Abstract
Glycogen storage disease type II (GSDII) is an autosomal recessive lysosomal disorder caused by mutations in the gene encoding alpha-glucosidase (GAA). The disease can be clinically classified into three types: a severe infantile form, a juvenile and an adultonset form. Cases with juvenile or adult onset GSDII mimic limb-girdle muscular dystrophy or polymyositis and are often characterized by respiratory involvement. GSDII patients are diagnosed by biochemical assay and by molecular characterization of the GAA gene. Ascertaining a natural history of patients with heterogeneous late-onset GSDII is useful for evaluating their progressive functional disability. A significant decline is observed over the years in skeletal and respiratory muscle function. Enzyme replacement therapy (ERT) has provided encouraging results in the infantile form. It is not yet known if ERT is effective in late-onset GSDII. We examined a series of 11 patients before and after ERT evaluating muscle strength by MRC, timed and graded functional tests, 6-minute walk test (6MWT), respiratory function by spirometric parameters and quality of life. We observed a partial improvement during a prolonged follow-up from 3 to 18 months. The use of different clinical parameters in the proposed protocol seems crucial to determine the efficacy of ERT, since not all late-onset patients respond similarly to ERT.
Keywords: glycogen storage disease type II, trial, protocol
Introduction
Glycogenosis type II (GSDII) is an autosomal recessive disorder which can be clinically classified into three types (severe or infantile, juvenile and adult onset). In the severe infantile form of GSDII (Pompe disease) there is a marked hypotonia and cardiomyopathy due to acid alpha-glucosidase (GAA) deficiency. In the juvenile or adult forms, named late-onset GSDII, a residual enzyme activity is usually found [Angelini and Engel, 1972]. Late-onset GSDII mimics limbgirdle dystrophy or polymyositis [Angelini and Nascimbeni, 2007] and might also be frequently characterized by a marked respiratory involvement with restrictive respiratory insufficiency [Pellegrini et al. 2005; Laforet et al. 2000; Margolis and Hill, 1986]. The diagnosis can be missed in a routine muscle biopsy, since the classical vacuolar pathology, revealed only by acid phosphatase and PAS stain, might be scarcely detectable in which case additional biochemical and molecular analysis are required [Nascimbeni et al. 2008]. Sometimes GSDII is revealed by drug use such as statin [Voermans et al. 2005]. The natural history of the late-onset disease is still poorly investigated, because of its heterogeneous course, and because available studies have collected data only through a questionnaire [Hagemans et al. 2005a] or have investigated retrospectively the disorder [Hagemans et al. 2005b]. In several late-onset cases there is a lack of correlation between limb muscle weakness and respiratory function [Pellegrini et al. 2005].
The gene encoding GAA is 20 kb in size, maps to chromosome 17q22-qter [D'Ancona et al. 1979], and is translated into a 110 kDa peptide, the inactive precursor, which is translocated from the Golgi apparatus to lysosomes, where it is shortened to 76 and 70 kDa peptides, forming the mature enzyme. Proteolytic processing appears to be required for optimal activity towards the natural substrate, glycogen. During this process, there is a 7- to 10-fold increase activity of the enzyme. The mature form is assembled in a larger molecular mass multicomponent enzyme complex [Moreland et al. 2005]. There is an inverse correlation between the level of residual enzyme activity and clinical manifestations [Reuser et al. 1987; Mehler and DiMauro, 1977]. Enzyme level and lysosomal glycogen content appear to be useful to predict the age of onset in GSDII patients. ERT is currently available for late-onset GSDII patients, but the study of the natural history of such patients should be conducted before ERT in order to evaluate the efficacy of ERT and because the treatment is expensive and needs strict selection criteria. We report the results of a study on a cohort of 11 late-onset GSDII patients, describing their clinical response and changes of functional ability during a prolonged period of ERT.
Methods
Inclusion criteria
This clinical study was conducted according to the principles of the Declaration of Helsinki, and informed consent was obtained from participating patients. The inclusion criteria for GSDII patients in the study were:
Age at treatment ranging from 20 to 70 years.
