Abstract
Primary CNS Lymphoma (PCNSL) accounts for 3% of all primary brain tumors with a median age at onset of about 62 years. In the vast majority of cases, PCNSL presents as unifocal or multifocal enhancing lesions on MRI, frequently adjacent to the ventricles. Stereotactic biopsy is the diagnostic procedure of choice revealing high-grade malignant non-Hodgkin's B-cell lymphoma in more than 90% of cases. Therapy is not evidence based. When eligible, patients should be included in clinical trials. In patients younger than 60 years cure is the aim. Polychemotherapy based on high-dose methotrexate (MTX) or alternatively high-dose chemotherapy with autologous stem cell rescue should be offered to patients eligible for this regimens. For patients over 60 years of age no curative regimen with acceptable toxicity has yet been established. An MTX-based chemotherapy, for example, in combination with temozolomide, is recommended. The role of radiotherapy as part of the initial treatment is not established; however, the combination of radiotherapy with MTX-based chemotherapy potentially leads to severe long-term neurotoxic sequelae. Therefore, radiotherapy as part of the initial therapy is not recommended by the author outside clinical trials. At relapse or in cases of refractory disease, patients will frequently benefit of salvage therapy, which depends on the initial treatment.
Keywords: CNS lymphoma, brain tumors, non-Hodgkin's lymphoma, methotrexate, temozolomide
Introduction
Treatment of primary CNS lymphoma (PCNSL) has improved substantially within the last two decades by the implementation of high-dose systemic methotrexate (MTX)-based chemotherapy, such that a substantial fraction of patients may even be cured with this disease [Batchelor and Loeffler, 2006; Pels and Schlegel, 2006]. In this review, recent developments will be highlighted with a particular focus on chemotherapeutic and experimental approaches.
Epidemiology
The incidence of PCNSL has increased significantly in immunocompromised as well as in immunocompetent individuals and accounts for approximately 3% of primary intracranial tumors [Central Brain Tumor Registry of the United States, 2005]. It had become the most frequent brain tumor in AIDS patients; however, the introduction of highly active antiretroviral therapy (HAART) has reduced the occurrence of all non-Hodgkin's lymphomas (NHL), including primary and secondary brain lymphomas, dramatically [Antinori et al. 2001]. PCNSL may affect all age groups with a peak incidence in the fifth to seventh decade in non-AIDS patients [Batchelor and Loeffler, 2006].
Clinical presentation and imaging
Most commonly, PCNSL presents as diffuse and multifocal supratentorial brain masses. As a peculiarity of PCNSL, involvement of the vitreous, retina and optic nerves may be found in about 10-15% at presentation [Jahnke etal. 2006b]. Lymphomatous infiltration of the leptomeninges or ependymal surfaces and radicular or plexus invasion may occur as well [Pels and Schlegel, 2006]. By systemic staging, occult systemic lymphoma may be detected in up to 8% of patients initially presenting with brain lymphoma. Therefore, bone marrow biopsy, CT scan of chest and abdomen, testicular ultrasound and careful physical examination to detect occult systemic lymphoma is recommended [Abrey et al. 2005]. In a monocentric series, whole body 18F-fluorodeoxyglucose (FDG) PET has been shown to disclose systemic NHL in 7% at presentation of lymphoma primarily affecting the CNS and in 27% at relapse [Mohile etal. 2008]; however, its general diagnostic role is not yet established. Routine diagnostic evaluation in suspected PCNSL comprises ophthalmologic investigation including slit lamp examination, CSF analysis (see below), HIV serology, and serum lactate dehydrogenase (LDH) level [Batchelor and Loeffler, 2006]. Clinical symptoms of PCNSL comprise cognitive dysfunction, psychomotor slowing, personality changes, and disorientation in the majority of patients; raised intracranial pressure and focal symptoms affect about half of them; while brainstem, cerebellar signs and cranial nerve dysfunction and seizures are present only in a minority [Pels and Schlegel etal. 2006]. Magnetic resonance imaging is the most sensitive radiological procedure: the densely cellular tumor appears as single (65%) or multiple lesions on nonenhanced T1-weighted images (Figure 1), hyperintense tumor and edema on T2 or FLAIR images and densely enhancing masses after administration of gadolinium. Fifty per cent or more of the lesions are in contact with the meninges, and meningeal enhancement appears in 10-20% [Küker etal. 2005].
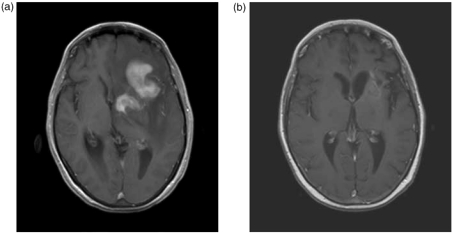
Figure 1.
Contrast-enhanced T1-weighted MR image of a 64-year old lady with primary CNS lymphoma at presentation (a) and after six cycles of MTX-based polychemotherapy (b) showing complete resolution of enhancing tumor lesions. (The hyperintense areas at the initial tumor location are also visible without administration of gadolinium).
Diagnosis and pathology
Stereotactic biopsy is the diagnostic procedure of choice and can be targeted to CTor MRI-defined masses. Glucocorticoids should be withheld if possible, since steroid treated lesions may disappear within a few days and ‘nondiagnostic’ biopsies may result. However, a recent retrospective analysis of cases eventually confirmed as PCNSL has shown, that in this selection repeated biopsy for a nondiagnostic first procedure was infrequent irrespective of former steroid treatment (12%) or no treatment (13%) and that complete regression of a lesion under steroid treatment had been exceptional [Porter et al. 2008]. Steroid response is not diagnostic of PCNSL, since many types of brain infiltrates improve with steroid therapy - for example, sarcoidosis, multiple sclerosis (MS) plaques and even infectious lesions [Porter etal. 2008]. In a prospective series the evaluation of 116 spinal fluid specimens revealed pathologic findings in more than half of the cases analyzed and CSF cytomorphology was positive for lymphoma cells in 18% [Fischer etal. 2006]. Immunohistochemical studies of CSF cells using antibodies to lymphocytes (LCA) or B cells (CD20) are not specific for diagnosis of a clonal proliferate in the CSF. The detection of B-cell monoclonality by PCR amplification of immunoglobulin heavy chain gene rearrangements can be performed in specialized laboratories [Gleisner etal. 2002], but was found less sensitive than cytomorphology in a prospective series on 282 PCNSL patients [Fischer et al. 2008]. Therefore, these techniques cannot be considered established for routine diagnostics.
