Abstract
A novel composite endpoint, sustained pain-free/no adverse events, was recently proposed as a more rigorous means of capturing in a single measure the attributes of migraine pharmacotherapy that patients consider most important: rapid and sustained pain-free response with no side-effects. Using pooled data from two replicate randomized, double-blind, parallel-group, placebo-controlled studies, this post hoc analysis compared the fixed-dose combination tablet sumatriptan/naproxen sodium (n = 726) with sumatriptan monotherapy (n = 723), naproxen sodium monotherapy (n = 720), and placebo (n = 742) with respect to sustained pain-free/no adverse events and closely related composite measures. Sustained pain-free/no adverse events was defined as having both a sustained pain-free response from 2 through 24 hours post-dose with no use of rescue medication and having no adverse events within up to 5 days after dosing with study medication. The percentage of patients with sustained pain-free/no adverse events was 16% with sumatriptan/naproxen sodium compared with 11%, 9% and 7% for sumatriptan, naproxen sodium and placebo, respectively (p<0.01 sumatriptan/naproxen sodium versus each other treatment). Sumatriptan/naproxen sodium was also significantly more effective than sumatriptan, naproxen sodium, and placebo for other composite endpoints including the percentages of patients with (1) sustained pain-free/no adverse events within 1 day; (2) sustained pain-free/no drug-related adverse events within up to 5 days; (3) sustained pain-free/no drug-related adverse events within 1 day; (4) sustained pain relief/no adverse events within up to 5 days; and (5) sustained pain relief/no adverse events within 1 day. The results demonstrate the superiority of sumatriptan/naproxen sodium to sumatriptan monotherapy, naproxen sodium monotherapy and placebo with respect to the rigorous and clinically relevant endpoint of sustained pain-free/no adverse events and reinforce the usefulness of utilizing this new composite endpoint.
Keywords: migraine, headache, sumatriptan/naproxen sodium, sustained pain-free, tolerability, safety, efficacy
Introduction
The past decade and a half has witnessed remarkable advances in migraine pharmacotherapy with the introduction in the early 1990s of sumatriptan, the first medication specifically developed to treat migraine, followed in subsequent years by the licensing of six additional triptans and, most recently, the introduction of the fixed-dose combination tablet sumatriptan/naproxen sodium.
All of these pharmacotherapies have been demonstrated effective versus placebo in randomized, double-blind clinical trials, and all are generally well tolerated [Pascual et al. 2007; Ferrari et al. 2001]. Although the performance of these medications with respect to standard clinical endpoints such as rates of pain relief or freedom from pain 2 hours post-dose is well documented [Pascual et al. 2007; Ferrari et al. 2001], choosing among them in managing migraine can be challenging.
It has been argued that clinical decision-making about migraine pharmacotherapy could be enhanced with better information about how therapies compare with respect to patients’ treatment goals and preferences [Dodick et al. 2007]. Results of surveys demonstrate that, for patients, the ideal migraine pharmacotherapy confers a pain-free response that is both rapid and sustained with an absence of side effects [Lanteri-Minet, 2005; Lipton et al. 2002; Silberstein, 1995]. A novel composite endpoint, sustained pain-free/no adverse events, has recently been proposed as a means of capturing more comprehensively these key attributes of migraine pharmacotherapy in a single measure [Dodick et al. 2007; Williams and Reeder, 2004, 2003]. Sustained pain-free/no adverse events has been defined as freedom from pain with no use of rescue medication or a second dose of study medication from 2 hours through 24 hours post-dose with no adverse events through 24 hours postdose. For a response to be counted as sustained pain-free/no adverse events, patients must experience rapid freedom from pain (as reflected in pain-free response 2 hours post-dose), sustained freedom from pain (as reflected in pain-free response sustained through 24 hours post-dose with no additional therapy), and sustained tolerability (as reflected in no adverse events through 24 hours post-dose). This measure constitutes a very rigorous criterion for evaluating a drug's therapeutic utility in that it requires that high standards of both efficacy and tolerability be met concurrently and in that it counts as treatment failures both partial responses (e.g. a reduction in headache pain from severe to mild intensity) and any post-dose adverse event regardless of whether the event is considered to be caused by study medication.
