Abstract
Over 320 years after Thomas Sydenham described the condition labelled Sydenham’s chorea, it remains poorly understood. The disorder is an antineuronal antibody-mediated neuropsychiatric disorder caused by a poststreptococcal, autoimmune condition affecting control of movement, mood, behaviour and potentially the heart. The treatment remains empirical, and is less than optimal. There are few large clinically controlled trials. Recommendations for optimal management remain inconsistent and are hampered by the side effects from pharmacotherapy. Care for patients should be targeted at primary treatment (penicillin and bed rest), secondary palliation (symptomatic medication) and supportive (social) care. Small studies have demonstrated trends to support the use of immunoglobulins and steroids as therapeutic interventions for children affected by Sydenham’s chorea.
Keywords: carbamazepine, epidemiology, haloperidol, immunomodulatory, pimozide, Sydenham’s chorea, treatment, valproate
Introduction
Sydenham's chorea (SC) was first described in 1686 by Thomas Sydenham [Al-Eissa, 1993]. Despite some progress in understanding the pathogenesis and pathophysiology, the treatment remains largely symptomatic and not evidence based. SC is a major criterion for the diagnosis of acute rheumatic fever and according to the modified Jones’ criteria, its presence alone is sufficient to make this diagnosis [Garvey and Swedo, 1997].
Literature review/data analysis
This review used articles from the peer-reviewed literature, screens of on-going clinical trials and the author’s experience. Medline search extended from 1948 until 2010. The search terms ‘Sydenham's chorea’, ‘rheumatic chorea’, ‘treatment’ and ‘children’ yielded 665 articles from Medline. From these, 195 articles with relevance to the review topic were selected and 86 of these were in languages other than English and no abstracts were available. One hundred and nine articles were studied in more detail and 59 articles were referenced in this review. Twenty articles which focused on the management are summarized in Table 1. A search of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, issue 3, 2008, http://www.mrw.interscience.wiley.com/cochrane/cochrane clcentral articles fs.html) using ‘treatment of Sydenham’s chorea’ as keywords identified three comparative trials which were reviewed. There were no Cochrane reviews. A search in NLM Gateway (http://gateway.nlm.nih.gov/gw/Cmd) using general terms identified 4068 records but using the specific keywords itemized, less than 179 articles were identified. Case reports, clinical trials and review articles were selected and if available were studied and referenced.
Table 1.
Pharmacotherapy of Sydenham's chorea.
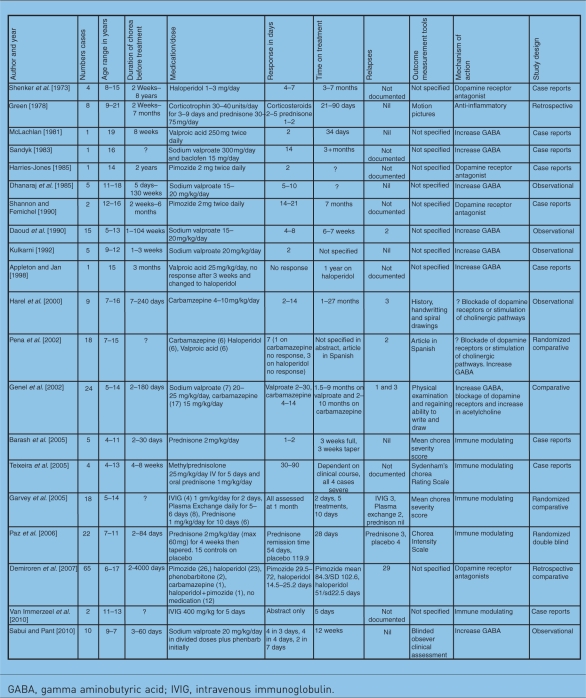 |
GABA, gamma aminobutyric acid; IVIG, intravenous immunoglobulin.
Relevant articles in English were reviewed in full and those in other languages were assessed based on the abstracts if available.
Epidemiology
Rheumatic fever is currently the major cause of acquired heart disease in children [Manyemba and Mayosi, 2003]. Up to 60% of people who present with SC will later develop rheumatic heart disease [Carapetis and Currie, 1999]. Hence, when SC is diagnosed, treatment strategies must include the prevention of rheumatic heart disease.