Levels of GAA activity below 30% of control, measured either in skeletal muscle or leukocytes. All centers diagnosed their patients on the basis of biochemical assay. Molecular analysis was also performed in six patients from various centers.
Patients were symptomatic; that is, at least grade 2 on the Gardner-Medwin and Walton functional scale, with the exception of one asymptomatic patient with only hyperCKemia.
Clinical and instrumental examinations
Patients were evaluated with routine blood exams, ECG, echocardiography, spirometry, and a series of clinical functional tests (Table 1).
Table 1.
Protocol for glycogenosis type II.
| Pre | to | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | W | |
| ERT | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 28 | 20 | 22 | 24 | 26 | 28 | 30 | 32 | 34 | 36 | 38 | 40 | 42 | 44 | 46 | 48 | 50 | 52 | ||
| Informed consent | X | |||||||||||||||||||||||||||
| Inclusion criteria | X | |||||||||||||||||||||||||||
| Medical history | X | |||||||||||||||||||||||||||
| Nutritional evaluation | X | X | X | |||||||||||||||||||||||||
| 6MWT | X | X | X | X | X | X | X | |||||||||||||||||||||
| FVC | X | (X) | X | X | X | X | ||||||||||||||||||||||
| MMT | X | X | X | X | X | X | ||||||||||||||||||||||
| Functional activity | X | X | X | X | X | |||||||||||||||||||||||
| (GSGC) | ||||||||||||||||||||||||||||
| RHS | ||||||||||||||||||||||||||||
| SF-36 Health survey | X | X | X | X | ||||||||||||||||||||||||
| Gene GAA mutation | X | |||||||||||||||||||||||||||
| GAA activity | X | |||||||||||||||||||||||||||
| in muscle | ||||||||||||||||||||||||||||
| Vital signs | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Blood exams | X | X | X | X | X | X | X | X | X | |||||||||||||||||||
| Urine exams | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| EGG | X | X | X | |||||||||||||||||||||||||
| Echocardiography | X | X | ||||||||||||||||||||||||||
| Weight | X | X | X | X | X | |||||||||||||||||||||||
| Anti-rhGAA | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Antibody-IgG | ||||||||||||||||||||||||||||
| Drug infusion | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Ventilation diary | Continuous | |||||||||||||||||||||||||||
| monitoring | ||||||||||||||||||||||||||||
| Adverse reactions | Continuous | |||||||||||||||||||||||||||
| monitoring |
6MWT, six-minute walk test; FVC, forced vital capacity; MMT, manual muscle testing; RHS, Rotterdam Handicap Scale; ECG, electrocardiogram.
Muscle strength
Manual muscle testing (MMT) was done in the following muscle groups: deltoid, biceps brachii, triceps brachii, intra/extra rotators of arms, iliopsoas, quadriceps femoris, thigh abductors, thigh adductors, tibialis anterior, tibialis posterior, neck flexors and neck extensors.
Muscle strength in the above-mentioned muscles was assessed according to the expanded Medical Research Council (MRC) scale. While this scale is satisfactory for routine clinical examination, in myopathic patients the subdivision in + or - is desirable in which a plus sign corresponds to an increase of one-third of the score point and the minus sign corresponds to a decrease of one-third of the score point.
Functional performances (GSGC score)
We obtained a baseline score of late-onset GSDII patients by timing and grading the four GSGC functional performances; that is, walking for 10 meters (Gait), climbing four steps on a stair (Stair), Gower's manoeuvre (Gower's), rising from a chair (Chair) (Table 2, Figure 1) and also examined the lifting of proximal upper limbs according to a standardized protocol (Table 2). Similar data were previously collected in an open trial with albuterol in late-onset GSDII patients [Angelini et al. 2004]. The GSGC score obtained adding the grades of the four functional tests varied from a minimum of 4 (normal performances) to a maximum of 27 (worst score) and was validated in the clinical follow-up of GSDII cases as well as in Duchenne muscular dystrophy [Angelini, 2007].
Table 2.