Several of the triptans have recently been compared with respect to sustained pain-free/no adverse events [Dodick et al. 2007], but the performance of sumatriptan/naproxen sodium - the newest addition to the migraine armamentarium - has not been evaluated to date on this endpoint. Sumatriptan/naproxen sodium, the first single-tablet product to target multiple putative mechanisms of migraine, contains sumatriptan 85 mg formulated with RT Technology and the nonsteroidal anti-inflammatory drug (NSAID) naproxen sodium 500 mg. Sumatriptan/naproxen sodium was generally well tolerated and was significantly more effective than monotherapy with sumatriptan 85 mg or naproxen sodium 500 mg and to placebo with respect to 2-hour pain relief, 2-hour pain-free response, and 24-hour sustained pain-free response in two replicate randomized, double-blind studies involving 2956 patients who treated a single migraine attack [Brandes et al. 2007]. Using pooled data from these two studies, the post hoc analysis described herein was conducted to compare sumatriptan/naproxen sodium with sumatriptan monotherapy, naproxen sodium monotherapy and placebo with respect to the endpoint of sustained pain-free/ no adverse events and closely related composite measures reflecting concurrent rapid and sustained efficacy and sustained tolerability.
Methods
This post hoc analysis was based on data from two replicate randomized, double-blind, parallel-group, placebo-controlled studies (GSK protocol numbers TXA112495 and TXA112496, also known as POZEN protocol numbers MT400:301 and MT400:302, respectively) of a single, fixed-dose tablet of sumatriptan 85 mg/naproxen sodium 500 mg (sumatriptan/ naproxen sodium) versus placebo or sumatriptan or naproxen sodium monotherapy in the acute treatment of migraine. The methods and results of the studies are fully described elsewhere [Brandes et al. 2007]. The protocols for the studies were approved by ethics committees or institutional review boards for all study sites. All patients provided written informed consent prior to study participation.
Sample
Males and nonpregnant, nonlactating females 18-65 years old with at least a 6-month history of migraine with or without aura as defined by the International Headache Society criteria [Headache Classification Committee, 2004, 1988] were eligible for the studies. Exclusion criteria included, but were not limited to, >6 migraine attacks monthly during either of the 2 months before screening, =15 days per month of nonmigraine headaches during each of the 3 months before screening, uncontrolled hypertension (diastolic blood pressure >95 mmHg or systolic blood pressure >160 mmHg), and confirmed or suspected cardiovascular or cerebrovascular disease.
Study treatment and measures
Patients meeting eligibility criteria were instructed to treat, on an outpatient basis, a single migraine attack with study medication when pain was moderate or severe. Study medication was a single tablet containing sumatriptan 85mg formulated with RT Technology/naproxen sodium 500mg, a single tablet containing sumatriptan 85mg formulated with RT Technology, a single tablet of naproxen sodium 500mg, or placebo. RT Technology is a fast-disintegrating, rapid-release formulation designed to facilitate tablet disintegration and drug dispersion and to mitigate the effects of gastric stasis that can accompany migraine [Carpay et al. 2004]. Rescue medication (with the exception of an ergot-containing medication, a serotonin agonist, an NSAID-containing product, or a second dose of study medication) was permitted beginning 2 hours post-dose as prescribed or recommended by the physician.
Patients used a four-point scale (0 = none; 1 = mild; 2 = moderate; 3 = severe) to record on diary cards headache pain severity immediately before dosing; 0.5, 1, 1.5 hours post-dose; and hourly from 2 to 24 hours post-dose. Tolerability was assessed by calculating the incidence of specific adverse events, defined as any untoward medical occurrences regardless of their suspected cause, that were reported by a patient or noted by a clinician from study initiation through a post-treatment follow-up visit that occurred 1 to 5 days after administration of study medication. For each adverse event, investigators recorded whether they considered it to be possibly related to study medication.
Data analysis
Data from the studies were pooled for analysis. The main endpoint of interest was the percentage of patients with sustained pain-free/no adverse events, a composite endpoint defined as having both a sustained pain-free response from 2 through 24 hours post-dose with no use of rescue medication and having no adverse events within up to 5 days after administration of study medication. Five days, rather than 1 day as in previous studies [Dodick et al. 2007; Williams and Reeder, 2004, 2003], was chosen as a cutoff for this endpoint because adverse-event data were collected for up to 5 post-treatment days in these studies and the 5-day cutoff was considered to be a more stringent test of tolerability. Data were also summarized for the two components of sustained pain-free/no adverse events: the percentage of patients with sustained pain-free response from 2 through 24 hours post-dose and the percentage of patients with no adverse events within 5 days of treatment with study medication.
