Abstract
Migraine is considered a neurovascular disease affecting more than 10% of the general population. Currently available drugs for the acute treatment of migraine are vasoconstrictors, which have limitations in their therapeutic use. The calcitonin gene-related peptide (CGRP) has a key role in migraine, where levels of CGRP are increased during acute migraine attacks. CGRP is expressed throughout the central and peripheral nervous system, consistent with control of vasodilatation and transmission of nociceptive information. In migraine, CGRP is released from the trigeminal system. At peripheral synapses CGRP results in vasodilatation via receptors on the smooth muscle cells. At central synapses, CGRP acts postjunctionally on second-order neurons to transmit pain centrally via the brainstem and midbrain to higher cortical pain regions. The recently developed CGRP-receptor antagonists have demonstrated clinical efficacy in the treatment of acute migraine attacks. A remaining question is their site of action. The CGRP-receptor components (calcitonin receptor-like receptor, receptor activity modifying protein 1 and receptor component protein) are found to colocalize in the smooth muscle cells of intracranial arteries and in large-sized neurons in the trigeminal ganglion. The CGRP receptor has also been localized within parts of the brain and the brainstem. The aim of this paper is to review recent localization studies of CGRP and its receptor components within the nervous system and to discuss whether these sites could be possible targets for the CGRP-receptor antagonists.
Keywords: brainstem, calcitonin gene-related peptide, calcitonin gene-related peptide antagonists, migraine, trigeminal ganglion
Introduction
Migraine
Migraine is defined as a neurovascular disorder affecting more than 10% of the global population with a prevalence of 15–18% in females and 6–9% in males. The disorder is characterized by attacks of severe pulsating headache associated with nausea and vomiting and, in some cases, preceded by an aura (migraine with aura). Neurological symptoms including photophobia, phonophobia, scintillations, numbness and weakness are common. Migraine can be triggered by both internal and external triggers. In early studies migraine was first seen as a vascular disorder [Wolff, 1938] and then as a neurogenic disorder [Moskowitz, 1993; Moskowitz et al. 1979]. Currently, migraine is considered as a neurovascular disorder involving activation of the trigemino-vascular system with disturbance in the brainstem/brain. Intense research has been carried out to identify signal molecules associated with the trigeminal system; the only neuronal messenger so far reliably demonstrated in migraine attacks is the neuropeptide, calcitonin gene-related peptide (CGRP) [Durham, 2006; Goadsby et al. 1988].
The role of CGRP in migraine
CGRP is a 37 amino acid neuropeptide and a potent vasodilator neuropeptide, which also has a role in the transmission of nociceptive information [Goadsby, 2007; Gulbenkian et al. 2001; Poyner, 1992; Edvinsson et al. 1987]. There are two forms of this peptide, αCGRP, which is predominantly expressed in the nervous system and βCGRP, which is primarily expressed in the enteric sensory system. In the central nervous system (CNS), CGRP is expressed in several regions such as the striatum, amygdale, hypothalamus, colliculi, brainstem, cerebellum and the trigeminal complex [Hokfelt et al. 1992; Skofitsch and Jacobowitz, 1985]. Moreover, CGRP is found in primary spinal afferent C and Aδ fibres, which project to the brainstem. CGRP acts at second-order neurons in the trigeminal nucleus caudalis (TNC) and at C1–2 levels, to transmit pain signals to the thalamus and higher cortical pain regions [Goadsby, 2007]. Early autoradiographic studies have shown CGRP-binding sites in the rat cerebellum, hippocampus, amygdale, cortex, brainstem and spinal cord [Sexton et al. 1988; Inagaki et al. 1986].
The potential role of CGRP in migraine pathophysiology was suggested 20 years ago [Edvinsson and Goadsby, 1990; Goadsby et al. 1990], and several studies have since then revealed the correlation between migraine and cranial release of CGRP. Experimental and clinical studies have shown that there is an increased level of trigeminal system-released CGRP during migraine attacks [Bellamy et al. 2006; Goadsby and Edvinsson, 1993; Goadsby et al. 1990]. The most important evidence for the role of CGRP in migraine pain came recently from the development of CGRP-receptor antagonists [Ho et al. 2008a, 2008b; Olesen et al. 2004].
CGRP-receptor antagonists
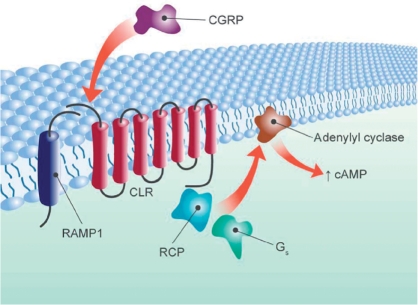
The CGRP-receptor antagonists are a new class of antimigraine drug, which act by blocking the action of CGRP on the CGRP-receptor complex (Figure 1). The receptor for CGRP has been identified as a G-protein-coupled receptor of the B-subtype [Hay et al. 2008]. The functional receptor consists of a complex of a seven transmembrane spanning protein, calcitonin receptor-like receptor (CLR), a single transmembrane-spanning protein designated receptor activity modifying protein (RAMP)1 [Mclatchie et al. 1998], and an intracellular protein, receptor component protein (RCP) [Evans et al. 2000]. RAMP1 is involved in receptor trafficking and is required for CGRP binding to CLR, whereas the interaction of CLR with other RAMP proteins, RAMP2 or RAMP3, forms adrenomedullin receptors [Zhang et al. 2007; Foord and Marshall, 1999; Mclatchie et al. 1998].
