Abstract
Optical coherence tomography (OCT) is a noninvasive tool used for measuring tissue at micrometer resolution. It has been extensively applied to ocular pathologies and is now being studied as a biomarker in various neurologic conditions. The retina represents a unique environment for study, with unmyelinated axons that directly synapse into the central nervous system. When trying to quantify axonal degradation in neurologic disease, the currently used imaging modalities are limited in sensitivity and specificity. Early data suggest that several neurologic conditions have pathologic changes in the retinal nerve fiber layer of the eye, creating a potential surrogate marker for neurodegeneration. OCT has the potential to become a noninvasive, reproducible test for axonal degeneration that could become an invaluable tool for measuring the efficacy of potential neuroprotective agents. If the natural history of neurodegeneration, as measured by OCT, can be documented in diseases such as Alzheimer’s, Parkinson’s and multiple sclerosis, then OCT can be used to measure alterations in the rate of degeneration when treatment is applied. Thus, OCT represents a new, promising technology for documenting outcomes in neuroprotection trials.
Keywords: macula, multiple sclerosis, neurodegeneration, optical coherence tomography, retinal nerve fiber layer
Introduction
Optical coherence tomography (OCT) was developed as a noninvasive technique for acquiring in vivo cross-sectional images during the 1980s and 1990s [Huang et al. 1991]. OCT generates high-resolution images of cell layers, allowing the practitioner and investigator to obtain precise detail about the microstructure of organs. Since its development, this technology has been applied to clinical questions in numerous medical subspecialties including ophthalmology, dermatology, cancer biology, cardiology, and neurology [Chang and Budenz, 2008; Gimbel, 2008; Goh et al. 2008; Guagliumi and Sirbu, 2008; Chen and Lee, 2007; Hsiung et al. 2007; Tearney et al. 2006; Gambichler et al. 2005]. Determining the biologic correlates of these structures and the dynamic changes that occur over time is vital for incorporating OCT into clinical research and patient care algorithms. The primary consumer of OCT technology over the last 10 years has been ophthalmologists, who have used the technique to study a variety of retinal disorders. It was only recently that this technology was applied to patients with neurologic disorders.
Utilizing data that can be gathered from examinations of the eye has allowed novel insights into neurologic disease to be garnered and modeling systems to be developed. OCT of the eye holds tremendous promise as both a research and a clinical tool. Exploring the pathologic retinal changes in patients with neurologic diseases can provide a greater understanding of disease pathobiology. Furthermore, this technology may have the capability to serve as a surrogate marker of disease activity that can be used when screening therapeutic agents for efficacy and safety.
The retina provides a unique window into the nervous system because of the absence of myelin and a reduced concentration of glial cells. Thus, when studying neurons and axons in a relative ‘vacuum’ we can begin to characterize measures of retinal structure–functional relationships which can serve as a practically useful surrogate marker for neurodegeneration, neuroprotection, and potentially even neurorestoration. Over the next 10 years a large number of neurologic clinical trials will incorporate OCT data in the outcome measures for drug validation. It is critical for physicians to have an understanding of the technology, what it offers, how data are acquired and how to interpret those data. Ultimately, OCT devices may become a mainstay of the neurologist’s office, just as ophthalmoscopes have been for decades.
Optical coherence tomography
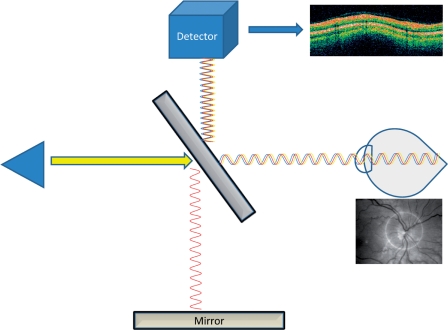
OCT is analogous to ultrasound in its operation, but uses light instead of ultrasonic waves. An OCT scan starts with a light source that emits broad-bandwidth light through a beam splitter. The light beam is split between the sample to be measured and a reference (usually a mirror). When the light beam hits the sample, most of the light is scattered, but some of the light reflects back (backscatter) towards the source. An interferometer is responsible for gathering data about the reflected light and filtering out the ‘noise’ of scattered light (Figure 1). Measurements of the reflected light allow for micrometer-level resolution of biologic structures. Various types of OCT devices allow for different levels of resolution and various methods of filtering the signal from the noise. Time domain OCT, spectral domain OCT, and time encoded frequency domain OCT are all variations on the basic OCT technology, and are all being studied in various clinical scenarios. Technologic advances are making it possible to resolve structures in the micrometer range, thus cell layers can be distinguished easily.