According to the World Health Organization (WHO) classification [Harris etal. 2000], the majority of PCNSL are classified as diffuse, large B-cell lymphomas (DLBCL). More than 95% of PCNSL correspond to non-Hodgkin's B-cell lymphomas as evidenced by expression of the B-cell markers CD19, CD20, and CD79a. The mitotic activity is generally high and necrosis may occur [Pels and Schlegel, 2006]. The vast majority of PCNSL are of germinal center (GC) B-cell origin: they exhibit bcl-6 gene over-expression and ongoing mutational activity [Montesinos-Rongen etal. 2004] and display an activated B-cell-like phenotype: PCNSL show molecular alterations characteristic for GC B-cell (GCB)-like and for activated B-cell (ABC)-like DLBCLs [Courts etal. 2007]. Primary T-cell lymphomas of the CNS account for 4% of all PCNSL [Shenkier etal. 2005]; with respect to prognosis and response to therapy they show no obvious difference to PCNSL of the B-cell type [Shenkier etal. 2005]. In less than 5%, low-grade PCNSL (of B-cell or T-cell origin) are found. They may display a more indolent clinical course, frequently present with seizures and show distinct radiological features like a less intense or heterogeneous enhancement [Jahnke etal. 2005b].
Treatment
Surgery
The role of surgery is restricted to stereotactic biopsy of a lesion suggestive of PCNSL in order to gain material for histopathological diagnosis [Batchelor and Loeffler, 2006]. Every attempt to completely resect this diffusely infiltrating lesion is contraindicated. Surgical removal as a part of multimodal therapy has been shown to be associated with a worse prognosis [Bellinzona etal. 2005].
Radiotherapy
Radiotherapy has been the mainstay of treatment for many years until the early, 1990s. The only prospective phase II study on brain irradiation (Radiation Therapy Oncology Group 83-15) in HIV-negative patients with PCNSL [Nelson etal. 1992] evaluated 41 patients who received 40 Gy whole brain radiotherapy (WBRT) plus a focal tumor boost of 20 Gy, each dose delivered in 1.8 Gy fractions. Median survival for the whole group was 12.2 months after diagnosis and 7.6 months for patients over 60 years of age; 4 out of 41 patients (10%) died during administration of therapy. In this trial, 28 patients developed recurrent lymphoma of whom 22 were within the ‘boost’ fields [Nelson etal. 1992]. In a retrospective analysis of patients being treated with radiotherapy alone in the, 1990s, the median survival for the whole group (median age 63 years) was 18 months and the 5-year-survival fraction was 18% [Shibamoto et al. 2005]. Therefore, despite the lack of ‘evidence-based’ recommendations, there is a broad consensus, that primary provision of brain irradiation alone is insufficient to provide either durable remission or cure of PCNSL.
Chemotherapy
Concerning the role of chemotherapy, a retrospective analysis of 370 patients treated for PCNSL with different therapies has shown a significantly better outcome for patients treated with combination chemotherapy and radiotherapy in comparison with radiotherapy alone, if chemotherapy had included high-dose intravenous MTX [Ferreri etal. 2002]. MTX at a dosage high enough to reach therapeutic levels in plasma, CSF, brain parenchyma and eye following parenteral administration, has to be given intravenously exceeding 1 g/m2; however, the optimal dosage or dosing schedule is not defined [Pels and Schlegel, 2006].
Two multicenter trials have evaluated high-dose MTX alone for PCNSL: in a German phase II study (NOA-03-trial) MTX was administered at a dosage of 8 g/m2 as a 4-hour infusion every 14 days for six cycles; however, the study was terminated after including 37 of 105 projected patients, since the overall response rate was only 35%. Even with the application of salvage WBRT in 20 out of these 37 patients either for progressive disease (PD) or for relapse, median survival did not exceed 25 months [Herrlinger etal. 2005]. The same protocol, in a single-arm multicenter trial (NABTT 96-07), achieved a 74% overall response rate (52% complete responses, 22% partial responses); however, the median progression-free survival was only 12.8 months [Batchelor etal. 2003] and the median overall survival of 55 months has been the result of efficient salvage therapy at progression or relapse [Gerstner etal. 2008].
Combination chemotherapy is probably more efficient than high-dose MTX alone; a retrospective analysis has identified the inclusion of high-dose cytarabine (ara-C) in MTX-based protocols as an independent positive prognostic predictor [Ferreri etal. 2002]. Polychemotherapy as a sole treatment has been evaluated in two prospective multicenter phase (I)/II trials. In a multidrug phase I/II trial [Pels etal. 2003a], 65 patients were treated with six chemotherapy cycles based on high-dose MTX, high-dose ara-C, vinca-alkaloids and alkylating agents in combination with intraventricular MTX, prednisolone and ara-C. A 61% complete response rate and a 10% partial response rate have been achieved; 9% treatment related deaths have been reported. The median event-free survival was 21 months and the median overall survival 50 months. Results have been particularly promising in 30 patients under 60 years of age included in this trial. In these patients neither median progression-free survival nor median overall survival had been reached after a median follow up of 26 months; the 5-year survival fraction was 75% [Pels etal. 2003a]. In patients over 60 years the overall survival was 34 months. However, the protocol resulted in an overall response rate of only 56% in patients over 60 years of age and was associated with 12% toxic deaths in this age group [Pels etal. 2003a]. In an EORTC trial, 52 patients 60 years and older (median age 72 years, median Karnofsky performance score 50) were treated with MTX as a 1 g/m2 infusion on days 1, 10, and 20, combined with lomustine 40 mg/m2 day 1, procarbazine 60 mg/m2 days 1-7, methylprednisolone alternating days 120 g/m2 days 1-20 and 60 mg/m2 days 20-45, intrathecal MTX 15 mg and ara-C 40 mg days 1, 5, 10 and 15 [Hoang-Xuan etal. 2003]. In case of complete or partial response, documented by MRI or CT, after the first cycle, five more cycles ('maintenance therapy') were applied every 6 weeks with only one MTX infusion and only one intrathecal MTX and ara-C application on day 1 of each cycle. The overall response rate was 48%, median overall survival 14.3 months and the 1-year progression-free survival fraction 40% [Hoang-Xuan etal. 2003]. In a more recent French consecutive case series of 23 patients older than 60 years, MTX 3 g/m2 on days 1, 10, and 20 was combined with temozolomide 100 mg/m2 on days 1-5; in patients with partial or complete response to this induction chemotherapy (17 out of 20 evaluable patients) a maintenance therapy was provided with MTX 3 g/m2 and temozolomide 100 mg/m2 on days 1-5 every month up to five times. The median event-free survival was 8 months in this study and the overall survival 35 months, comparing favorably with results of more complicated regimens [Omuro etal. 2007].