Figure 1.
Schematic view of the CGRP receptor with its components. The receptor for CGRP is a G-protein-coupled receptor of the B-subtype. The functional receptor consists of CLR, RAMP1 and RCP. CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like receptor; RAMP1, receptor activity modifying protein 1; RCP, receptor component protein.
Olcegepant (BIBN4096BS) was the first developed CGRP-receptor antagonist that showed clinical efficacy in intravenously administered treatment of acute migraine [Doods et al. 2007]. However, due to its low oral bioavailability, the development of this compound was terminated. Recently, telcagepant (MK-0974) was developed and is the first orally active CGRP-receptor antagonist that is effective in the acute treatment of migraine [Edvinsson and Linde, 2010]. In phase III trials, acute use of telcagepant was shown to have fewer side-effects than the currently used antimigraine drugs, 5-hydroxytryptamine (HT)1B/1D agonists (triptans) [Ho et al. 2008a, 2008b]. The most important difference from the triptans is that telcagepant does not appear to constrict intracranial [Edvinsson et al. 2010] or coronary blood vessels [Chan et al. 2010; Lynch et al. 2010].
The CGRP-receptor antagonists have opened a possible new option in migraine treatment [Edvinsson, 2008]. Consequently, many scientific questions have arisen, which need to be addressed. It is of great importance to clarify where the CGRP receptor is expressed and on which possible sites telcagepant has its therapeutic effect. The trigemino-vascular system is without doubt an interesting area for this because of its important role in migraine pathology [May and Goadsby, 1999]. Recent data demonstrate that there are several regions in the CNS that could play a role in nociception and in migraine pathology. The distribution of the CGRP receptors within these areas is discussed in the following.
Distribution of the CGRP receptor
Vascular sites
As described before, CGRP is one of the most potent vasodilators and almost all vasculatures are innervated by CGRP-containing nerve fibres [Edvinsson et al. 1988]; the intracranial CGRP fibres originate in the trigeminal ganglion. Distribution studies have shown the presence of the receptor components, CLR and RAMP1, in human middle meningeal, middle cerebral, pial and superficial temporal arteries [Oliver et al. 2002], which is in accordance with CLR and RAMP1 mRNA expression in these vessels [Sams and Jansen-Olesen, 1998; Edvinsson et al. 1997]. In rat dura mater, CLR and RAMP1 have been localized to the smooth muscle layer of arterial blood vessels and mast cells [Lennerz et al. 2008]. In human cranial vessels, the receptor components are expressed in the smooth muscle layer (Figure 2) [Edvinsson et al. 2010; Oliver et al. 2002]. In some studies, the presence of CLR mRNA has also been demonstrated in primary cultures of endothelial cells [Moreno et al. 1999], there is observation of ‘CGRPR-1’ immunoreactivity in the endothelium of human coronary and bronchial vessel endothelial cells [Hagner et al. 2001]. However, RAMP1 expression is absent in most endothelial cells [Edvinsson et al. 2010; Oliver et al. 2002], which reflects a minor role of the endothelial cells since CLR and RAMP1 must be expressed together to act as a functional CGRP receptor. This is in agreement with functional studies where CGRP-induced relaxation is independent of the endothelium [Edvinsson et al. 1998, 1985]. Recently, the effect of telcagepant was studied on CGRP-induced vasodilatation on human cerebral and meningeal arteries, where telcagepant was able to block the vasodilatory effect of αCGRP on these blood vessels [Edvinsson et al. 2010]. The distributional and functional studies together suggest that the cranial arteries are a possible site of action of telcagepant in migraine treatment.
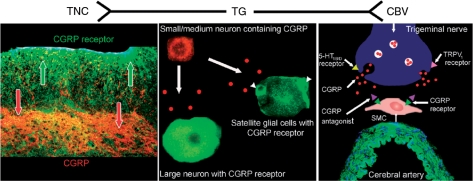
Figure 2.
Expression of CGRP and its receptor within the trigemino-vascular system. TG is in the centre and peripheral parts with CBV to the right and TNC to the left. In the TG, the CGRP is stored in small to medium-sized neurons whereas large neurons do not express CGRP. The CGRP receptor is localized in large neurons and satellite glial cells (arrowheads). The wall of the CBV has CGRP-containing nerves that transmit CGRP TO receptors in the SMC. In the TNC, fibres express CGRP and the CGRP receptor, where CGRP may act presynaptically. CBV, cerebral or meningeal blood vessel; CGRP, calcitonin gene-related peptide; SMC, smooth muscle cells; TG, trigeminal system with ganglion; TNC, trigeminal nucleus caudalis.
