Abstract
Available symptomatic therapies for the treatment of Alzheimer's disease (AD) have been based on known neurotransmitter dysfunctions associated with the illness. The second-generation cholinesterase inhibitors and the N-methyl D-aspartate receptor antagonist memantine have been widely prescribed and studied. Meta-analyses of these therapies were reviewed, focusing on effectiveness and tolerability. Although many of the meta-analyses demonstrate statistically significant improvements, some question if these benefits are sufficient to justify their current widespread and protracted use. This has spurred the development of new disease-modifying therapies that aim to have a greater impact on this debilitating illness.
Keywords: Alzheimer's disease, cholinesterase inhibitors, meta-analysis, donepezil, galantamine, rivastigmine, memantine, tacrine, beta amyloid, tau protein
Introduction
Alzheimer's disease (AD), the most common cause of dementia [Blennow et al. 2006], is characterized clinically by ongoing declines in cognitive and functional ability and the emergence of behavioural and psychological symptoms. With estimated health costs reaching billions of dollars per year and afflicting over 5 million people in North America alone [2008], effective symptomatic and disease-modifying therapies are urgently needed.
The causes of AD have not been fully elucidated and are a focus of current research. Early research, which demonstrated disruptions in the cholinergic [Davies and Maloney, 1976] and glutamatergic [Bleich et al. 2003] neurotransmitters, led to currently available symptomatic treatments. Emerging data on risk factors for the development of AD, including the APOE e4 allele [Raber et al. 2004], cardiovascular risk factors [Cechetto et al. 2008] and diabetes [Xu et al. 2009] have provided the rationale for other targeted treatments. Recently, more attention has been placed on the role of beta-amyloid and tau proteins since they are recognized as the critical neuropathological findings of the illness. The conversion of the amyloid precursor protein (APP) to the more toxic and highly aggregating
Aβ42 form [Jarrett et al. 1993] is thought to contribute to amyloid plaque formation and ultimately to neuronal death [Hardy and Selkoe, 2002]. A case has also been made for hyperphosphorylation of the tau protein and subsequent neurofibrillary tangles as a cause for neuronal cell death [Anderton et al. 2001]. Recent hypotheses recognize the complimentary role of both proteins in the pathogenesis of AD based on animal models demonstrating that amyloid aggregation promotes the hyperphosphorylation of tau protein [King et al. 2006; Oddo et al. 2003; Gotz et al. 2001]. Emerging therapies with proposed disease-modifying effects have targeted these findings.
Presently, the only approved therapies for AD are the cholinesterase inhibitors (ChEIs) and an N-methyl-D-aspartate (NMDA) receptor antagonist. While these agents are being used frequently, and for increasingly long periods of time [Herrmann et al. 2007], numerous controversies exist about the clinical significance of their therapeutic effect and thus, their resulting cost-effectiveness [Herrmann and Lanctôt, 2007].
Evidence-based medicine guidelines suggest that the strongest support for interventions is based on meta-analyses of high-quality randomized controlled trials (RCTs) [Godloe, 2007]. The purpose of this review was therefore to examine the safety and efficacy of currently available treatments for AD, focussing on meta-analyses of cholinesterase inhibitors and memantine. In addition to this, we will also explore new types of therapies and their potential to become new ‘gold standard’ treatments.
Methods
We performed a literature search using MEDLINE, Pubmed and the Cochrane Library, specifically searching for tacrine, donepezil, galantamine, rivastigmine or memantine and treatment of AD. Meta-analyses looking at other compounds for the treatment of AD were also examined. We have reviewed all meta-analyses that have been published up to October 2008. We used the national clinical trial registry clinicaltrials.gov as a guide to studies that have recently been completed or are currently recruiting patients.
Chollnesterase inhibitors: efficacy and tolerability
Measurement of outcomes
RCTs for AD have measured cognitive ability, functional ability, behavioural symptoms and overall global function. Cognitive function was a primary outcome variable and predominantly measured using the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog) [Rosen et al. 1984] with the Mini Mental Status Exam (MMSE) [Folstein et al. 1975] as a secondary cognitive outcome. The Clinician's Interview Based Impression of Change (CIBIC) [Schneider et al. 1997] was the scale most commonly used to measure global change, while the Alzheimer's Disease Cooperative Studies Activities of Daily Living (ADCS-ADL) [Galasko et al. 1997] measured functional ability and the Neuropsychiatric Inventory (NPI) [Cummings et al. 1994] measured behavioural and psychological symptoms associated with dementia. Table 1 summarizes the meta-analyses that were reviewed.
Table 1.
Meta-analyses of AD treatments.
Tacrine
The first of the ChEIs approved for AD treatment, tacrine has been replaced as a first-line treatment since the introduction of the second-generation ChEIs. A meta-analysis looking at five studies found that subjects on tacrine had a greater MMSE score compared with placebo after 12 weeks treatment and a significant improvement in global assessment [Qizilbash et al. 1998]. Unfortunately this analysis was criticized for ignoring the fact that nonindustrysponsored studies reported no benefits associated with tacrine treatment [Koepp and Miles, 1999] while another analysis that included five studies comparing tacrine to lecithin and/or placebo found no significant long-term efficacy. Tacrine has been reported to have a high incidence of side-effects [Koepp and Miles, 1999], where a significantly higher proportion of subjects taking the medication discontinued treatment compared to placebo [Qizilbash et al. 1998]. In addition, hepatoxicity is a concern [Lagadic-Gossmann et al. 1998]. While still available commercially in some countries like the US, tacrine is not available in the UK and is rarely prescribed today, its use supplanted by the better-tolerated ‘second-generation’ ChEIs [Ringman and Cummings, 2006].