The incidence and prevalence of acute rheumatic fever and rheumatic heart disease in industrialized countries with market economies has been reduced significantly. In resource-poor countries, the burden of disease remains high. A minimum of 15.6 million people worldwide are estimated to have established rheumatic heart disease and close to 300,000 new cases are identified each year with 233,000 directly attributable deaths [Carapetis et al. 2005b]. The prevalence of rheumatic heart disease in children aged 5–14 years is highest in sub-Saharan Africa (5–7 per 1000), the Pacific and indigenous populations of Australia and New Zealand (3–5 per 1000) and South–Central Asia (2.2 per 1000) and lowest in developed countries (0.5 per 1000) [Carapetis et al. 2005b].
The World Health Organization (WHO) criteria using an echocardiogram to diagnose subclinical rheumatic heart disease is suboptimal when compared with the combined criteria of valve morphology in addition to the assessment of regurgitation [Marijon et al. 2009]. As such, prevalence data based on the WHO criteria alone will underestimate the true figures.
SC is the commonest form of acquired chorea. This is supported by case series from Tunisia, Iran and Turkey [Demiroren et al. 2007; Ghram et al. 1999; Ben Hamida and Hamza, 1979; Gharagozloo et al. 1976]. Large series over prolonged periods have noted a decline in the number of cases [Nausieda et al. 1980]. Whilst a reduction in the prevalence of pure SC is welcomed chorea remains a major manifestation in 20–40% of cases of acute rheumatic fever and there are reports of increasing numbers of sporadic cases in the United States [Ayoub, 1992; Jamal and Abbas, 1989].
Aetiology, histopathology and pathophysiology
SC is an antineuronal antibody-mediated neuropsychiatric disorder [Husby et al. 1976]. Antibodies which arise in response to group A beta-haemolytic streptococcus (GABAS) infection cross react with epitopes on neurons within the basal ganglia, frontal cortex and other regions. Children with SC have elevated serum antineuronal antibody titres [Swedo, 1994]. Immunoglobulin G from patients with SC cross reacts specifically with neuronal cytoplasmic antigens in subthalamic and caudate nuclei [Wolf and Singer, 2008]. A cerebral arteritis with cellular degeneration occurs. Hyperaemia, endothelial swelling, perivascular round cell infiltration and petechial haemorrhage are found on histological examination [Aron et al. 1965]. These changes are a result of the autoimmune process and result in dopaminergic dysfunction [Nausieda et al. 1983]. These insights are leading to targeted therapeutic interventions rather than symptomatic management alone.
Movements are controlled by two main systems: (a) through the motor cortex and cerebellum for simple movements and (b) through the striatum, substantia nigra, subthalamic nuclei and globus pallidus for complex movements [Edgar, 2003; Comings, 1994]. The second system (b) is relevant to movement disorders and it consists of three main pathways [Comings, 1994]. These pathways are mainly dopamine controlled and there is significant cross over between them. The basal ganglia is best considered as a relay station containing neurones with many different neurotransmitters that regulate and integrate sensory, emotional and voluntary inputs controlling motor activities. In addition, the limbic system and prefrontal cortex are seminal in the control of attention and emotion. In summary, movements, attention and emotion all result from a complex interaction of neurotransmitters in the basal ganglia, limbic systems and prefrontal cortex [Comings, 1994]. Gamma aminobutyric acid (GABA) dopamine, noradrenaline and serotonin all play a role. Symptomatic treatments act via these neurotransmitters [Edgar, 2003]. Certain medications act on the dopamine system, for example haloperidol, tetrabenazine and pimozide block dopamine receptors. Benzodiazepines facilitate the action of GABA. Valproate enhances the action of GABA. Carbamazepine modifies sodium channels which increase neuronal stability and it also acts by blockage at the level of dopaminergic postsynaptic receptors [Carapetis et al. 2005b; Edgar, 2003; Genel et al. 2002]. Carbamazepine can increase GABA levels or decrease glutamatergic activity, restoring disrupted interplay between basal ganglia and the cerebral cortex which further explains its role in the management of chorea [Feigin et al. 1995].