Moreover, CGRP is an important peptide in the cardiovascular system; that is, the human coronary circulation is innervated with CGRP-positive fibres [Gulbenkian et al. 1993]. As in the cranial arteries, CLR and RAMP1 are expressed in the coronary smooth muscle cell layer but not in the endothelial cells [Chan et al. 2010]. Our in vitro study has shown that telcagepant does not cause any vasoconstriction of the coronary arteries, which suggests that telcagepant is unlikely to cause coronary side effects, opposed to the 5-HT1B/D receptor agonists [Chan et al. 2010]. In vivo, CGRP antagonists had no effect on coronary vascular function in dogs with ‘ischaemia’ but a 5-HT1B/D receptor agonist caused more severe ischaemia [Lynch et al. 2009; Regan et al. 2009].
No difference in the expression of CGRP-receptor components was found when comparing human proximal and distal coronary arteries [Chan et al. 2010; Gupta et al. 2006]. It remains to be evaluated if there is any difference between cranial and coronary arteries in the expression of the CGRP receptor. The functional studies suggest more CGRP receptors in cranial arteries based on the pharmacology with more potent and stronger vasodilator effects in the cerebral and meningeal versus coronary arteries.
Trigeminal ganglion
The trigeminal ganglion consists of bipolar neurons of different cell sizes and two types of glial cells; satellite glial cells and Schwann cells. About 50% of the neurons in human and rat trigeminal ganglia express CGRP [Eftekhari et al. 2010b; Lennerz et al. 2008; Tajti et al. 1999]. CGRP co-expresses with substance P and 5-HT1B/D receptors [Hou et al. 2001]. The distribution of the receptor components in the rat trigeminal ganglion has been demonstrated by immunohistochemistry [Lennerz et al. 2008], and recently by our group in the human trigeminal ganglion [Eftekhari et al. 2010b]. We found that 37% of neurons express CLR and 36% express RAMP1 and the receptor components were co-expressed in most cases. In human and rat trigeminal ganglia, CGRP was expressed in small to medium-sized neurons. These cells lacked expression of the CGRP-receptor components (Figure 2). The receptor components were instead found in CGRP-negative, large neurons and some satellite glial cells [Eftekhari et al. 2010b]. The combination of CGRP and the receptor components was uncommon and then only found in small to medium-sized cells. This suggests a low presence of putative autoreceptors within the human and rat trigeminal ganglion. Neurons only expressing one of the receptor components were also observed, indicating that there might be situations when the CGRP receptor is not expressed as a functional receptor. However, this needs to be further evaluated.
Apart from the difference in cell size of neurons expressing CGRP, CLR and RAMP1, there is a difference in thickness of fibres expressing CGRP and the receptor components. CGRP is found in thin fibres with a ‘pearl-like’ structure, while the receptor components are expressed in thicker fibres. The receptor components co-expressed with a marker for neurofilaments of molecular weight 160–200 kDa, suggesting that the receptor-positive fibres belong to Aδ-fibres while the thin fibres may be of the C-type of unmyelinated sensory nerves [Eftekhari et al. 2010b].
Interestingly, both immunohistochemical and in vitro studies have shown that the glial cells of the trigeminal ganglion express CLR and RAMP1 [Eftekhari et al. 2010b; Vause and Durham, 2010; Lennerz et al. 2008; Li et al. 2008]. In our work we observed CLR and RAMP1 expression in the cytoplasm of satellite glial cells, but not in the Schwann cells. Several studies have pointed out a putative, essential role of glial cells in the trigeminal ganglion [Dublin and Hanani, 2007; Hanani, 2005], where this cell type communicates with the neurons via gap junctions [Thalakoti et al. 2007]. Furthermore, nitric oxide and pro-inflammatory cytokines can be released from the satellite glial cells when CGRP is released within the ganglion [Capuano et al. 2009; Li et al. 2008; Thalakoti et al. 2007]. In a recent study it was shown that CGRP treatment increased the release of many cytokines and mRNA levels of proteins involved in mitogen-activated protein kinase signalling in cultured trigeminal ganglia enriched with glia [Kristiansen and Edvinsson, 2010; Vause and Durham, 2010]. The function of the localization of CGRP receptor in satellite glial cells needs to be evaluated, however these results suggest that these cells could play a role in migraine pathology and, together with the trigeminal neurons, be a site of action for the CGRP-receptor antagonists.
Brainstem and the spinal cord
It is thought that CGRP acts postjunctionally on second-order neurons to transmit pain signals centrally via the brainstem and midbrain to the thalamus and higher cortical regions. Neurons of the trigeminal ganglion have further connections with neurons in the TNC in the brainstem and in related extensions down to the C1–2 level [Liu et al. 2009]. Several studies have investigated the role of the brainstem in migraine pathology and its possible site of action for the CGRP-receptor antagonist.
It has been hypothesized that brainstem stimulation can cause activation of the trigemino-vascular system, resulting in CGRP-dependent vasodilatation [Just et al. 2005]. In patients, a migraine-active region in the brainstem has been demonstrated with positron emission tomography (PET) [Goadsby, 2005; Diener, 1997; Weiller et al. 1995]. Consequently, studies have focused on detailed mapping of CGRP and its receptor in the brainstem.
