Introduction
Multiple sclerosis (MS) is an autoimmune disease in which autoreactive T cells cross the blood—brain barrier and attack the myelin sheath leading to a cascade of inflammation. The result is demyelination, acute axonal transection, gliosis and subsequent axonal degeneration [Trapp et al. 1998]. Unpredictable episodes of neurological disability in young adults are followed by a gradual accumulation of deficits over time as the disease switches from an inflammatory to a degenerative ‘secondary progressive’ phase. Monoclonal antibodies have been used as experimental treatments of MS since the 1980s. Their advantage is high specificity for their target; their disadvantages are that they (usually) require intravenous administration, often are associated with infusion reactions and, as large foreign proteins, they are immunogenic.
Past experience with monoclonal antibodies has taught researchers to interpret results seen in animal models with caution. Anti-TNFα antibodies are successful in the treatment of rheumatoid arthritis and work done using the animal model of MS (experimental autoimmune encephalomyelitis, EAE) suggested a beneficial effect on the disease. Early trials in MS patients, however, showed the opposite effect with worsening of disease activity [Lenercept Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group, 1999]. There have also been reports of de novo MS occurring in rheumatoid arthritis patients treated with anti-TNFα therapy [Sicotte and Voskuhl, 2001].
Data from clinical research trials has however given important insights in to the underlying disease mechanism and suggests that treating MS in the early inflammatory phase gives an opportunity to delay or prevent the onset of disease progression [Coles et al. 2006]. To date only one monoclonal antibody has been licensed as a treatment for MS: natalizumab. Here we review the current and future use of monoclonal antibodies in MS (Table 1).
Table 1.
Current and future monoclonal antibodies in MS.
| Name | Type of monoclonal Ab | Target molecule | Mechanism of action | Trial stage in MS |
| Natalizumab (Tysabri) | Humanised | a4-integrin | Prevention of migration of activated lymphocytes across the blood—brain barrier | Licensed |
| Alemtuzumab (Campath-1H) | Humanised | CD52 | Prolonged T cell lymphopaenia (and transient depletion of B cells and monocytes) | Phase III |
| Rituximab (Rituxan) | Chimeric | CD20 | B cell depletion | Phase II |
| Ocrelizumab | Humanised | CD20 | B cell depletion | Phase II |
| Daclizumab (Zenepax) | Humanised | CD25 (IL-2Rα) | Reduced survival of activated T cells, possibly by NK cells | Phase II |
| Ustekinumab (CNTO-1275) | Human | IL12 p40 + IL23p40 | Blockade of interleukin-12 and interleukin-23 | Phase II |
| ch5 D12 | Chimeric | CD40 | Antigen presenting cells | Preclinical |
Introduction to monoclonal antibodies
Kohler and Milstein's seminal work in 1975 heralded the development of monoclonal antibodies that could be used to treat human diseases [Kohler and Milstein, 1975]. The technique generates ‘hybridomas’ by the fusion of a plasma cell from the spleen of an animal (usually a rodent) immunised with the antigen of interest together with a myeloma cell. The resulting hybrid cells are then screened and a cell producing the required antibody with good specificity for the antigen is selected and cloned. The resulting cell line will continually grow in culture and produce large quantities of the same antibody. The history of subsequent events in the development of monoclonal antibodies is summarised in Table 2.
Table 2.
The history of subsequent events in the development of monoclonal antibodies.