GSGC score and proximal upper limb score.
| 1. GAIT (G) | 3. GOWER'S MANOEUVRE (G) |
| 1= Normal 2= Mild waddling, lordosis and/or toe walking 3= Moderate waddling, lordosis and/or toe walking 4= Severe waddling, lordosis and/or toe walking 5= Walks only with assistance (i.e braces, cane, crutches) 6= Stands, but unable to walk 7= Confined to wheelchair |
1= Normal 2= Butt first manoeuvre, one hand on floor 3= Butt first manoeuvre, two hands on floor 4= Unilateral hand support on thigh 5= Bilateral hand support on thighs 6= Arises only with aid of an object (table, chair, etc.) 7= Unable to rise |
| Time to walk 10 meters:… s | Time to stand from the floor:… s |
| 2. CLIMBING STAIRS (S) | 4. ARISING FROM A CHAIR (C) |
| 1= Climbs four stairs without assistance 2= Supports one hand on thigh 3= Supports both hands on thighs 4= Climb stairs in upright position but with aid of railing 5= Climbs while clinging to the railing with both hands 6= Manages to climb only a few steps 7= Unable to climb steps |
1= Normal 2= With wide base and/or difficulty but without support 3= With support on one thigh 4= With support on both thighs 5= With support on arms of chair or on a table (Figures 1 and 2) 6= Not possible |
| Time to climb four steps:… s | Time to stand from sitting position:… s |
| PROXIMAL UPPER LIMBS | |
| 1= Starting from the arms at the sides, the patient can
move the arms in a full circle till they touch above the head and
canlift a glass of water to above eye level 2= Can range the arms as above but not lift weight 3= Lifts arms over head, but must flex elbows or use accessory muscles 4= Unable to lift arms above eye level 5= Unable to lift arms above shoulder level | |
| Time to lift arms:… s | |
Figure 1.
Patient 8, a 50-year-old woman, trying to raise from a chair (A) using both hands on the table or one hand on her thigh (B).
Six-minute walk test (6MWT)
This test was performed as a measurement of functional endurance during prolonged ambulation. The 6MWT was performed as a standard walk in a long hospital corridor. We choose a 25-meter course divided in squares to measure length covered during a 6-minute timed walk. The 6MWT is an important test in monitoring GSDII patients, and a well-established measurement of functional endurance that has been used to monitor changes resulting from various interventions in studies of cardiac and pulmonary disease [Wokke et al. 2008; Florence et al. 2006]. For the baseline, two administrations of the test were undertaken followed by one administration during the course of therapy, similar to that adopted by Wokke et al. [2008]. The healthy adult control subjects cover a distance of 698+/- 96 meters [Gibbons et al. 2001].
Additional instrumental tests
Spirometric evaluations were performed in sitting position and forced vital capacity (FVC) was expressed in litres. Serial evaluations of FVC were used in follow-up.
Quality of life
This was considered an additional useful parameter to evaluate these disabled patients. In order to measure the quality of life we used the 36-item short form (SF-36) scale in four of eleven patients. The SF-36 was administered by self-report at baseline and after treatment.
Statistical analysis
All statistically analyses were conducted by a staff statistician. For normally distributed variables (FCV, 6MWT), the repeated measures variance analysis (ANOVA test) was performed to disclose first order interaction effects (i.e. patient ventilation or overnight ventilation versus patients in spontaneous breathing). For ordinal variables (i.e. GSGC score) the Wilcoxon test for paired data was used. Significant level was set at p<0.05.
Results
A cohort of 11 late-onset Italian GSDII patients have been diagnosed in three collaborating University Centers (Padova, Verona and Brescia). We collected the most relevant clinical features in 11 patients and found a wide clinical heterogeneity (Table 3). The age of onset, characterized mostly by proximal lower-limb muscle weakness, ranged from 15 to 42 years. Most clinical diagnosis was done between 15 and 54 years. At the beginning of ERT, the disease duration ranged from 5 to 24 years. Only one patient (n.7) required overnight respiratory support. Nutritional and exercise therapy, consisting of low carbohydrate, hyperproteic diet and regular aerobic submaximal exercise, was followed by nine patients (two patients were wheelchair-bound).