Figure 1.
Diagram of optical coherence tomography.
The most widely used OCT technology to date has been based on time domain tomography, with a resolution of approximately 10 µm and a sampling rate of 400 scans per second. The newer, spectral domain OCT technology has a resolution of approximately 3 µm and performs 20,000–40,000 scans per second [Ahlers and Schmidt-Erfurth, 2009; Pierre-Kahn et al. 2005]. All of the variations of these technologies aim to reduce the signal-to-noise ratio (thereby improving resolution) and minimize the scan time for image acquisition.
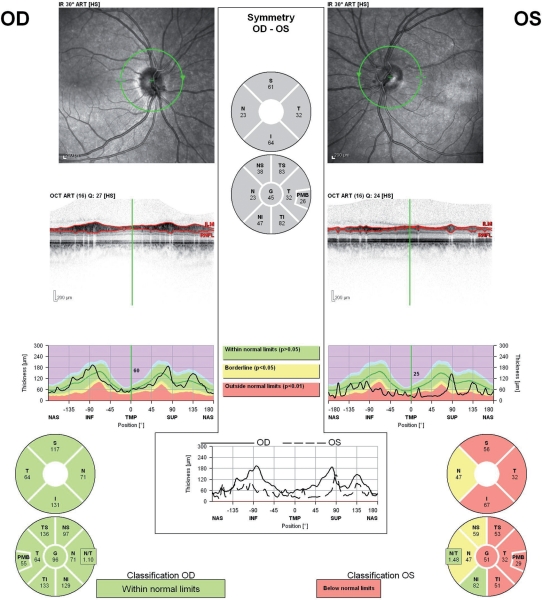
Coherence tomography techniques have been most widely used for the evaluation of ophthalmologic diseases such as macular degeneration and glaucoma [Chang and Budenz, 2008; Zhang et al. 2007]. Prior to the introduction of OCT, retinal photography was used to assess retinal nerve fiber layer (RNFL) thinning in glaucoma patients. Comparative studies between OCT and retinal photography confirmed concordance [Zangwill et al. 2000]. Extensive studies in these areas have confirmed the reproducibility of the technology between scans on the same patient and between testers, making the technology ideal for multicenter studies [Zangwill et al. 2000; Schuman et al. 1996]. RNFL metrics (e.g. average thickness, quadrant and sector analysis) can be reproducibly measured via OCT to both aid in the diagnosis and monitoring of progression of glaucoma. Normative ranges based on age, gender, and ethnicity are still being developed, but enough data already exists to make this technique clinically applicable (Figure 2) [Chang and Budenz, 2008].
Figure 2.
(a) Optic nerve head pictured on fundus photography. (b) Representative report from a time domain optical coherence tomography (OCT) device acquired on a Zeiss Cirrus™ model 4000 OCT device. (c) Representative report from a GDx polarimetry device acquired on a Zeiss GDx™ scanner.
Challenges in clinical trial design in neurology
A large of number of neurologic diseases pose unique clinical trial design challenges because of their slowly degenerative time courses, clinical heterogeneity, and lack of corresponding surrogate biomarkers. Diagnostically, neurology has been transformed with the advent and advancement of MRI technology, but its reproducibility as an outcome measure has been limited. There are countless instances of clinical–radiographic discordance (the so-called clinicoradiologic paradox). In some diseases, MRI is essentially useless for measuring disease progression. Thus, outcome measures in clinical trials have largely relied on clinical assessment scales that usually require years to measure meaningful differences.
A number of neurologic diseases follow a ‘degenerative’ course with patients having disability accumulate over many years. The therapeutic pipeline for these conditions has been limited by the expense associated with large, randomized, controlled clinical trials. Large numbers of subjects, followed for significant amounts of time are required to demonstrate statistically significant ‘neuroprotective’ qualities of medications. Thus, biomarkers are an area of intense research. These surrogate markers can have various qualities. Some biomarkers may fulfill criteria as a real-time surrogate marker of a pathologic process. For example, a circulating protein could be found to be elevated in states of neuronal apoptosis. This biomarker would be expected to decline in response to a neuroprotective agent and could be used to monitor response to therapy. Thus, a short-term trial that showed a profound impact on circulating levels of this protein biomarker could be used to justify larger, more costly trials.