In addition to the endpoint described above, five other composite endpoints reflecting sustained efficacy and tolerability were examined: the percentages of patients with (1) sustained pain-free/ no adverse events within 1 day; (2) sustained pain-free/no drug-related adverse events within up to 5 days; (3) sustained pain-free/no drug-related adverse events within 1 day; (4) sustained pain relief/no adverse events within up to 5 days; and (5) sustained pain relief/no adverse events within 1 day. Sustained pain relief was defined as reduction of moderate or severe predose pain to mild or no pain from 2 through 24 hours post-dose.
All endpoints were calculated at the level of the individual subject by using each patient's raw data to derive the endpoint and then averaging the data across patients. This approach, which is more rigorous than the population-level analysis used in some previous research [Dodick et al. 2007], accounts for the possibility that an individual patient's headache response to and tolerance of study medication are correlated. For all composite endpoints, Cochran-Maentel-Haenszel methods stratified by study were used to compare sumatriptan/naproxen sodium with each mono-therapy and placebo in the efficacy population, which was defined as all randomized patients who took at least one dose of study medication and provided at least one post-baseline efficacy assessment.
The percentage of patients with sustained pain-free response from 2 through 24 hours postdose, a component of the composite endpoint of sustained pain-free/no adverse events, was compared between sumatriptan/naproxen sodium and each monotherapy and placebo in the efficacy population with Cochran-Maentel-Haenszel methods stratified by study. The component endpoint of the percentage of patients with adverse events within up to 5 days dosing with study medication was summarized by treatment with descriptive statistics for the safety population, but hypothesis testing was not undertaken for this endpoint. The safety population was defined as all randomized patients who took at least one dose of study medication. Demographics were summarized with descriptive statistics for the efficacy population.
Results
Sample
Treatment groups were balanced with respect to numbers of patients in the pooled efficacy population (Table 1). Demographics were similar among treatment groups (Table 1). Patients were predominantly white females with a median age of approximately 40 years.
Table 1.
Demographics (efficacy population).
| Sumatriptan/naproxen sodium (n = 726) | Sumatriptan (n = 723) | Naproxen sodium (n = 720) | Placebo (n = 742) | |
| Female, n (%) | 633 (87) | 625 (86) | 630 (88) | 644 (87) |
| White, n (%) | 650 (90) | 635 (88) | 647 (90) | 656 (88) |
| Median age, years (range) | 40 (18-65) | 40 (18-65) | 40 (18-65) | 41 (18-65) |
The time between dosing with study medication and the final follow-up visit for collecting adverse-event data was within 1 day for 8.0% of patients, within 2 days for 29.0% of patients, within 3 days for 49.0% of patients, and within 4 days for 68.5% of patients. The time between dosing with study medication and the final follow-up visit for collecting adverse-event data was >4 days for 31.5% of patients. Some patients may have not had all adverse events collected within 5 days of treatment because their follow-up visit occurred earlier than 5 days post-treatment. It is expected that any missing adverse events were balanced among the treatment groups by virtue of randomization and thus should not have affected the interpretation of the results.
Sustained pain-free/no adverse events and its component endpoints
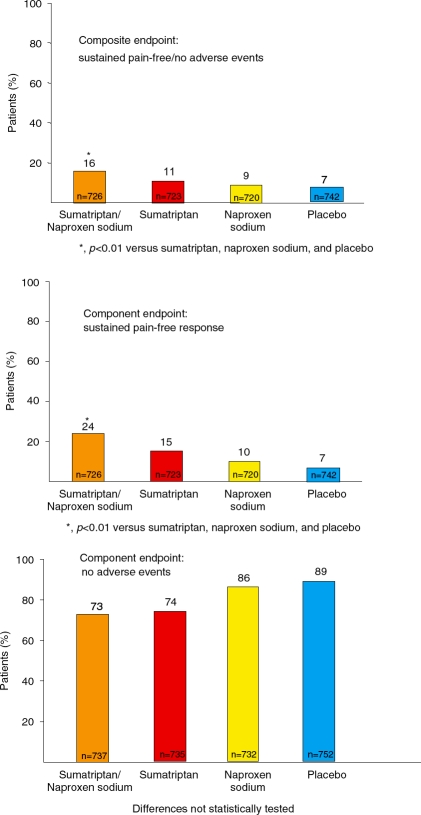
Sumatriptan/naproxen sodium was significantly more effective than sumatriptan monotherapy, naproxen sodium monotherapy and placebo for the incidence of sustained pain-free/no adverse events within up to 5 days post-dose (p<0.01 for each comparison) (Figure 1). The percentage of patients with sustained pain-free/no adverse events was 16% with sumatriptan/naproxen sodium compared with 11%, 9%, and 7% for sumatriptan monotherapy, naproxen sodium monotherapy and placebo, respectively. On the component endpoints, sumatriptan/naproxen sodium performed better than both of the monotherapies and placebo for the incidence of sustained pain-free response (p<0.01 for each comparison) and was associated with an incidence of freedom from adverse events similar to that of sumatriptan but slightly lower than those of naproxen sodium monotherapy and placebo (not statistically tested) (Figure 1).