Intrathecal chemotherapy
The role of intrathecal/intraventricular chemotherapy in PCNSL is not defined. MTX, ara-C and steroids have been given by lumbar or ventricular (via a subgaleal reservoir) routes as part of systemic chemotherapy regimens: MTX 12 mg twice a week was given by lumbar route in two trials [Poortmans etal. 2003; DeAngelis etal. 2002], but intraventricular applications provide lower daily doses to achieve sustained CSF levels [Pels etal. 2003b]. In general these levels likely exceed 10 p.M concentrations. Leaving the systemic polychemotherapy unchanged in comparison with the original protocol [Pels etal. 2003a] but omitting intraventricular therapy, a subsequent trial in patients < 60 years led to significantly worse results [Pels etal. 2008], such that at least with this polychemotherapy protocol intraventricular chemotherapy seems indispensible. The benefits of CSF drug application have to be weighted against the risk of iatrogenic ventriculitis [Pels etal. 2003a], the problems of repeated access to the CSF, and the leukoencephalopathic effects of loculated drug within the neuraxis. In a small, case-controlled retrospective study, no differences in survival, disease control or neurotoxicity could be found between recipients and nonrecipients of intrathecal therapy [Khan etal. 2002], and a retrospective analysis of prognostic factors for 370 PCNSL did not find any influence of intrathecal therapy upon outcome [Ferreri etal. 2002]. However, only a small minority of patients having received intrathecal chemotherapy had not been treated with whole brain radiation chemotherapy (WBRT), an efficient treatment of the CSF compartment, such that a possible impact of intrathecal chemotherapy may have been obscured by this fact [Ferreri etal. 2002]. At this time prophylactic intrathecal or intraventricular chemotherapy should still be considered investigational.
Myeloablative high-dose chemotherapy
This achieves therapeutic drug levels in brain, CSF and throughout the neuraxis. Autologous stem cells are provided to rescue the patients from drug-induced leukopenia and thrombocytopenia. Treatment has been provided to recurrent [Soussain et al. 2008] (see below) or newly diagnosed PCNSL [Abrey etal. 2003, Illerhaus etal. 2006, 2008]. ‘Induction therapy’ to achieve remission is provided with MTX and/or ara-C and followed by thiotepa-based regimens [Soussain et al. 2008; Illerhaus et al. 2008, 2006] or by ara-C, melphalan, carmustine and etoposide [Abrey etal. 2003]. While the latter has produced disappointing results [Abrey et al. 2003], combination of high-dose chemotherapy with WBRT (50 Gy after partial and 45 Gy after complete response to chemotherapy, respectively) within a phase II trial for patients up to 65 years has resulted in a 69% 5-year-survival fraction on an intent-to-treat analysis [Illerhaus etal. 2006]. First monocentric experience with an intensified regimen based on this protocol but without radiotherapy has revealed promising preliminary results [Illerhaus et al. 2008], and is currently subject of a multicenter, single-arm, open-label phase II trial. However, this approach should still be considered experimental as primary treatment. Response rates and event-free survival in newly diagnosed PCNSL are not better than with conventional therapy, deaths from drug toxicity or tumor progression occur in patients over 60 years, and longer follow-up of the above-mentioned trial [Illerhaus et al. 2006] shows late relapses [Illerhaus etal. 2008]. At PCNSL relapse, however, high dose chemotherapy with autologous stem cell transplantation appears to be an excellent option in younger individuals [Soussain etal. 2008].
Combined chemotherapy and radiotherapy
Systemic ‘high-dose’ MTX has been included in most combination chemo-and radiotherapy protocols evaluated in phase I/II trials. Three large prospective, multicenter phase II trials have combined MTX-based chemotherapy with WBRT: Within the Trans-Tasman Radiation Oncology Group a protocol has been applied to 46 patients (median age 58 years, range 25-76) consisting of two cycles of MTX 1 g/m2 as a 6 h infusion on days 1 and 8 followed by WBRT with 45 Gy and a 5.4 Gy tumor boost delivered in 1.8 Gy fractions starting on day 15. There was only one toxic death, and 45 patients proceeded to radiotherapy. In 39 assessable patients the complete response rate was 82% and the partial response rate 13%. Median overall survival was 33 months and the 2-year survival probability was 62% [O'Brian, 2000]. More complex protocols have been used by the Radiation Therapy Oncology Group (RTOG 93-10) and by the European EORTC (Trial, 20962). In the latter MTX 3 g/m2 (days 1, 15) was combined with methylprednisolone 60 mg/m2 (days 1-5), teniposide 100 mg/m2 (days 2, 3) and carmustine 100 mg/m2 (day 4) and with intrathecal MTX 15 mg, ara-C 40 mg and hydrocortisone 25 mg (days 1, 15) followed by radiotherapy of the brain with a total dose of 40 Gy in 1.5-1.8 Gy fractions [Poortmans etal. 2003]. Fifty-two patients with an upper age limit of 65 years were included; one died prior to, and five died during, treatment. In the intent-to-treat group, 69% had a complete response and 12% a partial response. Median estimated overall survival was 46 months; the 2-year survival estimate was 69% [Poortmans etal. 2003]. Taking into consideration that only patients up to 65 years of age have been included in this EORTC study, the data are not better than that of the trans-Tasman trial. In the RTOG study 93-10 [DeAngelis etal. 2002] 102 patients with a median age of 56.5 years received systemic MTX 2.5 g/m2, vincristine 1.4 mg/m2 and procarbazine 100 mg/m2/day in weeks 1, 3, 5, 7 and 9, alternating with intraventricular MTX 12 mg once in weeks 2, 4, 6, 8 and 10, followed by WBRT from week 11 to 15 with 45 Gy or-for evidence of permanent neurotoxicity of this regimen - only 36 Gy in case of complete response after chemotherapy for 16 patients being accrued in the second half of the trial. Radiotherapy was followed by systemic ara-C 3 g/m2 days 1 and 2 in week 16 and 19 [DeAngelis etal. 2002]. There was no treatment-related death reported; however, four patients have been excluded from the analysis. Among the 98 remaining patients, 82 proceeded to radiotherapy and 50 of these were assessed for treatment response with a 58% complete response rate, a 36% partial response rate and 6% treatment failures. The median overall survival was 37 months and the 2-year-survival fraction 64% [DeAngelis etal. 2002]. Since radiotherapy as part of the initial treatment is still a matter of debate, its role is currently being investigated in a large German multicenter phase IV trial (German PCNSL Study Group 1 study) comparing immediate WBRT (30 × 1.5 Gy) after complete remission (CR) to six cycles of MTX (4 g/m2) in comparison with deferred radiotherapy at relapse after CR.