Table 3.
Clinical and molecular data of 11 adult GSDII patients.
| Pt, sex | Age at onset (years) | Symptoms at onset | Age at diagnosis (years) | Disease duration (years) | Enzyme residual activity in muscle | GAA gene mutation | Nutrition/excercise therapy | Months of ERT | Ambulation possible | Respiratory support |
| 1, F | 40 | Lower limbs proximal weakness, episodic dyspnea | 54 | 8 | 1% | IVS1/ | Yes | 18 | No | No |
| 2, F | 15 | Lower limbs proximal weakness | 15 | 22 | 18% | n.a. | Yes | 18 | Yes | No |
| 3, M | 25 | Difficulty in climbing stairs | 26 | 5 | 0 (leukocytes) | G1465A/C2014T | Yes | 18 | No | No |
| 4, M | 30 | Asthenia, cramps | 38 | 10 | 30% | n.a. | No | 3 | Yes | No |
| 5, M | 26 | Weakness | 28 | 13 | 10% | n.a. | No | 3 | Yes | No |
| 6, F | 42 | Lower limbs proximal weakness | 45 | 24 | 5% | IVS1/307T>G | Yes | 6 | With support | No |
| 7, F | 33 | Lower limbs proximal weakness | 49 | 28 | 7% | IVS1/2219delTG | Yes | 12 | Yes | At night only |
| 8, F | 39 | Lower limbs proximal weakness | 42 | 10 | 12% | IVS1/546+1G>T | Yes | 3 | Yes | No |
| 9, F | 30 | Difficulty in climbing stairs | 39 | 9 | 11% | n.a. | Yes | 18 | Yes | No |
| 10, F | – | Asymptomatic hyperCKemia | 22 | – | 12% | n.a. | Yes | 12 | Yes | No |
| 11, F | 2nd decade | Weakness | 30 | 15 | n.a. | IVS1/C1836G | Yes | 18 | Yes | No |
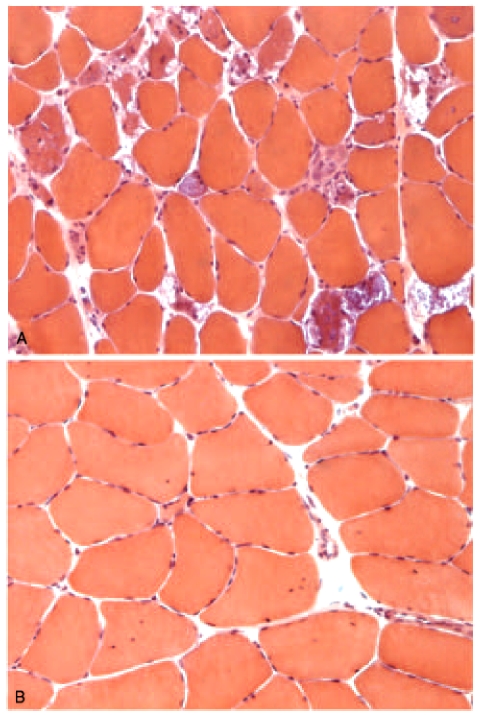
We investigated whether the clinical phenotype of our patients was correlated with the level of residual enzyme activity or to the type of the mutations in both mutant alleles. We found no firm genotype-phenotype correlations, except for the presence of the common leaky splice mutation IVS1 (-13T!G) that was found in five of six patients with complete molecular characterization. One patient (n.3) was a compound heterozygote for two different mutations. Therefore, molecular data do not seem to be useful in predicting the natural course of the disease. The extent of muscle fibre vacuolization varied in different patients from a widespread vacuolar myopathy to a mild nonspecific myopathy (Figure 2).
Figure 2.
Muscle biopsies from patients with GSDII might show evident fibre vacuolization (panel A, patient 7) or few atrophic fibres or central nuclei but nonevident vacuoles (panel B, patient 8). Hematoxylin and eosin stain. Microscope magnification x400.
