Abstract
The incidence of trigeminal neuralgia (TN) is 4.3 per 100,000 persons per year, with a slightly higher incidence for women (5.9/100,000) compared with men (3.4/100,000). There is a lack of certainty regarding the aetiology and pathophysiology of TN. The treatment of TN can be very challenging despite the numerous options patients and physicians can choose from. This multitude of treatment options poses the question as to which treatment fits which patient best. The preferred medical treatment for TN consists of anticonvulsant drugs, muscle relaxants and neuroleptic agents. Large-scale placebo-controlled clinical trials are scarce. For patients refractory to medical therapy, Gasserian ganglion percutaneous techniques, gamma knife surgery and microvascular decompression are the most promising invasive treatment options.
Keywords: current treatment, facial pain, future treatment, treatment options, trigeminal neuralgia
Introduction
Trigeminal neuralgia (TN) is defined by the International Headache Society (IHS) as “unilateral disorder characterized by brief electric shock-like pains, abrupt in onset and termination, and limited to the distribution of one or more divisions of the trigeminal nerve” [International Headache Society, 2004]. The IHS suggests a classification of TN as either classic (essential or idiopathic) TN (CTN) or symptomatic TN (STN; pain indistinguishable from that of CTN, but caused by a demonstrable structural lesion other than vascular compression). The diagnosis of CTN requires the absence of a clinically evident neurological deficit. CTN starts in the second or third divisions, affecting the cheek or the chin [International Headache Society, 2004]. The ophthalmic division alone is involved in less than 5% of cases [De Simone et al. 2005]. The single attack generally lasts from less than a second to a few seconds, but it may present in clusters of variable intensity with up to 2 minutes duration. In many cases it is followed by a brief refractory period during which a new stimulation is not able to evoke another attack. Between paroxysms the patient is usually pain free, but a dull background pain may persist in some cases [International Headache Society, 2004]. Growing neurosurgical data advocate the distinction of these two subtypes of TN into type 1 as defined as >50% episodic onset of TN pain and type 2 defined by >50% constant pain [Tatli et al. 2008; Limonadi et al. 2006]. The mechanisms associated with the development of this persistent pain are not well understood but concomitant background pain is associated with poor medical and surgical outcome [Obermann et al. 2008; Sandell and Eide, 2008; Szapiro et al. 1985]. Recent investigations focused on the suspected central component in the pathophysiology of TN, which could involve central allodynic mechanisms that may also engage the nociceptive neurons at thalamic and cortical level [Obermann et al. 2007].
Methods
In this review we summarize current knowledge about the established treatment options for TN on the basis of recent reports of the Quality Standards Subcommittee of the American Academy of Neurology (AAN) [Gronseth et al. 2008] and the European Federation of Neurological Societies (EFNS) [Cruccu et al. 2008]. An additional MEDLINE search (25 August 2009) for articles was performed using the search term ‘trigeminal neuralgia’. The search was limited to the past 3 years and returned 525 articles of which 90 were marked as review articles. The focus was to find more recent, experimental and pilot studies with regards to the treatment options of TN.
Disease burden
The pain resulting from TN imposes a substantial burden on patients. During severe attacks, affected patients may be unable to speak or eat. Even between attacks, some patients are gripped by an overwhelming fear that the pain could suddenly return at any time [Cheshire, 2003]. This poses serious impairment in daily function and reduces quality of life. Pain severity correlated with reduced measures of daily functioning, quality of life, well-being, sleep, mood and overall health status [Tolle et al. 2006]. TN impacted employment in 34% of patients. Two-thirds of patients reported moderate to severe pain within the previous 24 h. Depressive symptoms are frequent in patients suffering from TN [Zakrzewska, 2006; Zakrzewska et al. 1999; Marbach and Lund, 1981].
Diagnosis
The correct clinical diagnosis is the most important factor for sufficient treatment. History remains the essential tool for diagnosis. It is important to differentiate trigeminal autonomic cephalalgias (e.g. cluster headache, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing [SUNCT], paroxysmal hemicrania), especially in patients with first division pain only [Cohen et al. 2006]. There is a lack of evidence for diagnostic criteria [Zebenholzer et al. 2006; Zakrzewska, 2002]. The main objective of special diagnostic procedures is the differentiation of CTN from STN. Clinical presentation with bilateral TN as well as trigeminal sensory deficits are indicative of STN, but due to low specificity, their absence does not rule in out completely [Cruccu et al. 2008; Gronseth et al. 2008]. Routine head imaging identifies structural causes in up to 15% (95% confidence interval [CI], 11–20%) of patients (excluding nerve vessel conflict). The most commonly identified abnormalities were cerebello-pontine angle tumours and multiple sclerosis (MS) plaques. Electrophysiological examination can reliably distinguish STN from CTN. The diagnostic accuracy of trigeminal reflex testing including the blink reflex for identifying patients with STN was relatively high with a pooled sensitivity over five studies of 94% (95% CI, 91–97%) and pooled specificity of 87% (95% CI, 77–93%). Evoked potentials on the other hand did not distinguish STN from CTN sufficiently (pooled sensitivity 84% [95% CI, 73–92%]; pooled specificity 64% [95% CI, 56–71%]) and are not recommended to determine the differentiation [Cruccu et al. 2008; Gronseth et al. 2008].
