteria invade from the blood stream to the central nervous system (CNS). Alternatively, direct accesses to the CNS through dural defects or local infections are potential entrance routes. In the clinical setting, such defects should be identified by CCT or MRI scans.
The anatomical site of bacterial invasion from the bloodstream remains unidentified. Experimental evidence suggests that the choroid plexus may be a site of invasion [Daum et al. 1978]. Meningococci are found in the choroid plexus as well as in the meninges [Pron et al. 1997] and pneumococci infiltrate the leptomeningeal blood vessels [Zwijnenburg et al. 2001; Rodriguez et al. 1991] in meningitis. These data suggest that several highly vascularized sites are potential entry locations. In order to cross the blood—brain or the blood—CSF barrier and to overcome sophisticated structures such as tight junctions, meningeal pathogens must carry effective molecular tools.
Streptococcal proteins such as CbpA interact with glycoconjugate receptors of phosphorylcholine with platelet activating factor (PAF) on the eukaryotic cells and promote endocytosis and crossing the blood—brain barrier [Radin et al.. 2005; Orihuela et al.. 2004; Ring et al. 1998; Cundell et al.. 1995]. Meningococci's PilC1 adhesin interacts with CD46 and the outer membrane protein connects to vitronectin and integrins [Unkmeir et al.. 2002; Kallstrom et al.. 1997]. Bacteria causing meningitis in newborns, most importantly group B streptococcal (GBS) and E. coli, are also well equipped with adhesive proteins allowing them to invade the CNS [Maisey et al. 2007; Prasadarao et al.. 1997]. Detailed knowledge of how bacteria activate and invade cells may allow to block these interactions and therefore to prevent disease progression.
Inflammatory response
Inflammatory activation of endothelial cells seems to be a prerequisite for bacterial invasion but also results in the regulation of adhesion molecules as ICAM-1 [Freyer et al. 1999]. Subsequently, these molecules promote the multistep process of leukocyte invasion. Leukocytes, in particular the presence of granulocytes in the CSF, are the diagnostic hallmark of meningitis. Early inflammatory response and bacterial invasion seem to progress in parallel and products of activated leukocytes such as MMPs [Kieseier et al. 1999] and NO [Koedel et al. 1995] and others contribute to early damage of the blood—brain and blood—CSF barrier. Once bacteria have entered the subarachnoidal space, they replicate, undergo autolysis and cause further inflammation.
Several cell types seem to be involved and as mentioned endothelial cells, perivascular macrophages and mast cells may play a crucial role [Polfliet et al. 2001; Weber et al. 1997]. Heat killed bacteria and pathogen-associated molecular patterns (PAMP) of meningitis pathogens as lipoprotein (LP), lipoteichoic acid (LTA), peptidoglycan (PG), and lipopolysaccarid (LPS) cause meningitis indistinguishable from living bacteria [Hoffmann et al. 2007a; Ivey et al. 2005; Tuomanen et al. 1985]. Immune pattern recognition molecules as CD14 and LBP function as sensors in identifying PAMPs [Beutler, 2003]. Pneumococcal PG and LP are recognized by TLR2 [Weber et al. 2003; Aliprantis et al. 1999] whereas LPS, and interestingly the pneumococcal toxin pneumolysin, signal through TLR4 [Malley et al. 2003]. TLR signals are conveyed by the intracellular adapter protein MyD88 downstream to a multitude of inflammatory signaling cascades including NFkB and MAP kinases leading to a rapid inflammatory response in meningitis [Lehnardt et al. 2006].
Neuronal damage
Up to 50% of survivors of bacterial meningitis suffer from disabling neuropsychological deficits [van de Beek et al. 2002; Merkelbach et al. 2000]. Clinically as well as experimentally, the hippocampus seems to be the most vulnerable area of the brain [Nau et al. 1999a; van Wees et al. 1990]. Neuronal loss translates into hippocampal atrophy and has been reported on MRI scans in survivors of bacterial meningitis [Free et al. 1996].
The predisposition of the hippocampus for neuronal damage remains unclear. The extracellular fluid around brain cells is contiguous with the CSF and the proximity to the ventricular system allows diffusion between these compartments [Rennels et al. 1985] that could deliver soluble bacterial and inflammatory toxic mediators.
Neuronal damage in meningitis is clearly multi-factorial, involving bacterial toxins, cytotoxic products of immune competent cells, and indirect pathology secondary to intracranial complications (Figure 1). In the case of S. pneumoniae, the pathogen associated with the highest frequency of neuronal damage, two major toxins have been identified, H2O2 and pneumolysin, a pore-forming cytolysin. In experimental meningitis induced by toxin-deficient pneumococcal mutants, neuronal damage was reduced by 50% compared to wild-type bacteria [Braun et al. 2002]. The proof of direct bacterial toxicity underlines the critical importance of rapid antibiotic elimination of living bacteria and their metabolism. In insufficiently treated patients or resistant bacteria toxic activity may be significantly prolonged and harm neuronal functions. Mechanistically, these toxins seem to cause programmed death of neurons and microglia by inducing rapid mitochondrial damage.
Figure 1.
Two major routes involving bacterial toxins and cytotoxic products of the inflammatory response lead to intracranial complications and brain damage. Peptidoglycan (PG), bacterial lipopeptide (LP), lipopolysaccharide (LPS), apoptosis inducing factor (AIF), intracranial pressure (ICP).