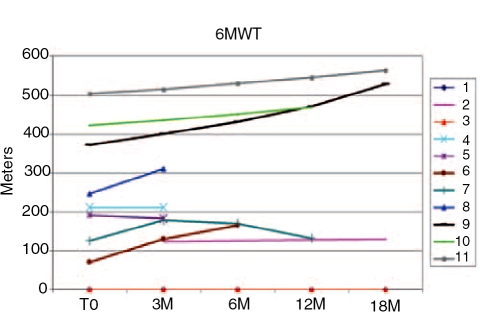
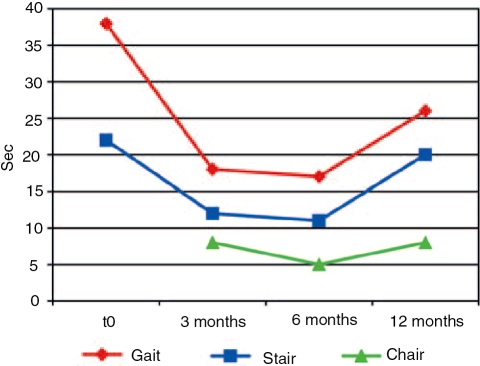
Our clinical protocol has been used to collect data from adult-onset GSDII patients. The overall tests were done in about 2 hours. In this protocol, we determined the clinical phenotype of GSDII by various parameters including age of disease onset and muscle group involvement. The measurement of muscle strength by manual muscle testing did not reveal any significant improvement during ERT. The 6MWT was performed every 3 months, to record meaningful functional data for ambulating late-onset patients (Figure 3). This was the most useful test in indicating changes during treatment since several patients improved in the 6MWT (Figure 3). Two patients had a walking time over 400 meters, two patients were unable to walk and one had to stop the test for secondary reaction to treatment. The only patient who, after an initial improvement, walked a shorter distance at 12 months had an accidental fall, followed by 1 month of forced immobility. The same patient is shown on the functional analysis in Figure 4. This patient improved for up to 6 months (decreasing performance time), and then increased after 6 months for her accidental fall. GSGC score was followed in six patients, who after a variable period of ERT showed a decreased functional performance time.
Figure 3.
6MWT of 11 patients treated with ERT for 3 to 18 months. T0 indicates initial treatment. Patient 8 stopped treatment for adverse reaction. Normal range of healthy controls = 400-700 m. Two patients were unable to walk.
Figure 4.
Time to complete functional performances in GSGC score of patient 7. T0 indicates initial treatment. The patient fell accidentally after 10 months of therapy and was subsequently unable to raise from bed for 1 month. At the last examination her motor function was still affected by the month of forced immobility.
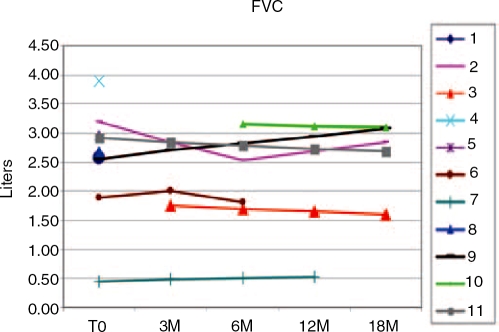
In late-onset GSDII patients, respiratory muscles are often severely involved. We therefore periodically monitored FVC by spirometry to careful monitor the diaphragmatic weakness (Figure 5). There was a wide range of variation in individual respiratory capacity. We observed only a partial response in FVC. Most patients remained stable, and one patient had a slight increase from 0 to 18 months. One patient in overnight ventilation was monitored through a diary but she did not improve.
Figure 5.
Forced vital capacity (FVC) in sitting position in 11 patients treated with ERT for 3 to 18 months. T0 indicates initial treatment.
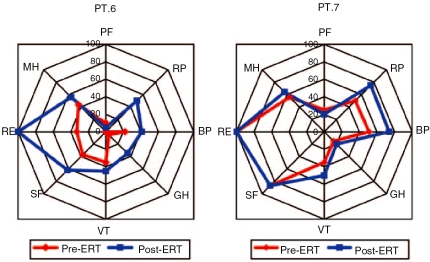
Three patients underwent an evaluation of quality of life by SF-36 (Figure 6). Patient n.6 had a mild improvement of mental health but not of bodily pain; conversely, patient n.7 had a mild improvement in bodily pain but not in mental health and role physical. One patient (n.8) stopped ERT after 3 months due to a moderate adverse reaction consisting of facial edema, oral paresthesia and tachycardia.