Early immunohistochemical studies revealed the presence of strongly positive CGRP fibres in the TNC and spinal cord in different species [Smith et al. 2002; Unger and Lange, 1991; Gibson et al. 1984]. The CGRP-receptor components have been localized in the superficial laminae (in glomeruli-like structures), partially colocalizing with CGRP. No CGRP, CLR or RAMP1 positive neurons are found in the rat TNC [Lennerz et al. 2008], which is in agreement with a study of rat spinal cord [Cottrell et al. 2005]. Positive neurons for CGRP and its receptor have been found in the dorsal root of ganglia (DRG), where low levels of CLR and RAMP1 were expressed in the soma of neurons. The receptor components were mostly expressed in larger neurons, co-expressed with a marker for Aδ fibres, and the combination of CGRP with CLR occurred in small-sized neurons and bound to a marker for C fibres [Cottrell et al. 2005]. The difference in cell size expressing CGRP and the receptor components in DRG is similar to the findings in rat and human trigeminal ganglia. In the dorsal horn of rat spinal cord, CLR and RAMP1 were detected in fibres within laminae I and II. The presence of receptor components in the spinal cord is supported by the mRNA expression of RAMP1 and RCP, detected with specific oligonucleotides for in situ hybridization [Oliver et al. 2001].
In rat TNC, it is suggested that CGRP and its receptor components are localized in terminals from primary afferents [Lennerz et al. 2008]. In contrast, CLR did not colocalize with neuropeptides of primary spinal afferents in the dorsal horn of rat [Cottrell et al. 2005].
We are presently examining the expression of CLR and RAMP1 in the human TNC. Preliminary results show that the receptor components are distributed predominantly on fibres in the spinal trigeminal tract, with some fibres spanning into the superficial laminae, where a strong CGRP-positive network is found (Figure 2). No neuronal cell bodies positive for CGRP or its receptor were found within the human TNC [Eftekhari and Edvinsson, 2010]. These results suggest that CGRP and the receptor components occur on nerve terminals, where CGRP may act presynaptically. However, a better understanding of the different fibre types expressing CGRP, CLR and RAMP1 in brainstem is needed.
Cerebellum
The cerebellum is important in modulating many cortical motor and sensory inputs. The cerebellum exerts an inhibitory control in the cerebral cortex and thus may play an important role in the filtering of sensory inputs [Strata et al. 2009]. A reduction in cerebellar inhibition in migraineurs with aura has been demonstrated [Brighina et al. 2009].
Interestingly, the activation of cerebellar regions has been demonstrated by PET of patients during migraine attacks [Bahra et al. 2001; Weiller et al. 1995]. However, no explanation for the activation has emerged. The cerebellum is of interest in migraine research since upcoming studies suggest a role of the cerebellum in migraine pathology [Vincent and Hadjikhani, 2007]. In a review Vincent and Hadjikhani describe how spreading depression, cerebellar dysfunction and familial hemiplegic migraine (FHM) are connected to the cerebellum and migraine [Vincent and Hadjikhani, 2007].
Spreading depression, which consists of a spreading wave of depolarization associated with a reduction of cortical activity, has been related to migraine. This has been recorded in various tissues including the cerebellum [Vincent and Hadjikhani, 2007]. FHM is a rare autosomal dominant type of migraine. FHM1 is one subtype that is related to mutations of the CACNA-1A gene, coding for a subunit of the high voltage-gated P/Q calcium channels [Pietrobon, 2007]. This channel plays a pivotal role in neurotransmitter release and influences neuronal excitability. P/Q calcium channels are expressed throughout the CNS, particularly in the Purkinje cells of the cerebellum [Vincent and Hadjikhani, 2007]. Interestingly, it has been shown that CGRP and its receptor are expressed in the cerebellum and the Purkinje cells [Eftekhari et al. 2010a].
CGRP expression in cerebellar Purkinje cells and its elaborated dendrite tree has previously been demonstrated by immunohistochemistry [Kawai et al. 1985]. Furthermore, CGRP distribution in different developmental stages in rats has been investigated [Morara et al. 2001, 2000, 1989]. It has been shown that CGRP is transiently expressed in cerebellar climbing fibres [Morara et al. 2001; Gregg et al. 1999], while its receptor is suggested to be expressed in cerebellar glial cells [Morara et al. 2008, 1998]. The receptor localization within the cerebellum reported above is not in agreement with a recent detailed immunohistochemial study on rat cerebellum, performed in our laboratory [Eftekhari et al. 2010a]. Our study revealed that CGRP immunoreactivity is only observed in the cerebellar Purkinje cell bodies as intracellular granular staining, similar to the staining pattern of the neurons in the trigeminal ganglion [Eftekhari et al. 2010b]. The receptor components, CLR and RAMP1, were detected on the surface of the Purkinje cell bodies and in their processes. The receptor components co-expressed in the Purkinje cell bodies suggests the presence of functional CGRP receptors within the cerebellum. The glial cells of the cerebellum were also studied in detail with stacking images confocal microscopy (a three-dimensional study). There receptor components were not expressed in the glial cells.