Imaging is important in the pre-surgical assessment of the presence of nerve vessel conflicts. Sensitivities and specificities vary widely (sensitivity 52–100%; specificity 29–93%) probably due to the difference in the MRI techniques employed in these studies [Cruccu et al. 2008; Gronseth et al. 2008]. Consequently there is insufficient evidence to support or refute the usefulness of MRI in identifying vascular contact. A very recent study employed the combination of three-dimensional (3D) reconstructed high-resolution balanced fast-field echo (BFFE) images, 3D time-of-flight (TOF) magnetic resonance (MR) angiography, and gadolinium (Gd)-enhanced 3D spoiled gradient recalled sequence to identify vascular conflict in 15 out of 18 CTN patients. Two out of the remaining three did not show a nerve vessel conflict in surgery and one patient had a small vein embedded in the trigeminal nerve that was too small for MR resolution to be able to detect it [Miller et al. 2008]. Hamlyn and King performed an interesting in vivo simulation of nerve vessel conflict on cadavers with a special technique of in situ blood vessel perfusion that enabled the authors to observe the normal neurovascular arrangement post-mortem at physiological pressures. They found that 39 of 41 TN patients had a nerve vessel conflict and the vessel created a groove in the trigeminal nerve. That groove was not present in 50 control subjects, even though 40% of them did show a nerve vessel conflict when physiological blood pressures were applied [Hamlyn and King, 1992]. Unfortunately, modern imaging techniques are unable to pick up this groove within the trigeminal nerve.
Medical treatment
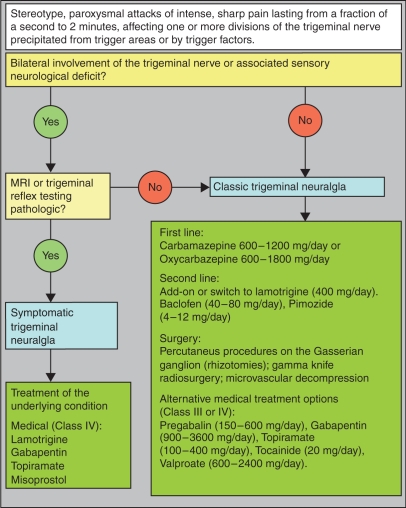
There is a huge variety of pharmacological and surgical treatment options for TN that are effective and widely used. The general recommendation is to start with medical therapy and consider surgical procedures in patients who are refractory to medical treatment (Figure 1). Studies that compare medical and surgical treatment directly are still missing. Active participation in support groups may help many patients in dealing better with their disease and with suggested therapy [Zakrzewska et al. 2009].
Figure 1.
Workup and management of trigeminal neuralgia.
First-line treatment
First-line therapy should be carbamazepine (CBZ; 200–1200 mg/day) and oxcarbazepine (OXC; 600–1800 mg/day) according to current evidence-based treatment guidelines [Cruccu et al. 2008; Gronseth et al. 2008]. Although the evidence for the efficacy of CBZ is stronger [Nicol, 1969; Killian and Fromm, 1968; Campbell et al. 1966; Rockliff and Davis, 1966], OXC has a better safety profile [Beydoun, 2000]. The mechanism of analgesia is unknown. Its effect may relate to the blockade of voltage-sensitive sodium channels resulting in the stabilization of hyperexcited neural membranes, inhibition of repetitive firing or reduction of propagation of synaptic impulses. The effective dose in newly diagnosed TN may be less than that required to treat epilepsy. Pain in some patients may resolve on as little as 100 mg two to three times a day. For the remaining patients the daily dose should be increased by 100 mg every other day until adequate pain relief is established or until intolerable side effects prevent further upward titration. Typical maintenance doses range from 300 to 800 mg/day divided into two to three daily doses. Efficacy is approximately 80% initially. Over time, higher doses may be needed to maintain efficacy, which declines to approximately 50% of patients due to autoinduction of CBZ [Campbell et al. 1966]. Common initial side effects include drowsiness, nausea, dizziness, diplopia, ataxia, elevation of transaminases and hyponatremia. Potentially serious but uncommon side effects are allergic rash, myelosuppression, hepatotoxicity, lymphadenopathy, systemic lupus erythematosus, Stevens–Johnson syndrome and aplastic anaemia. The US Food and Drug Administration (FDA) made recommendations about genetic testing for patients with Asian ancestry that are at highest risk for the development of Stevens–Johnson syndrome. Complete blood count, serum sodium and liver function test should be performed within several weeks after initiation of treatment to detect complications in a timely manner. OXC is a keto-analogue of CBZ that is rapidly converted into its pharmacologically active 10-monohydroxy metabolite and only weakly induces hepatic enzymes. This leads to a much better side effect profile [Martinez et al. 2006]. OXC is an acceptable alternative to CBZ and is initiated at 150 mg twice daily and increased as tolerated by 300 mg every 3 days until pain relief is accomplished. Maintenance doses range between 300 and 600 mg twice daily.