Figure 6.
SF-36 scale in two treated patients before and after ERT. PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Discussion
The results of treatment of late-onset GSDII are still poorly documented. Several therapeutic strategies are becoming available in the treatment of GSDII; for example, ERT, nutritional studies and use of chaperones. ERT with recombinant alpha-glucosidase (rGAA) became available in 2006, based on studies in infants both in the US and Europe [Hagemans et al. 2007; Klinge et al. 2005; Raben et al. 2004]. The results of an 18-month, randomized, placebo-controlled trial in a large cohort of juvenile and adult patients have been recently presented at the Annual meeting of the 60th American Academy of Neurology [van der Ploeg et al. 2008a] and at the 12th Annual meeting of the European Federation of Neurological Societies [van der Ploeg et al. 2008b]. A series of 90 patients older than 8 years of age, randomized between ERT and placebo were analyzed. In this study, ERT improved walking up to a certain period and prevented pulmonary decline compared with placebo. Only a few late-onset cases have been fully reported.
This trial was planned to determine the efficacy of ERT versus placebo infusion and used both the 6MWT and respiratory function as primary end-points. Our study was not sponsored by the enzyme-producing company (Genzyme) and our multicentric evaluation is worthy as an independent study to evaluate the response of adult-onset patients. The primary end-points of our protocol were the improvement in 6MWT, the functional tests and respiratory activity. The 6MWT has previously been administered to 40 late-onset GSDII patients participating in an observational study by the LOPOS (Late-Onset Pompe disease Observational Study) group. Florence et al. [2006] found a significant correlation of this validated test with QMT leg score, physical function scale and SF-36. This large multicenter trial had inclusion criteria that differ from those proposed in the present protocol, since it included only ambulating patients, while our study had two wheelchair-bound patients, and with one exception, the patients enrolled in our study were all variably symptomatic. Furthermore, our trial was done in the open, since the European Agency for Drug Studies approved in March 2006 the use of Myozyme from Genzyme for all GSDII cases. Only relatively few adult cases treated with ERT have so far been extensively reported in Europe [Winkel et al. 2003]. The fact that only few reports have appeared shows that it is difficult to compare the effect of ERT in late-onset cases, because of the heterogeneous course of late-onset cases. Our protocol takes advantage of the condition in which Myozyme ERT (Genzyme) is given to any late-onset GSDII patients and it is important since three neuromuscular centers evaluated his efficacy independently. Furthermore, this study is mainly directed to verify the protocol use in the follow-up of GDSII cases. This protocol in late-onset cases is novel and relevant for future application in Europe. ERT is extremely expensive and labour intensive, involving infusion every 2 weeks for the remainder of the patient's life.
The metabolic condition of each patient (nutritional and exercise therapy) might determine different responses in individual patients. We have also to consider possible individual idiosyncratic reactions to Myozyme - this indeed happened in one of our patients that had to discontinue the treatment.
A goal of our protocol was to carefully monitor the disease in patients with late-onset GSDII. The progression of the disease might be due not only to the increasing accumulation of glycogen, which is focal in some fibers, but also to the disuse muscle fiber atrophy and to organelle membrane remodelling that may contribute to the overall disability. The use of ERT may not be the only way to manage late-onset GSDII cases. Indeed, the efficacy of such therapy was not clearly observed in advanced cases with extensive vacuolization and interstitial tissue replacement, due to the progress of irreversible damage on muscle fibres and tissue structure. It seems that the most responsive patients were those able to do physical exercise and did not have extensive connective tissue replacement.