Donepezil
Donepezil, a specific selective reversible inhibitor of acetylcholinesterase, has been widely studied. A pooled analysis of 15 studies of donepezil compared with placebo looked at treatment outcomes including cognitive function, activities of daily living and behavioural symptoms in mild-to-moderate AD subjects [Birks et al. 2006]. This Cochrane Database review found that donepezil demonstrated significant improvement in scores of the ADAS-Cog and MMSE at both 5 and 10mg/day doses and for treatment periods lasting 12, 24 and 52 weeks. There were also some benefits noted in global function and behavioural symptoms. Those authors concluded that both doses of donepezil demonstrated similar efficacy and that the average improvement in cognitive scores was below what would be considered as clinically beneficial (>4 point on ADAS-Cog [Rockville, 1989]). Other meta-analyses of ChEIs found similar improvements in cognitive outcomes with donepezil treatment [Hansen et al. 2008; Takeda et al. 2006; Thompson et al. 2004]. Hansen et al. [2008] pooled data from eight studies (four studies using 5mg/day, four studies using 10mg/day for cognitive function). Weighted mean scores favoured donepezil over placebo, although this change in ADAS-Cog score was less than the clinically beneficial threshold. While 5 and 10mg/day doses were combined in this analysis, heterogeneity was not significant, indicating that there were no significant between study differences in this outcome. With regard to functional outcomes, a modest improvement was observed in eight studies, however it must be noted that the weighted mean difference was calculated using seven different functional scales. Behavioural symptoms (four studies) and global assessment of change (six studies) scores improved, favouring treatment over placebo. Raina et al. [2008] included donepezil versus placebo studies in all severities of AD (n = 5) and other types of dementia as well. This extensive review found a significant mean decrease (−2.80, 95% CI −3.28 to −2.33, p<0.001) in ADAS-Cog scores, but restricted their analysis to the 10mg/day dose. MMSE scores were also evaluated in 14 studies, but they found no significant changes with treatment. With regard to global assessments, donepezil also displayed a significant improvement in CIBIC-plus scores (−0.45, 95% CI −0.54 to −0.36, p<0.001) and Clinical Dementia Rating - sum of boxes (CDR) (−0.44, 95% CI −0.65 to −0.23, p<0.001) [Raina et al. 2008]. Takeda et al. [2006] reviewed donepezil studies, primarily evaluating cognitive outcomes, and to a lesser extent quality of life. However, for quality of life, scales that were used had not been validated in a dementia population. Both ADAS-Cog (n = 6) and MMSE (n = 9) scores decreased significantly from baseline compared with placebo in the majority of studies that this group compiled, but a quantitative meta-analysis was not conducted [Takeda et al. 2006]. Tolerability was also evaluated in many of the meta-analyses and donepezil was found to be well-tolerated at both the 5 and 10mg/day [Pratt et al. 2002]. Significantly more subjects taking donepezil discontinued treatment due to an adverse event compared with placebo [Birks, 2006], though one meta-analysis found that this was only significant for the higher dose of donepezil [Ritchie et al. 2004].
Rivastigmine
Less frequently prescribed and studied compared to donepezil, rivastigmine is a potent inhibitor for both acetylcholinesterase and butylcholinesterase. Pooled analysis from two studies indicated an improvement in ADAS-Cog scores, for both lower (1-4mg/day) and higher (6-12mg/day) doses [Ritchie et al. 2004]. Pooled safety data indicated that subjects on the higher doses of rivastigmine were more likely to drop out of the study compared with placebo. A more extensive meta-analysis reviewed 11 RCTs of rivastigmine, dividing the studies into lower (1-4mg/day) and higher (6-12mg/day) doses and 12, 18 and 26 week durations [Birks et al. 2000]. Improvements in both ADAS-Cog and MMSE scores were noted with both doses, although the 6-12mg/day dose at 26 weeks had the greatest improvement in scores, compared with placebo. With regard to global assessment, an improvement was noted with higher doses at all time points, whereas the lower doses only exhibited an improvement at 26 weeks. An improvement in activities of daily living was only observed with the higher doses. Subjects were more likely to discontinue the medication due to an adverse event compared with placebo with the higher doses. One meta-analysis compared subjects who were experiencing rapid disease progression to subjects who had a slower cognitive decline. Based on the results of four studies, the rapidly progressing group appeared to have a greater cognitive improvements following treatment with rivastigmine [Farlow et al. 2005].
Galantamine
In addition to its ability to inhibit cholinesterase, galantamine can also stimulate nicotinic receptors that release acetylcholine. Trials using galantamine were divided into 3-month and 6-month durations in a meta-analysis by Loy and Schneider [2004]. Statistically significant improvements in cognitive outcomes were observed in doses ranging from 18 to 32mg/day in 3-month and 6-month trials. The proportion of patients who saw an improvement in ADAS-Cog scores greater than 4 points was significantly greater in one 3-month study and at doses of 16 and 32mg/day in studies lasting 6 months [Loy and Schneider 2004]. Other meta-analyses have confirmed the benefits of galantamine. Hansen et al. [2008] pooled data from seven studies that used doses ranging from 16 to 32mg/ day and determined that subjects had significant improvements in the cognitive domain. Galantamine also demonstrated efficacy in functional ability, global assessment of change and behaviour.
Cholinesterase inhibitors: which one is better?
A meta-analysis performed by our own group found that there was a significant improvement in global and cognitive responses compared with placebo when studies using all three ChEIs in mild-to-moderate AD were combined [Lanctôt et al. 2003] In that meta-analysis, 9% more subjects taking a ChEI had a global response compared with placebo, where response was defined as any improvement >minimal improvement on the CIBIS+ or the CGIC and the corresponding number needed to treat (NNT) was 12. Global improvement suggests that effects are clinically significant. For cognitive response, 10% more subjects ‘responded’ while on treatment compared with placebo, where cognitive response was defined as a >4 improvement in ADAS-Cog score, and the NNT for that outcome was 10. There was no significant heterogeneity suggesting ChEIs were similar. A Cochrane Database review of all three ChEIs looked at 13 studies comparing ChEIs with placebo in all three disease severities [Birks 2006]. The major finding was that treatment with donepezil, galantamine and rivastigmine demonstrated improvement in cognition, activities of daily living, behaviour and overall global function for the mild and moderate AD groups, although these improvements were characterized as modest at best. The authors found that there was no difference in efficacy between the three ChEIs, but donepezil was better tolerated than rivastigmine. One meta-analysis by Rockwood et al. [2004] took a unique approach to investigating the therapeutic potential of ChEIs by pooling data from six different ChEIs and grouping them based on dosing strength (low, mid and high). Effect sizes were greatest in the high doses of ChEIs (n = 9) for both cognitive (ADAS-Cog) and global (CIBIC+) outcomes [Rockwood 2004]. Despite pooling data from different ChEIs, that analysis demonstrates in a more general manner that this group of drugs, irrespective of dose strength, displayed at least 20% improvement in ADAS-Cog and CIBIC+ scores over placebo. Hansen et al. [2008] compared efficacy between donepezil, rivastigmine and galantamine by combining data from the small number of head-to-head comparative trials with data calculated by adjusted indirect comparison. That method can be used to calculate the relative benefits of different drugs when trials have a common comparator. The results, which must be interpreted with caution due to the lack of rigorous head-to-head data, suggest no significant differences in efficacy in the cognitive domains, although donepezil was more efficacious than galantamine for the treatment of behavioural symptoms. These behavioural data must be taken with some caution, as only four studies were examined and there was a moderate amount of heterogeneity between these studies [Hansen et al. 2008]. The lack of superiority of one ChEI over another in cognitive outcomes was supported by a meta-analysis comparing donepezil and galantamine. Effect sizes of ADAS-Cog score change after treatment was described as small and neither drug had an advantage over the other [Harry and Zakzanis, 2005]. In addition to this, the pooled data favoured both donepezil and rivastigmine over galantamine in global assessment of change. In terms of adverse events reported, donepezil was also found to have the least amount while rivastigmine had the greatest. Limitations, as pointed out by the authors include the pooling of AD severity, although there was a lack of viable studies looking at severe AD and dosing strengths.
In many of the RCTs and open-label trials examining ChEIs, behavioural symptoms are often secondary outcomes. A recent review by Cummings et al. [2008] demonstrated a large proportion of these studies have reported improvements in NPI scores with treatment, suggesting the benefit for use of ChEIs for the treatment of behavioural symptoms. The effects of ChEIs on neuropsychiatric symptoms was evaluated in a meta-analysis and the results showed that subjects taking any of the three ChEIs improved in NPI and ADAS-non cognitive scores [Trinh et al. 2003]. A major shortcoming of these data was that study subjects generally had little in the way of baseline neuropsychiatric symptoms.