In particular, pneumolysin was shown to translocate to mitochondria and induce pore formation in mitochondrial membranes [Braun et al. 2007; Bermpohl et al. 2005; Braun et al. 2001]. Release of apoptosis inducing factor (AIF) from damaged mitochondria leads to large-scale fragmentation of the DNA and apoptosis-like cell death. This type of cell death is executed in a caspaseindependent manner. As a consequence, cells exposed to live pneumococci or pneumolysin in vitro cannot be rescued by caspase inhibitors [Bermpohl et al. 2005; Braun et al. 2001]. In vivo, however, intrathecal application of the broad spectrum caspase inhibitor, z-VAD-fmk, prevents about 50% of neuronal damage in experimental meningitis [Braun et al. 1999]. Further studies in caspase-3 deficient mice revealed that late but not early neuronal damage is dependent on caspase activity [Mitchell et al. 2004]. The interpretation of these findings is that early caspase-independent cell death may be induced by bacterial toxins while delayed, caspase-mediated apoptosis occurs as a consequence mostly of the host immune response. The in vivo findings can be modeled in cell culture systems, and the coexistence of different forms and time courses of cell damage has been confirmed in vitro [Bermpohl et al. 2005; Colino and Snapper, 2003].
Antibiotic treatment results in an increase of bacterial debris, including bacterial DNA and extremely powerful stimuli of the immune response such as PG and LPS [Fischer and Tomasz, 1984]. The concentration of PG in the CSF correlates with the clinical outcome of pneumococcal meningitis [Schneider et al. 1999], just as LPS concentrations in body compartments are linked to the severity of meningococcal disease [Brandtzaeg et al. 1992]. While they are extremely potent inflammatory stimuli [Hoffmann et al. 2007a], these bacterial cell wall components have no direct toxic effect on cultured neurons [Lehnardt et al. 2006, 2003]. This resistance of neurons to TLR ligands can be explained by the absence of TLR4 and TLR2 on these cells. Instead, PAMPs induce indirect neurotoxicity by activation of pattern recognition receptors (PRRs) present on microglia, as has been shown elegantly in coculture systems. Neuronal death is efficiently induced by TLR ligands in the presence of microglia, and it is strictly dependent on the presence of the exact matching TLR and an intact downstream MyD88 pathway in microglia [Lehnardt et al. 2006, 2003].
Taken together, bacterial components activate microglia in a TLR-dependent fashion and microglia releases cell death signals such as NO to neighboring neurons. Bacterial and host-derived reactive oxygen and nitrogen species coalesce to form highly reactive, tissue damaging intermediates [Hoffmann et al. 2006]. Moreover, dying parenchymal cells release TLR ligands as endogenous ‘danger signals’, leading to a vicious circle of inflammatory tissue damage [Lehnardt et al. 2008]. These mechanisms are important and clearly add to our understanding of how activated microglia can damage surrounding neurons and expand the importance of the findings beyond meningitis.
Leukocyte influx as the hallmark of acute meningitis contributes to neuronal damage and may in fact be more detrimental than beneficial to the host [Tuomanen et al. 1989]. Neutrophils, which form the first line of defense in bacterial meningitis, are equipped with cytotoxic and proinflammatory activity, placing these cells both as direct effectors of tissue damage and as orchestrators of further immune activation. Granulocyte depletion is neuroprotective in experimental meningitis, while persistence of granulocytes was associated with more pronounced neuronal damage [Hoffmann et al. 2007b]. Especially in the context of sufficient antibiotic therapy, it may therefore be desirable to limit granulocyte activity and to speed up granulocyte clearance from the CSF. The cytokine TRAIL was recently identified as a factor that reduces the life span of activated granulocytes [Renshaw et al. 2003]; TRAIL deficient mice displayed prolonged CSF pleocytosis and increased neurotoxicity in experimental meningitis, while therapeutic application of recombinant TRAIL reversed this effect and provided neuroprotection [Hoffmann et al. 2007b].
Clinical features and diagnosis
Clinical features
Early clinical features of bacterial meningitis are nonspecific and include fever, malaise and headache; and later on, meningismus (neck stiffness), photophobia, phonophobia and vomiting develop as signs of meningeal irritation [van de Beek et al. 2004]. Headache and meningismus indicate inflammatory activation of the trigeminal sensory nerve fibers in the meninges and can be blocked experimentally by 5-HT1B/D/F receptor agonists (triptans) [Hoffmann et al. 2002]. However the role of triptans for headache control in patients with bacterial meningitis remains to be clarified [Lampl et al. 2000].
Meningismus may be absent very early in the disease, in deeply comatose patients, in children and in immunocompromised patients such as in liver cirrhosis [Cabellos et al. 2008]. It is important to consider that the classical triad of fever, neck stiffness and altered mental state is present in less than 50% of adults with proven bacterial meningitis [Heckenberg et al. 2008; van de Beek et al. 2004]. Approximately 33% of patients develop focal neurological signs, such as epileptic seizures or paresis of a limb, and up to 69% present with impaired consciousness or 14% with coma [van de Beek et al. 2004].
Inspection of the integument may reveal petechiae suggestive of meningococcal infection or Osler's nodes indicative of bacterial endocarditis. Meningitis occurs in about 7% of patients with bacterial endocarditis, often as a presenting symptom [Angstwurm et al. 2004; Jones et al. 1969]. The most frequent pathogens in this context are S. aureus, otherwise uncommon in bacterial meningitis, and pneumococci. Occasionally, pneumococcal meningitis, endocardits and pneumonia may be diagnosed simultaneously (Austrian's syndrome) [Dalal and Ahmad, 2008]. Meningococcal disease may present as a fulminant gram-negative sepsis with prominent cardiovascular insufficiency and disseminated intravascular coagulation, threatening ischemic tissue damage. Notably, a petechial skin rash is not unique to meningococcal disease but may also be present in septicemia caused by, amongst others, streptococci or S. aureus.