In the juvenile and adult forms of GSDII, Slonim et al. [2007] has advocated a high-protein diet as an effective treatment. Proteins are important to antagonize muscle protein waste, due to cathepsin activation and consequent hypercatabolism of skeletal muscle. Slonim et al. [2007] reported a beneficial effect of a high-protein diet, supplemented by L-alanine and exercise therapy (NET). According to their observations, NET stopped deterioration in muscle function and caused either improvement or stabilization in compliant patients. The main problem of this retrospective study is that two factors (exercise and diet) were simultaneously analysed, and that the criteria to identify compliant and non-compliant cases were uncertain; however this treatment might be particularly useful to manage muscle fiber atrophy and wasting and possibly combine with ERT.
Overall, maintaining good diet with attention to macro and micro nutrition is important. We therefore advised diet and exercise protocol in all GSDII patients that are in treatment, although such a strict regimen was not followed.
However, we observed that the best responders were the patients that were able to constantly follow both an exercise protocol and an accurate diet.
Conflict of interest statement
None declared.
Contributor Information
Corrado Angelini, Department of Neurosciences. University of Padova. Padova. Italy corrado.angeLini@unipd.it.
Claudio Semplicini, Department of Neurosciences. University of Padova. Padova. Italy.
Paola Tonin, Department of Neurological Sciences. University of Verona.
Massimiliano Filosto, Department of Neurology. University of Brescia. Brescia. Italy.
Elena Pegoraro, Department of Neurosciences. University of Padova. Padova. Italy.
Gianni Sorarù, Department of Neurosciences. University of Padova. Padova. Italy.
Marina Fanin, Department of Neurosciences. University of Padova. Padova. Italy.
References
- Angelini C., Engel A.G. (1972) Comparative study of acid maltase deficiency. Biochemical differences between infantile, childhood and adult types. Arch Neurol 26: 344–349 [DOI] [PubMed] [Google Scholar]
- Angelini C., Pegoraro E., Zambito-Marsala S., Vergani L., Nascimbeni A.C., Fulizio L.et al. (2004) Adult acid maltase deficiency: an open trial with albuterol and branched-chain aminoacids. Basic Appl Myol 14: 71–78 [Google Scholar]
- Angelini C. (2007) The role of corticosteroids in muscular dystrophy: A critical appraisal. Muscle Nerve 36: 424–435 [DOI] [PubMed] [Google Scholar]
- Angelini C., Nascimbeni A.C. (2007) Late-onset GSDII with novel GAA gene mutation. Clin Genet 71: 374–375 [DOI] [PubMed] [Google Scholar]
- D'Ancona G.G., Wurn J., Croce C.M. (1979) Genetics of type II glycogenosis: assignment of the human gene for acid alpha-glucosidase to chromosome 17. Proc Nat Acad Sci 76: 4526–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence J.M., Mayhew J.E., Skrinar A.M. LOPOS Clinical Evaluators (2006) Use of the six minute walk test as an endpoint in late-onset Pompe disease. Neuromusc Disord 16: S184 [Google Scholar]
- Gibbons W.J., Fruchter N., Sloan S., Levy R.D. (2001) Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil 21: 87–93 [DOI] [PubMed] [Google Scholar]
- Hagemans M.L.C., Winkel L.P.F., Van Doorn A.P., Hop W.J.C., Loonen M.C.B., Reuser A.J.J.et al. (2005a) Clinical manifestation and natural course of late-onset Pompe's disease in 54 Dutch patients. Brain 128: 671–677 [DOI] [PubMed] [Google Scholar]
- Hagemans M.L.C., Winkel L.P.F., Hop W.C.J., Reuser A.J.J., Van Doorn P.A., Van der Ploeg A.T. (2005b) Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology 64: 2139–2141 [DOI] [PubMed] [Google Scholar]
