Abstract
More than 30% of epilepsy patients remain refractory despite the advent of new antiepileptic drugs (AEDs) over two decades. Although a small percentage of these refractory patients may become seizure free when a new AED is added, combined administration of AEDs or the application of novel AEDs is the most common therapeutic option when surgical treatment cannot be offered. The most recently approved AED in Europe and the USA is lacosamide (LCM), which offers new mechanisms of action and favorable safety profiles. This article reviews LCM’s molecular mechanisms of action, pharmacokinetic profiles as well as efficacy and safety from phase II and III clinical trials. In addition, comparison between LCM and other existing AEDs is discussed.
Keywords: partial seizures, new anticonvulsant, lacosamide, Vimpat, sodium channels, slow inactivation
Introduction
Epilepsy is one of the most common neurological disorders affecting up to two percent of the population worldwide [Centers for Disease Control and Prevention, 1994]. Treatment of epilepsy often imposes an exposure to various antiepileptic drugs (AEDs) and requires long-term commitment and compliance from the patient [Chung et al. 2007b]. Despite the advent of new AEDs over the past 15 years, approximately 30% of epilepsy patients experience recurrent seizures [Perucca, 2007; Kwan and Brodie, 2000] and many experience undesirable side effects [Guevara et al. 2005; Deckers et al. 2003]. Therefore there are still unmet needs for the treatment of epilepsy and there remains a need to develop new AEDs that could reduce seizure frequency and severity as well as improve tolerability and safety. For those patients with medically refractory epilepsy, combined administration of AEDs or the use of new AEDs is an appropriate therapeutic option.
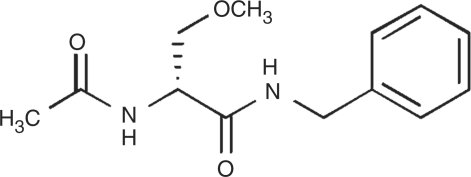
Lacosamide (LCM), (R)-2-acetamido-N-benzyl-3-methoxypropionamide, is a functionalized amino acid with a novel anticonvulsant activity (Figure 1) [Hovinga, 2003; Andurkar et al. 1999]. Based on the efficacy and therapeutic index observed in a range of animal models of epilepsy at the NIH Anticonvulsant Screening Program, LCM was subsequently developed as an AED for both oral and intravenous use. Additionally, LCM is available as oral syrup (15 mg/mL) in Europe. LCM was approved as an adjunctive treatment for partial-onset seizures in patients ≥16 years by the European Commission (August 2008) and in patients ≥17 years by the U.S. Food and Drug Administration (October 2008). It is also undergoing clinical evaluation for the monotherapy treatment of diabetic neuropathic pain, fibromyalgia and migraine prophylaxis [Bialer et al. 2009].
Figure 1.
Chemical structure of lacosamide.
Pharmacokinetics
LCM has a linear pharmacokinetic profile with high oral bioavailability [Horstmann et al. 2002]. Studies in healthy volunteers demonstrated that LCM is rapidly and completely absorbed [Doty et al. 2007; Thomas et al. 2006; Cawello et al. 2004]. The rate and extent of absorption are not affected by the presence of food [Cawello et al. 2004]. Peak serum concentrations occur at 1–2 hours after oral intake, and the elimination half-life of LCM is about 13 hours, allowing convenient twice-daily dosing [Bialer et al. 2007; Hovinga, 2003; Horstmann et al. 2002]. LCM intravenous solution is typically administered over 30 minutes and the Cmax is reached at the end of infusion. Studies in healthy volunteers demonstrate bioequivalence for Cmax and AUC for both the 30 and 60 min infusion durations [Kropeit et al. 2004]. Infusion over 15 min was near bioequivalent, with a slightly higher Cmax and equivalence for AUC [Bialer et al. 2009].