Second-line treatment
Second-line treatment is based on very little evidence and includes add-on therapy with lamotrigine (400 mg/day) [Zakrzewska et al. 1997], or a switch to lamotrigine, baclofen (40–80 mg/day) [Fromm et al. 1984] or pimozide (4–12 mg/day). Pimozide is seldom in clinical use and poses concern about possible long-term side effects such as extrapyramidal symptoms. Baclofen is a GABAB receptor agonist and thus depresses excitatory neurotransmission. It has been shown in double-blind studies to be effective in 70% of patients at doses of 10–60 mg per day [Fromm et al. 1984]. Follow-up of 60 patients over 1–5 years showed that efficacy was maintained in only 30% of patients, while 17% experienced pain recurrence within 3–6 months, and 22% became refractory after 1–18 months [Fromm et al. 1984]. Potential side effects include lassitude, drowsiness, dizziness and gastrointestinal discomfort. To date, baclofen has the strongest scientific evidence for efficacy in the treatment of TN next to CBZ. Lamotrigine acts at voltage-sensitive sodium channels, stabilizes neural membranes and inhibits the release of excitatory neurotransmitters. It was shown to be superior to placebo in a randomized, controlled trial investigating 14 patients with TN refractory to CBZ [Zakrzewska et al. 1997]. The initial dose of 25 mg/day is slowly increased to a target dose of 200–400 mg/day divided between two doses. Potential side effects include dizziness, nausea, blurred vision and ataxia. Approximately 7–10% of patients will report a skin rash during the first 4–8 weeks of therapy [Wiffen and Rees, 2007] that most often resolves with continued therapy. With severe rash, desquamation or associated symptoms of fever or lymphadenopathy indicative of Stevens–Johnson syndrome requires prompt discontinuation. The slower the titration, the less likely it is that these side effects will occur, but most patients will not accept an overly cautious and long titration phase [Ketter et al. 2005].
Alternative treatment options
Other antiepileptic drugs (AEDs) have been studied in small controlled or open-label studies. Benefit was suggested for phenytoin, clonazepam, gabapentin, pregabalin, topiramate, levetiracetam and valproate as well as tocainide (12 mg/day) [Lindstrom and Lindblom, 1987]. In particular, the newer AEDs with less interaction with other medications and fewer side effects will be worth further investigation. As the incidence of TN increases with age [Khan, 1998], age-related physiologic changes that alter pharmacokinetics, such as reduced hepatic and renal function, blood flow decline, less predictable drug protein binding and interactions with multiple other medications required due to concomitant illness, will come increasingly into focus. Approximately 6–10% of patients cannot tolerate CBZ [Taylor et al. 1981]. Multiple pharmacologic interactions and a narrow therapeutic window of tolerability further limit the use of CBZ. Promising in this regard are lamotrigine, pregabalin, gabapentin, topiramate and levetiracetam. The newer AEDs tested within the past 2 years are topiramate, levetiracetam, gabapentin and pregabalin. Gabapentin showed adequate efficacy alone and in combination with local injections of ropivacaine used to block trigger points in TN patients. Even though only 36 patients were investigated, this combination seems safe and effective in the treatment of TN [Lemos et al. 2008]. Gabapentin is initiated at 300 mg daily and may be gradually increased by 300 mg each 2–3 days as tolerated. Gabapentin has no interaction with other drugs and relatively minor side effects that may include dizziness, somnolence, headache, diarrhoea, confusion, nausea and ankle swelling. Pregabalin was tested in an open-label study including 53 patients (14 with concomitant constant facial pain) with 1-year follow-up. Pregabalin (150–600 mg/day) proved to be effective in reducing TN pain by over 50% in 74% of patients with minor efficacy reduction over the 1-year observational period. Patients without concomitant facial pain showed better response rates (32 out of 39, 82%) compared with patients with concomitant chronic facial pain (7 out of 14, 50%, p = 0.020) [Obermann et al. 2008]. Topiramate (100–400 mg/day) was effective in 75% of patients in a very small sample of only eight patients [Domingues et al. 2007]. A very recent pilot study investigated the efficacy and tolerability of levetiracetam in 10 patients with TN over a period of 10 weeks in an open-label prospective design. Patients were treated with up to 4000 mg daily and 40% (n = 4) reported an improvement of 50–90% [Jorns et al. 2009]. Further randomized, controlled trials will have to follow to confirm these preliminary findings.