Immunotherapy
Since PCNSL represent high-grade malignant B-cell NHL in more than 90% of cases, tumor cells express the CD20 antigen. Treatment with the anti-CD20 antibody rituximab is effective in B-cell NHL and several reports on rituximab therapy in PCNSL refractory to established therapy have been published [Rubenstein et al. 2007; Enting et al. 2004; Pels etal. 2003b]. Rituximab is obviously able to clear the CSF from floating tumor cells if administered intraventricularly [Rubenstein etal. 2006; Pels etal. 2003b]; however, its efficacy to control parenchymal lesions after intravenous or intraventricular administration has been demonstrated in single cases only [Rubenstein et al. 2007]. As part of a multimodal protocol comprising systemic MTX, vincristine and procarbazine, rituximab has been administered at a systemic dosage of 500 mg/m2 per cycle for five to seven cycles, followed by WBRT and by two consecutive cycles of systemic ara-C in a prospective multicenter trial; WBRT was ‘prophylactic’ with 23.4 Gy in case of complete response after five to seven cycles immunochemotherapy and ‘standard’ with 45 Gy in all other cases [Shah etal. 2007]. The regimen was well tolerated and the two-year progression-free and overall survival rates were 57% and 67%, respectively [Shah etal. 2007]. It is difficult to judge a possible therapeutic impact of rituximab within this multimodal regimen in a single-arm trial. It is of note, however, that in this trial rituximab levels in the CSF did not exceed 4.4% of the corresponding serum levels at any timepoint measured [Shah etal. 2007]. Including rituximab in treatment concepts directed against PCNSL might have another yet more important rationale: it has been shown that in up to 27% of relapsed PCNSL, systemic NHL is detectable [Mohile et al. 2008]; in 24% of another series PCNSL patients developed disease outside the CNS [Gerstner et al. 2008] and in an elegant study, occult NHL cells in peripheral blood samples have been detected by PCR amplification of clonally rearranged immunoglobulin heavy-chain (IgH) genes in at least 2 out of 24 PCNSL patients being in full remission of their CNS disease at the time of this analysis [Jahnke etal. 2006a]. Given the rather insensitive nature of this method, the real number of patients harboring occult NHL - with cells showing a tropism to CNS vasculature - may even be underestimated. Therefore, systemic administration of rituximab might well be toxic to occult systemic disease. Two case series of patients with refractory or relapsed PCNSL have shown that the application of radioconjugated CD20 antibodies like [90Y] ibritumomab (Zevalin(r)) and [131I] tositumomab (Bexxar(r)) is feasible and has resulted in responses in single cases [Doolittle etal. 2007; Iwamoto etal. 2007]. However, the future role of these radioconjugated CD20 antibodies in PCNSL needs to be defined [Wong etal. 2005].
Treatment-related late neurotoxicity
Deleterious long-term treatment related neurotoxicity after combination chemotherapy and radiotherapy has been reported in a single-center analysis in, 1998 [Abrey etal. 1998]. After having survived 4 years without detectable tumor, 100% of the patients being 60 years or older showed cognitive dysfunction. Younger individuals were affected less frequently and with a longer delay by this complication. Affected patients showed leukoencephalopathy and cortical/subcortical atrophy, which may leave them demented, ataxic, incontinent and dependent on custodial care [Abrey etal. 1998]. Pathologic investigation of the brains from patients with fatal neurotoxicity revealed no tumor cells, but myelin and axonal loss, gliosis, spongiosis and rarefication of the white matter [Lai etal. 2004]. In a retrospective analysis on 183 patients treated for PCNSL in a single center for the development of long-term treatment related neurotoxicity, only the administration of radiotherapy was identified as an independent risk factor [Omuro etal. 2005]. This finding is supported by a recent literature review suggesting that PCNSL patients having been treated with chemotherapy alone carry less risk of developing late neurotoxicity [Correa etal. 2007]. Therefore, formal neuropsychological testing in any prospective therapeutic trial in PCNSL is recommended and guidelines for testing have been established [Correa et al. 2007] . Taking into consideration the risk of long-term neurotoxicity due to ‘consolidating’ radiotherapy after complete response to chemotherapy, a retrospective single center analysis on 122 patients has addressed this issue. No difference in overall survival has been found between complete responders to MTX-based chemotherapy having received upfront ‘consolidating’ whole brain radiotherapy and those with deferred radiotherapy at relapse [Ekenel et al. 2008]. Therefore, and for the sake of avoidance of neurotoxicity, it is justified to withhold radiotherapy after complete response to chemotherapy for salvage at relapse (see below).