A controversial review by Kaduszkiewicz et al. [2005] criticized many of the RCTs and the meta-analyses in this area, citing improper study designs that overstated the benefits observed. ‘Shortcomings’ included a large proportion of trials using observed cases (OC) instead of intent-to-treat analysis (ITT) and a failure to correct for multiple comparisons. Although the majority of the trials included in this review reported significant improvements over placebo, the author points out that these gains should be considered minimal at best, thus putting into question the recommendation of ChEIs for the treatment of AD. Many groups have since contested the criticisms put forth by that review. The use of ITT analysis using last observation carried forward (LOCF) for study dropouts instead of an OC analysis is not without criticism. Molnar et al. [2008] outlined drawbacks associated with ITT analysis in a dementia population, citing that it assumed that patients would remain ‘stabilized’ if they continued participation in the study, when it could be possible that they would decline further. The authors concluded that ITT analysis could artificially increase or decrease the therapeutic benefit of a drug based on its tolerability profile. Other drawbacks of the review by Kaduszkiewicz et al. have been noted, including the failure to pool the data from included RCTs [Herrmann, 2007] and an improper recommendation for a correction for multiple comparisons. In a response by Birks [2008], the author felt that a correction for multiple comparisons only applied to exploratory post hoc analysis, not for predetermined outcomes, which applied to most of the RCTs.
Efficacy of memantine in the treatment of AD
Another target in AD therapy has been the glutamatergic system. Research has indicated that activation of the NMDA receptor by excessive amounts of glutamate, as found in AD, can lead to neuronal cell death [Shah et al. 2008]. Memantine hydrocholoride, a noncompetitive NMDA receptor antagonist, prevents excitatory activity and has been shown to have a neuroprotective role by preserving and restoring long-term potentiation (LTP) in vivo [Frankiewicz and Parsons 1999; Zajaczkowski et al. 1997]. It has also been suggested that memantine can influence tau, by decreasing levels of phosphorylated tau protein in CSF [Li et al. 2004], but a correlation between decreased levels of phosphorylated tau and an improvement in cognitive scores was not observed [Degerman Gunnarsson et al. 2007]. To date, it remains one of the few medications approved for treatment in moderate-to-severe AD in North America and Europe. Pooled analysis by Emre et al. [2008] found there was a significant improvement in cognitive domains, specifically memory, language and praxis compared with placebo with 24 weeks of treatment in a total of six studies. In addition to this, a greater percentage of subjects taking memantine had less cognitive decline compared with placebo. Another meta-analysis of six studies examined clinical worsening, defined as a drop in either cognitive, global assessment or functional ratings. The proportion of patients experiencing either any ‘clinical worsening’ or ‘marked clinical worsening’ was significantly lower in patients taking memantine versus placebo [Wilkinson and Anderson, 2007]. While it is unclear what the magnitude of change was and which domains are actually improving, those results suggest that changes are clinically relevant. A second meta-analysis of six phase III clinical trials of moderate-to-severe AD subjects evaluated cognitive, global, functional and behavioural domains. Pooled analysis showed significant improvement in all four domains and the effect sizes were described as similar in magnitude to the ChEIs for cognitive outcomes [Winblad et al. 2007]. A Cochrane Database meta-analysis included studies looking at both mild to moderate AD and moderate to severe AD, as well as studies using subjects with either vascular dementia or mixed dementia. The most benefit was observed in the moderate-to-severe AD cohort, as it was determined that subjects taking memantine saw a significant improvement in the four major outcomes compared with placebo, while subjects with mild-to-moderate AD improved significantly only in the cognitive and global assessment outcomes [McShane et al. 2006]. The authors concluded that memantine demonstrated the most efficacy in the moderate-to-severe subject cohort, although there is some cognitive benefit in the mild-to-moderate AD group. This is consistent with the Raina et al. [2008] meta-analysis of six studies in all three severities of AD, which found memantine demonstrated efficacy only in global and cognitive domains, but the improvement in scores should not be considered clinically significant. A low NNT was found for cognitive, global and functional response in an NNT analysis of memantine; however, only two studies were examined [Livingston and Katona 2004]. For behavioural outcomes, a meta-analysis evaluated pooled data from two studies, one with monotherapy and the other in combination with donepezil, and found a significant improvement in NPI agitation/aggression scores from baseline, compared with placebo [Gauthier et al. 2005]. A more recent metaanalysis using six studies at 12 and 24-28 week treatment periods focused exclusively on the treatment of behavioural symptoms in a moderate-to-severe AD population. A significant improvement in NPI total was observed for both time points using OC and LOCF. When looking at individual items of the NPI, delusions and agitation/aggression improved significantly at 12 and 24-28 weeks, while irritability improved only at 24-28 weeks [Gauthier et al. 2008]. That analysis also examined symptom emergence in patients who had not reported behavioural symptoms at baseline. Significantly more subjects taking memantine remained asymptomatic for specific behaviours including agitation/aggression, delusions and irritability, compared to placebo [Gauthier et al. 2008]. These findings were confirmed in another meta-analysis by Wilcock et al. [2008], who examined agitation/aggression and psychosis in moderately severe and severe AD subjects. The three 6-month studies that were included in the analysis demonstrated that behaviourally disturbed patients taking memantine (a score >0 on NPI subscales agitation/ aggression, hallucination or delusions) improved significantly in this NPI cluster score at 12 and 24-28 weeks, compared with placebo [Wilcock et al. 2008].
Data regarding adverse events have indicated that memantine is well tolerated, as there have been no significant differences between reported adverse events (AEs) between the treatment and placebo groups [Wilcock et al. 2008; Winblad et al. 2007; Areosa et al. 2003]. In summary, studies to date demonstrate a modest improvement in cognitive impairment in moderate-to-severe AD in addition to benefits on global assessment, functional ability and behavioural symptoms with excellent tolerability. It is unclear whether some of these improvements are clinically relevant.
Statistical significance versus clinical significance
A general issue that has arisen in many of the meta-analyses reviewed is the importance of clearly understanding the differences between statistical significance and clinical significance. The ChEIs and memantine have demonstrated statistically significant improvements in outcomes including cognitive and behaviour when compared with placebo, but how do these improvements translate in a clinical setting? A recent review by Hogan [2007] cautioned that many of the reported results from RCTs may be statistically significant but researchers must go a step further to prove that these benefits are clinically relevant. In order to avoid this exaggeration of results, methods including the reporting of effect sizes, NNT, or setting a minimum change in score in order to be labelled a ‘responder’ should be considered [Hogan, 2007].
