Abstract
The great majority of randomised controlled trials (RCTs) that compare antiepileptic drugs are industry sponsored and have the objective of obtaining a monotherapy license for a drug. Such trials do not inform everyday clinical practice as they tend to be too short and to depart from clinical practice by restricting clinicians in their choice of actions. The data that exists provides evidence that drugs with actions on voltage-gated sodium channels provide best seizure control for localised onset seizures and epilepsy syndromes, while valproate provides best seizure control for generalised epilepsy and unclassified syndromes. Drugs do, however, vary in their tolerability over the short term and in their risk for rare serious idiosyncratic adverse events, chronic toxicity and teratogenicity; issues that cannot be examined within the scope of RCTs.
Keywords: epilepsy, antiepileptic drugs, partial epilepsies, idiopathic generalised epilepsy, risk/benefit
Introduction
Well-educated medical students would, in this evidence-based medicine age, expect to be able to find abundant evidence from randomised controlled trials (RCTs) that would allow them to compare the benefits and harms of different antiepileptic drugs (AEDs) quickly, with a simple search strategy. They would expect to find trials that compare AEDs in head-to-head comparisons as monotherapy (relevant to 60-70% of patients who develop epilepsy) and other trials that compare drugs when they are added to existing one or two drug treatment regimens. They will be disappointed in both respects.
They might then turn to guidelines, where they would find that the NICE guidelines for the diagnosis and management of epilepsy were published in October 2004 (http://www.nice.org.uk/ nicemedia/pdf/CG020fullguideline.pdf). These are undoubtedly the most methodologically sound epilepsy guidelines available, but are remarkably nonspecific about comparisons of AEDs, only recommending newer AEDs where older drugs (carbamazepine or valproate) have failed. So we are faced with going back to first principles and asking what the characteristics of an ideal drug might be, how closely available drugs approach this and what new evidence is available since the NICE guideline.
Epilepsy is a varied disorder with many causes ranging from genetic causes through to acquired brain damage and insults. Disease outcomes are also heterogeneous. Most people have a relatively short-lasting susceptibility to seizures and enter remission shortly after starting treatment on small doses of AEDs [Marson et al. 2007a, 2007b; Annegers et al. 1979]. However, 20-30% of people who develop epilepsy will have a chronic epilepsy that responds incompletely to AED therapy, who will require treatment with one or more drugs through their life.
So, some of the fundamental questions with regard to efficacy are as follows. First, do individual drugs differ in their efficacy in suppressing different types seizures within different epilepsy syndromes? Second, do individual drugs exacerbate certain seizure types? And finally, do any of the drugs used to treat epilepsy do more than simply suppress seizures: are they antiepileptogenic and can they modify the natural history of epilepsy?
The ideal drug for someone with epilepsy would not only suppress seizures but also reduce the susceptibility to seizures in the future (both antiseizure and antiepileptogenic). Epileptogenesis is a process in which structural and functional changes occur after a brain insult that can lead to epilepsy, but epileptogenicity may also describe some processes that contribute to the progression that is observed in some types of epilepsy. There is much animal data that supports epileptogenesis as a process [Goddard et al. 1969]. This evidence is most clearly seen in the kindling model of epilepsy and in various chronic animal models of epilepsy in which spontaneous seizures may develop after a latent period following an acute brain insult such as induced status epilepticus, neonatal hypoxia or traumatic brain injury. Despite these models, there is a lack of any firm evidence that the drugs used to treat epilepsy in human beings are antiepileptogenic. Temkin [2001] reviewed many clinical trials in which treatment was compared with no treatment in individuals with significant risk for the development of later epilepsy. Whereas the drugs that were tested consistently suppressed seizures in the short term, they did not seem to influence the long-term risk ofseizures. These data are supported by a first-seizure studies, which showed that treatment reduced seizure recurrence after a first unprovoked seizure but did not affect long-term remission rates [Marson et al. 2005; Musicco et al. 1997]. Thus, while a search for genuinely antiepileptogenic drugs continues, current decision making about the choice of drug treatment needs to be based on the relative ability of drugs to suppress seizures in the short term.