Tizanidine is a centrally acting alpha-adrenergic agonist and has proven efficacy in a double-blind crossover study in 8 out of 10 patients with TN. All patients that were followed-up after 1–3 months experienced recurrence of pain [Fromm et al. 1993]. Tizanidine is less efficacious than CBZ [Vilming et al. 1986].
Several descriptions postulated an analgesic effect of botulinum neurotoxin type A (BoNT-A) through local release of anti-nociceptive neuropeptides such as substance P, glutamate and calcitonin-gene related peptide (CGRP) inhibiting central and possibly peripheral sensitization [Aoki, 2005]. Reports of isolated TN patients treated with BoNT-A and a small, uncontrolled clinical trial (n = 13) showed significant relief from symptoms after treatment with BoNT-A. Mean BoNT-A dose was 3.22 units/cm2 administered directly into the affected facial regions subcutaneously. At 60 days follow-up the pain began to slowly return in most patients [Piovesan et al. 2005].
Surgical treatment
Surgical treatments are generally reserved for patients with debilitating pain refractory to an adequate trial of at least three drugs including CBZ in sufficient dosage. The decision to perform an invasive neurosurgical procedure or minimally invasive stereotactic radiotherapy procedure should be based on the clinical presentation and not primarily on neuroimaging findings [Cheshire, 2005]. Some patients may request surgical intervention despite nearly complete pain relief by medication, in fear of eventual return or progression of pain over time. When patients (n = 156) were asked hypothetical questions about possible treatment options they did not prefer or refute anything in particular, but medical treatment was the least favourite option [Spatz et al. 2007]. Side effects of medication may also lead patients to think about surgical intervention. Surgeons who perform trigeminal nerve procedures frequently achieve the greatest margin of safety and efficacy [Kalkanis et al. 2003]. Zakrzewska and Lopez introduced a checklist to use before surgical intervention to improve the evaluation quality of surgical treatment of TN [Zakrzewska and Lopez, 2003].
Percutaneous procedures on the Gasserian ganglion, gamma knife and microvascular decompression are recommended, efficacy-proven surgical treatment options for medical refractory TN. Surgery for TN is either destructive (ablative), where the trigeminal nerve sensory function is intentionally destroyed, or non-destructive, where the trigeminal nerve is decompressed preserving its normal function. Gasserian ganglion percutaneous techniques are all destructive and include radiofrequency thermocoagulation (RFT), balloon compression (BC) and percutaneous glycerol rhizolysis (PGR). Ninety percent of patients report pain relief following these procedures. After 1 year, 68–85% of patients are still pain free, after 3 years this is reduced to 54–64% and after 5 years only 50% of patients are still pain free following RFT. The most common side effects are sensory loss (50%) which extremely decreases the quality of life [Zakrzewska et al. 1999], dysesthesias (6%), anaesthesia dolorosa (4%), corneal numbness with risk of keratitis (4%). Gasserian ganglion therapies require short acting anesthetics, are primarily overnight minor procedures with extremely low mortality [Cruccu et al. 2008; Gronseth et al. 2008].
In gamma knife surgery, a focused beam of radiation is aimed at the trigeminal root in the posterior fossa. One year after gamma knife surgery, 69% of patients are pain free without additional medication. At 3 years, 52% are still pain free. The development of pain relief can be delayed (mean 1 month). Side effects are sensory complications in 6% that may develop with a delay of up to 6 months, facial numbness in 9–37% which improves over time and paresthesias in 6–13% [Cruccu et al. 2008; Gronseth et al. 2008]. Quality of life improves by 88% [Zakrzewska et al. 1999]. The main disadvantage of gamma knife surgery is the treatment expense that limits widespread usage making it a reserve treatment option for patients that cannot undergo open surgery or have blood coagulation problems (e.g. are receiving warfarin).
Microvascular decompression achieves the most sustained pain relief with 90% of patients reporting initial pain relief and over 80% still pain free after 1 year, with 75% after 3 years and 73% after 5 years remaining pain free. It is, however, a major surgical procedure that entails craniotomy to reach the trigeminal nerve in the posterior fossa. The average mortality rate ranges from 0.2% to 0.5%, and up to 4% of patients suffer from major problems such as cerebrospinal fluid (CSF) leakage, infarcts or haematomas. The most common complications are aseptic meningitis (11%), sensory loss (7%) and hearing loss (10%) as long-term complications [Cruccu et al. 2008; Gronseth et al. 2008].