Other therapies
Besides the ChEIs and memantine, meta-analyses have been conducted looking at other forms of AD treatments. A Cochrane Database review looking at the benefits of huperzine A, a plant-derived ChEI found that there were improvements in MMSE with this compound alone or in conjunction with vitamin E, compared with placebo [Li et al. 2008]. Improvements in other outcomes were also observed, but further studies will need to be conducted in order to confirm this, as many of the trials were excluded from their final analysis due to questionable quality of data. Pooled data from RCTs investigating the effects of hydergine have demonstrated efficacy in global assessment, but many of the outcomes used were not standard scales like the CGI-C or the ADAS-Cog [Olin et al. 2001]. Meta-analyses have also been done on metrifonate [Lopez-Arrieta and Schneider, 2006], vitamin E [Isaac et al. 2008] and ginkgo biloba [Kurz and Van Baelen, 2004].
Future directions
What's old is new again
Because it may be many years before new interventions are approved, further clinical trials are being conducted with the available therapies in an attempt to clarify their spectrum of activity. Trials of donepezil hydrocholoride now underway are evaluating long-term efficacy across the spectrum of AD severities. Other trials include comparative designs with memantine or galantamine. Recently completed or currently recruiting studies with galantamine have looked at the efficacy over 2 years, and evaluation of an extended release formulation. With rivastigmine, efficacy studies are now focusing on the transdermal patch for delivery of the drug. A recent study comparing variable doses of the transdermal patch with the capsule found that the patch had the similar efficacy with significantly less adverse events [Mercier et al. 2007; Winblad et al. 2007]. Current studies of memantine are looking at comparative/adjunct therapy with vitamin E, off-label uses and switching from drugs with poorer tolerability profiles.
Therapies targeting amyloid plaque formation
The conversion pathway of the precursor protein APP to the more toxic, highly aggregating Aβ42 is a target being evaluated for future AD therapies. Past studies have linked plaque deposition to AD [Wilcock and Esiri, 1982; Blessed et al. 1968], although plaques have also been detected in healthy, nondemented controls [Henriksen et al. 2008; Knopman et al. 2003] and beta-amyloid as a predictor for AD has been challenged [Hansson et al. 2009]. High levels of Aβ42 in the plasma has been linked with an increased risk of developing AD and plasma and CSF Aβ42 concentrations have been shown to decrease with conversion from mild cognitive impairment to AD [Schupf et al. 2008].
APP is cleaved by either a or β then ? secretase, the latter two forming major products Aβ40 and Aβ42 [Blennow et al. 2006]. New therapies under development seek to disrupt this process through the inhibition of either isoform of secretase and, to date, both in vitro [Rajendran et al. 2008] and in vivo [Wong et al. 2004] studies have demonstrated promising results. Activation of a-secretase was shown to decrease levels of Aβ40 and Aβ42 in vivo [Etcheberrigaray et al. 2004]. Recently, testing on human subjects has begun, evaluating the tolerability and efficacy of a ?-secretase inhibitor LY450139. The medication was well-tolerated in a phase II safety trial with no significant changes in Aβ40 and Aβ42 CSF levels or significant improvements in cognitive or functional outcomes observed at 14 weeks of treatment [Fleisher et al. 2008]. Immunization against β-amyloid protein is another treatment that may prove beneficial. A number of studies using transgenic mouse models that overexpressed mutant human APP found that immunization against Aβ42 led to a decrease in amyloid plaque formation [Bacskai et al. 2002; Bard et al. 2000; Schenk et al. 1999] and protected neuronal synapses [Buttini et al. 2005]. Initial trials looking at immunization against β-amyloid in humans evaluated efficacy and tolerability using variable doses in 20 patients with AD [Bayer et al. 2005]. Due to the emergence of meningioencephalitis in 18/300 or 6% of patients, that study was ended prematurely [Gilman et al. 2005]. A number of newer studies using antibodies directed against fi-amyloid are in various phases and are currently recruiting patients.
Commonly prescribed for insulin resistance in the treatment of diabetes mellitus, the thiazolidinediones are peroxisomal proliferator-activated receptor-gamma (PPAR-?) agonists and have been evaluated in preliminary trials for the treatment of AD. Considered a risk factor for the eventual development of dementia [Ott et al. 1999], a strong association has been found between diabetes and AD pathology [Miklossy et al. 2008], as a recent study demonstrated that delivery of insulin intranasally led to both cognitive improvement in APOE4 e4- subjects and increased plasma levels of Aβ42 [Reger et al. 2008]. Studies have shown the efficacy of both pioglitazone [Nicolakakis et al. 2008; Heneka et al. 2005] and rosiglitazone [Pedersen et al. 2006] in mouse models of AD. One small scale RCT (n = 30) evaluating treatment with rosiglitazone for 6 months found a significant improvement in some aspects of memory, but standard cognitive tests like the ADAS-Cog were not utilized in this study [Watson et al. 2005] A larger scale, 24-week treatment RCT (n = 511) found no significant difference between rosiglitazone and placebo. When patients were stratified based on the APOE4 status, e4 negative subjects demonstrated a significant improvement in ADAS-Cog scores with the highest tested dose of drug, compared with e4 subjects, although a correction for multiple testing was not done and it is likely that the significance would subsequently be lost [Risner et al. 2006]. Nonetheless, further RCTs have since been conducted, although controversies surrounding the potential cardiac risk of rosiglitazone have recently arisen and may negatively impact its viability as a potential AD treatment [Selvin et al. 2008].
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, or ‘statins', typically used in lowering cholesterol, have also been tested as potential treatments for AD based on prospective studies that found an association between statin use and a decreased risk of developing AD [Haag et al. 2009]. Subsequent RCTs evaluating efficacy of various statins have found no decrease in CSF levels of Aβ42 [Carlsson et al. 2008; Riekse et al. 2006; Hoglund et al. 2005; Simons et al. 2002] and no significant improvement in ADAS-Cog scores at the 1-year-study endpoint [Sparks et al. 2008]. There are two clinical trials currently recruiting subjects looking at pitavastatin and simvastatin and a number of studies on simvastatin and atorvastatin that have recently been completed.
Finally, one phase II RCT (n = 58) looked at the efficacy of 3-amino-1-propanesulfonic acid (3APS), a compound that selectively binds to soluble forms of Aβ40 and Aβ42, in mild-to-moderate AD for a 3-month period [Aisen et al. 2006]. Although a dose dependent decrease was observed in Aβ42 levels following treatment, there were no significant improvements in either cognitive or global outcomes. Further tests in a larger sample size and over a longer treatment period are ongoing.
Therapies targeting tau protein
The phosphorylation of tau protein in frontal cortex regions of the brain has been observed in AD patients in various stages of the disease [Muntane et al. 2008] and is believed to lead to the neurofibrillary tangles that eventually lead to neuronal cell death [Alonso et al. 2001; Alonso et al. 1994]. One main target in the tau phosphorylation pathway is glycogen synthase kinase-3b (GSK-3b), a kinase that has been associated with an increased risk of AD [Schaffer et al. 2008]. This kinase has previously been shown to be upregulated in the frontal cortex of AD brains [Leroy et al. 2007]. Treatment with a GSK-3b inhibitor in a rat model was found to decrease levels of phosphorylated tau [Selenica et al. 2007]. In vivo studies have previously shown that lithium could decrease the expression of GSK-3b in specific brain regions [Mendes et al. 2008] and both lithium and sodium valproate had the ability to inhibit GSK-3b and decrease phosphorylated tau [De Sarno et al. 2002]. Lithium's ability to decrease Aβ levels can best be described as contradictory, where some studies have demonstrated an association [Su et al. 2004; Phiel et al. 2003], and other groups have found no significant association [Caccamo et al. 2007]. In a recent case-control study, bipolar geriatric subjects taking lithium had a decreased risk of developing AD over a 6-year period compared with an age-matched group not taking lithium [Nunes et al. 2007]. Further clinical trials in larger populations are warranted in order to confirm these results. Besides targeting GSK-3b in the tau phosphorylation pathway, immunization against tau protein has been investigated in an animal model; however, results demonstrated that tauopathies were induced in mice vaccinated against neuronal tau [Rosenmann et al. 2006].