One of the key features in the management of epilepsy has been the differentiation between seizures that are focal in onset and those which seem to be generalised from the start. There is a strong clinical belief, supported by some evidence, that different AEDs may be effective against different seizure types and different epilepsy syndromes (Table 1). Thus, drugs that have been shown to be effective against focal seizures may be relatively ineffective against some generalised seizures. Conversely, those effective against generalised seizures may be somewhat effective against seizures with focal onset. We should of course avoid prescription of drugs that might exacerbate seizures in a susceptible patient. A systematic review did show evidence of some specific drug effects in the exacerbation of seizures. Carbamazepine may exacerbate typical and atypical absence seizures and myoclonic seizures [Perucca et al. 1998]. Ethosuximide may exacerbate tonic-clonic seizures in children, and there is somewhat more consistent evidence that vigabatrin can precipitate myoclonus and absence seizures. It is perhaps here that, until recently, we have the most satisfactory evidence to prefer specific AEDs, such as valproic acid, for the treatment of adults with generalised epilepsies.
Table 1.
Antiepileptic drugs (AEDs) and their spectrum of efficacy.
| AEDs for localised-onset seizures and syndromes | Potential broad spectrum AEDs (localised onset and generalised onset seizures and syndromes) |
| Carbamazepine | Benzodiazepines |
| Gabapentin | Levetiracetam |
| Phenytoin | Phenobarbitone |
| Lamotrigine | Topiramate |
| Pregabalin | Valproate |
| Oxcarbazepine | Zonisamide |
| Tiagabine | |
| Vigabatrin |
So far in this article we have focused upon efficacy, but ultimately the choice of AED for an individual will be based upon an assessment of benefit and risk. For some individuals there may be significant trade-offs between benefit and risk such that a less effective but safer treatment may be chosen over a more effective but potentially more harmful treatment. The most important example here is of women of child-bearing age with and idiopathic generalised epilepsy, who may choose a less effective but less teratogenic drug (e.g. lamotrigine) over a more effective but more teratogenic drug (e.g. valproate). An assessment of risk will require an appraisal of data from RCTs as well of studies with an observational design and this is expanded upon later in the article.
Studies to inform the choice of a first drug for epilepsy
The most complete register of RCTs in epilepsy is the Cochrane Epilepsy Group Trial Register which is publicly available as part of the Cochrane Controlled Trial Register (http:// www.cochrane.org). It is derived from searching electronic databases (e.g. Medline, EMBASE) and from hand searching of the majority of relevant journals. This database identifies many RCTs that compare one AED with another. However, the great majority of these trials are sponsored by industry to support the licensing of their drugs. Such trials do not necessarily supply data that inform everyday clinical decision making. Few, if any, of these trials attempt to assess quality of life (QoL) outcomes (an important patient-centred issue), and none have included assessment of cost effectiveness. An important assessment from the perspective of healthcare guidelines were: (a) that trials were not designed and powered as noninferiority trials; (b) that they were not long enough to produce clinically relevant information; (c) that titrations schedules were fixed and forced; (d) that the trials included multiple age groups and seizure types that were not adequately recognised in the reporting and analysis; and (e) that the design, conduct and analysis of the trials were by industry [Glauser et al. 2006].
A recently reported study of Standard And New Antiepileptic Drugs Trial (SANAD) [Marson et al. 2007a, 2007b] set out to compare clinicians’ choices of one of the standard drug treatments (carbamazepine or valproate) versus comparator new drugs as monotherapy, and examined the outcomes with respect to: (a) time to treatment failure, (b) time to 12 month remission, (c) QoL, and (d) cost effectiveness. How does its methodology hold up given the criticisms in the ILAE guidelines [Glauser et al. 2006]? First, it was powered not to detect differences in efficacy between drugs, but rather to address issues of equivalence, a more demanding outcome. The calculations of sample size were based on the aim of establishing that the 95% confidence limit for the old-new treatment comparisons did not exceed 10% for the two primary outcomes - retention time and time to achieve 12-month remission. It includes many more patient-years of follow-up than any previous study. The titration and dosing regimens were not fixed, but followed clinicians’ everyday clinical practice. While the study included multiple ages and seizure types, these variables are identified so that testing for interactions between treatments and subgroups could be carried out. The valproate arm of the study allows a comparison of relevant drugs in patients with idiopathic generalised epilepsies (IGE) for the first time. The study was sponsored by the Health Technology Assessment Programme of the UK National Health Service and had limited financial support from industry.
The study was, however, designed as a pragmatic clinical trial [Hotopf et al. 1999; Roland and Torgerson, 1998], so as to mimic everyday clinical practice and so to have strong ‘external validity’. It randomised over 2400 patients and achieved a high level of completeness of follow-up (95% of possible follow-up was available for analysis, involving close to 8000 patient-years).