Laboratory tests
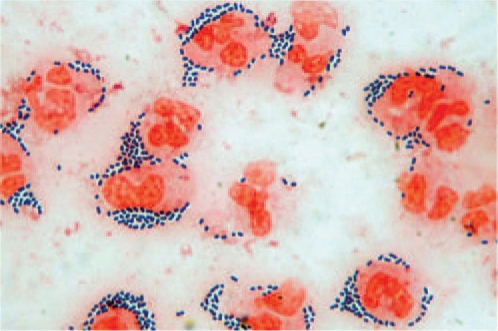
The key to the diagnosis of bacterial meningitis is the proof of bacteria in the CSF by Gram-staining (Figure 2) or a positive bacterial culture. Detection rates in the CSF may be as high as 90%, while about 50% positive results are observed in blood cultures. The diagnostic yield of CSF microscopy can be improved by centrifugation of a larger sample and experience. Polymerase chain reaction (PCR) may be attempted if microscopic and cultural identification of the pathogen fail but is not yet a routine test. PCR has an important role in strain identification mostly in meningococcal disease [Fox et al. 2007]. Latex agglutination-based rapid tests are available for major meningitis pathogens, but imperfect sensitivity and specificity argue against routine clinical use at this time [Hayden and Frenkel, 2000].
Figure 2.
Diagnostic Gram stain of CSF from a patient with pneumococcal meningitis. Neutrophils (stained red) are surrounded by Gram-positive dipolcocci (stained blue).
The CSF in bacterial meningitis is characterized by a strongly elevated white blood cell count (<500 cells/μl) with predominant neutrophils and a strongly elevated protein (< 1 g/l), indicating severe blood—CSF barrier disruption. Increased lactate (<0.3g/l) and decreased glucose CSF/blood ratio (>0.4) support the diagnosis of acute bacterial meningitis [Straus et al. 2006]. Use of urine dipsticks for semiquantitive detection of glucose and leukocyte concentrations in the CSF has been suggested for resource-limited conditions when elaborated CSF studies and microscopy are not available [Moosa et al. 1995].
Lower cell counts and a mixed pleocytosis are observed with L. monocytogenes, M. tuberculosis and fungi; also, they may be found in partially or insufficiently treated meningitis. Cerebral malaria, an important clinical differential diagnosis in endemic regions, is not usually associated with pronounced CSF pleocytosis. As a caveat, low-CSF white blood cell counts may confound the diagnosis in bacterial meningitis of immunocompromised, leukopenic patients or in overwhelming bacterial infection (‘apurulent bacterial meningitis’), further emphasizing the importance of Gram stains.
Peripheral white blood cells, erythrocyte sedimentation rate, serum C-reactive protein, procalcitonin [Hoffmann et al. 2001] and other acute phase proteins are usually elevated in bacterial meningitis but are of limited diagnostic value especially in atypical cases. Additionally, the above-mentioned typical CSF results can be different very early in the disease and in patients insufficiently treated by antibiotics.
CT
A cranial CT provides information concerning intracranial complications such as brain edema, hydrocephalus and infarcts. Moreover, bone window imaging identifies parameningeal foci such as sinusitis, mastoiditis or odontogenic abscess. Local infections are especially frequent in pneumococcal meningitis and may require surgical treatment.
There is an ongoing controversy about a cranial CT before lumbar puncture, the concern being the risk of cerebral herniation due to raised intracranial pressure. In particular, those patients who present with focal neurological deficits or seizures and those who have a disturbed consciousness should have a cranial CT before lumbar puncture. If the technique is not available treatment must be initiated based on clinical suspicion and without CSF examination. Of those patients without focal signs or seizures and with a normal level of consciousness, CT abnormalities are found in less than 3% [Joffe, 2007; Hasbun et al. 2001]; here, CSF can be drawn without prior CT scanning. However, a normal CT does not rule out intracranial hypertension and a residual risk of herniation [Oliver et al. 2003].
Treatment
Antibiotics
Immediate antibiotic therapy is imperative and must not be postponed by diagnostic delays; for example, waiting for a CT scan. Prehospital antibiotic treatment is advised in cases of suspected meningococcal disease but depends on local resistance situation and the medical environment [Sudarsanam et al. 2008]. Prior to treatment, a blood culture should be obtained. Since microbiological identification of the pathogen is not immediately available, the initial choice of antibiotics is usually empirical. Factors to consider include regional antibiotic resistance rates, patient age, predisposing conditions and resources (Table 1).
Table 1.
Empirical antibiotic therapy.
| Probable pathogens | Empirical therapy | |
| Neonates | Gram-negative Enterobacteriacae (E. coli, Klebsiella, Enterobacter, Proteus) Group B streptococci | CephaLosporin1 + ampicilLin |
| Infants and children | N. meningitides, S. pneumoniae (H. influenzae2) | Cephalosporin1 (+ vancomycin or rifampin3) |
| Adults | S. pneumoniae, N. meningitidis, L. monocyto-genes4, Aerobic streptococci (H. influenzae) | CephaLosporin1 (+ ampicilLin*) |
| Nosocomial, trauma, ventriculitis, shunt infection | Staphylococci, Gram-negative, Enterobacteriacae P. aeruginosa | Meropenem or cephalosporin5 + vancomycin (or rifampicin or fosfomycin or linezolid) |
| Immunocompromised patients | L. monocytogenes, Gram-negative Enterobacteriacae, S. pneumoniae, P. aeruginosa | CephaLosporin1 + ampicilLin (+ vancomycin3) |
| Resource-limited countries | N. meningitidis, S. pneumoniae, H. influenzae, L. monocytogenes | Ceftriaxone6 chloramphenicol7, penicillin G7, ampicillin/amoxicillin’ rifampicin8 |
| Chemo-prophylaxis of close contacts | N. meningitidis | Adult doses9: rifampicin (600mg b.i.d., 2 days), ciprofloxacin (500mg single dose), ceftriaxone (250 mg single dose) |
Cephalosporins group 3a (e.g. ceftriaxone or cefotaxime) or group 4 (e.g. cefepime) are recommended.
H. influenzae is unlikely if the child has been vaccinated.
Cephalosporin- and penicillin-resistant pneumococci are increasingly frequent (e.g. in areas of the US, Australia, South Africa and Spain). In these regions, vancomycin or rifampicin should be included in the initial antibiotic regimen.
Listeriae may occasionally cause meningitis in immunocompetent patients. Addition of ampicillin should be considered especially in patients with atypical CSF findings (mixed pleocytosis).
Cephalosporins with activity against P. aeruginosa include (e.g. ceftazidime and cefepime).