| 1982 | The therapeutic potential of monoclonal antibodies is shown by a complete response of a patient with a B cell lymphoma to an anti-idiotype antibody [Miller et al. 1982] |
| 1986 | OKT3 (anti-CD3 monoclonal antibody) is approved by FDA for acute renal transplant rejection |
| 1986 | Greg Winter and his team produce the first humanised monoclonal antibody by grafting the CDR regions from a mouse antibody [Jones et al. 1986]. Herman Waldmann's group in collaboration with the Winter group develop Campath-1H which is subsequently shown to be successful in the treatment of chronic lymphocytic leukaemia. |
| 1997 | Daclizumab is the first humanised monoclonal antibody (anti-IL2R) approved by the FDA for acute renal transplantation rejection |
| 1997 | First humanised oncology monoclonal antibody approved (rituximab) |
| 1998 | Antibodies to TNF-α (infliximab) and IgG-TNFR (etanercept) approved for Crohns disease and rheumatoid arthritis. |
| 2000 | First toxin-linked monoclonal antibody (gemtuzumab ozogamicin) approved by the FDA |
| 2002 | First radionuclide-linked monoclonal antibody (ibritumomab) approved by the FDA |
Generic problems with monoclonal antibodies 1: immunogenicity
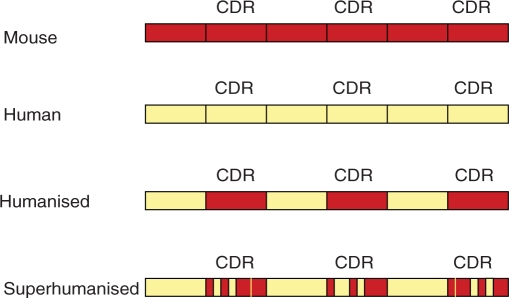
When mouse monoclonal antibodies are used as therapies, they are recognised as foreign proteins and may induce neutralising anti-mAb antibodies. To reduce immunogenicity, variable amounts of the murine immunoglobulin have been replaced by the human equivalent. Thus ‘chimeric antibodies’ have the murine antigen binding domain fused to a human IgG framework. Humanised antibodies go a step further with the only murine element being the complementarity determining regions (Figure 1). The extreme form of this technology is ‘superhumanisation’ where even parts of the murine antigen-binding site are humanised [Tan et al. 2002].
Figure 1.
Humanised antibodies are developed by combining the complementarity-determining regions (CDRs) of a murine mAb with the framework of a human antibody variable domain. Superhumanised antibodies go a step further incorporating human regions into the CDR. Modified from Tan J Immunol 2002; 169, 1120.
Another aspect to immunogenicity is dose and route of administration. In general intravenous administration is less immunogenic than subcutaneous. In addition, following some classical observations, either very low or very high doses of any foreign protein can achieve low or high ‘zone tolerance’ when given intravenously [Parish and Liew, 1972]. It is probably for this reason that 1 g rituximab i.v., a chimeric antibody, is less immunogenic than 20mg Campath-1H i.v., a humanised antibody. An ingenious solution to this problem has been proposed by Herman Waldmann and colleagues. They have shown that a variant of Campath-1H, in which one amino acid within the antigen-binding site has been altered to abolish cell binding and so can be given in very large doses, successfully induces high-zone tolerance to subsequent doses of Campath-1H in huCD52-transgenic animals [Gilliland et al. 1999].
Generic problems with monoclonal antibodies 2: infusion reactions
From the first use of OKT3, cell-depleting antibodies have been associated with an acute infusion reaction attributable to cytokine release: specifically TNF-a after anti-CD3 antibodies [Ferran et al. 1991] and cytokines other than TNF-a after Campath [Coles et al. 1999]. Pretreatment with corticosteroids can reduce the cytokine release and associated symptoms. The Fc portion of the antibody can also be altered so that it no longer induces cytokine release, as in the aglyosyl anti-CD3 [Bolt et al. 1993].
Generic problems with monoclonal antibodies 3: CNS penetration
It is very unlikely that significant amounts of monoclonal antibodies, injected intravenously, penetrate the CNS even in patients with active MS, in whom there will be several sites of breakdown of the blood—brain barrier. We have consistently been unable to detect Campath-1H in the cerebrospinal fluid of our patients (unpublished observations). There have also been many reports of induction of brain metastases in women with a good systemic response to trastuzumab for HER-2 positive breast cancer [Lindrud et al. 2003], suggesting not only that the antibody does not penetrate the blood—brain barrier, but also that a ‘safe haven’ for metastases is promoted.