Another form of a biomarker is a measure that tracks disease progression in such a sensitive and specific way, that measuring the rate of change could be meaningful. For example, if atrophy on brain MRI correlated perfectly with disease progression in Alzheimer’s disease (AD), then annual MRIs could be used to track disease progression and response to therapy. MRI, however, does not possess these characteristics and, as such, is a poor biomarker for disease progression in this disorder. Notwithstanding the well-characterized MRI changes that contribute to the diagnostic confirmation of a disorder such as multiple sclerosis (MS), MRI unfortunately has a poor correlation with disease outcome over short-term measures [Daumer et al. 2009]. Identifying a reproducible, sensitive measure of degeneration in neurologic disease would allow for accelerated drug studies where promising therapeutic candidates could be screened more efficiently.
Applications of OCT to neurologic disease
OCT has been studied in a variety of neurologic conditions including AD, Parkinson’s disease (PD), MS, and neuromyelitis optica [Altintas et al. 2008; de Seze et al. 2008; Paquet et al. 2007; Pulicken et al. 2007]. Depending on the condition, OCT can provide different insights into the pathogenesis of the specific disease entity. While the most extensive studies have been performed in MS, OCT validation as a biomarker for neurodegeneration is ongoing in each of these conditions. Alternative applications of this technology in neurologic disease have been studied in vitro, including the utilizing of an OCT probe to better position electrical leads during deep brain stimulator placement, while utilizing OCT has been recently studied to help diagnose Creutzfeldt–Jacob disease in vivo [Jafri et al. 2005].
Visual symptoms are frequent early complaints in AD patients [Berisha et al. 2007]. Some pathologic studies have found correlations between the visual complaints and regional pathologic changes in the occipital lobes [Trick et al. 1995]. While other studies have identified axonal degeneration within the optic nerve, vacuolar degeneration of retinal ganglion cells within the macula was noted in patients with AD in the 1980s [Sadun and Bassi, 1990; Blanks et al. 1989; Hinton et al. 1986]. When patients with AD were studied with OCT and pattern electroretinography, both structural and functional deficits were noted [Parisi et al. 2001]. Patients with mild cognitive impairment and AD have been compared with healthy controls with OCT technology, and statistically significant differences were identified between healthy controls and those with mild cognitive impairment. Alternately, those with severe dementia could not adequately complete the test [Paquet et al. 2007].
In another neurodegenerative disease, PD, pathologic changes in the retina, such as reduced dopamine content, have been documented [Djamgoz et al. 1997]. When PD patients were studied with OCT, selective thinning of the RNFL in the inferotemporal region was noted [Inzelberg et al. 2004]. In one study, there were correlations between macular changes and motor disability, but this has not been reproduced in larger studies [Altintas et al. 2008]. There are no longitudinal studies documenting progressive changes in PD patients over time. Demonstrating progressive thinning of RNFL over time in a neurodegenerative disorder will be critical for validating the technology as a viable biomarker.
The most extensively studied neurologic disorder with OCT has been MS. The technique has been studied in the setting of optic neuritis (ON) and as a biomarker for the degenerative processes inherent in MS. ON is a frequent event in relapsing–remitting MS (RRMS) and often heralds the onset of disease. During the inflammatory component of the ON, the optic nerve can swell secondary to inflammation, but over months after the acute event, the swelling subsides and the optic nerve decreases in size. Clinically, patients can be left with loss of vision and optic pallor on physical examination. The affected eye begins to show thinning at approximately 2 months, with changes occurring up to 6 months after the event. Subsequently there is relative stability as compared with the unaffected eye [Costello et al. 2008]. Thinning of the RNFL thickness (signifying axonal degeneration) correlates with newer MRI diffusion tensor imaging studies and clinical outcomes, as measured by low-contrast sensitivity eye charts [Naismith et al. 2009].