Specific situations
The impact of age
The prognosis and response to therapy in patients 60 years of age or older is significantly worse irrespective of the treatment modality applied [Pels etal. 2003a; DeAngelis etal. 2002; Nelson etal. 1992]. It has also been proposed that, irrespective of their Karnofsky Performance Score, patients younger than 50 years do better than older ones [Abrey etal. 2006]. However, the median age in PCNSL is over 60 years and a ‘specific treatment situation’ has to be considered for the majority of patients, since older individuals frequently show comorbidity, tolerate treatment less well and carry a high risk of neurotoxicity. Thus, specific precautions have to be undertaken. The MTX dosage is to be adjusted to the glomerular filtration rate. In 89 patients over 60 years of age treated within a German trial, 4 g/m2 MTX have been applied as a 4 hour infusion every 2 weeks for a maximum of six cycles, together with dexamethasone 3 × 8 mg dexamethasone for 10 days in the first cycle. Prior to each cycle the creatinine clearance was measured and the dosage of MTX was reduced according to creatinine clearance values from 80 ml/min onwards; for example, by 20% at 80 ml/min and by 40% at 60 ml/min; a creatinine clearance value less than 50 ml/min was an exclusion criteria for high-dose MTX [Jahnke etal. 2005a]. Dose reduction was necessary in 44% of patients over 60 years, and termination of therapy due to nephrotoxicity in only 3%. General toxicity, a WHO score greater than 2, occurred in less than 10% of these patients; treatment results were not given [Jahnke etal. 2005a]. Taking these data together, it is recommended to treat older patients upfront with a high-dose MTX-based regimen; for example, 4 g/m2 for six cycles (adjusted to the glomerular filtration rate) in combination with dexamethasone 3 × 8 mg dexamethasone for 10 days during the first cycle, because this has been shown to be feasible [Jahnke etal. 2005a]. Alternatively MTX 3 g/m2 on days 1, 10, and 20 can be combined with temozolomide 100 mg/m2 on days 1-5 with a maintenance therapy (MTX 3 g/m2 and temozolomide 100 mg/m2 on days 1-5) every month up to five times in responding patients [Omuro etal. 2007]. According to published results from other studies [Hoang-Xuan etal. 2003; Pels etal. 2003a], however, a response rate of less than 60% is anticipated and a salvage therapy should be initiated in refractory patients (see below).
HIV-positive patients and post-transplant lymphoproliferation (PTLD)
Prior to the era of HAART, HIV-infected patients with PCNSL usually carried a dismal prognosis: one-third of them died while receiving radiation for their brain lymphoma [Pels and Schlegel, 2006]. This situation has substantially improved: first, the incidence of PCNSL has decreased dramatically with HAART [Antinori etal. 2001]; and second, AIDS patients treated concomitantly with HAART and chemotherapy for NHL are more likely to respond to chemotherapy when HAART induces a reduction of HIV viral load [Hoffmann etal. 2001]. In a multicenter retrospective analysis, patients with PCNSL and AIDS showed the best outcome when treated with cranial radiation and HAART (median survival, 1093 days). The survival was 132 days after cranial radiation alone and 33 days without specific therapy [Hoffmann etal. 2001]. Selected AIDS-PCNSL patients may be candidates for aggressive chemo or chemo/radiotherapy if: (1) their performance status has a KPI greater than 50; (2) their CD4+ cell count is above 200/p.l; (3) comorbidities of AIDS are limited and non-neurologic [Pels and Schlegel, 2006]. For the severely ill, comfort care only may be a therapeutic option. Non-AIDS PTLD patients tend to have EBV-induced PCNSL and demonstrate elevated loads of EBV in CSF [Pels and Schlegel, 2006]. Usually demonstrable is reactivation of latent EBV or newly acquired seroconversion. The PCNSL which emerges cannot easily be distinguished from the EBV or other infectious complications of transplant, although PCNSL may be accompanied by lymphoma invasion of the transplanted organ. Therapy is based on reduction or discontinuation of immunosuppression in favor of low-dose steroid use with chemotherapy [Pels and Schlegel, 2006].
Intraocular lymphoma
About 10-20% of PCNSL patients show ocular involvement at presentation or during the course of the disease: lymphomatous uveitis, vitrous and/or optic nerve infiltration [Batchelor and Loeffler, 2006; Pels and Schlegel, 2006]. Intraocular involvement may occur as an isolated site of relapse after successful treatment of PCNSL or in combination with CNS relapse [Jahnke etal. 2006b; Pels etal. 2003a]. Treatment with high-dose MTX-based chemotherapy, with ifosfamide or with oral trofosfamide is efficient and frequently results in CR or PR [Jahnke etal. 2006b]. Alternatively, radiation to the posterior two-thirds of the eye chamber with 30-45 Gy can be applied; however, this is frequently complicated by the occurrence of cataracts and uncertain control of coincident optic nerve and brain involvement by tumor. Intraocular MTX with 400 mg in a 0.1ml volume instillation into the vitreous achieves cytotoxic drug levels and may lead to clearance of ocular tumor as well, but is complicated by a rate of 73% cataracts, 58% corneal epitheliopathy, 42% maculopathy and other serious complications [Smith etal. 2002]. Therefore, this modality is considered experimental. It is recommended to treat ocular involvement at presentation of a PCNSL with high-dose MTX-based chemotherapy alone and in case of isolated ocular relapse with oral trofosfamide or intravenous ifosfamide [Jahnke etal. 2006b]. Ocular radiotherapy should be reserved to cases refractory to this treatment. It is of note that patients treated for intraocular lymphoma with systemic chemotherapy relapse less frequently in the CNS than patients treated with local therapy [Jahnke etal. 2006b].