Natalizumab
Natalizumab (Tysabri, Biogen Idec) is the only monoclonal antibody currently licensed for relapsing-remitting MS. It works by targeting lymphocyte migration across the blood—brain barrier, an early step in MS lesion formation. The protein a4 ß1 integrin is present on lymphocytes and interacts with vascular-cell adhesion molecule 1 (VCAM-1) on brain and spinal blood vessels to allow migration in to the central nervous system (CNS). Natalizumab binds the a 4 subunit (very late antigen 4) preventing this interaction and halting passage of lymphocytes in to the CNS. Two large phase III double-blind trials; AFFIRM (natalizumab versus placebo) and SENTINEL (natalizumab plus interferon-ß1a versus placebo plus interferon-ß1a) assessed safety and efficacy over a 2-year period.
In AFFIRM 942 patients were randomised in a 2: 1 ratio to receive either natalizumab 300 mg every 4 weeks or placebo infusion, 856 patients completed the study [Polman et al. 2006]. Patients had RRMS (EDSS 0—5) with one relapse in the preceding 12 months, no interferon or glatiramer acetate therapy for 6 months and no other immunosuppression for 12 months. At 2 years there was a 68% relative reduction in annualised relapse rates in the natalizumab group. Natalizumab reduced the risk of sustained progression of disability by 42% at 2 years. New or enlarging T2 MRI lesions were reduced by 83% and the mean number of gadolinium enhancing lesions by 92%.
In SENTINEL patients had RRMS (EDSS 0—5) and one or more relapses during a minimum of 12 months on IFN therapy in the preceding year [Rudick et al. 2006]. One-thousand-one-hun-dred-and ninety-six patients were randomised in a 1: 1 ratio to receive either natalizumab four times weekly or placebo in addition to weekly IFN-ß1a (Avonex). Data from 25 patients was excluded and 14% of the remaining patients withdrew from the study. Combination therapy resulted in a 24% reduction in the relative risk of sustained progression of disability. Annualised relapse rate was 0.34 on combination therapy compared to 0.75 on IFN-ß1a alone. New or enlarging T2 MRI lesions were reduced by 83% with combination therapy.
Natalizumab was approved by the FDA in November 2004 after efficacy in reducing relapse rate was shown at 1 year, confirmatory trial evidence showing 2 year efficacy was required [US Food and Drug Administration, 2009]. Two patients on the SENTINEL trial subsequently developed progressive multifocal leukoencephalopathy (PML) and died. One had received 28 doses of natalizumab [Langer-Gould et al. 2005] and the other had finished the study and received a total of 37 doses [Kleinschmidt-DeMasters and Tyler, 2005] with no signs of MS at post mortem. These cases lead to the suspension of natalizumab marketing and the clinical trials in February 2005. A further case of PML in a patient treated with natalizumab for Crohn's disease was later identified [Van et al. 2005].
No further PML cases in MS patients were identified at this time and the suspension of clinical trials with natalizumab was lifted in February 2006. In June 2006 the marketing suspension was lifted with the condition that in the US appropriate patients only receive natalizumab monotherapy under the risk minimisation scheme ‘TOUCH’ (Tysabri Outreach: Unified Commitment to Health). TYGRIS-ROW (Tysabri Global Observational Program in Safety — Rest of World) is a safety observational study of patients treated with natalizumab in any country outside of the US. The UK guidelines currently recommend natalizumab only in patients with rapidly evolving severe relapsing-remitting MS defined by two relapses within 12 months plus one gadolinium-enhancing lesion on MRI or a significant increase in T2 lesion load compared to a previous scan [NICE, 2007].
Since the reapproval of natalizumab a further five cases of PML have occurred; these are all in MS patients receiving monotherapy [Biogen Idec, 2009]. Two of these were reported in July 2008; one after 17 doses of natalizumab and the second case in a patient treated with 14 doses of natalizumab [Hartung, 2009]. The third case reported in October 2008 occurred in a patient who had 14 doses of natalizumab [Hartung, 2009]. The fourth case reported in December 2008 occurred after 26 months of natalizumab treatment and the fifth case was confirmed in February 2009 after 12 months of therapy.
Other side effects of natalizumab treatment include infusion reactions and hypersensitivity. In the AFFIRM and SENTINEL trial 6% of patients treated with natalizumab developed persistent antibodies which result in reduced efficacy and increased infusion reactions. There is post-marketing evidence that natalizumab can cause clinically significant liver injury and this can occur as early as 6 days after first treatment as well as after multiple doses [US Food and Drug Administration, 2009]. Patients should be made fully aware of the risks of natalizumab before starting treatment and closely monitored.