Figure 1.
Results for the composite endpoint sustained pain-free/no adverse events and its component endpoints.
Other composite endpoints
Results for the other composite endpoints mirrored those for sustained pain-free/no adverse events. Sumatriptan/naproxen sodium was significantly more effective than sumatriptan monotherapy, naproxen sodium monotherapy and placebo for the percentages of patients with (1) sustained pain-free/no adverse events within 1 day; (2) sustained pain-free/no drug-related adverse events within up to 5 days; (3) sustained pain-free/no drug-related adverse events within 1 day; (4) sustained pain relief/no adverse events within up to 5 days; and (5) sustained pain relief/no adverse events within 1 day (Table 2).
Table 2.
Results for secondary composite endpoints. Data are expressed as percentages of patients.
| Sumatriptan/naproxen sodium (n = 726) | Sumatriptan (n=723) | Naproxen sodium (n = 720) | Placebo (n=742) | |
| Sustained pain-free/no adverse events within 1 day | 17∗ | 11 | 10 | 7 |
| Sustained pain-free/no drug-related adverse events within up to 5 days | 21∗ | 14 | 10 | 7 |
| Sustained pain-free/no drug-related adverse events within 1 day | 21∗ | 14 | 10 | 7 |
| Sustained pain relief/no adverse events within up to 5 days | 31† | 24 | 25 | 15 |
| Sustained pain relief/no adverse events within 1 day | 32∗ | 24 | 25 | 16 |
p<0.001 versus sumatriptan, naproxen sodium, and placebo.
p<0.001 versus sumatriptan and placebo and p<0.005 versus naproxen sodium.
Discussion
In this analysis of pooled data from two replicate randomized, double-blind, parallel-group studies, the fixed-dose combination tablet sumatriptan/naproxen sodium performed significantly better than sumatriptan monotherapy, naproxen sodium monotherapy and placebo on the composite endpoint of sustained pain-free/no adverse events within up to 5 days of dosing. These results extend the previously reported findings in these studies, in which sumatriptan/naproxen sodium was well tolerated and was significantly more effective than sumatriptan monotherapy, naproxen sodium monotherapy and placebo with respect to the standard clinical endpoints of 2-hour pain relief, 2-hour pain-free response, and 2- to 24-hour sustained pain-free response [Brandes et al. 2007]. Compared with standard clinical endpoints that reflect single efficacy parameters or single tolerability parameters, the composite endpoint assessed in the current study is a more rigorous test of therapeutic benefit. Moreover, the composite endpoint of sustained pain-free/no adverse events is arguably more clinically relevant than standard clinical endpoints because it more comprehensively incorporates all of the main treatment attributes that determine patient satisfaction (i.e. rapid freedom from pain, sustained freedom from pain and absence of side-effects) as well as both of the main factors that healthcare providers consider in weighing risks and benefits of therapy: treatment success and absence of treatment-related harm [Dodick et al. 2007]. The results reported herein extend previous work with the composite endpoint [Dodick et al. 2007]. The previous work employed data from a meta-analysis to compare several triptans, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan and zolmitriptan, with respect to the composite endpoint [Dodick et al. 2007]. The current study employed data from randomized, doubleblind studies and assessed only three active treatments (sumatriptan monotherapy, naproxen sodium monotherapy and sumatriptan/naproxen sodium).