In the arm comparing new drugs (gabapentin, lamotrigine, oxcarbazepine and topiramate) to carbamazepine, lamotrigine had the lowest incidence of treatment failure and was statistically superior to all drugs for this outcome with the exception of oxcarbazepine (smaller numbers of patients were randomised to oxcarbazepine as it joined the study later). Fewer patients experienced treatment failure on lamotrigine than carbamazepine (the standard drug), at 1 year (12% fewer) and 2 years (8% fewer) after randomisation. The superiority of lamotrigine over carbamazepine was due to its better tolerability; however, the data also indicate that lamotrigine is not clinically inferior to carbamazepine for measures of its efficacy, time to achieving a 12-month remission, and time to treatment failure due to inadequate seizure control (a secondary efficacy outcome). No consistent differences in QoL outcomes were found among drug-treatment groups, although patients achieving a 12-month remission by 2 years after randomisation had superior QoL outcomes to those who had not, and patients who had experienced a treatment failure outcome exhibited poorer QoL than those who remained on their randomised treatment. Health economic analysis supported lamotrigine being preferred to carbamazepine for both cost per seizure avoided and cost per quality-life years(QALY) gained. There is therefore a high probability that lamotrigine is a cost-effective alternative to carbamazepine. SANAD is unique in allowing examination of treatment effects across age ranges from childhood to the elderly age. While age was an important determinant of seizure outcome (both children and the elderly had a better seizure outcome compared with patients recruited during adult life), there were no interactions between age and treatment group, indicating that the relative treatment effects are as applicable to children and the elderly with partial epilepsy as they are for the larger group of adults with epilepsy.
In contrast, in the arm comparing valproate to new drugs for time to treatment failure, valproate was preferred to both topiramate and lamotrigine. Valproate was the drug least likely to be associated with treatment failure for inadequate seizure control and was the preferred drug for time to achieving a 12-month remission. QoL assessments did not show any between treatment differences, though patients achieving a 12-month remission by 2 years after randomisation again had superior QoL outcomes to those who had not, and patients who had experienced a treatment failure outcome exhibited poorer QoL. The health economic assessment supported the conclusion that valproate should remain the drug of first choice for idiopathic generalised or unclassified epilepsy, although there is a suggestion that topiramate is a cost-effective alternative to valproate. The benefits of valproate over and above other drugs were larger within the patients with IGE, who made up more than 60% of patients recruited to this arm of the study. The fact that lamotrigine was the preferred drug in comparisons in patients with focal epilepsies, but the poorest for patients with generalised epilepsies, emphasises the importance of seizure and syndrome classification in deciding treatments. Lamotrigine should not now be considered a broad-spectrum antiseizure drug.
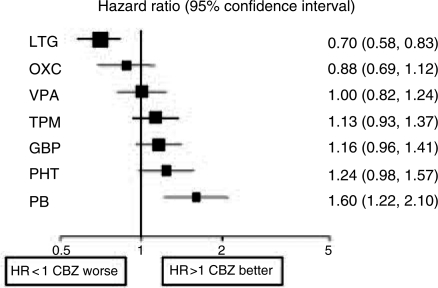
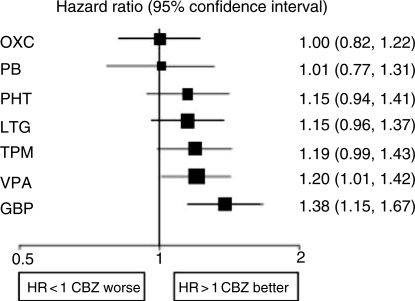
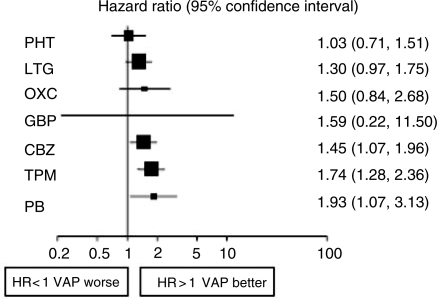
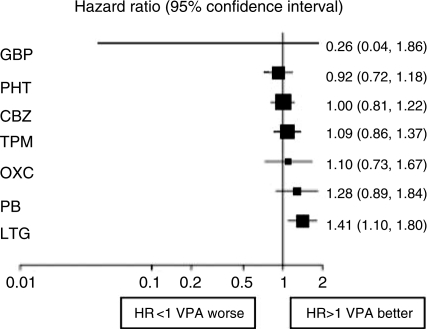