LCM has low plasma protein binding (≤15%) and the volume of distribution is approximately 0.6 L/kg, which is similar to body water [Thomas et al. 2006]. The pharmacokinetics of both oral and intravenous LCM are dose-proportional (up to 800 mg), with low intra- and inter-subject variability. Following twice-daily administration of oral LCM, steady-state plasma concentrations are reached after 3 days. The low protein binding of LCM minimizes the potential for displacement of other drugs [Ben-Menachem, 2008], and thus, low potential for drug–drug interactions. Furthermore, LCM has minimal interaction with CYP-450 isoforms [US FDA, 2008], making an effect on the metabolism of other drugs unlikely. In clinical efficacy and safety trials, LCM did not alter drug plasma levels of other AEDs (carbamazepine, oxcarbazepine, gabapentin, lamotrigine, levetiracetam, phenytoin, topiramate, valproic acid and zonisamide). Specific drug interaction studies involving carbamazepine, valproic acid, omeprazole, metformin, digoxin and an oral contraceptive (ethinylestradiol and levonorgestrel) also demonstrated no relevant interactions influence on the pharmacokinetics of these drugs or LCM [Beydoun et al. 2009; Ben-Menachem, 2008].
Lacosamide is primarily eliminated renally as unchanged drug (>40%) and an inactive O-desmethyl metabolite (<30%) [Bialer et al. 2007; Hovinga, 2003; Horstmann et al. 2002]. Although the hepatic isoenzyme 2C19 is mainly responsible for the formation of the O-desmethyl metabolite, co-administration of CYP2C19 inducers or inhibitors did not cause clinically relevant differences in the pharmacokinetics of LCM, indicating that the metabolic pathway involving CYP2C19 is minor. For patients with severe renal impairment (creatinine clearance of ≤30 mL/min) and in patients with endstage renal disease, a maximum dose of 250 mg/day (E.U.) or 300 mg/day (U.S.) is recommended.
Mechanisms of action
LCM demonstrated potent anticonvulsant activity in broad range of animal models of partial onset and pharmacoresistant seizures, generalized tonic-clonic seizures, as well as status epilepticus. Intraperitoneal LCM was effective in preventing seizures in the 6 Hz psychomotor seizure model (ED50 9.99 mg/kg) and audiogenic seizure model (ED50 0.63 mg/kg). LCM of 20 and 50 mg/kg completely prevented tonic convulsions induced by maximal electroconvulsive shock (MES) and 50 mg/kg provided partial protection against clonic convulsions induced by N-methyl-D-aspartate in mice [Beyreuther et al. 2007; Stöhr et al. 2007a]. LCM was also effective in amygdala and hippocampal kindling models. In hippocampal kindling rats, the activity of LCM (25 mg/kg) was superior to that of maximally effective doses of phenytoin (150 mg/kg), carbamazepine (50 mg/kg), valproic acid (250 mg/kg) and ethosuximaide (250 mg/kg) [Beyreuther et al. 2007]. However, LCM was less effective against clonic seizures induced by pentylenetetrazole (EC50 ∼25 mg/kg), bicuculline (EC50 >50 mg/kg), or picrotoxin (EC50 >30 mg/kg) in rodents [Beyreuther et al. 2007; Stöhr et al. 2007a]. LCM was effective in models of status epilepticus, stopping limbic seizures induced by self-sustaining status epilepticus in rats within 15 minutes of administration and preventing their recurrence over the next 24 hours [Beyreuther et al. 2007].
The precise mechanisms by which LCM exerts its antiepileptic effect in humans are not fully understood, but a novel mode of action has been suggested. LCM selectively enhances slow inactivation of voltage-dependent sodium channels without affecting fast inactivation, which may normalize neuronal firing thresholds [Beyreuther et al. 2007]. Classical anticonvulsant drugs such as carbamazepine, phenytoin and lamotrigine act on fast inactivation of voltage-dependent sodium channels [Beyreuther et al. 2007]. In preclinical experiments, lacosamide has also been shown to bind to collapsin response mediator protein 2 (CRMP-2), which is involved in neuronal differentiation, regulation of gene expression, polarization and axonal outgrowth [Beyreuther et al. 2007]. The role of CRMP-2 binding in seizure control is unknown at this time, but it may be a factor in the disease-modifying potential of LCM.
Clinical studies
Three pivotal studies (one phase II and two phase III studies) have been conducted to establish the efficacy and safety of LCM [Ben-Menachem et al. 2007; Chung et al. 2007a; Halasz et al. 2009]. Three doses of LCM (200, 400 and 600 mg/day) were administered as adjunctive therapy for patients with partial epilepsy with or without secondary generalization, with a starting dosage of 50 mg BID, followed by a weekly increase of 100 mg/day to the target dose. Titration phase was followed by 12 week maintenance phase with an option for continued open-label treatment. Patients experiencing intolerable adverse events were allowed one down-titration of 100 mg/day at the end of the titration period. A total of 1294 patients were randomized in three studies with a median age of 38.6 years. The studies were conducted in a refractory population, with 84.4% of subjects taking two or three concomitant AEDs (including substantial numbers on newer AEDs) and 17% being additionally treated with the vagus nerve stimulator. In addition, approximately half of the participants had tried seven or more AEDs in the past [Chung et al. 2008].