Other potential therapies
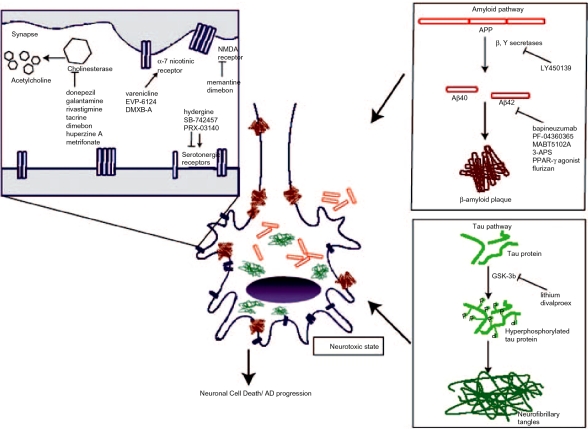
Other drugs that may or may not act through the amyloid or tau pathways (outlined in Figure 1) are being studied for their potential to be used in AD therapy and are outlined in Table 2. One such drug is the H1 antagonist dimebon [Doody et al. 2008]. Once used as an antihistamine, it is believed that this drug has multiple mechanisms of action that may improve symptoms associated with AD [Burns et al. 2008].
Figure 1.
Targets of Alzheimer's disease (AD) therapies. APP, amyloid precursor protein; 3-APS, 3-amino-1-propanesulfonic acid; GSK-3b, glycogen synthase kinase 3b; NMDA: N-methyl-D aspartate; PPAR-y agonist, peroxisomal proliferator-activated receptor-gamma.
Table 2.
New AD therapies under development.
| Intervention | Proposed mechanism of action | Sponsor | Phase of study |
| Therapies targeting β-amyloid | |||
| Antibody targeting amyloid plaque/vaccine | Monoclonal antibody targeting A-beta 42, disruption of amyloid plaque | ||
| bapineuzumab | Elan/Wyeth | III | |
| PF-04360365 | Pfizer | II | |
| MABT5102A | Genentech | I | |
| tarenflurbil | Selective Aβ42 lowering agent | Myriad Pharmaceuticals | III |
| y-secretase inhibitor LY450139 | Inhibition of y-secretase. Block conversion of APP to Aβ42 | Eli Lilly | III |
| PAZ-417 | Plasminogen activator | Wyeth | I |
| 3-APS (homotaurine) | Selective binding to soluble Aβ42 and Aβ40 | Bellus Health Inc. | III |
| HMG CoA reductase inhibitors | Reduction of Aβ42 levels | National institute on Aging (NIA) | II |
| PPAR-γ agonist | Reduction of A-beta plaque formation and Aβ42 brain levels in-vivo | GlaxoSmithKline | III |
| Therapies targeting the tau pathway | |||
| lithium and divalproex | GSK-3b | National Institute of Neurological Disorders and Stroke (NINDS) | II |
| Other therapies | |||
| a-7 nicotinic receptor partial agonist | ↑acetylcholine, neuroprotective | ||
| varenicline | Pfizer | I | |
| EVP-6124 | EnVivo Pharmaceuticals | I | |
| DMXB-A | CoMentis | II | |
| PRX-03140 | 5-HT4 agonist, ↑acetylcholine, ↑soluble APP | Epix | II |
| dimebon | Cholinesterase inhibition, NMDA receptor antagonist | Medivation | II |
| T-817MA | Neurotrophin | Toyama | II |
| ZT-1 (Huperzine A) | Cholinesterase inhibition | Debiopharm | II |
| SB-742457 | 5HT6 receptor antagonist | GlaxoSmithKline | II |
Conclusions
The meta-analyses of current AD treatments, including the ChEIs and memantine, have examined numerous trials that included thousands of AD patients. While the results of these analyses consistently show efficacy with statistical significance, conclusions about their clinical significance differs between clinicians. Some believe that ChEIs have demonstrated important improvements for most outcomes including cognition, global assessment, behaviour and function. Even these proponents agree that a limitation of many of these studies is that the treatment period (usually 6 months) is too short, and studies looking at the long-term effects of ChEIs are needed. Others believe that much of the reported data are flawed based on methodological limitations of the pivotal studies, and the improvements documented cannot be considered clinically significant. Adding to these controversies are the results and interpretation of cost-benefit analyses. For example, a costbenefit analysis led to the recommendation that ChEIs should not be used in the treatment of mild AD by the National Institute for Health and Clinical Excellence (NICE) in England, citing that the benefits gained did not justify the cost of the medication. As of May 2008, this decision was overturned by the appeal court [Dyer, 2008] and ChEI use in mild AD patients continues, for now.
A review of new drugs has shown an exciting array of possibilities with the potential for disease-modifying therapies. Unfortunately, it will likely be many years before any of these therapies are deemed efficacious and approved.
Until then, new strategies using currently available treatments will need to be devised in order to optimize treatment response in this growing population.
Conflict of interest statement
Dr Nathan Herrmann has received research support and/or speaker's honoraria from Lundbeck Canada Inc., Pfizer Canada Inc., Janssen Ortho, Neurochem, Novartis and Eli Lilly. Dr. Krista Lanct()t has received research support and/or speaker's honoraria from Abbott Laboratories, Lundbeck Canada Inc., Neurochem, Pfizer Canada Inc., Janssen Ortho, Eli Lilly and Wyeth. Ryan Rajaram has no financial disclosures to make.