Ceftriaxone may be administered i.m. or i.v. WHO recommends a single dose for epidemic meningococcal disease. At least 5 days of treatment are recommended for uncomplicated courses of bacterial meningitis in immunocompetent patients, and longer durations in immunocompromised patients or with persistent fever, seizures or coma.
Emerging resistance of S. pneumoniae and H. influenzae in certain regions.
Avoid first-line use to prevent resistance of M. tuberculosis.
Rifampicin and ciprofloxacin are not recommended in pregnancy. Recommended dose of rifampicin is 5mg/kg for neonates and 10mg/kg for children older than 1 month; alternatively, 125 mg ceftriaxone can be used. Ciprofloxacin should not be given below age 18.
Microbiological identification and susceptibility testing of the causative agent are key determinants of successful antibiotic therapy. In view of emerging resistances, antibiotic chemotherapy should be adjusted according to the cultural results in order to provide highly active yet narrowly targeted coverage. However, penicillin G monotherapy for meningococci or pneumococci is advisable only after susceptibility has been confirmed. Treatment durations of 10–14 days are adequate for most pathogens; a shorter course of 5–7 days will be sufficient for uncomplicated meningococcal disease, while 3–4 weeks of treatment are recommended for L. monocytogenes and Enterobacteriacae. The data for treatment durations are very limited and mostly based on expert opinion [Tunkel et al. 2004]. Suspected or proven meningococcal meningitis requires patient isolation during the first 24 h of treatment; chemoprophylaxis is recommended for close contacts (Table 1). Cerebral imaging and repeat lumbar puncture should be considered in patients who fail to improve clinically after 48 h of treatment to assess antibiotic failure.
Corticosteroids
Corticosteroids reduce brain edema, intracranial hypertension and meningeal inflammation in experimental models of bacterial meningitis. Subsequent clinical studies have led to conflicting results concerning potential benefits of steroid use in patients with meningitis. Currently available evidence supports a reduced incidence of severe hearing loss in children with H. influenzae meningitis [Odio et al. 1991; Lebel et al. 1988], while information on other pediatric pathogens is incomplete. In adults, a single double-blind RCT of 301 adult patients reported reduced mortality and lower frequency of hearing loss and neuropsychological sequelae [de Gans and van de Beek, 2002]. Subgroup analysis suggested that protective effects of dexamethasone are limited to pneumococcal meningitis (death: 34% versus 14%; unfavorable outcome: 52% versus 26%) [van de Beek et al. 2006]. Expert opinion and several societal guidelines recommend routine treatment with dexamethasone for community-acquired meningitis of children (0.15mg/kg every 6 hours for 2–4 days) and adults (10 mg every 6 hours for 4 days). Discontinuation of this therapy is advisable if H. influenzae (children) and S. pneumoniae (adults and children) can be ruled out as the underlying pathogen. Notably, H. influenzae and S. pneumoniae infections are declining in the pediatric population in those countries promoting immunization. The first steroid dose should be administered 10–20 min before initiating antibiotic treatment, or at least concomitantly. Delayed treatment is not beneficial as dexamethasone does not reverse existing brain edema or intracranial hypertension in later stages of meningitis. Conversely, there is concern about aggravated neurotoxicity which seems to have no clinically relevance [Weisfelt et al. 2006; Zysk et al. 1996] and may impair antibiotic penetration into the CSF [Paris et al. 1994] as a consequence of dexamethasone treatment. Current data do not support the routine use of corticosteroids in countries with limited resources [Scarborough and Thwaites, 2008].
Other symptomatic therapy
Severe headache requires generous analgesia, often including opioids. Antiepileptic treatment is indicated if seizures occur; prophylactic treatment is not recommended.
Complications
Mortality from bacterial meningitis may reach 34% [van de Beek et al. 2006] and is highest with S. pneumoniae and L. meningitidis. Long-term neurological sequelae are found in up to 50% of survivors [Weisfelt et al. 2006; de Gans and van de Beek, 2002; Bohr et al. 1984; Schuchat et al. 1997]. Both intracranial and systemic complications contribute to this negative outcome. Complications are most likely to occur during the first few days of therapy. Sensorineural hearing loss or vestibular dysfunction are the most frequent problems. They are most frequent with H. influenzae and S. pneumoniae. As outlined above, the incidence of these complications is reduced by adjunctive dexamethasone therapy. The most threatening intracranial complications are brain edema, vascular alterations and hydrocephalus, which all contribute to increased intracranial pressure and parenchymal damage [Pfister et al. 1992]. Clinically, patients may display prolonged or progressive alteration of their mental state or level of consciousness. CT imaging should be performed if patients fail to improve within 48 h of antibiotic treatment or if new focal signs develop. In general, a head elevation (30°) of the bed is recommended in patients with meningitis. Treatment options for brain edema include osmotherapy. Therapeutic hypothermia, which is effective in experimental models of bacterial meningitis [Angstwurm et al. 2000], has not been investigated in patients but lowering of increased body temperature seems advisable.
Hydrocephalus develops in up to 15% of patients, usually in the form of malresorption due to increased outflow resistance of the CSF. Patients with hydrocephalus and impaired consciousness should be closely monitored on follow-up CTs; eventually, they may require external ventricular drainage (EVD). EVD offers the additional benefit of ICP monitoring. The amount of drainage is determined using ICP, clinical improvement and CT follow up. With normalization of CSF protein and leukocyte concentrations, EVD usually becomes expendable; otherwise, a ventriculoperitoneal shunt should be placed.