The primary assessment of efficacy was based on the change in partial-onset seizure frequency and was evaluated in two ways: (1) the change in seizure frequency per 28 days from baseline to the maintenance period, and (2) the proportion of patients who experienced a 50% or greater reduction in seizure frequency from baseline to maintenance period (50% responder rate). The primary efficacy analysis was conducted on the intent-to-treat (ITT) population, which is defined as all randomized patients who received at least one dose of the trial medication and had at least one post-baseline efficacy assessment.
Efficacy
In the phase II study, the 50% responder rates were 32.7% for 200 mg/d (p = 0.090), 41.1% for 400 mg/d (p = 0.004), and 38.1% for 600 mg/day (p = 0.014), compared with 21.9% for the placebo group [Ben-Menachem et al. 2007]. Percent reduction in seizure frequency per 28 days over placebo was 14.6% in the 200 mg/day group (p = 0.101) and reached statistical significance for both the LCM 400 mg/day (28.4%, p = 0.002) and 600 mg/day (21.3%, p = 0.008) groups.
One of two phase III trials [Halász et al. 2009] was conducted in Europe and Australia, evaluating LCM 200 mg/day and 400 mg/day compared to placebo in a total of 485 randomized patients. Median percent reduction in seizure frequency in the ITT population was 20.5% for placebo, 35.3% for LCM 200 mg/day (p = 0.02) and 36.4% for 400 mg/day (p = 0.03). The 50% responder rate for LCM 400 mg/day (40.5%) was significant (p = 0.01) over placebo (25.8%), but was not for 200 mg/day (35.0%).
The more recent phase III trial [Chung et al. 2007a] was conducted in the US and evaluated LCM 400 mg/day and 600 mg/day compared with placebo in a total of 405 randomized patients. Significant differences in the 50% responder rates from baseline to maintenance were observed in both the 400 and 600 mg/day LCM treatment groups (38.3% and 41.2%; p < 0.001 for both) compared with placebo (18.3%). The median percent reduction in seizure frequency was also significant for both doses in the ITT population: 20.8% for placebo, 37.3% for LCM 400 mg/day (p = 0.008) and 37.8% for LCM 600 mg/day (p = 0.006).
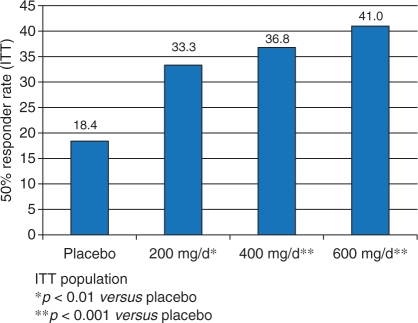
Subsequent analysis of pooled efficacy data from these trials further supports the overall efficacy of LCM at doses of 200–600 mg/day [Ben-Menachem et al. 2009; Chung et al. 2008]. For the pooled analysis, the 50% responder rates per 28 days from baseline to the maintenance period were 22.6% for placebo, 34.1% for LCM 200 mg/day, and 39.7% for LCM 400 mg/day. The median percent reduction in seizure frequency was 18.4% for placebo, 33.3% for LCM 200 mg/day, and 36.8% for LCM 400 mg/day. Overall, the LCM 600 mg/day group showed similar efficacy to the 400 mg/day group (Figure 2). Pooled analysis demonstrates that complete seizure freedom during the maintenance period was achieved in 2.7%, 3.3% and 4.8% of patients randomized to LCM 200, 400 and 600 mg/day, respectively, compared with 0.9% in the placebo group. Data from open-label extension trials suggests that the efficacy of LCM is maintained over time and the retention rate in 12 months was 77%.
Figure 2.
Efficacy of LCM: 50% responder rate from baseline to maintenance period (pooled data).
LCM onset of action appears rapid, since a significant seizure reduction compared with placebo was observed as early as in the first week when patients were receiving 100 mg/day regardless of assigned dose group in the pooled analysis (median percent reduction in seizure frequency: 33.0% versus 19.4%, p < 0.01) [Sperling et al. 2008].