Treatment in the refractory patient and at relapse
The optimal therapy of recurrent tumor is not established, but drug resistance is seldom documented and most patients benefit from reinduction with chemotherapeutic agents [Reni et al. 2007; Pels and Schlegel, 2006]. Patients with recurrent lymphoma after initial long-lasting response to chemotherapy are at least 50% likely to achieve a complete reinduction with MTX [Plotkin et al. 2004] suggesting that brain lymphoma may not recur as a function of drug resistance alone. In patients younger than 65 years and eligible for myeloablative therapy, intensive chemotherapy followed by hematopoietic stem-cell rescue is strongly recommended as a potentially curative salvage treatment option analogous to the situation in systemic relapsed high-grade malignant B-cell NHL. In 39 patients with relapse (n = 21) or refractory disease (n = 17) to first-line therapy, thiotepa/busulfane/cyclophosphamide-based high-dose chemotherapy was administered after two cycles of ara-C and etoposide induction. CR(PR) was achieved in 15(5) cases and median overall survival after initiation of salvage therapy was 18.3 months [Soussain etal. 2008]. Responses to other agents are seen in 25-40% of the cases as to temozolomide alone [Reni et al. 2004] or in combination with the CD20 antibody rituximab [Enting etal. 2004], and to topotecan 1.5 g/m2/day, days 1-5, every 4 weeks [Fischer etal. 2004]. After primary or secondary failure to high-dose MTX in 27 patients, WBRT with a median dose of 36 Gy and a facultative boost to the initial tumor region has resulted in 37% long-lasting complete remissions (median duration 57.6 months) and in another 37% short-lasting partial remissions (median duration 9.7 months). A total dose of more than 36 Gy, a single fraction higher than 1.5 Gy and age over 60 were predictors of treatment-related neurotoxicity at follow up [Ngyen etal. 2005]. These findings have been confirmed in another retrospective single-center analysis showing a CR rate (PR rate) of 58% (21%) in 48 patients treated with a median dose of 40 Gy WBRT at relapse (n = 24) or progression (n = 24); long-term disease control was seen in 31% of the patients [Hottinger etal. 2007].
Follow up
MRI after successful treatment of PCNSL may reveal small enhancing lesions in regions of initial tumor or surgical manipulation. These lesions may not reflect active tumor and are classified as complete response/unconfirmed [Abrey etal. 2005]. Follow-up MRI in these cases may reveal a decrease in size or even a disappearance of these lesions. If PET scanning will be helpful in the future in distinguishing these ‘scars’ from active tumor or if it will assist in detecting relapse early, remains to be seen [Palmedo etal. 2006]. MRI of the brain and neurological examination should be carried out every 3 months for 2 years after treatment and every 6 months for the next 3 years [Abrey etal. 2005]. Examination of the CSF, ophthalmologic investigation and other tests should be carried out only in case of clinical symptoms; serial neuropsychological testing is advisable since late neurotoxicity remains an important treatment complication.
Treatment recommendations
Unfortunately, the substantial progress in PCNSL treatment has not yet translated into broad clinical practice. A recent population-based analysis of survival data in PCNSL in the US has revealed no difference in survival numbers in the late nineties in comparison with the early seventies [Panageas etal. 2005]. Therefore the following is recommended:
When eligible, patients should be included in clinical trials.
The role of surgery is restricted to stereotactic biopsy for histopathological diagnosis.
In patients younger than 60 years cure is the aim. Polychemotherapy based on high-dose MTX with deferred radiation [Pels et al. 2003a] or alternatively high-dose chemotherapy with autologous stem cell rescue [Illerhaus et al. 2006] should be offered to patients eligible for this regimen.
For patients over 60 years, no curative regimen with acceptable toxicity has yet been established. An MTX-based chemotherapy; for example, in combination with temozolomide [Omuro et al. 2007] is recommended.
The combination of radiotherapy with MTX-based chemotherapy potentially leads to severe long-term neurotoxic sequelae. Therefore, radiotherapy as part of the initial therapy is not recommended outside clinical trials.
At relapse after long-lasting response, reinduction of MTX-based chemotherapy, having been effective as initial treatment, is recommended as salvage therapy in older patients. Temozolomide or topotecan may be administered in cases of short-lasting responses to initial therapy or in cases of initial failure to MTX. Radiation may be reserved for patients not responding to these regimens. In patients younger than 60 years of age intensive chemotherapy with autologous stem cell transplantation is recommended.
Conflict of interest statement
None declared.
References
- Abrey L.E., DeAngelis L.M., Yahalom J.(1998) Long-term survival in primary CNS lymphoma, J Clin Oncol 16: 859–863 [DOI] [PubMed] [Google Scholar]
- Abrey L.E., Moskowitz C.H., Mason W.P., Crump M., Stewart D., Forsyth P.et al. (2003) Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis, J Clin Oncol 21: 4151–4156 [DOI] [PubMed] [Google Scholar]
- Abrey L.E., Batchelor T.T., Ferreri A.J.M., Gospodarowicz M., Pulczynski E.J., Zucca E.et al. (2005) Report of an international workshop to None declared. standardize baseline evaluation and response criteria for primary CNS lymphoma, J Clin Oncol 23: 5034–5043 [DOI] [PubMed] [Google Scholar]
- Abrey L.E., Ben-Porat L., Panageas K.S., Yahalom J., Berkey B., Curran W.et al. (2006) Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model, J Clin Oncol 24: 5711–5715 [DOI] [PubMed] [Google Scholar]