Alemtuzumab
Alemtuzumab (Campath-1H, Genzyme and Bayer Schering Pharma) is a humanised monoclonal antibody against CD52 found on lymphocytes, monocytes and eosinophils. The function of the CD52 antigen however is still unknown. Alemtuzumab results in a rapid and profound cell lysis and lymphopaenia. It is licensed for treatment of chronic lymphocytic leukaemia and has been used in research trials in MS since 1991. When used in MS B cell and monocyte numbers recover within 3 months, however CD4+ T cells remain low for a median of 5 years [Coles et al. 2006]. Early studies showed that when used in secondary progressive MS, relapses and MRI disease activity were effectively suppressed; however, accumulation of disability over time continued [Coles et al. 2006]. In contrast it has been shown to be highly effective in early relapsing-remitting MS in comparison to interferon-b1a [Coles et al. 2008].
The CAMMS223 phase II study of 334 patients with early relapsing-remitting MS randomised patients to receive either high or low dose annual alemtuzumab infusions, or three times per week injections of interferon-b1a, over a 3-year period [Coles et al. 2008]. Alemtuzumab was shown to be highly effective and resulted in a reduced rate of sustained accumulation of disability as well as reduction in annualised relapse rate. Annualised relapse rate was 0.1 in alemtuzumab treated patients compared to 0.36 in the interferon-b1a group. The EDSS points in the alemtuzumab group improved by 0.39 points and worsened by 0.38 points in the interferon group (p<0.001). MRI T2 lesion volume was reduced more in the alemtuzumab group compared with the interferon-b1 a group, this was significant at 12 months (p=0.01) and 24 months (p=0.005) though not at 36 months. Overall no significant differences in outcomes were seen between the high and low dose of alemtuzumab.
In September 2005 alemtuzumab dosing was suspended following the development of immune thrombocytopenic purpura (ITP) in three patients, one of whom did not report their symptoms and subsequently died from an intra-cerebral haemorrhage. In the alemtuzumab group 75% of patients did not receive the third cycle of treatment due to the suspension. A total of six patients (2.8%) receiving alemtuzumab developed ITP compared to one patient receiving interferon-b1a.
Autoimmune thyroid disease was seen in 22.7% of patients treated with alemtuzumab (majority were hyperthyroid) compared with 2.8% of the interferon-b1a. Mild-to-moderate infections were also increased in the alemtuzumab group. Infusion reactions to alemtuzumab were common though serious reactions were only seen in 1.4%. A case of non-EBV-associated Burkitt's lymphoma has been reported in one patient treated with alemtuzumab. There have also been two reports of Goodpasture's disease occurring in MS patients treated with alemtuzumab on other clinical trials.
Despite the adverse event profile of alemtuzumab, 83% of treated patients completed the 36 months of the CAMMS223 trial compared to only 59% of interferon-b1a patients. The main reasons for discontinuation in the interferon-b1a group were lack of efficacy and adverse events. The extremely promising clinical and radiological efficacy of alemtuzumab is currently being tested in two phase III trials with careful monitoring for its potential adverse events.
Rituximab
Rituximab (Rituxan, Genentech and Biogen Idec) is a chimeric monoclonal anti-CD20 antibody that is approved for rheumatoid arthritis. It results in depletion of CD20 positive B cells (pre-B cells and mature B cells) but does not deplete plasma cells or progenitor cells in the bone marrow. Previously, most MS treatments targeted T cells, but the success of rituximab in rheumatoid arthritis and the growing evidence for antibody involvement in the pathogenesis of MS led to trials of the use of rituximab in the treatment of MS.