Beyond ON, there are correlations between OCT findings and progression of disease in MS patients. The first report of OCT in MS patients was published in 1999 by Parisi and colleagues [Parisi et al. 1999]. In this study MS patients who had recovered from ON were compared with healthy controls. When the two groups’ RNFL measurements were compared, there was a significant reduction in the RNFL thickness of ON affected eyes, compared with healthy controls, and a relative thinning of the unaffected eyes of MS patients compared with healthy controls (Figure 3). These findings correlated with functional changes as measured by pattern electroretinograms (PERGs), but not visual evoked potentials (VEPs) [Parisi et al. 1999]. Thus, this study provided the earliest evidence of clinical value from OCT in the evaluation of MS patients that was distinct from evaluating the afferent visual pathway with the classically used VEPs.
Figure 3.
Findings in a multiple sclerosis patient with previous right eye optic neuritis.
The study of the retina in MS provides an opportunity to separate demyelinating pathology from axonal pathology. In 2006, Trip and colleagues published research correlating RNFL thickness with MRI measures of optic nerve atrophy and visual acuity. When VEP results were segregated into amplitude and latency, there was only a correlation with amplitude [Trip et al. 2006]. Thus, the classic surrogate marker of demyelination, latency as measured by VEP, did not correlate with OCT findings, even though OCT correlated with functional outcomes and other measures of axonal integrity. These findings support the notion that OCT is a rather selective measure of axonal integrity, regardless of the level of demyelination.
Further value for OCT measurements are as a potential surrogate marker for global neuronal degeneration in MS patients, regardless of the presence of ON. Several studies have found correlations between cerebral atrophy, progressive disease and thinning of RFNL as measured by OCT. Correlations between RNFL measurements and cerebral atrophy, quantified by MRI measures of brain parenchymal fraction were demonstrated by Gordon-Lipkin and colleagues in 2007 [Gordon-Lipkin et al. 2007]. The same group identified a correlation between progressive forms of the disease and greater thinning of the RNFL as measured by OCT when compared with relapsing forms of the disease [Pulicken et al. 2007].
Pearls and pitfalls
There are a number of potential sources of error when performing and interpreting OCT. The normative values that account for differences in gender-, ethnicity- or age-related changes need to be verified in larger cohorts. If OCT is to become a biomarker for neurodegenerative disease, the normal rate of thinning also needs to be established. A cross-sectional normative database has been created, but needs validation by prospectively studying healthy controls [Sergott et al. 2007].
Identifying comorbid ocular disease is crucial for interpreting data gathered via OCT. Cataracts, changes in the vitreous (hemorrhage or inflammation), glaucoma, primary retinal disease, retinal edema, or optic nerve edema will alter the data [Sergott et al. 2007]. Careful acquisition of an ophthalmologic history is important prior to interpreting OCT data. Standard ophthalmologic screens, such as pressure testing, should be incorporated into OCT testing. In fact, even high myopia is a confounding factor in OCT metrics. Specifically, the RNFL thickness is reduced as the axial length and spherical equivalent of the eye becomes more negative (i.e. more myopic). This is particularly the case in those individuals who are highly myopic (less than –6 diopters) [Leung et al. 2006].
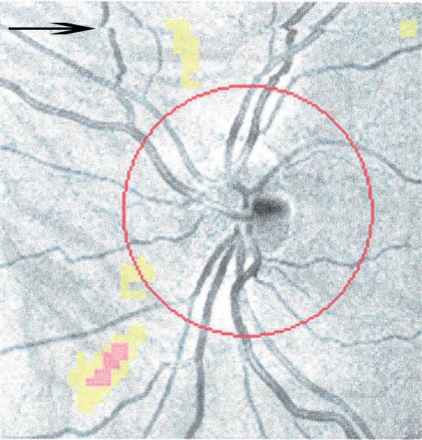
While the efforts to improve scan time and resolution are helpful for improved data, they potentially introduce more errors from eye movements. Newer devices that sample data at very high rates (up to 40,000 scans/second) may substantially reduce the impact of unwanted eye movements on scan quality and serial study coregistrations. Most devices will report ‘signal strength’ (Figure 2) which is a crude measure of the study’s validity. It should be above 7 in order for the data to be interpreted. OCT readers should become accustomed to recognizing some of the findings in substandard OCT (Figure 4).