The discrepancies of the CGRP-receptor components localization in the cerebellar glial cells could be due to several factors. In the early studies of Morara and colleagues (Morara et al. 2000, 1998), they used an antibody against the CGRP receptor. At that time the CGRP receptor was not well characterized [Hay et al. 2008]. Subsequently, it has been shown that the functional CGRP receptor consists of three different components, CLR, RAMP1 and RCP. In our study, we were able to use newly produced specific antibodies against two of the receptor components; CLR and RAMP1 (Eftekhari et al. 2010b). Morara and colleagues later used an antibody against RCP. However, this study was performed on cerebellar astrocytes from neonatal mice [Morara et al. 2008], which raises the question about species and methodology differences. It remains to study the expression of CGRP and its receptor in other species. It is important to note that Morara and colleagues studied the expression of the CGRP receptor in the glial cells during development stages of the rat. We performed our study on adult rats. Despite the discrepancies, the studies clearly point towards a functional role of CGRP in the cerebellum, where a CGRP-receptor antagonist could act on a cerebellar site.
Conclusions
Migraine is a complex neurovascular disorder with the neuropeptide CGRP being implicated in the underlying migraine pathology. The CGRP receptor has long been regarded as an important target for the development of antimigraine therapies. Telcagepant is an orally available CGRP-receptor antagonist currently in late stage clinical testing for the treatment of acute migraine [Ho et al. 2008a, 2008b]. It remains to be determined if the antimigraine action of this CGRP-receptor antagonist is mediated via central or peripheral mechanisms, or both. Drug studies have shown that high doses of CGRP-receptor antagonists are needed to elicit therapeutic effects. It has been suggested therefore that the CGRP antagonists need to penetrate the blood–brain barrier to access their target and achieve acute antimigraine efficacy [Edvinsson and Ho, 2010; Edvinsson and Tfelt-Hansen, 2008]. Telcagepant is a P-glycoprotein substrate, which thereby reduces its brain penetration [Ho et al. 2010]. In clinical trials it was shown that 150 mg and 300 mg were effective doses of telcagepant [Ho et al. 2010; Connor et al. 2009]. However, at this time, it is not known if these clinical doses are enough for central action and to what extent the substance is able to penetrate the blood–brain barrier. RAMP1 mRNA has been demonstrated in the subfornical organ and area postrema, which are circumventricular organs lacking blood–brain barrier characteristics [Barth et al. 2004; Ueda et al. 2001]. Such sites may not need full penetration of telcagepant and could be a site of action. However, functional CGRP receptor (CLR and RAMP1) expression in these areas must be clarified.
Localization studies of CGRP and its receptor components are discussed in this review. Sites of CGRP and its receptor expression are found both in the periphery and CNS, suggesting both vascular and neuronal actions. In summary, we suggest that the trigemino-vascular system is the key target together with parts of the brain in migraine pathology and that the CGRP-receptor antagonists may act on these sites in the treatment of migraine. The combination of localization studies and CGRP-receptor PET-tracer experiments may help to clarify further the exact sites of action of the newly developed CGRP-receptor antagonists.
Acknowledgments
Thanks are due to Christopher A. Salvatore, Merck & Co. and Karin Warfvinge, Warfvinge Science Support (www.sciencesupport.se) for valuable comments on the manuscript.
Funding
This research was supported by Swedish Research Council and Lundbeck Foundation.
Conflicts of interest statement
None declared.
References
- Bahra A., Matharu M.S., Buchel C., Frackowiak R.S., Goadsby P.J. (2001) Brainstem activation specific to migraine headache. Lancet 357: 1016–1017 [DOI] [PubMed] [Google Scholar]
- Barth S.W., Riediger T., Lutz T.A., Rechkemmer G. (2004) Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/B subtypes and receptor-activity modifying proteins in the rat. Brain Res 997: 97–102 [DOI] [PubMed] [Google Scholar]
- Bellamy J.L., Cady R.K., Durham P.L. (2006) Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 46: 24–33 [DOI] [PubMed] [Google Scholar]
- Brighina F., Palermo A., Panetta M.L., Daniele O., Aloisio A., Cosentino G., et al. (2009) Reduced cerebellar inhibition in migraine with aura: A TMS study. Cerebellum 8: 260–266 [DOI] [PubMed] [Google Scholar]
- Capuano A., De Corato A., Lisi L., Tringali G., Navarra P., Dello Russo C. (2009) Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: Relevance for migraine pathology. Mol Pain 5: 43–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Edvinsson L., Eftekhari S., Kimblad P.O., Kane S., Lynch J., et al. (2010) Characterization of the CGRP receptor antagonist telcagepant (MK-0974) in human isolated coronary arteries. J Pharmacol Exp Ther 334: 746–752 [DOI] [PubMed] [Google Scholar]