Clinical presentation
The clinical features of SC include both neurological abnormalities and psychiatric disorders. The former comprise involuntary choreatic movements, voluntary movement incoordination, muscular weakness and hypotonia [Gowers, 1881]. Psychiatric disorders include emotional lability, hyperactivity, distractibility, obsessions and compulsions [Garvey and Swedo, 1997]. This constellation of features results in difficulty in the execution of activities of daily living with the result that the condition impacts negatively on the quality of life of children. At the peak of their illness children may become totally dependent on their families.
Choreatic movements are involuntary, irregular, purposeless, non-rhythmic, abrupt, rapid and unsustained [Edgar, 2003]. Movements disappear with sleep and rest [Edgar, 2003]. Voluntary movements make the chorea worse and are themselves incoordinated making activities such as writing, dressing and eating difficult [Garvey and Asbahr, 2002]. The hypotonia and weakness have a range of severity from mild to severe. The severe form is termed chorea mollis or chorea paralytica and may be confused with the clinical appearance of a stroke [Garvey et al. 2005; Al-Eissa, 1993]. Such children may be mute and confined to a wheel chair [Garvey and Swedo, 1997]. Obsessions may include causing harm to loved ones, separation anxiety and fear of contamination, resulting in compulsive washing [Swedo, 1994]. Children may have severe chorea (ballistic movements) and/or hypotonia with few psychiatric symptoms or mild chorea with pronounced psychiatric symptoms [Garvey and Swedo, 1997]. A change in behaviour may precede the chorea [Asbahr et al. 1998].
Classical descriptions of SC indicate that it is benign and self-limiting [Carapetis et al. 2005b]. At best the condition lasts for 6 months but more usually it has a relapsing course for up to 2 years [Paz et al. 2006; Walker et al. 2005]. At worst it may evolve into a chronic movement disorder [Paz et al. 2006; Walker et al. 2005]. Variation in the duration of chorea and a lack of methods to quantify the severity together with the lack of a therapeutic index has made evaluation of therapy difficult [Aron et al. 1965]. To date Aron and colleagues’ clinical classification appears to be most widely used [Aron et al. 1965]. This refers to ‘mild’ in the presence of minimal movements, ‘moderate’ in the presence of movements of obvious inconvenience to the patient but which do not interfere with self-care and ‘severe’ if there are movements sufficiently incapacitating for the patient to require assistance for the activities of daily living [Al-Eissa, 1993; Aron et al. 1965]. Notably this scoring system does not include the neuropsychiatric implications of the disorder.
Therapeutic interventions for patients with Sydenham’s chorea
Based on the aetiology, pathology, pathophysiology and clinical presentation of SC, the treatment has four main tenets: elimination of the streptococcus, symptomatic treatment of the involuntary movements, incoordination and psychiatric symptoms, treatment of the immune and inflammatory response and supportive measures.
Primary treatment: elimination of streptococcus
First episodes of chorea tend to be ‘pure chorea’, but this varies in different communities [Carapetis and Currie, 1999]. Evidence of recent streptococcal infection as reflected by a raised antistreptolysin titre or a raised anti-D-Nase titre varies in different regions, but was demonstrated in studies from Turkey and Australia [Demiroren et al. 2007; Carapetis and Currie, 1999]. Furthermore 70.5% of 65 cases from Turkey and 58% of 108 cases from Australia developed cardiac involvement [Demiroren et al. 2007; Carapetis and Currie, 1999]. Thus, treatment with penicillin is mandatory to eliminate the streptococcus. Secondary prophylaxis with long-term penicillin is primarily given to protect the heart; whether it prevents relapses of SC is still debatable [Berrios et al. 1985].
Penicillin 500 mg twice daily for 10 days should be given together with rest. This is largely based on anecdotal evidence but it is common clinical practice. Physical activity should be restricted until the acute phase reactants have normalized and then restarted gradually [Cilliers, 2006]. Adverse outcomes and a chronic relapsing course of SC were more common in children who did not receive 10 days of penicillin, hospitalization and bed rest [Walker et al. 2005].