Figure 4.
Evidence of a saccadic eye movement occurring during optical coherence tomography (OCT) acquisition. The arrow denotes the point where the saccade occurred. If this had happened at the point of the optic nerve, the retinal nerve fiber layer (RNFL) measurement would be affected.
Future of OCT as a readout in clinical trials
OCT is a promising tool for the evaluation of retinal changes in a variety of neurologic conditions. As a measure of neuronal degeneration, longitudinal OCT measures of RNFL change can act as a surrogate marker of axonal health. When designing neuroprotection trials it will be important to have verified objective measures of neuronal health that can be serially followed in patients with high precision and low test–retest variability (e.g. coefficient of variation). If the natural history of RNFL thinning can be established in patients with AD, PD, or MS, then OCT could act as a sensitive biomarker in trials that attempt to halt neurodegeneration. Having a quick, noninvasive, reproducible measure of axonal health will be invaluable for screening therapeutic agents. Ultimately, OCT may become a standard part of drug trials and a standard part of the evaluation of patients in neurology practice.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
References
- Ahlers C., Schmidt-Erfurth U. (2009) Three-dimensional high resolution OCT imaging of macular pathology. Opt Express 17: 4037–4045 [DOI] [PubMed] [Google Scholar]
- Altintas O., Iseri P., Ozkan B., Caglar Y. (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 116: 137–146 [DOI] [PubMed] [Google Scholar]
- Berisha F., Feke G.T., Trempe C.L., McMeel J.W., Schepens C.L. (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48: 2285–2289 [DOI] [PubMed] [Google Scholar]
- Blanks J.C., Hinton D.R., Sadun A.A., Miller C.A. (1989) Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 501: 364–372 [DOI] [PubMed] [Google Scholar]
- Chang R., Budenz D.L. (2008) New developments in optical coherence tomography for glaucoma. Curr Opin Ophthalmol 19: 127–135 [DOI] [PubMed] [Google Scholar]
- Chen J., Lee L. (2007) Clinical applications and new developments of optical coherence tomography: an evidence-based review. Clin Exp Optom 90: 317–335 [DOI] [PubMed] [Google Scholar]
- Costello F., Hodge W., Pan Y.I., Eggenberger E., Coupland S., Kardon R.H. (2008) Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler 14: 893–905 [DOI] [PubMed] [Google Scholar]
- Daumer M., Neuhaus A., Morrissey S., Hintzen R., Ebers G.C. (2009) MRI as an outcome in multiple sclerosis clinical trials. Neurology 72: 705–711 [DOI] [PubMed] [Google Scholar]
- de Seze J., Blanc F., Jeanjean L., Zephir H., Labauge P., Bouyon M., et al. (2008) Optical coherence tomography in neuromyelitis optica. Arch Neurol 65: 920–923 [DOI] [PubMed] [Google Scholar]
- Djamgoz M.B., Hankins M.W., Hirano J., Archer S.N. (1997) Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res 37: 3509–3529 [DOI] [PubMed] [Google Scholar]
- Gambichler T., Moussa G., Sand M., Sand D., Altmeyer P., Hoffmann K. (2005) Applications of optical coherence tomography in dermatology. J Dermatol Sci 40: 85–94 [DOI] [PubMed] [Google Scholar]
- Gimbel C. (2008) Optical coherence tomography diagnostic imaging. Gen Dent 56: 750–757; quiz 758–759, 768 [PubMed] [Google Scholar]
- Goh A.C., Tresser N.J., Shen S.S., Lerner S.P. (2008) Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology 72: 133–137 [DOI] [PubMed] [Google Scholar]
- Gordon-Lipkin E., Chodkowski B., Reich D.S., Smith S.A., Pulicken M., Balcer L.J., et al. (2007) Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69: 1603–1609 [DOI] [PubMed] [Google Scholar]
- Guagliumi G., Sirbu V. (2008) Optical coherence tomography: high resolution intravascular imaging to evaluate vascular healing after coronary stenting. Catheter Cardiovasc Interv 72: 237–247 [DOI] [PubMed] [Google Scholar]
- Hinton D.R., Sadun A.A., Blanks J.C., Miller C.A. (1986) Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315: 485–487 [DOI] [PubMed] [Google Scholar]