Invasive ICP monitoring should be considered in comatose patients with generalized brain edema. Vascular complications include vasculitis, vasospasm and septic thrombosis of dural sinuses and cortical veins [Haring et al. 1998, 1993; Pfister et al. 1992], often leading to the infarction of large cerebral territories. Clinically new focal neurological deficits in the course of meningitis should lead to such diagnostic considerations. MR, CT and MR or CT angiograms are of specific diagnostic use. The risks and benefits of anticoagulation in septic sinus thrombosis are uncertain in the absence of controlled trials. Likewise, no evidence-based therapies exist for meningitis-associated vasculitis or vasospasm. Hemodilution and nimodipine may be given in analogy to subarachnoid hemorrhage, and dexamethasone has been suggested for suspected vasculitis. Extracranial complications include sepsis, disseminated coagulopathy, multiorgan failure, arthritis and electrolyte imbalance, usually due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion.
Neuropsychological deficits are frequently found in survivors of bacterial meningitis. In adults, long-lasting cognitive impairment is most prominent after pneumococcal meningitis, with lower incidence after meningococcal meningitis [van de Beek et al. 2002]. Short-term and working memory, executive functions, and associative learning of verbal material were specifically affected in adults 1–12 years after bacterial meningitis [Schmidt et al. 2006]; other authors emphasize psychomotor slowing as a primary feature [Hoogman et al. 2007; Merkelbach et al. 2000]. Children may show persistent difficulties in learning, impaired short-term memory and behavioral deficits, leading to poorer academic performance [Grimwood et al. 2000].
Acknowledgement
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB-TRR43/A1 and a grant from the Wlillhelm Sanderstiftung Stiftung.
Conflict of interest statement
None declared.
Contributor Information
Olaf Hoffman, Department of Neurology, Charité – Universitaetsmedizin Berlin, Berlin, Germany.
R. Joerg Weber, Department of Cell Biology and Neurobiology, Charité —Universitaetsmedizin Berlin, Berlin, Germany and Department of Neurology, LKH Klagenfurt, Klagenfurt, Austria joerg.weber@charite.de.
References
- Aliprantis A.O., Yang R.B., Mark M.R., Suggett S., Devaux B., Radolf J.D.et al. (1999) Cell activation and apoptosis by bacterial lipoproteins through tolllike receptor-2. Science 285:736–739 [DOI] [PubMed] [Google Scholar]
- Angstwurm K., Borges A.C., Halle E., Schielke E., Weber J.R. (2004) Neurological complications of infective endocarditis. Nervenarzt 75:734–741 [DOI] [PubMed] [Google Scholar]
- Angstwurm K., Reuss S., Freyer D., Arnold G., Dirnagl U., Schumann R.R.et al. (2000) Induced hypothermia in experimental pneumococcal meningitis. J Cereb Blood Flow Metab 20:834–838 [DOI] [PubMed] [Google Scholar]
- Berg S., Trollfors B., Claesson B.A., Alestig K., Gothefors L., Hugosson S.et al. (1996) Incidence and prognosis of meningitis due to Haemophilus influenzae. Streptococcus pneumoniae and Neisseria meningitidis in Sweden. Scand J Infect Dis 28:247–252 [DOI] [PubMed] [Google Scholar]
- Bermpohl D., Halle A., Freyer D., Dagand E., Braun J.S., Bechmann I.et al. (2005) Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest 115:1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. (2003) Not ‘molecular patterns’ but molecules. Immunity 19:155–156 [DOI] [PubMed] [Google Scholar]
- Bohr V., Paulson O.B., Rasmussen N. (1984) Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol 41:1045–1049 [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Ovsteboo R., Kierulf P. (1992) Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis 166:650–652 [DOI] [PubMed] [Google Scholar]
- Braun J.S., Hoffmann O., Schickhaus M., Freyer D., Dagand E., Bermpohl D.et al. (2007) Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun 75:4245–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J.S., Novak R., Herzog K.H., Bodner S.M., Cleveland J.L., Tuomanen E.I. (1999) Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med 5:298–302 [DOI] [PubMed] [Google Scholar]
- Braun J.S., Novak R., Murray P.J., Eischen C.M., Susin S.A., Kroemer G.et al. (2001) Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by pneumococcus. J Infect Dis 184:1300–1309 [DOI] [PubMed] [Google Scholar]
- Braun J.S., Sublett J.E., Freyer D., Mitchell T.J., Cleveland J.L., Tuomanen E.I.et al. (2002) Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest 109:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabellos C, Viladrich P.F., Ariza J., Maiques J.M., Verdaguer R., Gudiol F. (2008) Community-acquired bacterial meningitis in cirrhotic patients. Clin Microbiol Infect 14:35–40 [DOI] [PubMed] [Google Scholar]
- Colino J., Snapper CM. (2003) Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol 171:2354–2365 [DOI] [PubMed] [Google Scholar]
- Cundell D.R., Gerard N.P., Gerard C, Idanpaan-Heikkila I., Tuomanen E.I. (1995) Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438 [DOI] [PubMed] [Google Scholar]
- Dalal A., Ahmad H. (2008) Austrian syndrome (pneumococcal pneumonia, meningitis, and endocarditis): a case report. Am J Med Sci 336:354–355 [DOI] [PubMed] [Google Scholar]
- Daum R.S., Scheifele D.W., Syriopoulou V.P., Averill D., Smith A.L. (1978) Ventricular involvement in experimental Hemophilus influenzae meningitis. J Pediatr 93:927–930 [DOI] [PubMed] [Google Scholar]
- de Gans J., van de Beek D. (2002) Dexamethasone in adults with bacterial meningitis. N Engl J Med 347:1549–1556 [DOI] [PubMed] [Google Scholar]