Prevention of recurrent attacks of rheumatic fever is the most cost-effective way of preventing rheumatic heart disease. Penicillin remains the antibiotic of choice [Cilliers, 2006]. Intramuscular benzylpenicillin every 28 days or oral penicillinVK 250 mg twice daily is advocated as secondary prevention of rheumatic fever. Patients must also be empowered to seek primary treatment for future streptococcal sore throats. Secondary penicillin prophylaxis is given to protect the heart and does not necessarily prevent relapses of abnormal movements [Berrios et al. 1985].
Symptomatic treatment
Despite more than three centuries of experience there is still no globally accepted protocol to treat SC. Treatments have ranged from bleeding, purging, hyperthermia, anti-inflammatory agents, sedation and currently a variety of oral pharmacotherapy is prescribed [Gordan, 2009]. The use of sedation was based on the observation that excitement and stress aggravated symptoms whilst sleep abolishes them. Barbiturates, bromides and chloral hydrate were therefore utilized in the 1960s [Aron et al. 1965]. The increased understanding of the pathophysiology has lead to the use of agents which affect the neurotransmitters dopamine and GABA.
Dopamine receptor antagonists include haloperidol, pimozide and risperidone. Haloperidol is an effective symptomatic medication but it must be titrated slowly to reach maximum effect with minimal toxic manifestations [Shenker et al. 1973].
Table 1 summarizes the larger cases series which have addressed the efficacy and safety of treatment options for SC. In a review of 65 children with SC from Turkey, comparisons of treatments showed that haloperidol was superior to pimozide for controlling chorea, both in time to onset of recovery and time to complete remission, but haloperidol was associated with more side effects [Demiroren et al. 2007]. In a retrospective study of 42 patients with SC from South Africa, 39 were treated with haloperidol. Twenty five of these patients reported side effects severe enough to cause the physician or parent to discontinue treatment or to reduce the dose [Walker et al. 2005]. In a retrospective study, patients with SC had an increased susceptibility to develop drug-induced Parkinsonism on neuroleptics [Teixeira et al. 2003]. Underlying nigrostriatal dysfunction in SC was hypothesised. Pimozide has a more selective antidopaminergic action with fewer side effects and as such is recommended [Demiroren et al. 2007; Edgar, 2003]. However, in many resource-poor countries haloperidol is first-line therapy as it is cost effective and readily available. Use of haloperidol should be based on a regimen of ‘start low and go slow’: 0.025 mg/kg/day in divided doses going up to a maximum of 0.05 mg/kg/day in divided doses [Walker et al. 2006]. Cardoso recommended that patients who present with severe chorea (particularly chorea paralytica, in which the muscle tone is so decreased that patients are bedridden) should be treated with risperidone [Cardoso, 2008]. The efficacy of this agent would be based on its role as a dopamine D2 receptor blocker [Edgar, 2003].
GABA is a neurotransmitter which inhibits dopaminergic overactivity [Edgar, 2003]. Benzodiazepines facilitate the action of GABA and valproate enhances the action on GABA, hence these agents are used to treat chorea [Edgar, 2003]. Ten patients were treated with sodium valproate, 20 mg/kg/day in two or three divided doses, for 12 weeks [Sabui and Pant, 2010]. The intervention was considered effective and safe in the treatment of SC [Sabui and Pant, 2010]. Other small case series reported similar results [Davutoglu et al. 2004; Daoud et al. 1990; Dhanaraj et al. 1985]. However, other researchers did not find valproic acid to be effective [Appleton and Jan, 1998]. Valproic acid is recommended as the first-line agent in the treatment of SC [Cardoso, 2008], especially in severe cases of SC where trials with haloperidol and diazepam have failed [Alvarez and Novak, 1985]. Carbamazepine is used in some institutions to treat SC [Carapetis et al. 2005b; Edgar, 2003; Genel et al. 2002].