- Antinori A., Cingolani A., Alba L.R., Ammassari A., Serraino D., Ciancio B.C.et al. (2001) Better response to chemotherapy and prolonged survival in AIDS-related lymphomas responding to highly active antiretroviral therapy, AIDS 15: 1483–1491 [DOI] [PubMed] [Google Scholar]
- Batchelor T., Loeffler J.S.(2006) Primary CNS lymphoma, J Clin Oncol 24: 1281–1288 [DOI] [PubMed] [Google Scholar]
- Batchelor T., Carson K., O'Neill A., Grossman S.A., Alavi J., New P.et al. (2003) Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07, J Clin Oncol 21: 1044–1049 [DOI] [PubMed] [Google Scholar]
- Bellinzona M., Roser F., Ostertag H., Gaab R.M., Saini M.(2005) Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases, Eur J Surg Oncol 31: 100–105 [DOI] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States (2005)Statistical Report: Primary Brain Tumors in the United States, 1998-2002. Central Brain Tumor Registry of the United States, Chicago, IL [Google Scholar]
- Correa D.D., Maron L., Harder H., Klein M., Armstrong C.L., Calabrese P.et al. (2007) Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines, Ann Oncol 18: 1145–1151 [DOI] [PubMed] [Google Scholar]
- Courts C., Montesinos-Rongen M., Martin-Subero J.I., Brunn A., Siemer D., Zuhlke-Jenisch R.et al. (2007) Transcriptional profiling of the nuclear factor-kappaB pathway identifies a subgroup of primary lymphoma of the central nervous system with low BCL10 expression, J Neuropathol Exp Neurol 66: 230–237 [DOI] [PubMed] [Google Scholar]
- DeAngelis L.M., Seiferheld W., Schold S.C., Fisher B., Schultz C.J.(2002) Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10, J Clin Oncol 20: 4643–4648 III [DOI] [PubMed] [Google Scholar]
- Doolittle N.D., Jahnke K., Belanger R., Ryan D.A., Nance R.W., Lacy C.A.et al. (2007) Potential of chemo-immunotherapy in relapsed primary central nervous system (CNS) lymphoma, Leuk Lymphoma 48: 1712–1720 [DOI] [PubMed] [Google Scholar]
- Ekenel M., Iwamoto F.M., Ben-Porat L.S., Panageas K.S., Yahalom J., Deangelis L.M.et al. (2008) Primary central nervous system lymphoma: The role of consolidation treatment after a complete response to high-dose methotrexate-based chemotherapy, Cancer 113: 1025–31 [DOI] [PubMed] [Google Scholar]
- Enting R.H., Demopoulos A., DeAngelis L.M., Abrey L.E.(2004) Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide, Neurology 63: 901–903 [DOI] [PubMed] [Google Scholar]
- Ferreri A.J., Reni M., Pasini F., Calderoni A., Tirelli U., Pivnik A.et al. (2002) A multicenter study of treatment of primary CNS lymphoma, Neurology 58: 1513–1520 [DOI] [PubMed] [Google Scholar]
- Fischer L., Jahnke K., Martus P., Weller M., Thiel E., Korfel A.(2006) The diagnostic value of cerebrospinal fluid pleocytosis and protein in the detection of lymphomatous meningitis in primary central nervous system lymphomas, Haematologica 91: 429–430 [PubMed] [Google Scholar]
- Fischer L., Martus P., Weller M., Klasen H.A., Rohden B., Roth A.et al. (2008) Meningeal dissemination in primary CNS lymphoma: Prospective evaluation of 282 patients, Neurology 71: 1102–1108 [DOI] [PubMed] [Google Scholar]
- Fischer L., Thiel E., Klasen H.A., Kirchen H., Jahnke K., Korfel A.(2004) Response of relapsed or refractory primary CNS lymphoma (PCNSL) to topotecan, Neurology 62: 1885–1888 [DOI] [PubMed] [Google Scholar]
- Gerstner E.R., Carson K.A., Grossman S.A., Mrugala M.M., Nugent W., Nierenberg K.et al. (2008) Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation, Neurology 70: 401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleissner B., Siehl J., Korfel A., Reinhardt R., Thiel E.(2002) CSF evaluation in primary CNS lymphoma patients by PCR of the CDR III IgH genes, Neurology 58: 390–396 [DOI] [PubMed] [Google Scholar]
- Harris N.L., Jaffe E.S., Diebold J., Flandrin G., Muller-Hermelink H.K., Vardiman J.et al. (2000) The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting - Airlie House, Virginia, November, 1997, Hematol J 1: 53–66 [DOI] [PubMed] [Google Scholar]
- Herrlinger U., Küker W., Uhl M., Blaicher H.P., Karnath H.O., Kanz L.et al. (2005) NOA-03 multicenter trial of high-dose methotrexate therapy in primary CNS lymphoma: final report, Ann Neurol 57: 843–847 [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan K., Taillandier L., Chinot O., Soubeyran P., Bogdhan U., Hildebrand J.et al. (2003) Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group, J Clin Oncol 21: 2726–2731 [DOI] [PubMed] [Google Scholar]
- Hoffmann C., Tabrizian S., Wolf E., Eggers C., Stoehr A., Plettenberg A.et al. (2001) Survival of AIDS patients with primary central nervous system lymphoma is dramatically improved by HAART-induced immune recovery, AIDS 15: 2119–2127 [DOI] [PubMed] [Google Scholar]
- Hottinger A.F., DeAngelis L.M., Yahalom J., Abrey L.E.(2007) Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma, Neurology 69: 1178–1182 [DOI] [PubMed] [Google Scholar]
- Illerhaus G., Marks R., Ihorst G., Guttenberger R., Ostertag C., Derigs G.et al. (2006) High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma, J Clin Oncol 24: 3865–3870 [DOI] [PubMed] [Google Scholar]
- Illerhaus G., Muller F., Feuerhake F., Schafer A.O., Ostertag C., Finke J.et al. (2008) High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system, Haematologica 93: 147–148 [DOI] [PubMed] [Google Scholar]
- Iwamoto F.M., Schwartz J., Pandit-Taskar N., Peak S., Divgi C.R., Zelenetz A.D.et al. (2007) Study of radiolabeled indium-111 and yttrium-90 ibritumomab tiuxetan in primary central nervous system lymphoma, Cancer 110: 2528–2534 [DOI] [PubMed] [Google Scholar]
- Jahnke K., Korfel A., Martus P., Weller M., Herrlinger U., Schmittel A.et al. (2005a) High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma, Ann Oncol 16: 445–449 [DOI] [PubMed] [Google Scholar]
- Jahnke K., Thiel E., Schilling A., Herrlinger U., Weller M., Coupland S.E.et al. (2005b) Low-grade primary central nervous system lymphoma in immunocompetent patients, Br J Haematol 128: 616–624 [DOI] [PubMed] [Google Scholar]