Safety and tolerability was initially shown in a phase I study of 26 patients [Bar-Or et al. 2008]. A subsequent phase II, double-blind trial randomised 104 relapsing-remitting MS patients in a 2: 1 ratio to receive either two doses of rituximab or placebo infusions [Hauser et al. 2008]. Patients had at least one relapse in the previous year and were off disease modifying therapy for at least 60 days prior to treatment. Relapses were reduced in the rituximab group compared with placebo. The proportion of patients with relapses were 15.4% versus 34.3% respectively at 24 weeks, and 20.3% versus 40% at 48 weeks. Total gadolinium-enhancing lesions were reduced in the rituximab group at 12, 16, 20, 24 and 48 weeks.
Infusion reactions were common (78%) in the rituximab-treated patients. Infection rates were similar in the two groups; however, urine infections and sinusitis were more common in the rituximab group. At 48 weeks a quarter of rituximab treated patients were positive for antihuman chimeric antibodies. The authors report that a reduction in inflammatory lesions was seen as early as 4 weeks after the first dose. As rituximab does not deplete plasma cells and antibody levels are not significantly affected it is unlikely that it exerts its effect through the reduction of pathogenic antibodies. Other mechanisms such as reduced antigen presentation or reduced proinflammatory B cell cytokine secretion are more plausible [Bar-Or et al. 2008]. Physicians using rituximab should be alert to the possible development of PML; cases have been reported in patients treated for rheumatoid arthritis, systemic lupus erythematosus and non-Hodgkin's lymphoma.
Rituximab has also been used in a phase II trial in primary progressive MS, the results of which were presented at the World Congress on Treatment and Research in Multiple Sclerosis in Montreal, September 2008. In this trial, 439 patients with primary progressive MS were randomised in a 2:1 ratio to either rituximab or placebo. The percentage of patients with confirmed disease progression at 96 weeks (defined as an increase in the disability score maintained for 12 weeks) was not statistically different between the two groups (38.5% for placebo versus 30.2% for rituximab). The median change in T2 lesion volume on MRI from baseline to week 96 was significantly lower for rituximab (p=0.0008). Given the lack of efficacy and the risk of increased serious infections seen in the rituximab group (4.5% versus <1.0% for placebo) the use of rituximab cannot be justified in primary progressive MS.
Ocrelizumab
This is a humanised anti-CD20 monoclonal antibody which is currently being tested in MS in a phase II trial. The trial aims to investigate the efficacy (measured by MRI lesion activity) and safety of two dose regimens of ocrelizumab over 24 weeks. Patients with relapsing-remitting MS and evidence of recent disease activity, who have been off disease modifying therapy for at least 12 weeks, are randomised to one of two dose regimens of ocrelizumab, or placebo, or weekly Avonex.
Daclizumab
Interleukin-2 expands T cells, an effect which can be limited by blocking its receptor (the IL-2 Ra-chain, CD25) on activated T cells. Interleukin-2 receptor antibodies are licensed for prevention of organ transplant rejection [Adu et al. 2003]. Daclizumab (Zenepax) is an anti-CD25 antibody which has been tested in an open-label phase II study of patients who have clinically failed to respond to interferon therapy [Bielekova et al. 2004]. Prior to treatment patients were followed for 4 months on their interferon therapy and had to have at least 0.67 new contrast enhancing lesions per month. A total of 11 patients received seven doses each (five with SPMS, six RRMS), the first two doses were given fortnightly and then every 4 weeks.
Overall the drug was well tolerated; a slight increase in mild infections and two cases of transient liver function abnormalities were seen. Daclizumab resulted in a 78% reduction in new contrast-enhancing lesion formation; this occurred over 1.5—2 months. An 80% reduction in exacerbation rate was also seen.
The mechanism of action of daclizumab is intriguing. The authors initiated the clinical trial based on the hypothesis that lymphocytes in MS patients are chronically activated and dependent on high affinity IL2R signalling [Bielekova et al. 2006]. However when the effect of daclizumab was studied little change was seen in the function of the patients’ T cells. Only a 20% reduction in CD4+ proliferation was seen and there was no effect on CD8+ cell proliferation or T cell cytokine secretion. Circulating patient CD4+ and CD8+ cell counts were only slightly reduced. In contrast a gradual expansion of CD56bright NK cells was seen and this correlated with the reduction in MRI disease activity. In vitro, these CD56 bright NK cells were shown to limit the survival of activated T cells by a contact dependent mechanism. An NK cell mediated regulation of activated T cells was also indirectly suggested in vivo as the expansion in NK cell numbers correlated with the small but significant reduction in CD4+ and CD8+ cells.