Of course it may be misleading to base judgements on a single RCT. The individual patient data from SANAD has now been added to similar data from other monotherapy comparative RCTs [Tudur Smith et al. 2007]. Multiple treatment comparisons from epilepsy monotherapy trials were synthesised in a single stratified Cox regression model adjusted for treatment by epilepsy type interactions and making use of direct and indirect evidence. Individual patient data for 6418 patients from 20 randomised trials comparing eight antiepileptic drugs were synthesised, including studies of older drugs such as phenobarbitone and phenytoin. For partial onset seizures [4628 (72%) patients], lamotrigine, carbamazepine and oxcarbazepine provide the best combination of seizure control and treatment failure. Lamotrigine is clinically superior to all other drugs for treatment failure but estimates suggest a disadvantage compared with carbamazepine for time to 12-month remission (Figures 1 and 2). For generalised onset tonic clonic seizures [1790 (28%) patients] estimates suggest valproate or phenytoin may provide the best combination of seizure control and treatment failure but some uncertainty remains about the relative effectiveness of other drugs (Figures 3 and 4). Conclusions are less definite here, because of uncertainty about the classification of patients as having generalised tonic-clonic seizures in older studies.
Figure 1.
Time to treatment failure for partial onset seizures (Hazard Ratio for each AED compared to standard CBZ) [Tudur Smith et al. 2007]. CBZ, carbamazepine; VPA, sodium valproate; PHT, phenytoin; PB, phenobarbitone; LTG, lamotrigine; OXC, oxcarbazepine; GBP, gabapentin; TPM, topiramate.
Figure 2.
Time to 12-month remission for partial onset seizures (Hazard Ratio for each AED compared to standard CBZ) [Tudur Smith et al. 2007]. CBZ, carbamazepine; VPA, sodium valproate; PHT, phenytoin; PB, phenobarbitone; LTG, lamotrigine; OXC, oxcarbazepine; GBP, gabapentin; TPM, topiramate.
Figure 3.
Time to treatment failure for generalised onset seizures (Hazard Ratio for each AED compared to standard VPA) [Tudur Smith et al. 2007]. CBZ, carbamazepine; VPA, sodium valproate; PHT, phenytoin; PB, phenobarbitone; LTG, lamotrigine; OXC, oxcarbazepine; GBP, gabapentin; TPM, topirimate.
Figure 4.
Time to 12-month remission for generalised onset seizures (Hazard Ratio for each AED compared to standard VPA) [Tudur Smith et al. 2007]. CBZ, carbamazepine; VPA, sodium valproate; PHT, phenytoin; PB, phenobarbitone; LTG, lamotrigine; OXC, oxcarbazepine; GBP, gabapentin; TPM, topiramate.
Polytherapy trials
While many patients with epilepsy are treated with two or more drugs there is little or no direct evidence that particular combinations are effective in different patients. Some indirect comparisons can be made from RCTs of new AEDs used as add-on therapy in patients with drug resistant focal seizures. These licensing studies make comparisons against placebo. Table 2 summarises the results of intention-to-treat analyses from systematic reviews in patients with drug-refractory localisation-related seizures [McCorry et al. 2004]. Although all these drugs show efficacy, caution should be used in comparing outcomes as populations differed and doses used varied.
Table 2.
Results of the systematic reviews of new drugs as add-on therapy in partial-onset epilepsy [McCorry et al. 2004].
| AED | Number of trials | Daily dose range (mg/day) | Effect estimate (95% CI) for a 50% reduction in seizures compared with placebo∗ | Relative risk (95% CI) of treatment withdrawal compared with placebo∗ |
| Gabapentin | 5 | 600-1800 | OR 1.93 (1.37-2.7) | 1.05 (0.68-1.61) |
| Tiagabine | 3 | 16-56 | RR 3.16 (1.97-5.07) | 1.80 (1.2-2.7) |
| Topiramate | 9 | 200-1000 | RR 3.32 [2.52-4.39] | 2.06 (1.38-3.08) |
| Lamotrigine | 11 | 200-500 | OR 2.71 (1.87-3.61) | 1.10 (0.81-1.50) |
| Levetiracetam | 4 | 1000-3000 | OR 3.78 (2.62-5.44) | 1.21 (0.88-1.66) |
| Oxcarbazepine | 2 | 600-2400 | OR 2.51 (1.88-3.33) | 1.72 (1.35-2.18) |
| Zonisamide | 3 | 400 | OR 2.46 (1.61-3.74) | 1.46 (1.02-2.62) |
All results are calculated for all doses. Note that 95% CIs that include 1 indicate no statistically significant difference. OR, odds ratio; RR, risk ratio.