Safety and tolerability
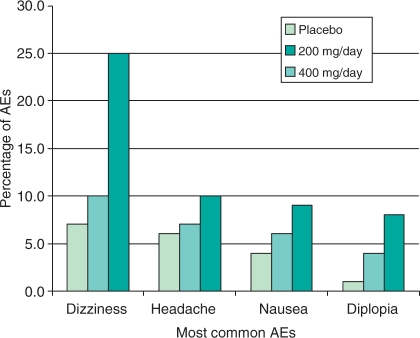
LCM was generally well tolerated in patients with partial-onset seizures, with most treatment-emergent adverse events (TEAEs) being of mild or moderate severity [US FDA, 2008]. The most common TEAEs of oral LCM were dizziness, headache, nausea and diplopia (Figure 3). All of these TEAEs were dose-related except for headache, and reported more often during titration rather than during the maintenance phase. Overall, discontinuation rates due to TEAEs were 8% in LCM 200 mg/day, 17% in 400 mg/day, and 29% in 600 mg/day, compared with 5% of placebo recipients [US FDA, 2008]. According to the interim analysis of the long-term (up to 5.5 years) safety data of LCM, TEAEs were similar to that reported in the initial phase II/III with 11.1% discontinuation rate in LCM recipients [Rosenfeld et al. 2007]. The incidence of somnolence during the treatment period was approximately 7% for placebo and 9% for the total LCM groups, and did not appear to be dose-related. The incidence of rash was low for patients randomized to LCM similar to that reported with placebo (3%). No rashes were serious and all were assessed as mild to moderate in intensity.
Figure 3.
Most common adverse events (AEs) occurring in at least 10% of patients at the approved daily dosages of 200 mg/day and 400 mg/day (pooled data).
Results of clinical laboratory tests and vital sign measurements across treatment groups did not identify any changes that appeared to be associated with LCM. Evaluation of ECG readings demonstrated little change from baseline to the end of maintenance in heart rate, QTc interval or QRS duration for the placebo and LCM groups. A small increase in mean PR interval at the end of maintenance (1.4–6.6 ms increase) was noted. There were no reports of adverse events associated with PR interval prolongation, and the degree of increase is considered to be similar to other AEDs, such as carbamazepine (8–16 ms increase), lamotrigine (5 ms increase), and prebagalin (up to 5 ms increase) [Laville et al. 2008; Kennebäck et al. 1995, 1991; Matsuo et al. 1993].
The tolerability profile of short-term intravenous LCM was similar, and the injection site pain was low. In a 2 day, randomized, double-blind, placebo-controlled study, previously LCM treated patients (n = 60, aged 19–61years) were randomized to either oral LCM (plus placebo infusion) or 30 or 60 minute intravenous LCM infusions (plus oral placebo) [Biton et al. 2008]. The intravenous LCM dosage was the same as the previous oral dosage ranged (200–600 mg/day). TEAEs associated with intravenous LCM were mild or moderate in intensity and included dizziness, headache, back pain and somnolence. Infusion site-related pain was infrequent (0% in 60 min infusion and 11% in 30 min infusion), and did not result in discontinuations of LCM [Biton et al. 2008]. In another open-label study (n = 60), in which LCM was infused faster over 10, 15 or 30 minutes for 2–5 days (200–800 mg/day), the incidence of adverse events was similar with headache (5%, 7%, 8%) and dizziness (5%, 6%, 8%) being most commonly reported [Krauss et al. 2007].
In a human abuse potential study, single dose administration of LCM 800 mg produced subjective euphoria-type responses in 15% of subjects (5/34), compared with 0% in placebo. These euphoria-type responses were similar to those produced by alprazolam, but the duration of the euphoria was shorter. Two other pharmacokinetic studies also showed euphoria-type responses following single and multiple doses of LCM 300 mg and 800 mg (ranging from 6% [2/33] to 25% [3/12]) compared with placebo (0%) [UCB 2008a, 2008b]. Subsequently, LCM is designated as a Schedule IV drug even though the rate of euphoria at therapeutic doses in other clinical studies was less than 1% [US FDA, 2008].