More recent investigations have focused mainly on treatment evaluation in long-term follow-up studies [Kabatas et al. 2009; Little et al. 2008] and improvement of existing surgical techniques [Kanpolat et al. 2008; Sindou et al. 2008; Tatli and Sindou, 2008]. Even though this has been the most active field of TN research over recent years, the vast majority of studies remain on a descriptive level making evidence-based comparison and recommendation difficult. The right timing for surgical intervention is yet to be determined [Spatz et al. 2007]. Some TN experts suggest early surgical referral in patients who fail to respond to first-line medical therapy, while others request trialling of at least two different medical regimens including combination therapy before considering surgery. There is no supporting evidence for either of the two opinions. Referral for surgical intervention seems reasonable in TN patients refractory to medical therapy.
Acute neuralgia attack treatment
When initiating treatment it is often hard to find the balance between the need to achieve rapid pain relief and the goal of avoiding troublesome dose-related medication side effects. The intensity of pain may compel a rapid medical response, but rapid dose escalation could risk unpleasant dose-dependent side effects, persuading the patient to never again try a drug that might have proven remarkably helpful if only it had been initiated more slowly. Phenytoin has proven effective in managing neuralgia crisis in a small case series. A loading dose of 14 mg/kg applied intravenously was required to relieve the pain for 1–2 days, which is long enough for alternative oral drug therapy to kick in when initiated simultaneously [Cheshire, 2001]. Intranasal administered lidocaine 8% was effective in temporarily relieving second-division neuralgic pain [Kanai et al. 2006b]. Subcutaneous sumatriptan 3 mg was shown to be superior to placebo in providing prompt and marked analgesia in 80% of patients in a double-blind, placebo-controlled study of 24 patients with refractory TN. The median duration of pain relief was 8 h [Kanai et al. 2006a]. A different approach could be ganglionic local opioid analgesia (GLOA) at the superior cervical ganglion, which was evaluated retrospectively in 74 patients with neuropathic facial pain. A clinically relevant pain reduction was observed in 73% of the patients. The proportion of responders (pain reduction ≥50%) was 59% after the first blockade [Elsner et al. 2006].
Transcranial magnetic stimulation
Repetitive transcranial magnetic stimulation (rTMS) is an emerging technology that introduces the possibility of assessing whether patients with trigeminal neuropathic pain will respond to direct epidural cortical stimulation by first measuring their response to a trial of non-invasive cortical stimulation. In a study of 24 TN patients given rTMS to the motor cortex at 20 Hz daily for 5 days, pain ratings decreased by approximately 45% for 2 weeks [Khedr et al. 2005]. In a different study of 12 patients with chronic intractable TN who had failed surgical treatment, 58% experienced a greater than 30% reduction in pain after receiving repetitive TMS [Lefaucheur et al. 2004].
Treatment of trigeminal neuralgia in multiple sclerosis
The pharmacological treatment of TN in MS is similar to that of idiopathic TN. Randomized controlled treatment trials on this special subgroup of patients do not exist. Central nervous system (CNS) demyelination renders some patients with MS more sensitive to cognitive and motor side effects, which might lead to an earlier decision for surgery in these patients. However, surgical outcomes are less predictable and less durable in MS, presumably due to unrelieved central pain mechanisms [Athanasiou et al. 2005; Broggi et al. 2004]. Nevertheless, the possibility of additional nerve–vessel conflict in MS patients should be kept in mind. Microvascular decompression is associated with a favourable response in approximately 50% of patients [Monstad, 2007; Patwardhan et al. 2006; Cheng et al. 2005]. The major problem with microvascular decompression in this patient population is the observation that TN often occurs bilaterally and bilateral posterior fossa craniotomies entail a greater risk [Berk, 2001]. MS patients also have a considerably less-satisfactory long-term outcome following microvascular decompression, particularly when MRI finds demyelinating lesions in the brainstem trigeminal pathways of the painful side, indicating an ongoing role of central mechanisms [Patwardhan et al. 2006; Berk, 2001].
Conclusion
Among the very many diagnostic and treatment options in the management of TN only very few have proven their efficacy to modern evidence-based medicine standards. For thorough and accurate management, a stepwise diagnostic and treatment approach is recommended (Figure 1). In most cases the diagnosis can be made clinically. Bilateral involvements of the trigeminal nerve or sensory deficit are suspicious of STN and routine imaging (MRI) may be considered. Electrophysiological testing may also be considered to confirm STN but generally is restricted to qualified laboratories. First-line therapy is CBZ (600–1200 mg/day) or OXC (600–1800 mg/day), switching to or adding-on lamotrigine (200–400 mg/day), pregabalin (150–600 mg/day), gabapentin (1800–4200 mg/day) or topiramate (100–400 mg/day) may also be considered. If the combination therapy fails, a switch to baclofen (40–80 mg/day) can be tried. Surgical management should be discussed at this stage and should be recommended if sufficient and compliant medical therapy failed. Reluctance to refer for surgery is inappropriate at this stage of treatment and may be disadvantageous to the patient. The kind of intervention (Gasserian ganglion procedures, gamma knife surgery, microvascular decompression) should be discussed intensively with the patients with regards to their own individual wishes and overall medical condition. The medical status and biological age of the patient should be taken into consideration before a particular treatment is recommended (i.e. older patients with serious comorbidities should receive less-invasive treatment).