Comparing harms of AEDs
Although the overall aim of AED treatment is to control seizures with the minimum of adverse effects, adverse events in patients taking antiepileptic drugs are common and can have a significant impact on quality of life [Gilliam, 2003]. Adverse effects are an important consideration when choosing a treatment as many patients will take treatment for many years (even if their seizures go into remission), 30% of patients never achieve a remission and are exposed to combinations of AEDs, and one-third of people taking AEDs are women of child-bearing age whose offspring might be exposed to teratogenic effects. There are three commonly recognised types of adverse drug reactions, which can be classified as: type A, common and acute reactions related to the drugs mechanism of action; type B, idiosyncratic and related to the patient's genetic and immunological profile; and type C, which are long-term and delayed side effects. To this must be added the potential for teratogenicity.
Comparing adverse effects of AEDs poses a number of methodological challenges. While the RCT is the best methodology for assessing benefits associated with treatments, it is not always the most appropriate method to study adverse effects. RCTs are the most appropriate method to assess type A events, but they will never be sufficiently powered to assess the risk of rare events (type B), nor will follow-up be long enough to assess late events (type C), and it would not be ethical to recruit women to an RCT to assess teratogenic effects. The assessment of type C and teratogenic effects requires the use of observational methods such as case-control studies and pregnancy registries, making treatment comparisons from studies using such designs are problematic. Thus a comprehensive assessment of adverse effects requires an integration of evidence from both RCTs and observational studies, although the methods for doing so remain inadequately developed.
Although RCTs allow the prospective collection of adverse effects data within a design that allows comparisons among randomised treatment groups the methods currently used for assessing adverse events remain problematic. Adverse events are heterogeneous and there are a large number of potential events and most RCTs are not powered to assess the risk of specific events. There is no consensus in the way that data on adverse effects should be ascertained in antiepileptic drug trials; trials in which the patient is given a list of possible adverse events consider at study visits are likely to find a higher incidence of adverse effects than studies in which the clinician or patient are asked to volunteer events. In addition, there is little agreement on the best methods to assess or grade the severity of adverse events. Finally, the reporting of adverse event data in reports of RCTs has been inadequate, but hopefully this will improve as authors and journal editors adhere to the CONSORT recommendations [Moher et al. 2001]. Inability to reliably assess the incidence and severity of specific adverse events make any assessment of trade-offs between benefit and hard somewhat difficult.
Rather than assess the risk of specific adverse effects, another approach is to make an assessment of the overall impact of adverse effects. This is most commonly done using a patient completed questionnaire such as the Liverpool Adverse Event Profile [Baker et al. 1994]. Such scales ask patients to indicate how badly they are affected by adverse events commonly associated with antiepileptic drugs, and an overall score is generated allowing an overall comparison among drugs. These tools are commonly used in RCTs, but do not capture data on type B, C or teratogenic effects.
The trade-off between benefit and harm can be assessed in a number of ways. Within an RCT, time to treatment failure is an outcome that provides some assessment of this trade off as treatment may fail due to inadequate seizure control, adverse effects or a combination of both. This outcome has therefore been described as a global effectiveness outcome and is the primary outcome for monotherapy studies recommended by the International League Against Epilepsy. Assessment of quality of life using instruments such as the Liverpool battery [Baker et al. 1993] or the QOLIE 89 [Kim et al. 2003] will also provide an overall assessment of treatment effects including domains of efficacy and harm which are put in a wider context.
While risk-benefit assessments can be made within a single RCT, methods for quantifying a wider risk-benefit assessment utilising data from RCTs and observational studies (e.g. studies on teratogenic effects) have not been developed but are clearly needed. However, such data on benefit and risk have been considered in the preparation of guidelines (NICE - see above).