Discussion
When a new AED such as LCM is introduced, clinicians may raise several questions regarding the medication. The first question may be whether a new medication works better than any other existing medications. Other questions may include what is new about the medication in terms of mechanism of action (MOA), dosing schedule, pharmacokinetics and safety. These questions ultimately lead to a more clinically relevant question of whether a new medication can provide better seizure control and improve the quality of life for patients with epilepsy. Therefore, comprehensive understanding of the differences in efficacy, pharmacokinetics, MOA, potential drug-to-drug interactions, and tolerability may provide useful guidance when choosing a new AED for epilepsy patients. Favored AEDs should have 100% bioavailability, linear kinetics, low or no drug-to-drug interactions, low protein binding, renal clearance, longer half-life, convenient dosing, and preferably a novel MOA. Table 1 lists the main properties of LCM and Table 2 further describes strengths and limitations of LCM in clinical practice.
Table 1.
Main properties of lacosamide.
| Indications | Adjunctive therapy for partial seizures (≥16 years) |
| Approval status | Approved by both EMEA, FDA |
| Mode of action | Selective enhancement of slow inactivation of voltage-gated sodium channels |
| Starting dose | 100 mg/day |
| Therapeutic dose | 200–400 mg/day |
| Dosing schedule | BID |
| Half-life (h) | 13 |
| Time to Cmax (h) | 1–4 |
| Oral bioavailability (%) | ∼100 |
| Protein binding (%) | <15 |
EMEA, European Medicines Agency; FDA, Food and Drug Administration (U.S.).
Table 2.
Practical considerations of LCM for patients with epilepsy.
| Strength of lacosamide | Limitation of lacosamide |
|---|---|
| Novel mechanism of action Clean pharmacokinetics Rapid onset of action Low drug interactions Available intravenous solution No interaction with oral contraceptives Low incidence of sedation, rash or weight gain | High incidence of dizziness Required dose adjustment in patients with renal and hepatic impairment Potential PR prolongation on EKG Unknown efficacy and safety in children |
EKG, electrocardiogram; PR refers to PR interval in the EKG measurements.
Results from multiple clinical studies have demonstrated that LCM is a well-tolerated and effective treatment option in reducing partial onset seizures as an adjunctive agent. Although some preclinical studies suggest that LCM could be potentially effective against generalized onset seizures, there has been no human study yet to establish LCM as a broad spectrum AED. Despite the fact that LCM displays a unique and novel MOA for seizure treatment, the question still remains whether MOAs of any AEDs matter significantly in clinical practice. When combination therapy is considered, using AEDs with different MOAs may provide better efficacy and tolerability, and even possibly a synergistic (supra-additive) effect. On the other hand, using AEDs with similar MOAs may result in simple additive or even antagonistic (infra-additive) effects. More recently, isobolographic analysis in rodent models has been used to determine whether combination of two medications could be synergistic, additive, or antagonistic to each other [Stöhr et al. 2007b]. This study examined isobolographic analysis of LCM in order to evaluate pharmacodynamic interactions with lamotrigine, valproate, carbamazepine, phenytoin, levetiracetam, topiramate, and gabapentin, utilizing the mouse 6 Hz psychomotor seizure model. The authors found that the combination of LCM with levetiracetam or carbamazepine at fixed ratios of 1 : 3, 1 : 1, and 3 : 1 exerted synergistic interactions, while combinations with other AEDs produced additive or synergistic interactions depend on different fixed ratios. For example, the combination of LCM with gabapentin was synergistic at the fixed ratio of 1 : 3 and 1 : 1, while only additive effect was seen at 3 : 1 fixed ratio. Nonetheless, it is not yet clear how these combinations would impact the seizure control in clinical practice. To date, there are no clinical studies examining the pharmacodynamic interactions or clinical efficacy of LCM with other existing AEDs.
In summary, LCM is a new anticonvulsant with a proposed novel MOA, coupled with a favorable pharmacokinetic profile that includes absolute bioavailability, low protein binding, renal excretion, lack of hepatic enzyme induction or inhibition, low potential for drug-to-drug interactions, and a relatively long half-life. Efficacy data have shown a fast onset of anticonvulsant effects and a significant reduction of partial-onset seizures at 200 and 400 mg/day in a refractory population. LCM has been well tolerated with most common adverse event being dizziness, followed by headache, nausea and diplopia. LCM has been substantially less associated with sedation, cognitive dysfunction, rash and mood disorders when compared with many other existing AEDs.