- Connor K.M., Shapiro R.E., Diener H.C., Lucas S., Kost J., Fan X., et al. (2009) Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G.S., Roosterman D., Marvizon J.C., Song B., Wick E., Pikios S., et al. (2005) Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490: 239–255 [DOI] [PubMed] [Google Scholar]
- Diener H.C. (1997) Positron emission tomography studies in headache. Headache 37: 622–625 [DOI] [PubMed] [Google Scholar]
- Doods H., Arndt K., Rudolf K., Just S. (2007) CGRP antagonists: Unravelling the role of CGRP in migraine. Trends Pharmacol Sci 28: 580–587 [DOI] [PubMed] [Google Scholar]
- Dublin P., Hanani M. (2007) Satellite glial cells in sensory ganglia: Their possible contribution to inflammatory pain. Brain Behav Immun 21: 592–598 [DOI] [PubMed] [Google Scholar]
- Durham P.L. (2006) Calcitonin gene-related peptide (CGRP) and migraine. Headache 46(Suppl 1): S3–S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L. (2008) CGRP-receptor antagonism in migraine treatment. Lancet 372: 2089–2090 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Brodin E., Jansen I., Uddman R. (1988) Neurokinin a in cerebral vessels: Characterization, localization and effects in vitro. Regul Pept 20: 181–197 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Cantera L., Jansen-Olesen I., Uddman R. (1997) Expression of calcitonin gene-related peptide1 receptor mRNA in human trigeminal ganglia and cerebral arteries. Neurosci Lett 229: 209–211 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Chan K.Y., Eftekhari S., Nilsson E., De Vries R., Säveland H., et al. (2010) Effect of the calcitonin gene-related (CGRP) receptor antagonist telcagepant in human cranial arteries. Cephalalgia 30: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Ekman R., Jansen I., Mcculloch J., Uddman R. (1987) Calcitonin gene-related peptide and cerebral blood vessels: Distribution and vasomotor effects. J Cereb Blood Flow Metab 7: 720–728 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B.B., Hamel E., Jansen I., Verrecchia C. (1985) Perivascular peptides relax cerebral arteries concomitant with stimulation of cyclic adenosine monophosphate accumulation or release of an endothelium-derived relaxing factor in the cat. Neurosci Lett 58: 213–217 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Goadsby P.J. (1990) Extracerebral manifestations in migraine. A peptidergic involvement? J Intern Med 228: 299–304 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Gulbenkian S., Barroso C.P., Cunhae Sa E., Sa M., Polak J.M., Mortensen A., et al. (1998) Innervation of the human middle meningeal artery: Immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides 19: 1213–1225 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Ho T.W. (2010b) CGRP receptor antagonism and migraine. Neurotherapeutics 7: 164–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Linde M. (2010) New drugs in migraine treatment and prophylaxis: Telcagepant and topiramate. Lancet 376: 645–655 [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Tfelt-Hansen P. (2008) The blood–brain barrier in migraine treatment. Cephalalgia 28: 1245–1258 [DOI] [PubMed] [Google Scholar]
- Eftekhari, S. and Edvinsson, L. (2010) Immunoreactivity of calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus. In European Headache and Migraine Trust International Congress, October, abstract.
- Eftekhari, S., Edvinsson, L., Salvatore, C.A. and Warfvinge, K. (2010a) Cerebellar Distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. In European Headache and Migraine Trust International Congress, October, abstract. [DOI] [PubMed]
- Eftekhari S., Salvatore C.A., Calamari A., Kane S.A., Tajti J., Edvinsson L. (2010b) Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169: 683–696 [DOI] [PubMed] [Google Scholar]
- Evans B.N., Rosenblatt M.I., Mnayer L.O., Oliver K.R., Dickerson I.M. (2000) CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275: 31438–31443 [DOI] [PubMed] [Google Scholar]
- Foord S.M., Marshall F.H. (1999) RAMPs: Accessory proteins for seven transmembrane domain receptors. Trends Pharmacol Sci 20: 184–187 [DOI] [PubMed] [Google Scholar]
- Gibson S.J., Polak J.M., Bloom S.R., Sabate I.M., Mulderry P.M., Ghatei M.A., et al. (1984) Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci 4: 3101–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P.J. (2005) Advances in the understanding of headache. Br Med Bull 73–74: 83–92 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J. (2007) Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med 13: 39–44 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Edvinsson L. (1993) The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33: 48–56 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Edvinsson L., Ekman R. (1988) Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 23: 193–196 [DOI] [PubMed] [Google Scholar]
- Goadsby P.J., Edvinsson L., Ekman R. (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28: 183–187 [DOI] [PubMed] [Google Scholar]
- Gregg K.V., Bishop G.A., King J.S. (1999) Fine structural analysis of calcitonin gene-related peptide in the mouse inferior olivary complex. J Neurocytol 28: 431–438 [DOI] [PubMed] [Google Scholar]