Conclusions
Adequate comparison of AEDs is confounded greatly by the heterogeneity of epilepsy and by the different approaches to the use of AEDs (commonly as part of combined drug regimes). The majority of RCTs available are industry studies, which aim to provide evidence to support registration. There are few comparative studies that compare drugs head-to-head over clinically relevant periods of time. The best evidence is for patients with localised-onset seizures for whom treatment with a sodium channel drug (phenytoin, carbamazepine, oxcarbazepine or lamotrigine) would seem optimal, with newer drugs (oxcarbazepine or lamotrigine) being better tolerated. The evidence for patients with generalised epilepsies and seizures is sparse. Valproate appears to have greatest efficacy in RCTs, but is associated with significant weight gain and higher risks of foetal harm. With more AEDs becoming available there is a great need for clinically relevant head-to-head comparative RCTs that can inform the choice of clinicians, patients and providers of care.
Conflict of interest statement
None declared.
Contributor Information
David Chadwick, University of Liverpool, Liverpool, UK D.W.Chadwick@liverpool.ac.uk.
Arif Shukralla, University of Liverpool, Liverpool, UK.
Tony Marson, University of Liverpool, Liverpool, UK.
References
- Annegers J.F., Hauser W.A., Elverback L.R. (1979) Remission of seizures and relapse in patients with epilepsy. Epilepsia 20: 729–737 [DOI] [PubMed] [Google Scholar]
- Baker G.A., Jacoby A., Francis P., Chadwick D.W. (1994) Development of a patient-based adverse drug events profile as an outcome measure in epilepsy. Epilepsia 35: 80 [Google Scholar]
- Baker G.A., Smith D.F., Dewey M., Chadwick D.W. (1993) The initial development of a health-related quality of life model as an outcome measure in epilepsy. Epilepsy Res 16: 65–81 [DOI] [PubMed] [Google Scholar]
- Gilliam F. (2003) The impact of epilepsy on subjective health status. Curr Neurol Neurosci Rep 3: 357–362 [DOI] [PubMed] [Google Scholar]
- Glauser T., Ben-Menachem E., Bourgeois B., Cnaan A., Chadwick D., Guerreiro C.et al. (2006) ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 47: 1094–120 [DOI] [PubMed] [Google Scholar]
- Goddard G.V., McIntyre D.C., Leech C.K. (1969) A permanent change in brain function resulting form daily electrical stimulation. Exp Neurol 25: 295–330 [DOI] [PubMed] [Google Scholar]
- Hotopf M., Churchill R., Lewis G. (1999) Pragmatic randomised controlled trials in psychiatry. Br J Psychiatry 175: 217–223 [DOI] [PubMed] [Google Scholar]
- Kim S., Hays R.D., Birbeck G.L., Vickrey B.G. (2003) Responsiveness of the quality of life in epilepsy inventory (QOLIE-89) in an antiepileptic drug trial. Qual Life Res 12: 147–155 [DOI] [PubMed] [Google Scholar]
- Marson A., Jacoby A., Johnson A., Kim L., Gamble C., Chadwick D. (2005) Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet 365: 2007–2013 [DOI] [PubMed] [Google Scholar]
- Marson A.G., Al-Kharusi A.M., Alwaidh M., Appleton R., Baker G.A., Chadwick D.W.et al. (2007a) The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 369: 1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A.G., Al-Kharusi A.M., Alwaidh M., Appleton R., Baker G.A., Chadwick D.W.et al. (2007b) The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 369: 1000–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorry D., Chadwick D., Marson A. (2004) Current drug treatment of epilepsy in adults. Lancet Neurol 3: 729–735 [DOI] [PubMed] [Google Scholar]
- Moher D., Schulz K.F., Altman D.G. (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134: 657–662 [DOI] [PubMed] [Google Scholar]
- Musicco M., Beghi E., Solari A., Viani F. (1997) Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. Neurology 49: 991–998 [DOI] [PubMed] [Google Scholar]
- Perucca E., Gram L., Avanzini G., Dulac O. (1998) Antiepileptic drugs as a cause of worsening of seizures. Epilepsia 39: 5–17 [DOI] [PubMed] [Google Scholar]
- Roland M., Torgerson D.J. (1998) What are pragmatic trials? BMJ 316: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin N.R. (2001) Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42: 515–524 [DOI] [PubMed] [Google Scholar]
- Tudur Smith C., Marson A.G., Chadwick D.W., Williamson P.R. (2007) Multiple treatment comparisons in epilepsy monotherapy trials. Trials 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]