Contributor Information
Krista L. Lanctôt, Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto, ON, Canada krista.lanctot@sunnybrook.ca
Ryan D. Rajaram, Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Nathan Herrmann, Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
References
- (2008) 2008 Alzheimer's disease facts and figures. Alzheimers Dement 4: 110–133 [DOI] [PubMed] [Google Scholar]
- Aisen P.S., Saumier D., Briand R., Laurin J., Gervais F., Tremblay P.et al. (2006) A phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology 67: 1757–1763 [DOI] [PubMed] [Google Scholar]
- Alonso A.C., Zaidi T., Grundke-Iqbal I., Iqbal K. (1994) Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA 91: 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A.D., Zaidi T., Novak M., Barra H.S., Grundke-Iqbal I., Iqbal K. (2001) Interaction of Tau isoforms with Alzheimer's disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem 276: 37967–37973 [DOI] [PubMed] [Google Scholar]
- Anderton B.H., Betts J., Blackstock W.P., Brion J.P., Chapman S., Connell J.et al. (2001) Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp 67: 73–80 [DOI] [PubMed] [Google Scholar]
- Areosa S.A., Sherriff F. (2003) Memantine for dementia. Cochrane Database Syst Rev CD03154 [DOI] [PubMed]
- Bacskai B.J., Kajdasz S.T., Mclellan M.E., Games D., Seubert P., Schenk D.et al. (2002) Non-FC-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci 22: 7873–7878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F., Cannon C., Barbour R., Burke R.L., Games D., Grajeda H.et al. (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916–919 [DOI] [PubMed] [Google Scholar]
- Bayer A.J., Bullock R., Jones R.W., Wilkinson D., Paterson K.R., Jenkins L.et al. (2005) Evaluation of the safety and immunogenicity of synthetic Abeta42 (An1792) in patients with AD. Neurology 64: 94–101 [DOI] [PubMed] [Google Scholar]
- Birks J. (2006) Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev CD005593 [DOI] [PMC free article] [PubMed]
- Birks J. (2008) The evidence for the efficacy of cholinesterase inhibitors in the treatment of Alzheimer's disease is convincing. Int Psychogeriatr 6: 1–7 [DOI] [PubMed] [Google Scholar]
- Birks J., Grimley Evans J., Iakovidou V., Tsolaki M. (2000) Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev CD001191 [DOI] [PubMed]
- Birks J., Harvey R.J. (2006) Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev CD001190 [DOI] [PubMed]
- Bleich S., Romer K., Wiltfang J., Kornhuber J. (2003) Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psychiatry 18: S33–40 [DOI] [PubMed] [Google Scholar]
- Blennow K., De Leon M.J., Zetterberg H. (2006) Alzheimer's disease. Lancet 368: 387–403 [DOI] [PubMed] [Google Scholar]
- Blessed G., Tomlinson B.E., Roth M. (1968) The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 114: 797–811 [DOI] [PubMed] [Google Scholar]
- Burns A., Jacoby R. (2008) Dimebon in Alzheimer's disease: old drug for new indication. Lancet 372: 179–180 [DOI] [PubMed] [Google Scholar]
- Buttini M., Masliah E., Barbour R., Grajeda H., Motter R., Johnson-Wood K.et al. (2005) Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. J Neurosci 25: 9096–9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A., Oddo S., Tran L.X., Laferla F.M. (2007) Lithium reduces tau phosphorylation but not a beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol 170: 1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson C.M., Gleason C.E., Hess T.M., Moreland K.A., Blazel H.M., Koscik R.L.et al. (2008) Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer'disease. J Alzheimers Dis 13: 187–197 [DOI] [PubMed] [Google Scholar]
- Cechetto D.F., Hachinski V., Whitehead S.N. (2008) Vascular risk factors and Alzheimer's disease. Expert Rev Neurother 8: 743–750 [DOI] [PubMed] [Google Scholar]
- Cummings J.L., Mackell J., Kaufer D. (2008) Behavioral effects of current Alzheimer's disease treatments: a descriptive review. Alzheimers Dement 4: 49–60 [DOI] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. (1994) The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314 [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A.J. (1976) Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet 2: 1403. [DOI] [PubMed] [Google Scholar]
- De Sarno P., Li X., Jope R.S. (2002) Regulation of AKT and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology 43: 1158–1164 [DOI] [PubMed] [Google Scholar]
- Degerman Gunnarsson M., Kilander L., Basun H., Lannfelt L. (2007) Reduction of phosphorylated tau during memantine treatment of Alzheimer's disease. Dement Geriatr Cogn Disord 24: 247–252 [DOI] [PubMed] [Google Scholar]
- Doody R.S., Gavrilova S.I., Sano M., Thomas R.G., Aisen P.S., Bachurin S.O.et al. (2008) Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet 372: 207–215 [DOI] [PubMed] [Google Scholar]
- Dyer C. (2008) Appeal court rules that nice procedure was unfair. BMJ 336: 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M., Mecocci P., Stender K. (2008) Pooled analyses on cognitive effects of memantine in patients with moderate to severe Alzheimer's disease. J Alzheimers Dis 14: 193–199 [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Tan M., Dewachter I., Kuiperi C., Van Der Auwera I., Wera S.et al. (2004) Therapeutic effects of PKC activators in Alzheimer's disease transgenic mice. Proc Natl Acad Sci USA 101: 11141–11146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M.R., Small G.W., Quarg P., Krause A. (2005) Efficacy of rivastigmine in Alzheimer' disease patients with rapid disease progression: results of a meta-analysis. Dement Geriatr Cogn Disord 20: 192–197 [DOI] [PubMed] [Google Scholar]
- Fleisher A.S., Raman R., Siemers E.R., Becerra L., Clark C.M., Dean R.A.et al. (2008) Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol 65: 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. (1975) 'Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198 [DOI] [PubMed] [Google Scholar]
- Frankiewicz T., Parsons C.G. (1999) Memantine restores long term potentiation impaired by tonic N-methyl-D-aspartate (NMDA) receptor activation following reduction of Mg2+ in hippocampal slices. Neuropharmacology 38: 1253–1259 [DOI] [PubMed] [Google Scholar]
- Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M.et al. (1997) An inventory to assess activities of daily living for clinical trials in Alzheimer's Disease. The Alzheimer's disease cooperative study. Alzheimer Dis Assoc Disord 11(Suppl. 2): S33–39 [PubMed] [Google Scholar]
- Gauthier S., Loft H., Cummings J. (2008) Improvement in behavioural symptoms in patients with moderate to severe Alzheimer's disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry 23: 537–545 [DOI] [PubMed] [Google Scholar]
- Gauthier S., Wirth Y., Mobius H.J. (2005) Effects of memantine on behavioural Symptoms in Alzheimer's disease patients: an analysis of the neuropsychiatric inventory (NPI) data of two randomised, controlled studies. Int J Geriatr Psychiatry 20: 459–464 [DOI] [PubMed] [Google Scholar]
- Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C.et al. (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64: 1553–1562 [DOI] [PubMed] [Google Scholar]
- Godloe F. (2007) Milestones on the long road to knowledge. British Medical Journal 334: S2–S3 [DOI] [PubMed] [Google Scholar]