Controlled studies with long-term follow-up will be needed to compare surgical and medical therapy directly with one another and determine the optimal timing for surgical intervention. This also includes studies that investigate second-line medical therapy after the first line has failed in a stepwise, standardized regimen. The newer, antinociceptive drugs for the treatment of neuropathic pain need thorough investigation for treatment efficacy in TN. Emphasis should be placed on the importance of quality of life issues as an important outcome measure, as this is the core feature patients will measure treatment success on.
Conflict of interest statement
The author declares that there is no conflict of interest.
References
- Aoki K.R. (2005) Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 26: 785–793 [DOI] [PubMed] [Google Scholar]
- Athanasiou T.C., Patel N.K., Renowden S.A., Coakham H.B. (2005) Some patients with multiple sclerosis have neurovascular compression causing their trigeminal neuralgia and can be treated effectively with MVD: report of five cases. Br J Neurosurg 19: 463–468 [DOI] [PubMed] [Google Scholar]
- Berk C. (2001) Bilateral trigeminal neuralgia: a therapeutic dilemma. Br J Neurosurg 15: 198–198 [PubMed] [Google Scholar]
- Beydoun A. (2000) Safety and efficacy of oxcarbazepine: results of randomized, double-blind trials. Pharmacotherapy 20(8 Pt 2): 152S–158S [DOI] [PubMed] [Google Scholar]
- Broggi G., Ferroli P., Franzini A., Nazzi V., Farina L., La Mantia L., et al. (2004) Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery 55: 830–838;discussion 838–839. [PubMed] [Google Scholar]
- Campbell F.G., Graham J.G., Zilkha K.J. (1966) Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry 29: 265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.S., Sanchez-Mejia R.O., Limbo M., Ward M.M., Barbaro N.M. (2005) Management of medically refractory trigeminal neuralgia in patients with multiple sclerosis. Neurosurg Focus 18: e13–e13 [DOI] [PubMed] [Google Scholar]
- Cheshire W.P. (2001) Fosphenytoin: an intravenous option for the management of acute trigeminal neuralgia crisis. J Pain Symptom Manage 21: 506–510 [DOI] [PubMed] [Google Scholar]
- Cheshire W.P. (2003) Trigeminal neuralgia feigns the terrorist. Cephalalgia 23: 230–230 [DOI] [PubMed] [Google Scholar]
- Cheshire W.P. (2005) Can MRI distinguish injurious from innocuous trigeminal neurovascular contact? J Neurol Neurosurg Psychiatry 76: 1470–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.S., Matharu M.S., Goadsby P.J. (2006) Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) or cranial autonomic features (SUNA)–a prospective clinical study of SUNCT and SUNA. Brain 129: 2746–2760 [DOI] [PubMed] [Google Scholar]
- Cruccu G., Gronseth G., Alksne J., Argoff C., Brainin M., Burchiel K., et al. (2008) AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol 15: 1013–1028 [DOI] [PubMed] [Google Scholar]
- De Simone R., Marano E., Brescia Morra V., Ranieri A., Ripa P., Esposito M., et al. (2005) A clinical comparison of trigeminal neuralgic pain in patients with and without underlying multiple sclerosis. Neurol Sci 26(Suppl 2): s150–s151 [DOI] [PubMed] [Google Scholar]
- Domingues R.B., Kuster G.W., Aquino C.C. (2007) Treatment of trigeminal neuralgia with low doses of topiramate. Arq Neuropsiquiatr 65: 792–794 [DOI] [PubMed] [Google Scholar]
- Elsner F., Radbruch L., Gaertner J., Straub U., Sabatowski R. (2006) Efficacy of opioid analgesia at the superior cervical ganglion in neuropathic head and facial pain. Schmerz 20: 268–272, 274–266 [DOI] [PubMed] [Google Scholar]
- Fromm G.H., Aumentado D., Terrence C.F. (1993) A clinical and experimental investigation of the effects of tizanidine in trigeminal neuralgia. Pain 53: 265–271 [DOI] [PubMed] [Google Scholar]
- Fromm G.H., Terrence C.F., Chattha A.S. (1984) Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Ann Neurol 15: 240–244 [DOI] [PubMed] [Google Scholar]
- Gronseth G., Cruccu G., Alksne J., Argoff C., Brainin M., Burchiel K., et al. (2008) Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology 71: 1183–1190 [DOI] [PubMed] [Google Scholar]
- Hamlyn P.J., King T.T. (1992) Neurovascular compression in trigeminal neuralgia: a clinical and anatomical study. J Neurosurg 76: 948–954 [DOI] [PubMed] [Google Scholar]