Another small phase II trial involved nine RRMS patients who had at least one relapse in the prior 12 months while on interferon therapy and had two gadolinium enhancing lesions on any of four baseline MRI scans [Rose et al. 2007]. Interferon therapy was continued in addition to daclizumab. Patients stopped interferon if there were no contrast enhancing lesions at 5.5 months and continued on daclizumab alone for 10 months. If contrast-enhancing lesions returned interferon was restarted and the dose of daclizumab increased from 1mg/kg to 1.5 mg/kg. An extension study allowed treatment to continue for up to a total of 27.5 months.
Eleven patients were enrolled, although one stopped after two treatments due to urine infections and a severe relapse and another was withdrawn after developing transient thrombocytopenia while taking trimethoprim-sulfamethoxazole for a urine infection. The other nine patients all completed 27.5 months of therapy. All patients stopped interferon after 5.5 months although three subsequently developed new lesions and restarted it together with an increased daclizumab dose. A four-fold decrease in new contrast enhancing lesions compared with baseline was seen at all time intervals as well as a reduction in relapse rate. Therapy was well tolerated, one patient developed lymphadenopathy which receded on completion of the study.
Ustekinumab (CNTO-1275)
Ustekinumab is a monoclonal antibody against the p40 subunit which is a component of both interleukin-12 and interleukin-23 [Oppmann et al. 2000]. Interleukin-12 drives Th1 cell differentiation and the production of IFN-g [Mosmann et al. 1986] where as interleukin-23 drives the interleukin-17 producing CD4+ cells called ‘Th17’ [Aggarwal et al. 2003]. Work done in the animal model of MS has implicated a role of both of these cell types in the initiation of CNS lesions [Lees et al. 2008]. Ustekinumab has so far shown promising results for the treatment of psoriasis in clinical trials.
A phase II double-blind study randomised 246 RRMS patients to either one of four doses of ustekinumab subcutaneously (27 mg, 90 mg or 180 mg four times weekly or 90mg eight times weekly) or placebo [Segal et al. 2008]. Patients had experienced two relapses in the previous 2 years, or one in the last 6 months, and were off other treatments for at least 3 months. No significant differences were seen between the placebo and ustekinumab groups in the number of new gadolinium enhancing lesions at week 23 or relapse rate. Ustekinumab was generally well tolerated although 32% of patients reported injection site reactions and infections were increased. One possible hypothesis for the lack of efficacy of ustekinumab suggested by the authors is that the drug may not cross the blood—brain barrier and therefore be unable to exert an effect within the CNS.
ch5D12
This chimeric anti-human CD40 monoclonal antibody has been tested in the marmoset monkey experimental autoimmune encephalomyelitis model of MS. CD40 is a costimulatory molecule present on antigen presenting cells and B cells. The CD40 ligand is found on T cells, upon interaction with CD40 it results in activation of antigen presenting cells and B cell isotype switching. It has also recently been shown that CD40—CD40L interaction is important for the development of Th17 cells [Iezzi et al. 2009].
One study induced EAE in eight monkeys by injecting the recombinant myelin protein MOG in complete Freund's adjuvant [Boon et al. 2001]. Monkeys were treated with ch5D12 or placebo prior to and after induction. The four monkeys in the placebo group all developed EAE by day 41; by comparison, none of the treated monkeys developed clinical signs of EAE during the 50-day treatment period. However upon cessation of treatment the monkeys went on to develop EAE though this was not immediate.
In a subsequent study the authors tested the effect of ch5D12 on existing EAE in the marmoset model [’t Hart et al. 2005]. Treatment with ch5D12 (three monkeys) or placebo (four monkeys) was started once MRI lesions started to develop. The enlargement and intensity of T2 lesions was suppressed in treated monkeys; however, no clinical benefit was seen. No obvious side effects were seen in the small number of monkeys treated; however, previous studies using an anti-CD40L monoclonal antibody resulted in increased thromboembolic events [Kawai et al. 2000]. Given the uncertain clinical benefit and the potential for thromboembolic complications more evidence should be obtained before proceeding to trials in MS patients.