- Doern G.V., Heilmann K.P., Huynh H.K., Rhomberg P.R., Coffman S.L., Brueggemann A.B. (2001) Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000. Including a comparison of resistance rates since 1994-1995. Antimicrob Agents Chemother 45:1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore A.E., Moroney J.F., Farley M.M., Harrison L.H., Patterson J.E., Jorgensen J.H.et al. (2000) Clinical outcomes of meningitis caused by Streptococcus pneumoniae in the era of antibiotic resistance. Clin Infect Dis 30:71–77 [DOI] [PubMed] [Google Scholar]
- Fischer H., Tomasz A. (1984) Production and release of peptidoglycan and wall teichoic acid polymers in pneumococci treated with beta-lactam antibiotics. J Bacteriol 157:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner S. (1907) Experimental cerebrospinal meningitis in monkeys. J Exp Med 9:142–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.J., Taha M.K., Vogel U. (2007)Standardized nonculture techniques recommended for [DOI] [PubMed]
- European reference laboratories. FEMS Microbiol Rev 31:84–88 [DOI] [PubMed] [Google Scholar]
- Fraser D.W., Darby C.P., Koehler R.E., Jacobs CF., Feldman R.A. (1973) Risk factors in bacterial meningitis: Charleston County, South Carolina. J Infect Dis 127:271–277 [DOI] [PubMed] [Google Scholar]
- Free S.L., Li L.M., Fish D.R., Shorvon S.D., Stevens J.M. (1996) Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 37:400–405 [DOI] [PubMed] [Google Scholar]
- Freyer D., Manz R., Ziegenhorn A., Weih M., Angstwurm K., Docke WD.et al. (1999) Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol 163:4308–4314 [PubMed] [Google Scholar]
- Grimwood K., Anderson P., Anderson V, Tan L., Nolan T. (2000) Twelve year outcomes following bacterial meningitis: further evidence for persisting effects. Arch Dis Child 83:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring H., Kampfl A., Grubwieser G., Donnemiller E., Pfausler B., Schmutzhard E. (1998) Cerebral blood flow velocity and perfusion in purulent meningitis: a comparative TCD and 99 M-TC-HMPAO-SPECT study. Eur J Neurol 5:75–81 [DOI] [PubMed] [Google Scholar]
- Haring H.P., Rotzer H.K., Reindl H., Berek K., Kampfl A., Pfausler B.et al. (1993) Time course of cerebral blood flow velocity in central nervous system infections. A transcranial Doppler sonography study. Arch Neurol 50:98–101 [DOI] [PubMed] [Google Scholar]
- Hasbun R., Abrahams J., Jekel J., Quagliarello V.J. (2001) Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 345:1727–1733 [DOI] [PubMed] [Google Scholar]
- Hayden R.T, Frenkel L.D. (2000) More laboratory testing: greater cost but not necessarily better. Pediatr Infect Dis J 19:290–292 [DOI] [PubMed] [Google Scholar]
- Heckenberg S.G., de Gans J., Brouwer M.C, Weisfelt M., Piet J.R., Spanjaard L.et al. (2008) Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine 87:185–192 [DOI] [PubMed] [Google Scholar]
- Hoffmann O., Braun J.S., Becker D., Halle A., Freyer D., Dagand E.et al. (2007a) TLR2 mediates neuroinflammation and neuronal damage. J Immunol 178:6476–6481 [DOI] [PubMed] [Google Scholar]
- Hoffmann O., Keilwerth N., Bille M.B., Reuter U., Angstwurm K., Schumann R.R.et al. (2002) Triptans reduce the inflammatory response in bacterial meningitis. J Cereb Blood Flow Metab 22:988–996 [DOI] [PubMed] [Google Scholar]
- Hoffmann O., Priller J., Prozorovski T, Schulze-Topphoff U., Baeva N., Lunemann J.D.et al. (2007b) TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest 117:2004–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O., Reuter U., Masuhr F., Holtkamp M., Kassim N., Weber J.R. (2001) Low sensitivity of serum procalcitonin in bacterial meningitis in adults. Scand J Infect Dis 33:215–218 [DOI] [PubMed] [Google Scholar]
- Hoffmann O., Zweigner J., Smith S.H., Freyer D., Mahrhofer C, Dagand E.et al. (2006) Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect Immun 74:5058–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M., van de B.D., Weisfelt M., de Gans J., Schmand B. (2007) Cognitive outcome in adults after bacterial meningitis. JNeurol Neurosurg Psychiatry 78:1092–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.E., Shutt K.A., Moore M.R., Beall B.W., Bennett N.M., Craig A.S.et al. (2009) Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med 360:244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey N.S., Martin E.N., Scheld WM., Nathan B.R., Jr (2005) A new method for measuring blood-brain barrier permeability demonstrated with Europium-bound albumin during experimental lipopolysaccharide (LPS) induced meningitis in the rat. J Neurosci Methods 142:91–95 [DOI] [PubMed] [Google Scholar]
- Joffe A.R. (2007) Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 22:194–207 [DOI] [PubMed] [Google Scholar]
- Jones H.R., Jr, Siekert R.G., Geraci J.E. (1969) Neurologic manifestations of bacterial endocarditis. Ann Intern Med 71:21–28 [DOI] [PubMed] [Google Scholar]
- Kallstrom H., Liszewski M.K., Atkinson J.P., Jonsson A.B. (1997) Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol 25:639–647 [DOI] [PubMed] [Google Scholar]
- Kastenbauer S., Pfister H.W. (2003) Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126:1015–1025 [DOI] [PubMed] [Google Scholar]
- Kastenbauer S., Winkler F., Fesl G., Schiel X., Ostermann H., Yousry T.A.et al. (2001) Acute severe spinal cord dysfunction in bacterial meningitis in adults: MRI findings suggest extensive myelitis. Arch Neurol 58:806–810 [DOI] [PubMed] [Google Scholar]
- Kieseier B.C., Paul R., Koedel U., Seifert T., Clements J.M., Gearing A.J.et al. (1999) Differential expression of matrix metalloproteinases in bacterial meningitis. Brain 122:1579–1587 [DOI] [PubMed] [Google Scholar]