- Hagemans M.L., Laforêt P., Hop W.J., Merkies I.S., Van Doorn P.A., Reuser A.J.J.et al. (2007) Impact of late-onset Pompe disease on participation in daily life activities: Evaluation of the Rotterdam Handicap Scale. Neuromusc Disord 17: 537–543 [DOI] [PubMed] [Google Scholar]
- Klinge L., Straub V., Neudorf U., Voit T. (2005) Enzyme replacement therapy in classical infantile Pompe disease: results of a ten-month follow-up study. Neuropediatrics 36: 6–11 [DOI] [PubMed] [Google Scholar]
- Laforet P., Nicolino M., Eymard P.B., Puech J.P., Caillaud C., Poenaru L.et al. (2000) Juvenile and adult-onset acid maltase deficiency in France: genotype phenotype correlation. Neurology 55: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Margolis M.L., Hill A.R. (1986) Acid maltase deficiency in an adult: evidence for improvement in respiratory function with highprotein dietary therapy. Am Rev Resp Dis 134: 328–331 [DOI] [PubMed] [Google Scholar]
- Mehler M., DiMauro S. (1977) Residual acid maltase activity in late-onset acid maltase deficiency. Neurology 27: 178–184 [DOI] [PubMed] [Google Scholar]
- Moreland R.J., Jin X., Zhang X.K., Decker R.W., Albee K.L., Lee K.L.et al. (2005) Lysosomal acid alpha-glucosidase consists of four different peptides processed from a single chain precursor. J Biol Chem 280: 6780–6791 [DOI] [PubMed] [Google Scholar]
- Nascimbeni A.C., Fanin M., Tasca E., Angelini C. (2008) Molecular pathology and enzyme processing in various phenotypes of acid maltase deficiency. Neurology 70: 617–626 [DOI] [PubMed] [Google Scholar]
- Pellegrini N., Laforet P., Orlikowski D., Pellegrini M., Caillaud C., Eymard B.et al. (2005) Respiratory insufficiency and limb muscle weakness in adults with Pompe's disease. Eur Resp J 26: 1024–1031 [DOI] [PubMed] [Google Scholar]
- Raben N., Fukuda T., Gilbert A.L., de Jong D., Thurberg B.L., Mattaliano R.J.et al. (2004) Replacing acid alpha-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibres. Mol Ther 11: 48–53 [DOI] [PubMed] [Google Scholar]
- Reuser A.J.J., Kroos M., Willemsen R., Swallow D., Tager J.M., Galjaard H. (1987) Clinical diversity in glycogenosis type 11: biosynthesis and in situ localization of acid alpha-glucosidase mutant fibroblasts. J Clin Invest 79: 1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonim A.E., Bulone L., Goldberg T., Minikes J., Slonim E., Galanko J.et al. (2007) Modification of the natural history of adult-onset acid maltase deficiency by nutrition and exercise therapy. Muscle Nerve 35: 70–77 [DOI] [PubMed] [Google Scholar]
- Van der Ploeg A., Clemens P.R., Corzo D.et al. (2008a) Results from a randomized, double-blind, multicenter, multinational, placebo-controlled study of safety and efficacy of Myozyme, recombinant human acid alpha-glucosidase (rhGAA) for the treatment of Pompe disease in juveniles and adults. Neurology 71: 155(abstract) [Google Scholar]
- Van der Ploeg A., Clemens P.R., Corzo D.et al. (2008b) Safety and efficacy results from a randomized, double-blind, placebo-controlled study of alglucosidase alpha for the treatment of Pompe's disease in juvenile and adults. Eur J Neurol 15: 412–413(abstract) [Google Scholar]
- Voermans N.C, Lammens M., Weyers R.A., Hermus A.R., van Engelen B.G. (2005) Statin-disclosed acid maltase deficiency. J Intern Med 257: 313–314 [DOI] [PubMed] [Google Scholar]
- Winkel L.P., Van den Hout J.M., Kamphoven J.H., Disseldorp J.A., Remmerswaal M., Arts W.F.et al. (2003) Enzyme replacement therapy in late-onset Pompe's disease: a three-year follow-up. Ann Neurol 55: 495–502 [DOI] [PubMed] [Google Scholar]
- Wokke J.H.J., Escolar D.M., Pestronk A., Jaffe K.M., Carter G.T., van den Berg L.H.et al. (2008) Clinical features of late-onset Pompe disease: a prospective cohort study. Muscle Nerve 38: 1236–1245 [DOI] [PubMed] [Google Scholar]