- Jahnke K., Hummel M., Korfel A., Burmeister T., Kiewe P., Klasen H.A.et al. (2006a) Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes, J Clin Oncol 24: 4754–4757 [DOI] [PubMed] [Google Scholar]
- Jahnke K., Korfel A., Komm J., Bechrakis N.E., Stein H., Thiel E.et al. (2006b) Intraocular lymphoma, 2000-2005: results of a retrospective multicentre trial, Graefes Arch Clin Exp Ophthalmol 244: 663–669 [DOI] [PubMed] [Google Scholar]
- Khan R.B., Shi W., Thaler H.T., DeAngelis L.M., Abrey L.E.(2002) Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 58: 175–178 [DOI] [PubMed] [Google Scholar]
- Küker W., Nagele T., Korfel A.et al. (2005) Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients, J Neurooncol 72: 169–177 [DOI] [PubMed] [Google Scholar]
- Lai R., Abrey L.E., Rosenblum M.K., DeAngelis L.M.(2004) Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study, Neurology 62: 451–456 [DOI] [PubMed] [Google Scholar]
- Mohile N.A., DeAngelis L.M., Abrey L.E.(2008) The utility of body FDG PET in staging primary central nervous system lymphoma, Neuro Oncol 10: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos-Rongen M., Van Roost D., Schaller C., Wiestler O.D., Deckert M.(2004) Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation, Blood 103: 1869–1875 [DOI] [PubMed] [Google Scholar]
- Nelson D.F., Martz K.L., Bonner H., Nelson J.S., Newall J., Kerman M.D.et al. (1992) Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315, Int J Radiat Oncol Biol Phys 23: 9–17 [DOI] [PubMed] [Google Scholar]
- Nguyen P.L., Chakravarti A., Finkelstein D.M., Hochberg F.H., Batchelor T.T., Loeffler J.S.(2005) Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma, J Clin Oncol 23: 1507–1513 [DOI] [PubMed] [Google Scholar]
- O'Brien P., Roos D., Pratt G., Liew K., Barton M., Poulsen M.et al. (2000) Phase II multicenter study of brief single-agent methotrexate followed by irradiation in primary CNS lymphoma, J Clin Oncol 18: 519–526 [DOI] [PubMed] [Google Scholar]
- Omuro A.M., Ben-Porat L.S., Panageas K.S., Kim A.K., Correa D.D., Yahalom J.et al. (2005) Delayed neurotoxicity in primary central nervous system lymphoma, Arch Neurol 62: 1595–1600 [DOI] [PubMed] [Google Scholar]
- Omuro A.M., Taillandier L., Chinot O., Carnin C., Barrie M., Hoang-Xuan K.et al. (2007) Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly, J Neurooncol 85: 207–211 [DOI] [PubMed] [Google Scholar]
- Palmedo H., Urbach H., Bender H., Schlegel U., Schmidt-Wolf I.G., Matthies A.et al. (2006) FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up, Eur J Nucl Med Mol Imaging 33: 164–168 [DOI] [PubMed] [Google Scholar]
- Panageas K.S., Elkin E.B., DeAngelis L.M., Ben-Porat L., Abrey L.E.(2005) Trends in survival from primary central nervous system lymphoma, 1975-1999: a population-based analysis, Cancer 104: 2466–2472 [DOI] [PubMed] [Google Scholar]
- Pels H., Juergens A., Glasmacher A., Schulz H., Engert A., Linnebank M.et al. (2009) Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: results of a phase II study, J Neurooncol 91: 299–305 [DOI] [PubMed] [Google Scholar]
- Pels H., Schmidt-Wolf I.G., Glasmacher A., Schulz H., Engert A., Diehl V.et al. (2003a) Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy, J Clin Oncol 21: 4489–4495 [DOI] [PubMed] [Google Scholar]
- Pels H., Schulz H., Schlegel U., Engert A.(2003b) Treatment of CNS lymphoma with the anti-CD20 antibody rituximab: experience with two cases and review of the literature, Onkologie 26: 351–354 [DOI] [PubMed] [Google Scholar]
- Pels H., Schlegel U.(2006) Primary central nervous system lymphoma, Curr Treatment Opt Neurol 8: 346–357 [DOI] [PubMed] [Google Scholar]
- Plotkin S.R., Betensky R.A., Hochberg F.H., Grossman S.A., Lesser G.J., Nabors L.B.et al. (2004) Treatment of relapsed central nervous system lymphoma with high-dose methotrexate, Clin Cancer Res 10: 5643–5646 [DOI] [PubMed] [Google Scholar]
- Poortmans P.M., Kluin-Nelemans H.C., Haaxma-Reiche H., Van't Veer M., Hansen M., Soubeyran P.et al. (2003) High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial20962, J Clin Oncol 21: 4483–4488 III [DOI] [PubMed] [Google Scholar]
- Porter A.B., Giannini C., Kaufmann T., Lucchinetti C.F., Wu W., Decker P.A.et al. (2008) Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma, Ann Neurol 63: 662–667 [DOI] [PubMed] [Google Scholar]
- Reni M., Mason W., Zaja F., Perry J., Franceschi E., Bernardi D.et al. (2004) Salvage chemotherapy with temozolomide in primary CNS lymphomas: preliminary results of a phase II trial, Eur J Cancer 40: 1682–1688 [DOI] [PubMed] [Google Scholar]
- Reni M., Mazza E., Foppoli M., Ferreri A.J.(2007) Primary central nervous system lymphomas: salvage treatment after failure to high-dose methotrexate, Cancer Lett 258: 165–170 [DOI] [PubMed] [Google Scholar]
- Rubenstein J.L., Fridlyand J., Abrey L., Shen A., Karch J., Wang E.et al. (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma, J Clin Oncol 25: 1350–1356 [DOI] [PubMed] [Google Scholar]
- Shah G.D., Yahalom J., Correa D.D., Lai R.K., Raizer J.J., Schiff D.et al. (2007) Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma, J Clin Oncol 25: 4730–4735 [DOI] [PubMed] [Google Scholar]
- Shenkier T.N., Blay J.Y., O'Neill B.P., Poortmans P., Thiel E., Jahnke K.et al. (2005) Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group, J Clin Oncol 23: 2233–2239 [DOI] [PubMed] [Google Scholar]
- Soussain C., Hoang-Xuan K., Taillandier L., Fourme E., Choquet S., Witz F.et al. (2008) Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire, J Clin Oncol 26: 2512–2518 [DOI] [PubMed] [Google Scholar]
- Wong E.T.(2005) Monoclonal antibody therapy for central nervous system lymphomas: An emerging treatment paradigm, Expert Opin Pharmacother 6: 1107–1114 [DOI] [PubMed] [Google Scholar]