- Koedel U., Bernatowitz A., Paul R., Frei K, Fontana A., Pfister H.W. (1995) Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol 37:313–323 [DOI] [PubMed] [Google Scholar]
- Lampl C, Yazdi K, Buzath A., Klingler D. (2000) Migraine-like headache in bacterial meningitis. Cephalalgia 20:738–739 [DOI] [PubMed] [Google Scholar]
- Lebel M.H., Freij B.J., Syrogiannopoulos G.A., Chrane D.F., Hoyt M.J., Stewart S.M.et al. (1988) Dexamethasone therapy for bacterial meningitis. Results of two double-blind, placebo-controlled trials. N Engl J Med 319:964–971 [DOI] [PubMed] [Google Scholar]
- Lehnardt S., Henneke P., Lien E., Kasper D.L., Volpe J.J., Bechmann I.et al. (2006) A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol 177:583–592 [DOI] [PubMed] [Google Scholar]
- Lehnardt S., Massillon L., Follett P., Jensen F.E., Ratan R., Rosenberg P.A.et al. (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA 100:8514–8519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S., Schott E., Trimbuch T., Laubisch D., Krueger C, Wulczyn G.et al. (2008) A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 28:2320–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisey H.C., Hensler M., Nizet V., Doran K.S. (2007) Group B streptococcal pili proteins contribute to adherence and invasion of brain microvascular endothelial cells. J Bacteriol 189:1464–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R., Henneke P., Morse S.C., Cieslewicz M.J., Lipsitch M., Thompson CM.et al. (2003) Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA 100:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers J.A., English B.K., Novak R. (2000) Isolation and characterization of vancomycin-tolerant Streptococcus pneumoniae from the cerebrospinal fluid of a patient who developed recrudescent meningitis. J Infect Dis 181:369–373 [DOI] [PubMed] [Google Scholar]
- Merkelbach S., Sittinger H., Schweizer I., Müller M. (2000) Cognitive outcome after bacterial meningitis. Acta Neurol Scand 102:118–123 [DOI] [PubMed] [Google Scholar]
- Mitchell L., Hope Smith S., Braun J.S., Herzog K.H., Weber J.R., Tuomanen E.I. (2004) Dual phases of apoptosis in pneumococcal meningitis. J Infect Dis 190:2039–2046 [DOI] [PubMed] [Google Scholar]
- Molyneux E.M., Walsh A.L., Forsyth H., Tembo M., Mwenechanya J., Kayira K.et al. (2002) Dexamethasone treatment in childhood bacterial meningitis in Malawi: a randomised controlled trial. Lancet 360:211–218 [DOI] [PubMed] [Google Scholar]
- Moosa A.A., Quortum H.A., Ibrahim M.D. (1995) Rapid diagnosis of bacterial meningitis with reagent strips. Lancet 345:1290–1291 [DOI] [PubMed] [Google Scholar]
- Nau R., Soto A., Bruck W. (1999a) Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol 58:265–274 [DOI] [PubMed] [Google Scholar]
- Nau R., Soto A., Brück W. (1999b) Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J Neuropathol Exp Neurol 58:265–274 [DOI] [PubMed] [Google Scholar]
- Nguyen TH., Tran TH., Thwaites G., Ly V.C., Dinh X.S., Ho Dang T.N.et al. (2007) Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N Engl J Med 357:2431–2440 [DOI] [PubMed] [Google Scholar]
- O'Dempsey T.J., McArdle T.F., Lloyd-Evans N., Baldeh I., Lawrence B.E., Secka O.et al. (1996) Pneumococcal disease among children in a rural area of west Africa. Pediatr Infect Dis J 15:431–437 [DOI] [PubMed] [Google Scholar]
- Odio CM., Faingezicht I., Paris M., Nassar M., Baltodano A., Rogers J.et al. (1991) The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. NEnglJ Med 324:1525–1531 [DOI] [PubMed] [Google Scholar]
- Oliver W.J., Shope T.C., Kuhns L.R. (2003) Fatal lumbar puncture: fact versus fiction - an approach to a clinical dilemma. Pediatrics 112:e174–e176 [DOI] [PubMed] [Google Scholar]
- Orihuela C.J., Gao G., Francis K.P., Yu J., Tuomanen E.I. (2004) Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis 190:1661–1669 [DOI] [PubMed] [Google Scholar]
- Paris M.M., Hickey S.M., Uscher M.I., Shelton S., Olsen K.D., McCracken G.H.J. (1994) Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 38:1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H.W., Borasio G.D., Dirnagl U., Bauer M., Einhäupl K.M. (1992) Cerebrovascular complications of bacterial meningitis in adults. Neurology 42:1497–1504 [DOI] [PubMed] [Google Scholar]
- Polfliet M.M., Zwijnenburg P.J., van Furth A.M., van der Poll T, Döpp E.A., Renardel de Lavalette C.et al. (2001) Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol 167:4644–4650 [DOI] [PubMed] [Google Scholar]
- Prasadarao N.V., Wass C.A., Kim K.S. (1997) Identification and characterization of S fimbriabinding sialoglycoproteins on brain microvascular endothelial cells. Infect Immun 65:2852–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pron B., Taha M.K, Rambaud C, Fournet J.C., Pattey N., Monnet J.P.et al. (1997) Interaction of Neisseria maningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. J Infect Dis 176:1285–1292 [DOI] [PubMed] [Google Scholar]
- Radin J.N., Orihuela C.J., Murti G., Guglielmo C, Murray P.J., Tuomanen E.I. (2005) beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun 73:7827–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels M.L., Gregory T.F., Blaumanis O.R., Fujimoto K., Grady P.A. (1985) Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326:47–63 [DOI] [PubMed] [Google Scholar]
- Renshaw S.A., Parmar J.S., Singleton V., Rowe S.J., Dockrell D.H., Dower S.Ket al. (2003) Acceleration of human neutrophil apoptosis by TRAIL. J Immunol 170:1027–1033 [DOI] [PubMed] [Google Scholar]
- Richter S.S., Heilmann K.P., Coffman S.L., Huynh H.K., Brueggemann A.B., Pfaller M.A.et al. (2002) The molecular epidemiology of penicillin-resistant Streptococcus pneumoniae in the United States, 1994-2000. Clin Infect Dis 34:330–339 [DOI] [PubMed] [Google Scholar]