- Gotz J., Chen F., Van Dorpe J., Nitsch R.M. (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Haag M.D., Hofman A., Koudstaal P.J., Stricker B.H., Breteler M.M. (2009) Statins are associated with reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam study. J Neurol Neurosurg Psychiatry 80: 13–7 [DOI] [PubMed] [Google Scholar]
- Hansen R.A., Gartlehner G., Webb A.P., Morgan L.C., Moore C.G., Jonas D.E. (2008) Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. Clin Interv Aging 3: 211–225 [PMC free article] [PubMed] [Google Scholar]
- Hansson O., Buchave P., Zetterberg H., Blennow K., Minthon L., Warkentin S. (2009) Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol Aging 30: 165–173 [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- Harry R.D., Zakzanis K.K. (2005) A comparison of donepezil and galantamine in the treatment of cognitive symptoms of Alzheimer's disease: a metaanalysis. Hum Psychopharmacol 20: 183–187 [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C.et al. (2005) Acute treatment with the ppargamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain 128: 1442–1453 [DOI] [PubMed] [Google Scholar]
- Henriksen G., Yousefi B.H., Drzezga A., Wester H.J. (2008) Development and evaluation of compounds for imaging of beta-amyloid plaque by means of positron emission tomography. Eur J Nucl Med Mol Imaging 35(Suppl. 1): S75–81 [DOI] [PubMed] [Google Scholar]
- Herrmann N. (2007) Trials and tribulations of evidence-based medicine: the case of Alzheimer disease therapeutics. Can J Psychiatry 52: 617–619 [DOI] [PubMed] [Google Scholar]
- Herrmann N., Gill S.S., Bell C.M., Anderson G.M., Bronskill S.E., Shulman K.I.et al. (2007) A population-based study of cholinesterase inhibitor use for dementia. J Am Geriatr Soc 55: 1517–1523 [DOI] [PubMed] [Google Scholar]
- Herrmann N., Lanctôt K.L. (2007) Pharmacologic management of neuropsychiatric symptoms of Alzheimer disease. Can J Psychiatry 52: 630–646 [DOI] [PubMed] [Google Scholar]
- Hogan D.B. (2007) Improving drug trials for mild to moderate Alzheimer's disease. Can J Neurol Sci 34(Suppl. 1): S97–102 [DOI] [PubMed] [Google Scholar]
- Hoglund K., Thelen K.M., Syversen S., Sjogren M., Von Bergmann K., Wallin A.et al. (2005) The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer's disease. Dement Geriatr Cogn Disord 19: 256–265 [DOI] [PubMed] [Google Scholar]
- Isaac M.G., Quinn R., Tabet N. (2008) Vitamin E for Alzheimer's disease and mild cognitive impairment. Cochrane Database Syst Rev CD002854 [DOI] [PubMed]
- Jarrett J.T., Berger E.P., Lansbury P.T., Jr (1993) The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry 32: 4693–4697 [DOI] [PubMed] [Google Scholar]
- Kaduszkiewicz H., Zimmermann T., Beck-Bornholdt H.P., Van Den Bussche H. (2005) Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ 331: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.E., Kan H.M., Baas P.W., Erisir A., Glabe C.G., Bloom G.S. (2006) Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol 175: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Parisi J.E., Salviati A., Floriach-Robert M., Boeve B.F., Ivnik R.J.et al. (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Koepp R., Miles S.H. (1999) Meta-analysis of tacrine for Alzheimer disease: the influence of industry sponsors. JAMA 281: 2287–2288 [DOI] [PubMed] [Google Scholar]
- Kurz A., Van Baelen B. (2004) Ginkgo biloba compared with cholinesterase inhibitors in the treatment of dementia: a review based on metaanalyses by the Cochrane Collaboration. Dement Geriatr Cogn Disord 18: 217–226 [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Rissel M., Le Bot M.A., Guillouzo A. (1998) Toxic effects of tacrine on primary hepatocytes and liver epithelial cells in culture. Cell Biol Toxicol 14: 361–373 [DOI] [PubMed] [Google Scholar]
- Lanctôt K.L., Herrmann N., Yau K.K., Khan L.R., Liu B.A., Loulou M.M.et al. (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. CMAJ 169: 557–564 [PMC free article] [PubMed] [Google Scholar]
- Leroy K., Yilmaz Z., Brion J.P. (2007) Increased level of active GSK-3beta in Alzheimer's Disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol 33: 43–55 [DOI] [PubMed] [Google Scholar]
- Li J., Wu H.M., Zhou R.L., Liu G.J., Dong B.R. (2008) Huperzine a for Alzheimer's disease. Cochrane Database Syst Rev CD005592 [DOI] [PubMed]
- Li L., Sengupta A., Haque N., Grundke-Iqbal I., Iqbal K. (2004) Memantine inhibits and reverses the alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett 566: 261–269 [DOI] [PubMed] [Google Scholar]
- Livingston G., Katona C. (2004) The place of memantine in the treatment of Alzheimer's disease: a number needed to treat analysis. Int J Geriatr Psychiatry 19: 919–925 [DOI] [PubMed] [Google Scholar]
- Lopez-Arrieta J.M., Schneider L. (2006) Metrifonate for Alzheimer's disease. Cochrane Database Syst Rev CD003155 [DOI] [PubMed]
- Loy C., Schneider L. (2004) Galantamine for Alzheimer's disease Cochrane Database Syst Rev CD001747 [DOI] [PubMed]
- McShane R., Areosa Sastre A., Minakaran N. (2006) Memantine for dementia. Cochrane Database Syst Rev CD003154 [DOI] [PubMed]
- Mendes C.T., Mury F.B., De Sa Moreira E., Alberto F.L., Forlenza O.V., Dias-Neto E.et al. (2008) Lithium reduces GSK3b mRNA levels: implications for Alzheimer disease. Eur Arch Psychiatry Clin Neurosci [DOI] [PubMed] [Google Scholar]
- Mercier F., Lefevre G., Huang H.L., Schmidli H., Amzal B., Appel-Dingemanse S. (2007) Rivastigmine exposure provided by a transdermal patch versus capsules. Curr Med Res Opin 23: 3199–3204 [DOI] [PubMed] [Google Scholar]
- Miklossy J., Qing H., Radenovic A., Kis A., Vileno B., Laszlo F.et al. (2008) Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol Aging [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar F.J., Hutton B., Fergusson D. (2008) Does analysis using 'last observation carried forward' introduce bias in dementia research? CMAJ 179: 751–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntane G., Dalfo E., Martinez A., Ferrer I. (2008) Phosphorylation of tau and alpha-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer's disease, and in Parkinson's disease and related alpha-synucleinopathies. Neuroscience 152: 913–923 [DOI] [PubMed] [Google Scholar]
- Nicolakakis N., Aboulkassim T., Ongali B., Lecrux C., Fernandes P., Rosa-Neto P.et al. (2008) Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28: 9287–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes P.V., Forlenza O.V., Gattaz W.F. (2007) Lithium and risk for Alzheimer's disease in elderly patients with bipolar disorder. Br J Psychiatry 190: 359–360 [DOI] [PubMed] [Google Scholar]
- Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R.et al. (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39: 409–421 [DOI] [PubMed] [Google Scholar]
- Olin J., Schneider L., Novit A., Luczak S. (2001) Hydergine for dementia. Cochrane Database Syst Rev CD000359 [DOI] [PMC free article] [PubMed]
- Ott A., Stolk R.P., Van Harskamp F., Pols H.A., Hofman A., Breteler M.M. (1999) Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 53: 1937–1942 [DOI] [PubMed] [Google Scholar]