Conclusion
LCM is a new anticonvulsant with a favorable pharmacokinetic profile and a proposed novel MOA. Results from clinical studies demonstrate that LCM is well tolerated and effective in controlling partial-onset seizures as adjunctive therapy. LCM expands treatment options for patients with partial epilepsy and may provide significant benefit to patients with refractory seizures.
Conflict of interest statement
Steve Chung, MD, is a consultant for Medtronics, Inc., GlaxoSmithKline plc and UCB S.A., is on the speaker’s bureau of Cyberonics, Inc., GlaxoSmithKline plc, and UCB S.A., and receives grant and research support from Schwarz Pharma A.G., GlaxoSmithKline plc, UCB S.A., Valeant, Eisai Inc., Ortho-McNeil and Medtronics, Inc.
References
- Andurkar S.V., Stables J.P., Kohn H. (1999) The anticonvulsant activities of N-benzyl 3-methoxypropionamides. Bioorg Med Chem 7: 2381–9 [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. (2008) Lacosamide: an investigational drug for adjunctive treatment of partial-onset seizures. Drugs Today 44: 35–40 [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E., Biton V., Jatuzis D., Abou-Khalil B., Doty P., Rudd G.D. (2007) Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 48: 1308–17 [DOI] [PubMed] [Google Scholar]
- Ben-Menachem, E., Chung, S., Rudd, D., Hebert, D. and Doty, P. (2009) Evaluation of Lacosamide Efficacy in Subjects with Partial-Onset Seizures Across the Dose Range Used in Phase II/III Clinical Trials [abstract]. Poster Presentation, 28th International Epilepsy Congress 28 June – 2 July 2009.
- Beydoun A., D’Souza J., Hebert D., Doty P. (2009) Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother 9: 33–42 [DOI] [PubMed] [Google Scholar]
- Beyreuther B.K., Freitag J., Heers C., Krebsfanger N., Scharfenecker U., Stöhr T. (2007) Lacosamide: a review of preclinical properties. CNS Drug Rev 13: 21–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M., Johannessen S.I., Kupferberg H.J., Levy R.H., Perucca E., Tomson T. (2007) Progress report on the new antiepileptic drugs: a summary of the eighth Eilat conference (EILAT VIII). Epilepsy Res 73: 1–52 [DOI] [PubMed] [Google Scholar]
- Bialer M., Johannessen S., Levy R., Perucca E., Tomson T., White S. (2009) Progress report on new antiepileptic drugs: a summary of the ninth Eilat Conference (EILAT IX). Epilepsy Res 83: 1–43 [DOI] [PubMed] [Google Scholar]
- Biton V., Rosenfeld W.E., Whitesides J., Fountain N.B., Vaiciene N., Rudd G.D. (2008) Intravenous lacosamide as replacement for oral lacosamide in patients with partial-onset seizures. Epilepsia 49: 418–424 [DOI] [PubMed] [Google Scholar]
- Cawello W., Kropeit D., Schiltmeyer B., Hammes W., Horstmann R. (2004) Food does not affect the pharmacokinetics of SPM 927 [abstract]. Epilepsia 45: 307–307 [Google Scholar]
- Centers for Disease Control and Prevention (1994) Prevalence of self-reported epilepsy – United States, 1986–1990. JAMA 272: 1893–1893 [PubMed] [Google Scholar]
- Chung S., Sperling M., Biton V., Krauss G., Beaman M., Hebert D. (2007a) Lacosamide: efficacy and safety as oral adjunctive treatment for partial-onset seizures [abstract]. Epilepsia 48: 321–321 [Google Scholar]
- Chung S., Wang N., Hank N. (2007b) Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure 16: 296–304 [DOI] [PubMed] [Google Scholar]
- Chung S., Rudd R., Hebert D., Doty P. (2008) Evaluation of lacosamide efficacy in subjects with partial-onset seizures across the dose range used in phase II/III clinical trials [abstract]. Epilepsia 49: 426–426 [Google Scholar]
- Deckers L., Knoester D., de Haan G.J., Keyser A., Renier W.O., Hekster Y.A. (2003) Selection criteria for the clinical use of the newer antiepileptic drugs. CNS Drugs 17: 405–421 [DOI] [PubMed] [Google Scholar]