Conclusion
Experience with monoclonal antibodies has shown that early, effective treatment of MS has the potential to prevent or delay disability. Physicians and patients need to carefully weigh up the benefits and risks of monoclonal antibody therapy. The devastating clinical and social consequences of this disease, however, should not be underestimated.
Conflict of interest statement
AJC has received expenses and honoraria for speaking at meetings organised by Genzyme. His department has received funds from Genzyme to support research on alemtuzumab treatment of multiple sclerosis.
Contributor Information
Claire L. Helliwell, Department of Clinical Neurosciences, Addenbrooke's Hospital, Cambridge, UK clh74@cam.ac.uk
Alasdair J. Coles, Department of Clinical Neurosciences, Addenbrooke's Hospital, Cambridge, UK
References
- Adu D., Cockwell P., Ives N.J., Shaw J., Wheatley K. (2003) Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ 326: 789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., Gurney A.L. (2003) Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278: 1910–1914 [DOI] [PubMed] [Google Scholar]
- Bar-Or A., Calabresi P.A., Arnold D., Markowitz C., Shafer S., Kasper L.H.et al. (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63: 395–400 [DOI] [PubMed] [Google Scholar]
- Bielekova B., Catalfamo M., Reichert-Scrivner S., Packer A., Cerna M., Waldmann T.A.et al. (2006) Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2R alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Nati Acad Sei USA 103: 5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B., Richert N., Howard T., Blevins G., Markovic-Plese S., McCartin J.et al. (2004) Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proe Nati Aead Sei USA 101: 8705–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biogen Idec. (2009) Tysabri Update www.biogenidec.com/site/tysabri-information-center.html
- Bolt S., Routledge E., Lloyd I., Chatenoud L., Pope H., Gorman S.D.et al. (1993) The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur J Immunol 23: 403–411 [DOI] [PubMed] [Google Scholar]
- Boon L., Brok H.P., Bauer J., Ortiz-Buijsse A., Schellekens M.M., Ramdien-Murli S.et al. (2001) Prevention of experimental autoimmune encephalomyelitis in the common marmoset (Callithrix jaeehus) using a chimeric antagonist monoclonal antibody against human CD40 is associated with altered B cell responses. J Immunol 167: 2942–2949 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Compston D.A., Selmaj K.W., Lake S.L., Moran S., Margolin D.H.et al. (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359: 1786–1801 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Cox A., Le P.E., Jones J., Trip S.A., Deans J.et al. (2006) The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 253: 98–108 [DOI] [PubMed] [Google Scholar]
- Coles A.J., Wing M.G., Molyneux P., Paolillo A., Davie CM., Hale G.et al. (1999) Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 46: 296–304 [DOI] [PubMed] [Google Scholar]
- Ferran C, Dy M., Sheehan K., Schreiber R., Grau G., Bluestone J.et al. (1991) Cascade modulation by anti-tumor necrosis factor monoclonal antibody of interferon-gamma, interleukin 3 and interleukin 6 release after triggering of the CD3/T cell receptor activation pathway. Eur J Immunol 21: 2349–2353 [DOI] [PubMed] [Google Scholar]
- Gilliland L.K., Walsh L.A., Frewin M.R., Wise M.P., Tone M., Hale G.et al. (1999) Elimination of the immunogenicity of therapeutic antibodies. J Immunol 162: 3663–3671 [PubMed] [Google Scholar]
- Hartung H.P. (2009) New cases of progressive multifocal leukoencephalopathy after treatment with natalizumab. Lancet Neurol 8: 28–31 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Waubant E., Arnold D.L., Vollmer T, Antel J., Fox R.J.et al. (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676–688 [DOI] [PubMed] [Google Scholar]
- Iezzi G., Sonderegger I., Ampenberger F., Schmitz N., Marsland B.J., Kopf M. (2009) CD40-CD40 L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc NatlAcad Sci USA 106: 876–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.T., Dear P.H., Foote J., Neuberger M.S., Winter G. (1986) Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321: 522–525 [DOI] [PubMed] [Google Scholar]