- Gulbenkian S., Saetrum Opgaard O., Ekman R., Costa Andrade N., Wharton J., Polak J.M., et al. (1993) Peptidergic innervation of human epicardial coronary arteries. Circ Res 73: 579–588 [DOI] [PubMed] [Google Scholar]
- Gulbenkian S., Uddman R., Edvinsson L. (2001) Neuronal messengers in the human cerebral circulation. Peptides 22: 995–1007 [DOI] [PubMed] [Google Scholar]
- Gupta S., Mehrotra S., Villalon C.M., Garrelds I.M., De Vries R., Van Kats J.P., et al. (2006) Characterisation of CGRP receptors in human and porcine isolated coronary arteries: Evidence for CGRP receptor heterogeneity. Eur J Pharmacol 530: 107–116 [DOI] [PubMed] [Google Scholar]
- Hagner S., Haberberger R., Kummer W., Springer J., Fischer A., Bohm S., et al. (2001) Immunohistochemical detection of calcitonin gene-related peptide receptor (CGRP)-1 in the endothelium of human coronary artery and bronchial blood vessels. Neuropeptides 35: 58–64 [DOI] [PubMed] [Google Scholar]
- Hanani M. (2005) Satellite glial cells in sensory ganglia: From form to function. Brain Res Brain Res Rev 48: 457–476 [DOI] [PubMed] [Google Scholar]
- Hay D.L., Poyner D.R., Quirion R. (2008) International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev 60: 143–145 [DOI] [PubMed] [Google Scholar]
- Ho T.W., Edvinsson L., Goadsby P.J. (2010) CGRP receptor antagonism provides new insights into migraine pathophysiology. Nat Rev Neurol 6: 573–582 [DOI] [PubMed] [Google Scholar]
- Ho T.W., Ferrari M.D., Dodick D.W., Galet V., Kost J., Fan X., et al. (2008a) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: A randomised, placebo-controlled, parallel-treatment trial. Lancet 372: 2115–2123 [DOI] [PubMed] [Google Scholar]
- Ho T.W., Mannix L.K., Fan X., Assaid C., Furtek C., Jones C.J., et al. (2008b) Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70: 1304–1312 [DOI] [PubMed] [Google Scholar]
- Hokfelt T., Arvidsson U., Ceccatelli S., Cortes R., Cullheim S., Dagerlind A., et al. (1992) Calcitonin gene-related peptide in the brain, spinal cord, and some peripheral systems. Ann N Y Acad Sci 657: 119–134 [DOI] [PubMed] [Google Scholar]
- Hou M., Kanje M., Longmore J., Tajti J., Uddman R., Edvinsson L. (2001) 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: Co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 909: 112–120 [DOI] [PubMed] [Google Scholar]
- Inagaki S., Kito S., Kubota Y., Girgis S., Hillyard C.J., Macintyre I. (1986) Autoradiographic localization of calcitonin gene-related peptide binding sites in human and rat brains. Brain Res 374: 287–298 [DOI] [PubMed] [Google Scholar]
- Just S., Arndt K., Doods H. (2005) The role of CGRP and nicotinic receptors in centrally evoked facial blood flow changes. Neurosci Lett 381: 120–124 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Takami K., Shiosaka S., Emson P.C., Hillyard C.J., Girgis S., et al. (1985) Topographic localization of calcitonin gene-related peptide in the rat brain: An immunohistochemical analysis. Neuroscience 15: 747–763 [DOI] [PubMed] [Google Scholar]
- Kristiansen, K.A. and Edvinsson, L. (2010) Neurogenic inflammation, a study of rat trigeminal ganglion. J Headache Pain; DOI 10.1007/s10194-010-0260-x. [DOI] [PMC free article] [PubMed]
- Lennerz J.K., Ruhle V., Ceppa E.P., Neuhuber W.L., Bunnett N.W., Grady E.F., et al. (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507: 1277–1299 [DOI] [PubMed] [Google Scholar]
- Li J., Vause C.V., Durham P.L. (2008) Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res 1196: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Broman J., Zhang M., Edvinsson L. (2009) Brainstem and thalamic projections from a craniovascular sensory nervous centre in the rostral cervical spinal dorsal horn of rats. Cephalalgia 29: 935–948 [DOI] [PubMed] [Google Scholar]
- Lynch J., Regan C., Edvinsson L., Hargreaves R., Kane S. (2010) Comparison of the vasoconstrictor effects of the calcitonin gene-related peptide (CGRP) receptor antagonist telcagepant (MK-0974) and zolmitriptan in human isolated coronary arteries. J Cardiovasc Pharmacol 55: 518–521 [DOI] [PubMed] [Google Scholar]
- Lynch J.J., Shen Y.T., Pittman T.J., Anderson K.D., Koblan K.S., Gould R.J., et al. (2009) Effects of the prototype serotonin 5-HT(1B/1D) receptor agonist sumatriptan and the calcitonin gene-related peptide (CGRP) receptor antagonist CGRP(8–37) on myocardial reactive hyperemic response in conscious dogs. Eur J Pharmacol 623: 96–102 [DOI] [PubMed] [Google Scholar]
- May A., Goadsby P.J. (1999) The trigeminovascular system in humans: Pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 19: 115–127 [DOI] [PubMed] [Google Scholar]
- Mclatchie L.M., Fraser N.J., Main M.J., Wise A., Brown J., Thompson N., et al. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393: 333–339 [DOI] [PubMed] [Google Scholar]
- Morara S., Provini L., Rosina A. (1989) CGRP expression in the rat olivocerebellar system during postnatal development. Brain Res 504: 315–319 [DOI] [PubMed] [Google Scholar]
- Morara S., Rosina A., Provini L., Forloni G., Caretti A., Wimalawansa S.J. (2000) Calcitonin gene-related peptide receptor expression in the neurons and glia of developing rat cerebellum: An autoradiographic and immunohistochemical analysis. Neuroscience 100: 381–391 [DOI] [PubMed] [Google Scholar]