Results with the components of the composite endpoint of sustained pain-free/no adverse events suggest that both greater efficacy and the lack of a substantial adverse-event penalty contributed to the better outcome with sumatriptan/ naproxen sodium on the composite endpoint. The percentage of patients with sustained pain-free response was significantly (p<0.01) greater with sumatriptan/naproxen sodium (24%) than the other treatments (15% sumatriptan, 10% naproxen sodium, 7% placebo). The percentage of patients with no adverse events within up to 5 days of dosing was similar between sumatriptan/naproxen sodium (73%) and sumatriptan monotherapy (74%) and only slightly lower with sumatriptan/naproxen sodium compared with naproxen sodium monotherapy (86%) and placebo (89%) (differences not tested statistically). The greater efficacy of the combination tablet relative to its components has been explained in part by its multimechanism-targeted action [Brandes et al. 2007].
Besides assessing sustained pain-free/no adverse events within up to 5 days of treatment, this study explored the utility of several other composite measures reflecting sustained efficacy and tolerability. Results of these analyses suggest that defining tolerability in terms of drug-related adverse events rather than adverse events regardless of causality might improve the ability to gauge a benefit of active treatment. The difference between active treatment and placebo was larger for both sumatriptan/naproxen sodium and sumatriptan monotherapy for the two composite measures incorporating drug-related adverse events (i.e. sustained pain-free/no drug-related adverse events within up to 5 days and sustained pain-free/no drug-related adverse events within 1 day) than for the corresponding composite measures that incorporated adverse events, regardless of causality. Furthermore, the results suggest that a 5-day post-dose window for monitoring adverse events does not improve the ability to differentiate among treatments relative to a 1-day post-dose window. The percentage of patients with sustained pain-free/no adverse events within up to 5 days did not differ by more than 1% from the percentage with sustained pain-free/no adverse events within 1 day in any given treatment group for either adverse events of any causality or drug-related adverse events. Finally, the results with other composite endpoints demonstrate that, not unexpectedly, pain relief (moderate or severe pain reduced to mild or no pain) constitutes a less stringent criterion for treatment success than pain-free response. Success rates were substantially higher with all active treatments for the composite measures incorporating pain relief instead of pain-free response as the efficacy criterion.
Data from this analysis should be interpreted cautiously given its retrospective nature, which could introduce various biases stemming largely from the authors’ awareness of the results of the prospective double-blind studies. Data from this analysis should also be interpreted with the knowledge that patients were instructed to treat moderate or severe pain with study medication.
The rates of sustained pain-free/no adverse events with active treatment might have been greater had study medication been administered according to the now-prevalent practice of early intervention; that is, administration of medication early during the course of a migraine attack while pain is mild rather than delaying treatment until pain is moderate or severe. Pain-free efficacy of migraine pharmacotherapy appears to be enhanced, and the incidence of adverse events appears to be reduced by the practice of early intervention [Goadsby et al. 2008; Silberstein et al. 2008; Mathew et al. 2007; Carpay et al. 2004; Brandes et al. 2005; Loder et al. 2005; Mathew et al. 2004; Scholpp et al. 2004; Cady et al. 2000]. These caveats notwithstanding, the results demonstrate the better performance of sumatriptan/naproxen sodium to sumatriptan monotherapy, naproxen sodium monotherapy and placebo with respect to the rigorous clinical endpoint of sustained pain-free/no adverse events and reinforce the usefulness of utilizing this new composite endpoint.
Acknowledgments
The authors acknowledge Jane Saiers, PhD, for assistance with writing this manuscript. GlaxoSmithKline funded Dr Saiers’ work.
Conflict of interest statement
Three of the authors (White, Lener, McDonald) are employees of GlaxoSmithkline. Author Landy has served as an investigator for GlaxoSmithkline-funded studies.
Contributor Information
Stephen Landy, Wesley Headache Clinic, Memphis, TN, USA wesleyhead@aol.com.
Jonathan White, GlaxoSmithKline, Research Triangle Park, NC, USA.