- Kawai T, Andrews D., Colvin R.B., Sachs D.H., Cosimi A.B. (2000) Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 6: 114. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters B.K., Tyler K.L. (2005) Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1 a for multiple sclerosis. N Engl J Med 353: 369–374 [DOI] [PubMed] [Google Scholar]
- Kohler G., Milstein C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497 [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Atlas S.W., Green A.J., Bollen A.W., Pelletier D. (2005) Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 353: 375–381 [DOI] [PubMed] [Google Scholar]
- Lenercept Multiple Sclerosis Study Group, and the University of British Columbia MS/MRI Analysis Group (1999) TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53: 457–465 [PubMed] [Google Scholar]
- Lees J.R., Iwakura Y., Russell J.H. (2008) Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J Immunol 180: 8066–8072 [DOI] [PubMed] [Google Scholar]
- Lindrud S., Orlick M., Barnard N., Hait W.N., Toppmeyer D.L. (2003) Central nervous system progression during systemic response to trastuzumab, humanized anti-HER-2/neu antibody, plus paclitaxel in a woman with refractory metastatic breast cancer. Breast J 9: 116–119 [DOI] [PubMed] [Google Scholar]
- Miller R.A., Maloney D.G., Warnke R., Levy R. (1982) Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med 306: 517–522 [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Cherwinski H., Bond M.W, Giedlin MA., Coffman R.L. (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357 [PubMed] [Google Scholar]
- NICE (2007) Natalizumab for the treatment of adults with highly active relapsing-remitting multiple sclerosis. NICE Technology Appraisal Guidance 127.http://www.nice.org.uk/nicemedia/pdf/TA127publicinfo.pdf [Google Scholar]
- Oppmann B., Lesley R., Blom B., Timans J.C., Xu Y., Hunte B.et al. (2000) Novel p19 protein engages IL-12 p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725 [DOI] [PubMed] [Google Scholar]
- Parish C.R., Liew F.Y. (1972) Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med 135: 298–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., O'Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H.et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899–910 [DOI] [PubMed] [Google Scholar]
- Rose J.W, Burns J.B., Bjorklund J., Klein J., Watt H.E., Carlson N.G. (2007) Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 69: 785–789 [DOI] [PubMed] [Google Scholar]
- Rudick R.A., Stuart W.H., Calabresi P.A., Confavreux C, Galetta S.L., Radue E.Wet al. (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354: 911–923 [DOI] [PubMed] [Google Scholar]
- Segal B.M., Constantinescu C.S., Raychaudhuri A., Kim L., Fidelus-Gort R., Kasper L.H. (2008) Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol 7: 796–804 [DOI] [PubMed] [Google Scholar]
- Sicotte N.L., Voskuhl R.R. (2001) Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 57: 1885–1888 [DOI] [PubMed] [Google Scholar]
- Tan P., Mitchell D.A., Buss T.N., Holmes M.A., Anasetti C., Foote J. (2002) 'Superhumanized' antibodies: reduction of immunogenic potential by complementarity-determining region grafting with human germline sequences: application to an anti-CD28. J Immunol 169: 1119–1125 [DOI] [PubMed] [Google Scholar]
- 't Hart B.A., Blezer E.L., Brok H.P., Boon L., de B.M., Bauer J.et al. (2005) Treatment with chimeric anti-human CD40 antibody suppresses MRI-detectable inflammation and enlargement of pre-existing brain lesions in common marmosets affected by MOG-induced EAE. J Neuroimmunol 163: 31–39 [DOI] [PubMed] [Google Scholar]
- Trapp B.D., Peterson J., Ransohoff R.M., Rudick R., Mork S., Bo L. (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338: 278–285 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2009) Natalizumab (marketed as Tysabri) Information. http://www.fda.gov/Cder/drug/infopage/natalizumab/default.htm
- Van A.G., Van R.M., Sciot R., Dubois B., Vermeire S., Noman M.et al. (2005) Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med 353: 362–368 [DOI] [PubMed] [Google Scholar]