- Morara S., Van Der Want J.J., De Weerd H., Provini L., Rosina A. (2001) Ultrastructural analysis of climbing fiber-Purkinje cell synaptogenesis in the rat cerebellum. Neuroscience 108: 655–671 [DOI] [PubMed] [Google Scholar]
- Morara S., Wang L.P., Filippov V., Dickerson I.M., Grohovaz F., Provini L., et al. (2008) Calcitonin gene-related peptide (CGRP) triggers Ca2+ responses in cultured astrocytes and in Bergmann glial cells from cerebellar slices. Eur J Neurosci 28: 2213–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morara S., Wimalawansa S.J., Rosina A. (1998) Monoclonal antibodies reveal expression of the CGRP receptor in Purkinje cells, interneurons and astrocytes of rat cerebellar cortex. Neuroreport 9: 3755–3759 [DOI] [PubMed] [Google Scholar]
- Moreno M.J., Cohen Z., Stanimirovic D.B., Hamel E. (1999) Functional calcitonin gene-related peptide type 1 and adrenomedullin receptors in human trigeminal ganglia, brain vessels, and cerebromicrovascular or astroglial cells in culture. J Cereb Blood Flow Metab 19: 1270–1278 [DOI] [PubMed] [Google Scholar]
- Moskowitz M.A. (1993) Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 43: S16–S20 [PubMed] [Google Scholar]
- Moskowitz M.A., Reinhard J.F., Jr, Romero J., Melamed E., Pettibone D.J. (1979) Neurotransmitters and the fifth cranial nerve: Is there a relation to the headache phase of migraine? Lancet 2: 883–885 [DOI] [PubMed] [Google Scholar]
- Olesen J., Diener H.C., Husstedt I.W., Goadsby P.J., Hall D., Meier U., et al. (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 350: 1104–1110 [DOI] [PubMed] [Google Scholar]
- Oliver K.R., Kane S.A., Salvatore C.A., Mallee J.J., Kinsey A.M., Koblan K.S., et al. (2001) Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci 14: 618–628 [DOI] [PubMed] [Google Scholar]
- Oliver K.R., Wainwright A., Edvinsson L., Pickard J.D., Hill R.G. (2002) Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab 22: 620–629 [DOI] [PubMed] [Google Scholar]
- Pietrobon D. (2007) Familial hemiplegic migraine. Neurotherapeutics 4: 274–284 [DOI] [PubMed] [Google Scholar]
- Poyner D.R. (1992) Calcitonin gene-related peptide: Multiple actions, multiple receptors. Pharmacol Ther 56: 23–51 [DOI] [PubMed] [Google Scholar]
- Regan C.P., Stump G.L., Kane S.A., Lynch J.J., Jr (2009) Calcitonin gene-related peptide receptor antagonism does not affect the severity of myocardial ischemia during atrial pacing in dogs with coronary artery stenosis. J Pharmacol Exp Ther 328: 571–578 [DOI] [PubMed] [Google Scholar]
- Sams A., Jansen-Olesen I. (1998) Expression of calcitonin receptor-like receptor and receptor-activity-modifying proteins in human cranial arteries. Neurosci Lett 258: 41–44 [DOI] [PubMed] [Google Scholar]
- Sexton P.M., Mckenzie J.S., Mendelsohn F.A. (1988) Evidence for a new subclass of calcitonin/calcitonin gene-related peptide binding site in rat brain. Neurochem Int 12: 323–335 [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D.M. (1985) Calcitonin gene-related peptide: Detailed immunohistochemical distribution in the central nervous system. Peptides 6: 721–745 [DOI] [PubMed] [Google Scholar]
- Smith D., Hill R.G., Edvinsson L., Longmore J. (2002) An immunocytochemical investigation of human trigeminal nucleus caudalis: CGRP, substance P and 5-HT1D-receptor immunoreactivities are expressed by trigeminal sensory fibres. Cephalalgia 22: 424–431 [DOI] [PubMed] [Google Scholar]
- Strata P., Thach W.T., Ottersen O.P. (2009) New insights in cerebellar function. Introduction. Neuroscience 162: 545–548 [DOI] [PubMed] [Google Scholar]
- Tajti J., Uddman R., Moller S., Sundler F., Edvinsson L. (1999) Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 76: 176–183 [DOI] [PubMed] [Google Scholar]
- Thalakoti S., Patil V.V., Damodaram S., Vause C.V., Langford L.E., Freeman S.E., et al. (2007) Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 47: 1008–1023; discussion 1024–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Ugawa S., Saishin Y., Shimada S. (2001) Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res 93: 36–45 [DOI] [PubMed] [Google Scholar]
- Unger J.W., Lange W. (1991) Immunohistochemical mapping of neurophysins and calcitonin gene-related peptide in the human brainstem and cervical spinal cord. J Chem Neuroanat 4: 299–309 [DOI] [PubMed] [Google Scholar]
- Vause C.V., Durham P.L. (2010) Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: Findings from array analysis. Neurosci Lett 473: 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M., Hadjikhani N. (2007) The cerebellum and migraine. Headache 47: 820–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C., May A., Limmroth V., Juptner M., Kaube H., Schayck R.V., et al. (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1: 658–660 [DOI] [PubMed] [Google Scholar]
- Wolff H.G. (1938) Headache and cranial arteries. Trans Assoc Am Physicians 53: 193–198 [Google Scholar]
- Zhang Z., Winborn C.S., Marquez De Prado B., Russo A.F. (2007) Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 27: 2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]