- Hsiung P.L., Phatak D.R., Chen Y., Aguirre A.D., Fujimoto J.G., Connolly J.L. (2007) Benign and malignant lesions in the human breast depicted with ultrahigh resolution and three-dimensional optical coherence tomography. Radiology 244: 865–874 [DOI] [PubMed] [Google Scholar]
- Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W., et al. (1991) Optical coherence tomography. Science 254: 1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzelberg R., Ramirez J.A., Nisipeanu P., Ophir A. (2004) Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 44: 2793–2797 [DOI] [PubMed] [Google Scholar]
- Jafri M.S., Farhang S., Tang R.S., Desai N., Fishman P.S., Rohwer R.G., et al. (2005) Optical coherence tomography in the diagnosis and treatment of neurological disorders. J Biomed Opt 10: 051603–051603 [DOI] [PubMed] [Google Scholar]
- Leung C.K., Mohamed S., Leung K.S., Cheung C.Y., Chan S.L., Cheng D.K., et al. (2006) Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Invest Ophthalmol Vis Sci 47: 5171–5176 [DOI] [PubMed] [Google Scholar]
- Naismith R.T., Xu J., Tutlam N.T., Snyder A., Benzinger T., Shimony J., et al. (2009) Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology 72: 589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet C., Boissonnot M., Roger F., Dighiero P., Gil R., Hugon J. (2007) Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 420: 97–99 [DOI] [PubMed] [Google Scholar]
- Parisi V., Manni G., Spadaro M., Colacino G., Restuccia R., Marchi S., et al. (1999) Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 40: 2520–2527 [PubMed] [Google Scholar]
- Parisi V., Restuccia R., Fattapposta F., Mina C., Bucci M.G., Pierelli F. (2001) Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 112: 1860–1867 [DOI] [PubMed] [Google Scholar]
- Pierre-Kahn V., Tadayoni R., Haouchine B., Massin P., Gaudric A. (2005) Comparison of optical coherence tomography models OCT1 and Stratus OCT for macular retinal thickness measurement. Br J Ophthalmol 89: 1581–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulicken M., Gordon-Lipkin E., Balcer L.J., Frohman E., Cutter G., Calabresi P.A. (2007) Optical coherence tomography and disease subtype in multiple sclerosis. Neurology 69: 2085–2092 [DOI] [PubMed] [Google Scholar]
- Sadun A.A., Bassi C.J. (1990) Optic nerve damage in Alzheimer’s disease. Ophthalmology 97: 9–17 [DOI] [PubMed] [Google Scholar]
- Schuman J.S., Pedut-Kloizman T., Hertzmark E., Hee M.R., Wilkins J.R., Coker J.G., et al. (1996) Reproducibility of nerve fiber layer thickness measurements using optical coherence tomography. Ophthalmology 103: 1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergott R.C., Frohman E., Glanzman R., Al-Sabbagh A. (2007) The role of optical coherence tomography in multiple sclerosis: expert panel consensus. J Neurol Sci 263: 3–14 [DOI] [PubMed] [Google Scholar]
- Tearney G.J., Jang I.K., Bouma B.E. (2006) Optical coherence tomography for imaging the vulnerable plaque. J Biomed Opt 11: 021002–021002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick G.L., Trick L.R., Morris P., Wolf M. (1995) Visual field loss in senile dementia of the Alzheimer’s type. Neurology 45: 68–74 [DOI] [PubMed] [Google Scholar]
- Trip S.A., Schlottmann P.G., Jones S.J., Li W.Y., Garway-Heath D.F., Thompson A.J., et al. (2006) Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage 31: 286–293 [DOI] [PubMed] [Google Scholar]
- Zangwill L.M., Williams J., Berry C.C., Knauer S., Weinreb R.N. (2000) A comparison of optical coherence tomography and retinal nerve fiber layer photography for detection of nerve fiber layer damage in glaucoma. Ophthalmology 107: 1309–1315 [DOI] [PubMed] [Google Scholar]
- Zhang N., Hoffmeyer G.C., Young E.S., Burns R.E., Winter K.P., Stinnett S.S., et al. (2007) Optical coherence tomography reader agreement in neovascular age-related macular degeneration. Am J Ophthalmol 144: 37–44 [DOI] [PubMed] [Google Scholar]