Shelly E. Lener, GlaxoSmithKline, Research Triangle Park, NC, USA
Susan A. McDonald, GlaxoSmithKline, Research Triangle Park, NC, USA
References
- Brandes J.L., Kudrow D., Cady R., Tiseo P.J., Sun W., Sikes C.R. (2005) Eletriptan in the early treatment of acute migraine: influence of pain intensity and time of dosing. Cephalalgia 25: 735–742 [DOI] [PubMed] [Google Scholar]
- Brandes J.L., Kudrow D., Stark S.R., O'Carroll C.P., Adelman J.U., O'Donnell F.J.et al. (2007) Sumatriptan-naproxen for acute treatment of migraine. JAMA 297: 1443–1454 [DOI] [PubMed] [Google Scholar]
- Cady R.K., Sheftell F., Lipton R.B., O'Quinn S., Jones M., Diamond M.L.et al. (2000) Effects of early intervention with sumatriptan on migraine pain: Retrospective analyses of data from three clinical trials. Clin Ther 22: 1035–1048 [DOI] [PubMed] [Google Scholar]
- Carpay J., Schoenen J., Ahmad F., Kinrade F., Boswell D. (2004) Efficacy and tolerability of sumatriptan tablets in a fast-disintegrating, rapidrelease formulation for the acute treatment of migraine: results of a multicenter, randomized, placebo-controlled study. Clin Ther 26: 214–223 [DOI] [PubMed] [Google Scholar]
- Dodick D.W., Sandrini G., Williams P. (2007) Use of the sustained pain-free plus no adverse events endpoint in clinical trials of triptans in acute migraine. CNS Drugs 21: 73–82 [DOI] [PubMed] [Google Scholar]
- Ferrari M.D., Roon K.I., Lipton R.B., Goadsby P.J. (2001) Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 358: 1668–1675 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Zanchin G., Geraud G., de Klippel N., Diaz-Insa S., Gobel H.et al. (2008) Early vs non-early intervention in acute migraine - 'Act when Mild (AWM).' A double-blind, placebo-controlled trial of almotriptan. Cephalalgia 28: 383–391 [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (1988) Classification of headache disorders, cranial neuralgias and facial pain. Cephalalgia 8(Suppl. 7): 1–96 [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (2004) The International Classification of Headache Disorders. 2nd edn.Cephalalgia; (Suppl. 1): 9–160 [Google Scholar]
- Lanteri-Minet M. (2005) What do patients want from their acute migraine therapy? Eur Neurol 53(Suppl. 1): 3–9 [DOI] [PubMed] [Google Scholar]
- Lipton R.B., Hamelsky S.W., Dayno J.M. (2002) What do patients with migraine want from acute migraine treatment? Headache 42(Suppl. 1): 3–9 [DOI] [PubMed] [Google Scholar]
- Mathew N.T., Finlayson G., Smith T.R., Cady R.K., Adelman J., Mao L. (2007) Early intervention with almotriptan: results of the AEGIS trial (Axert Early Migraine Intervention Study). Headache 47: 189–198 [DOI] [PubMed] [Google Scholar]
- Mathew N.T., Kailasam J., Meadors L. (2004) Early treatment of migraine with rizatriptan: a placebo-controlled study. Headache 44: 669–673 [DOI] [PubMed] [Google Scholar]
- Loder E., Freitag F.G., Adelman J., Pearlmand S., Abu-Shakra S. (2005) Zolmitriptan study 014 group. Pain-free rates with zolmitriptan 2.5mg ODT in the acute treatment of migraine: results of a large double-blind placebo-controlled trial. Curr Med Res Opin 21: 381–389 [DOI] [PubMed] [Google Scholar]
- Pascual J., Mateos V., Roig C., Sanchez-Del-Rio M., Jiminez D. (2007) Marketed oral triptans in the acute treatment of migraine: a systematic review on efficacy and tolerability. Headache 47: 1152–1168 [DOI] [PubMed] [Google Scholar]
- Silberstein S.D. (1995) Migraine symptoms: results of a survey of self-reported migraineurs. Headache 35: 387–396 [DOI] [PubMed] [Google Scholar]
- Silberstein S.D., Mannix L.K., Goldstein J., Couch J.R., Byrd S.C., Ames M.H.et al. (2008) Multimechanistic (sumatriptan-naproxen) early intervention for the acute treatment of migraine. Neurology 71: 114–121 [DOI] [PubMed] [Google Scholar]
- Scholpp J., Schellenberg R., Moeckesch B., Banik N. (2004) Early treatment of a migraine attack while pain is still mild increases the efficacy of sumatriptan. Cephalalgia 24: 925–933 [DOI] [PubMed] [Google Scholar]
- Williams P., Reeder C.E. (2003) Cost-effectiveness of almotriptan and rizatriptan in the treatment of acute migraine. Clin Ther 25: 2903–2919 [DOI] [PubMed] [Google Scholar]
- Williams P., Reeder C.E. (2004) A comparison of the cost-effectiveness of almotriptan and sumatriptan in the treatment of acute migraine using a composite efficacy/ tolerability endpoint. J Manag Care Pharm 10: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]