- Doty P., Rudd G.D., Stöhr T., Thomas D. (2007) Lacosamide. Neurotherapeutics 4: 145–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara J., Carmona G., Ortega M.P., Iglesias A.A. (2005) Preliminary study on the efficacy and tolerability of newer anticonvulsants in a population of epileptic patients. Med Princ Pract 14: 31–34 [DOI] [PubMed] [Google Scholar]
- Halász P., Kälviäinen R., Mazurkiewicz-Beldzinska M., Rosenow F., Doty P., Hebert D., et al. (2009) Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 50: 443–453 [DOI] [PubMed] [Google Scholar]
- Horstmann R., Bonn R., Cawello W., Doty P., Rudd G.D. (2002) Basic clinical pharmacological investigations of the new antiepileptic drug SPM 927 (abstract). Epilepsia 43: 188–188 [Google Scholar]
- Hovinga C.A. (2003) SPM-927 (Schwarz Pharma). IDrugs 6: 479–485 [PubMed] [Google Scholar]
- Kennebäck G., Bergfeldt L., Tomson T. (1995) Electrophysiological evaluation of the sodium-channel blocker carbamazepine in healthy human subjects. Cardiovasc Drugs Ther 9: 709–714 [DOI] [PubMed] [Google Scholar]
- Kennebäck G., Bergfeldt L., Vallin H., Tomson T., Edhag O. (1991) Electrophysiologic effects and clinical hazards of carbamazepine treatment for neurologic disorders in patients with abnormalities of the cardiac conduction system. Am Heart J 121: 1421–1429 [DOI] [PubMed] [Google Scholar]
- Krauss G., Ben Menachem E., Mameniskiene R., Vaiciene N., Rudd D., Brock M. (2007) Lacosamide: safety and tolerability of adjunctive intravenous lacosamide as short-term replacement for oral lacosamide in subjects with partial-onset seizures during 30-, 15-, and 10-minute infusions. Eur J Neurol 14: 319–320 [Google Scholar]
- Kropeit D., Schiltmeyer B., Cawello W., Hammes W., Horstmann R. (2004) Bioequivalence of short-time infusions compared to oral administration of SPM 927 (abstract). Epilepsia 45: 123–123 [Google Scholar]
- Kwan P., Brodie M. (2000) Early identification of refractory epilepsy. N Engl J Med 342: 314–319 [DOI] [PubMed] [Google Scholar]
- Laville M.A., de la Gastine B., Husson B., Le Boisselier R., Mosquet B., Coquerel A. (2008) Should we care about pregabalin for elderly patients with a history of cardiac dysrhythmia? Rev Med Interne 29: 152–154 [DOI] [PubMed] [Google Scholar]
- Matsuo F., Bergen D., Faught E., Messenheimer J.A., Dren A.T., Rudd G.D., et al. (1993) Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures U.S. Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology 43: 2284–2291 [DOI] [PubMed] [Google Scholar]
- Perucca E. (2007) Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurology 6: 793–804 [DOI] [PubMed] [Google Scholar]
- Rosenfeld W., Fountaine N., Kaubrys G., Heinzen L., McShea C. (2007) Lacosamide: an interim evaluation of long-term safety and efficacy as oral adjunctive therapy in subjects with partial-onset seizures (abstract). Epilepsia 48: 318–318 [Google Scholar]
- Sperling M., Rudd D., Hebert D., Doty P. (2008) Early onset of efficacy in the initial weeks of treatment with lacosamide: a pooled analysis of three phase II/III trials (abstract). Epilepsia 49: 457–457 [Google Scholar]
- Stöhr T., Kupferberg H.J., Stables J.P., Choi D., Harris R.H., Kohn H., et al. (2007a) Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodent models for epilepsy. Epilepsy Res 74: 147–154 [DOI] [PubMed] [Google Scholar]
- Stöhr T., Shandra P., Kashenko O., Shandra A. (2007b) Synergism of lacosamide and first-generation and novel antiepileptic drugs in the 6 Hz seizure model in mice (abstract). Epilepsia 48: 252–252 [DOI] [PubMed] [Google Scholar]
- Thomas D., Scharfenecker U., Nickel B., Doty P., Cawello W., Horstmann R., et al. (2006) Low potential for drug–drug interaction of lacosamide (abstract). Epilepsia 47: 200–200 [Google Scholar]
- US FDA (2008) Vimpat (lacosamide) tablets and injection: US prescribing information [online]. Available from http://www.fda.gov/cder/foi/label/2008/022253lbl.pdf [Accessed 2008 Oct 30].