- International Headache Society (2004) The International Classification of Headache Disorders: 2nd Edition. Cephalalgia 24(Suppl 1): 9–160 [DOI] [PubMed] [Google Scholar]
- Jorns T.P., Johnston A., Zakrzewska J.M. (2009) Pilot study to evaluate the efficacy and tolerability of levetiracetam (Keppra) in treatment of patients with trigeminal neuralgia. Eur J Neurol 16: 740–744 [DOI] [PubMed] [Google Scholar]
- Kabatas S., Karasu A., Civelek E., Sabanci A.P., Hepgul K.T., Teng Y.D. (2009) Microvascular decompression as a surgical management for trigeminal neuralgia: long-term follow-up and review of the literature. Neurosurg Rev 32: 87–94 [DOI] [PubMed] [Google Scholar]
- Kalkanis S.N., Eskandar E.N., Carter B.S., Barker II F.G. (2003) Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery 52: 1251–1261;discussion 1261–1262. [DOI] [PubMed] [Google Scholar]
- Kanai A., Saito M., Hoka S. (2006a) Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache 46: 577–582;discussion 583–574. [DOI] [PubMed] [Google Scholar]
- Kanai A., Suzuki A., Kobayashi M., Hoka S. (2006b) Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth 97: 559–563 [DOI] [PubMed] [Google Scholar]
- Kanpolat Y., Kahilogullari G., Ugur H.C., Elhan A.H. (2008) Computed tomography-guided percutaneous trigeminal tractotomy–nucleotomy. Neurosurgery 63(1 Suppl 1): ONS147–153;discussion ONS153–145. [DOI] [PubMed] [Google Scholar]
- Ketter T.A., Wang P.W., Chandler R.A., Alarcon A.M., Becker O.V., Nowakowska C., et al. (2005) Dermatology precautions and slower titration yield low incidence of lamotrigine treatment-emergent rash. J Clin Psychiatry 66: 642–645 [DOI] [PubMed] [Google Scholar]
- Khan O.A. (1998) Gabapentin relieves trigeminal neuralgia in multiple sclerosis patients. Neurology 51: 611–614 [DOI] [PubMed] [Google Scholar]
- Khedr E.M., Kotb H., Kamel N.F., Ahmed M.A., Sadek R., Rothwell J.C. (2005) Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry 76: 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian J.M., Fromm G.H. (1968) Carbamazepine in the treatment of neuralgia. Use of side effects. Arch Neurol 19: 129–136 [DOI] [PubMed] [Google Scholar]
- Lefaucheur J.P., Drouot X., Menard-Lefaucheur I., Zerah F., Bendib B., Cesaro P., et al. (2004) Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry 75: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos L., Flores S., Oliveira P., Almeida A. (2008) Gabapentin supplemented with ropivacain block of trigger points improves pain control and quality of life in trigeminal neuralgia patients when compared with gabapentin alone. Clin J Pain 24: 64–75 [DOI] [PubMed] [Google Scholar]
- Limonadi F.M., McCartney S., Burchiel K.J. (2006) Design of an artificial neural network for diagnosis of facial pain syndromes. Stereotact Funct Neurosurg 84: 212–220 [DOI] [PubMed] [Google Scholar]
- Lindstrom P., Lindblom U. (1987) The analgesic effect of tocainide in trigeminal neuralgia. Pain 28: 45–50 [DOI] [PubMed] [Google Scholar]
- Little A.S., Shetter A.G., Shetter M.E., Bay C., Rogers C.L. (2008) Long-term pain response and quality of life in patients with typical trigeminal neuralgia treated with gamma knife stereotactic radiosurgery. Neurosurgery 63: 915–923;discussion 923–924. [DOI] [PubMed] [Google Scholar]
- Marbach J.J., Lund P. (1981) Depression, anhedonia and anxiety in temporomandibular joint and other facial pain syndromes. Pain 11: 73–84 [DOI] [PubMed] [Google Scholar]
- Martinez W., Ingenito A., Blakeslee M., Barkley G.L., McCague K., D’Souza J. (2006) Efficacy, safety, and tolerability of oxcarbazepine monotherapy. Epilepsy Behav 9: 448–456 [DOI] [PubMed] [Google Scholar]
- Miller J., Acar F., Hamilton B., Burchiel K. (2008) Preoperative visualization of neurovascular anatomy in trigeminal neuralgia. J Neurosurg 108: 477–482 [DOI] [PubMed] [Google Scholar]
- Monstad P. (2007) Microvascular decompression as a treatment for cranial nerve hyperactive dysfunction—a critical view. Acta Neurol Scand Suppl 187: 30–33 [DOI] [PubMed] [Google Scholar]
- Nicol C.F. (1969) A four year double-blind study of tegretol in facial pain. Headache 9: 54–57 [DOI] [PubMed] [Google Scholar]