- Ring A., Weiser J.N., Tuomanen E.I. (1998) Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest 102:347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A.F., Kaplan S.L., Hawkins E.P., Mason E.O., Jr (1991) Hematogenous pneumococcal meningitis in the infant rat: description of a model. J Infect Dis 164:1207–1209 [DOI] [PubMed] [Google Scholar]
- Scarborough M., Gordon S.B., Whitty C.J., French N., Njalale Y., Chitani A.et al. (2007) Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med 357:2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough M., Thwaites G.E. (2008) The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol 7:637–648 [DOI] [PubMed] [Google Scholar]
- Schmidt H., Heimann B., Djukic M., Mazurek C, Fels C., Wallesch C.W.et al. (2006) Neuropsychological sequelae of bacterial and viral meningitis. Brain 129:333–345 [DOI] [PubMed] [Google Scholar]
- Schneider O., Michel U., Zysk G., Dubuis O., Nau R. (1999) Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53:1584–1587 [DOI] [PubMed] [Google Scholar]
- Schuchat A., Robinson K, Wenger J.D., Harrison L.H., Farley M., Reingold A.L.et al. (1997) Bacterial meningitis in the United States in 1995. Active Surveillance Team. N Engl J Med 337:970–976 [DOI] [PubMed] [Google Scholar]
- Simberkoff M.S., Moldover N.H., Rahal J., Jr (1980) Absence of detectable bactericidal and opsonic activities in normal and infected human cerebrospinal fluids. A regional host defense deficiency. J Lab Clin Med 95:362–372 [PubMed] [Google Scholar]
- Stanek R.J., Mufson M.A. (1999) A 20-year epidemiological study of pneumococcal meningitis. Clin Infect Dis 28:1265–1272 [DOI] [PubMed] [Google Scholar]
- Stephens D.S., Greenwood B., Brandtzaeg P. (2007) Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196–2210 [DOI] [PubMed] [Google Scholar]
- Straus S.E., Thorpe K.E., Holroyd-Leduc J. (2006) How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA 296:2012–2022 [DOI] [PubMed] [Google Scholar]
- Sudarsanam T, Rupali P., Tharyan P., Abraham O.C., Thomas K. (2008) Pre-admission antibiotics for suspected cases of meningococcal disease.Cochrane Database Syst Rev CD005437 [DOI] [PubMed]
- Swartz M.N. (1984) Bacterial meningitis: more involved than just the meninges. N Engl J Med 311:912–914 [DOI] [PubMed] [Google Scholar]
- Tunkel A.R., Hartman B.J., Kaplan S.L., Kaufman B.A., Roos KL., Scheld W.M.et al. (2004) Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284 [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. (1985) The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis 151:859–868 [DOI] [PubMed] [Google Scholar]
- Tuomanen E.I., Saukkonen K, Sande S., Cioffe C., Wright S.D. (1989) Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med 170:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkmeir A., Latsch K, Dietrich G., Wintermeyer E., Schinke B., Schwender S.et al. (2002) Fibronectin mediates OPC-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol Microbiol 46:933–946 [DOI] [PubMed] [Google Scholar]
- van de Beek D., de Gans J., Spanjaard L., Weisfelt M., Reitsma J.B., Vermeulen M. (2004) Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 351:1849–1859 [DOI] [PubMed] [Google Scholar]
- van de Beek D., de Gans J., Tunkel A.R., Wijdicks E.F. (2006) Community-acquired bacterial meningitis in adults. N Engl J Med 354:44–53 [DOI] [PubMed] [Google Scholar]
- van de Beek D., Schmand B., de Gans J., Weisfelt M., Vaessen H., Dankert J.et al. (2002) Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis 186:1047–1052 [DOI] [PubMed] [Google Scholar]
- van Wees J., Tegtmeyer F.K, Otte J., Wood WG., Braun J. (1990) Proteinaseantiproteinase imbalance in meningitis: determination of alpha 1 proteinase inhibitor (alpha 1 PI), elastase-alpha 1 PI complex, and elastase inhibition capacity in cerebrospinal fluid. Klin Wochenschr 68:1054–1058 [DOI] [PubMed] [Google Scholar]
- Weber J.R., Angstwurm K, Rosenkranz T, Lindauer U., Bürger W, Einhäupl K.M.et al. (1997) Histamine (H1) receptor antagonist inhibits leukocyte rolling in pial vessels in the early phase of bacterial meningitis in rats. Neurosci Lett 226:17–20 [DOI] [PubMed] [Google Scholar]
- Weber J.R., Freyer D., Alexander C, Schroder N.W, Reiss A., Kuster C.et al. (2003) Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity 19:269–279 [DOI] [PubMed] [Google Scholar]
- Weisfelt M., Hoogman M., van de Beek D., de Gans J., Dreschler WA., Schmand B.A. (2006) Dexamethasone and long-term outcome in adults with bacterial meningitis. Ann Neurol 60:456–468 [DOI] [PubMed] [Google Scholar]
- Wenger J.D., Hightower A.W, Facklam R.R., Gaventa S., Broome C.V. (1990) Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. The Bacterial Meningitis Study Group. J Infect Dis 162:1316–1323 [DOI] [PubMed] [Google Scholar]
- Whitney C.G., Farley M.M., Hadler J., Harrison L.H., Bennett N.M., Lynfield R.et al. (2003) Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 348:1737–1746 [DOI] [PubMed] [Google Scholar]
- Whitney C.G., Farley M.M., Hadler J., Harrison L.H., Lexau C, Reingold A.et al. (2000) Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 343:1917–1924 [DOI] [PubMed] [Google Scholar]
- Zwijnenburg P.J., van der Poll T, Florquin S., van Deventer S.J., Roord J.J., van Furth A.M. (2001) Experimental pneumococcal meningitis in mice: a model of intranasal infection. J Infect Dis 183:1143–1146 [DOI] [PubMed] [Google Scholar]
- Zysk G., Bruck W, Gerber J., Bruck Y., Prange H.W., Nau R. (1996) Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol 55:722–728 [DOI] [PubMed] [Google Scholar]