- Pedersen W.A., Mcmillan P.J., Kulstad J.J., Leverenz J.B., Craft S., Haynatzki G.R. (2006) Rosiglitazone attenuates learning and memory deficits in TG2576 Alzheimer mice. Exp Neurol 199: 265–273 [DOI] [PubMed] [Google Scholar]
- Phiel C.J., Wilson C.A., Lee V.M., Klein P.S. (2003) GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature 423: 435–439 [DOI] [PubMed] [Google Scholar]
- Pratt R.D., Perdomo C.A., Surick I.W., leni J.R. (2002) Donepezil: tolerability and safety in Alzheimer's disease. Int J Clin Pract 56: 710–717 [PubMed] [Google Scholar]
- Qizilbash N., Whitehead A., Higgins J., Wilcock G., Schneider L., Farlow M. (1998) Cholinesterase inhibition for Alzheimer disease: a meta-analysis of the tacrine trials. Dementia Trialists' Collaboration. JAMA 280: 1777–1782 [DOI] [PubMed] [Google Scholar]
- Raber J., Huang Y., Ashford J.W. (2004) ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 25: 641–650 [DOI] [PubMed] [Google Scholar]
- Raina P., Santaguida P., Ismaila A., Patterson C., Cowan D., Levine M.et al. (2008) Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 148: 379–397 [DOI] [PubMed] [Google Scholar]
- Rajendran L., Schneider A., Schlechtingen G., Weidlich S., Ries J., Braxmeier T.et al. (2008) Efficient inhibition of the Alzheimer's disease beta-secretase by membrane targeting. Science 320: 520–523 [DOI] [PubMed] [Google Scholar]
- Reger M.A., Watson G.S., Green P.S., Baker L.D., Cholerton B., Fishel M.A.et al. (2008) Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis 13: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekse R.G., Li G., Petrie E.C., Leverenz J.B., Vavrek D., Vuletic S.et al. (2006) Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis 10: 399–406 [DOI] [PubMed] [Google Scholar]
- Ringman J.M., Cummings J.L. (2006) Current and emerging pharmacological treatment options for dementia. Behav Neurol 17: 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner M.E., Saunders A.M., Altman J.F., Ormandy G.C., Craft S., Foley I.M.et al. (2006) Efficacy of Rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J 6: 246–254 [DOI] [PubMed] [Google Scholar]
- Ritchie C.W., Ames D., Clayton T., Lai R. (2004) Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psychiatry 12: 358–369 [DOI] [PubMed] [Google Scholar]
- Rockville M. (1989) Peripheral and Central Nervous System Drugs Advisory Committee Meeting, 7 July 1989. Department of Health and Human Service, Food and Drug Administration, p. 227
- Rockwood K. (2004) Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer's disease. J Neurol Neurosurg Psychiatry 75: 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen W.G., Mohs R.C., Davis K.L. (1984) A new rating scale for Alzheimer's disease. Am J Psychiatry 141: 1356–1364 [DOI] [PubMed] [Google Scholar]
- Rosenmann H., Grigoriadis N., Karussis D., Boimel M., Touloumi O., Ovadia H.et al. (2006) Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch Neurol 63: 1459–1467 [DOI] [PubMed] [Google Scholar]
- Schaffer B.A., Bertram L., Miller B.L., Mullin K., Weintraub S., Johnson N.et al. (2008) Association of GSK3b with Alzheimer disease and frontotemporal dementia. Arch Neurol 65: 1368–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T.et al. (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173–177 [DOI] [PubMed] [Google Scholar]
- Schneider L.S., Olin J.T., Doody R.S., Clark C.M., Morris J.C., Reisberg B.et al. (1997) Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 11(Suppl. 2): S22–32 [DOI] [PubMed] [Google Scholar]
- Schupf N., Tang M.X., Fukuyama H., Manly J., Andrews H., Mehta P.et al. (2008) Peripheral abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci USA 105: 14052–14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenica M.L., Jensen H.S., Larsen A.K., Pedersen M.L., Helboe L., Leist M.et al. (2007) Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 152: 959–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E., Bolen S., Yeh H.C., Wiley C., Wilson L.M., Marinopoulos S.S.et al. (2008) Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med 168: 2070–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R.S., Lee H.G., Xiongwei Z., Perry G., Smith M.A., Castellani R.J. (2008) Current approaches in the treatment of Alzheimer's disease. Biomed Pharmacother 62: 199–207 [DOI] [PubMed] [Google Scholar]
- Simons M., Schwarzler F., Lutjohann D., Von Bergmann K., Beyreuther K., Dichgans J.et al. (2002) Treatment with simvastatin in normocholesterolemic patients with Alzheimer's disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol 52: 346–350 [DOI] [PubMed] [Google Scholar]
- Sparks D.L., Kryscio R.J., Sabbagh M.N., Connor D.J., Sparks L.M., Liebsack C. (2008) Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 5: 416–421 [DOI] [PubMed] [Google Scholar]
- Su Y., Ryder J., Li B., Wu X., Fox N., Solenberg P.et al. (2004) Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry 43: 6899–6908 [DOI] [PubMed] [Google Scholar]
- Takeda A., Loveman E., Clegg A., Kirby J., Picot J., Payne E.et al. (2006) A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer's disease. Int J Geriatr Psychiatry 21: 17–28 [DOI] [PubMed] [Google Scholar]
- Thompson S., Lanctôt K.L., Herrmann N. (2004) The benefits and risks associated with cholinesterase inhibitor therapy in Alzheimer's disease. Expert Opin Drug Saf 3: 425–440 [DOI] [PubMed] [Google Scholar]
- Trinh N.H., Hoblyn J., Mohanty S., Yaffe K. (2003) Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 289: 210–216 [DOI] [PubMed] [Google Scholar]
- Watson G.S., Cholerton B.A., Reger M.A., Baker L.D., Plymate S.R., Asthana S.et al. (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13: 950–958 [DOI] [PubMed] [Google Scholar]
- Wilcock G.K., Ballard C.G., Cooper J.A., Loft H. (2008) Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer's disease: a pooled analysis of 3 studies. J Clin Psychiatry 69: 341–348 [DOI] [PubMed] [Google Scholar]
- Wilcock G.K., Esiri M.M. (1982) Plaques, tangles and dementia. A quantitative study. J Neurol Sci 56: 343–356 [DOI] [PubMed] [Google Scholar]
- Wilkinson D., Andersen H.F. (2007) Analysis of the effect of memantine in reducing the worsening of clinical symptoms in patients with moderate to severe Alzheimer's disease. Dement Geriatr Cogn Disord 24: 138–145 [DOI] [PubMed] [Google Scholar]
- Winblad B., Grossberg G., Frolich L., Farlow M., Zechner S., Nagel J.et al. (2007) Ideal: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 69: S14–22 [DOI] [PubMed] [Google Scholar]
- Winblad B., Jones R.W., Wirth Y., Stoffler A., Mobius H.J. (2007) Memantine in moderate to severe Alzheimer's disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord 24: 20–27 [DOI] [PubMed] [Google Scholar]
- Wong G.T., Manfra D., Poulet F.M., Zhang Q., Josien H., Bara T.et al. (2004) Chronic treatment with the gamma-secretase inhibitor Ly-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem 279: 12876–12882 [DOI] [PubMed] [Google Scholar]
- Xu W., Qiu C., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L. (2009) Mid-and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes 58: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajaczkowski W., Frankiewicz T., Parsons C.G., Danysz W. (1997) Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology 36: 961–971 [DOI] [PubMed] [Google Scholar]