- Obermann M., Yoon M.S., Ese D., Maschke M., Kaube H., Diener H.C., et al. (2007) Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology 69: 835–841 [DOI] [PubMed] [Google Scholar]
- Obermann M., Yoon M.S., Sensen K., Maschke M., Diener H.C., Katsarava Z. (2008) Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia 28: 174–181 [DOI] [PubMed] [Google Scholar]
- Patwardhan R.V., Minagar A., Kelley R.E., Nanda A. (2006) Neurosurgical treatment of multiple sclerosis. Neurol Res 28: 320–325 [DOI] [PubMed] [Google Scholar]
- Piovesan E.J., Teive H.G., Kowacs P.A., Della Coletta M.V., Werneck L.C., Silberstein S.D. (2005) An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology 65: 1306–1308 [DOI] [PubMed] [Google Scholar]
- Rockliff B.W., Davis E.H. (1966) Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol 15: 129–136 [DOI] [PubMed] [Google Scholar]
- Sandell T., Eide P.K. (2008) Effect of microvascular decompression in trigeminal neuralgia patients with or without constant pain. Neurosurgery 63: 93–99;discussion 99–100. [DOI] [PubMed] [Google Scholar]
- Sindou M., Leston J.M., Decullier E., Chapuis F. (2008) Microvascular decompression for trigeminal neuralgia: the importance of a noncompressive technique—Kaplan–Meier analysis in a consecutive series of 330 patients. Neurosurgery 63(4 Suppl 2): 341–350;discussion 350–351. [DOI] [PubMed] [Google Scholar]
- Spatz A.L., Zakrzewska J.M., Kay E.J. (2007) Decision analysis of medical and surgical treatments for trigeminal neuralgia: how patient evaluations of benefits and risks affect the utility of treatment decisions. Pain 131: 302–310 [DOI] [PubMed] [Google Scholar]
- Szapiro Jr J., Sindou M., Szapiro J. (1985) Prognostic factors in microvascular decompression for trigeminal neuralgia. Neurosurgery 17: 920–929 [DOI] [PubMed] [Google Scholar]
- Tatli M., Satici O., Kanpolat Y., Sindou M. (2008) Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien) 150: 243–255 [DOI] [PubMed] [Google Scholar]
- Tatli M., Sindou M. (2008) Anatomoradiological landmarks for accuracy of radiofrequency thermorhizotomy in the treatment of trigeminal neuralgia. Neurosurgery 63(1 Suppl 1): ONS129–137;discussion ONS137–128. [DOI] [PubMed] [Google Scholar]
- Taylor J.C., Brauer S., Espir M.L. (1981) Long-term treatment of trigeminal neuralgia with carbamazepine. Postgrad Med J 57(663): 16–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolle T., Dukes E., Sadosky A. (2006) Patient burden of trigeminal neuralgia: results from a cross-sectional survey of health state impairment and treatment patterns in six European countries. Pain Pract 6: 153–160 [DOI] [PubMed] [Google Scholar]
- Vilming S.T., Lyberg T., Lataste X. (1986) Tizanidine in the management of trigeminal neuralgia. Cephalalgia 6: 181–182 [DOI] [PubMed] [Google Scholar]
- Wiffen P.J., Rees J. (2007) Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev 2: CD006044–CD006044 [DOI] [PubMed] [Google Scholar]
- Zakrzewska J.M. (2002) Diagnosis and differential diagnosis of trigeminal neuralgia. Clin J Pain 18: 14–21 [DOI] [PubMed] [Google Scholar]
- Zakrzewska J.M. (2006) Insights: facts and stories behind trigeminal neuralgia. Gainesville, FL: Trigeminal Neuralgia Association [Google Scholar]
- Zakrzewska J.M., Chaudhry Z., Nurmikko T.J., Patton D.W., Mullens E.L. (1997) Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain 73: 223–230 [DOI] [PubMed] [Google Scholar]
- Zakrzewska J.M., Jassim S., Bulman J.S. (1999) A prospective, longitudinal study on patients with trigeminal neuralgia who underwent radiofrequency thermocoagulation of the Gasserian ganglion. Pain 79: 51–58 [DOI] [PubMed] [Google Scholar]
- Zakrzewska J.M., Jorns T.P., Spatz A. (2009) Patient led conferences—who attends, are their expectations met and do they vary in three different countries? Eur J Pain 13: 486–491 [DOI] [PubMed] [Google Scholar]
- Zakrzewska J.M., Lopez B.C. (2003) Quality of reporting in evaluations of surgical treatment of trigeminal neuralgia: recommendations for future reports. Neurosurgery 53: 110–120;discussion 120–122. [DOI] [PubMed] [Google Scholar]
- Zebenholzer K., Wober C., Vigl M., Wessely P., Wober-Bingol C. (2006) Facial pain and the second edition of the International Classification of Headache Disorders. Headache 46: 259–263 [DOI] [PubMed] [Google Scholar]