A comparison study from Turkey compared the efficacy of carbamazepine (15 mg/kg/day) in 17 children with sodium valproate (20–25 mg/kg/day) given to 7 children with SC. There was no significant difference (p = 0.88) between the groups with respect to time of clinical improvement and time to complete remission, duration of therapy and the recurrence rates [Genel et al. 2002]. A group from Venezuela compared the efficacy of carbamazepine, haloperidol and valproic acid in the treatment of 18 patients with SC. The six children who received valproic acid and the five children treated with carbamazepine did well with no side effects compared with the seven patients on haloperidol of whom only three improved [Pena et al. 2002]. The remaining four were then given valproic acid and symptoms improved within a week [Pena et al. 2002].
Neither carbamazepine nor valproic acid have been assessed in controlled trials with significant numbers [Carapetis et al. 2005a].
It is important to stress that recommendations described relate to ‘off-label’ use of the cited drugs [Cardoso, 2010]. The US Food and Drug Administration (FDA) does not include SC in its guidelines list [Micro-Medics, 2010]. The FDA has the following recommendations for the medications discussed above. Haloperidol is indicated in Tourette’s disorder and the recommended paediatric dose is 0.025–0.05 mg/kg/day in divided doses, increasing gradually if necessary to a maximum of 0.15 mg/kg/day. It is not recommended in children less than 3 years old. Pimozide is indicated for Tourette’s disorder in children 12 years and older. There are no guidelines for use in children under 12 and there is a warning of cardiotoxicity and electrocardiogram is recommended before and during treatment. This is relevant in the context of rheumatic heart disease. Risperidone, sodium valproate, valproic acid and carbamazepine are not licensed for use in movement disorders and there are reports of chorea as a side effect to both carbamazepine and valproate [Lancman et al. 1994; Bimpong-Buta and Froescher, 1982]. To develop an evidence-based recommendation for the use of these medications in SC, double-blinded, placebo- and comparative-controlled trials are needed.
There are detailed descriptions of the psychiatric manifestations in SC but no reports of treatments specifically targeting these symptoms [Ridel et al. 2010; Moore, 1996].
Immunological treatments
The presence of antibodies reactive with neuronal tissue in the serum of patients with SC indicates that the condition is a humorally mediated autoimmune condition [Paz et al. 2006; Swedo, 1994]. Immunomodulatory therapies to shorten the course of the illness and to prevent complications are described using corticosteroids, intravenous immunoglobulins (IVIGs) and plasma exchange [Garvey et al. 2005]. The role for corticosteroids is further supported by the indolent inflammation of small vessels in the caudate putamen complex which occurs in SC [Green, 1978].
Improvements in symptoms with corticosteroids were reported in three studies but the group sizes were small (n = 4–8 patients) and conducted without controls [Barash et al. 2005; Teixeira et al. 2005; Green, 1978]. Twenty two children treated with steroids were compared with 15 placebo controls in a randomized, double-blind, parallel study [Paz et al. 2006]. Two milligrams per kilogram of prednisone was given for 4 weeks and then tapered. Chorea was evaluated using a chorea intensity score. Percentage decrease in chorea intensity scale was significantly better in the prednisone group (p ≤ 0.001) and chorea remission time was significantly shorter (p = 0.001). However recurrence rates were the same in the two groups [Paz et al. 2006]. Case reports have also shown that pulsed methylprednisolone followed by oral steroids was effective in severe cases [Teixeira et al. 2005] (Table 1).
Plasma exchange acts by removing the antineuronal antibodies. The first experience with plasma exchange was reported by Aron in 1991 (unpublished) [Garvey and Swedo, 1997]. A 12-year-old girl with severe chorea underwent five plasma exchanges over 2 weeks and the movements became imperceptible. Plasma exchange and IVIG were compared with prednisone [Garvey et al. 2005]. The results were not statistically significant but clinical improvement appeared to be more rapid and robust in the plasma exchange and IVIG groups [Garvey et al. 2005].
Inactivation of the antineuronal antibodies is the hypothesized effect of IVIG. Two studies are reported; namely the study cited above and another study which reported a successful outcome with two patients treated with IVIG 400 mg/kg/day for 5 days [Van Immerzeel et al. 2010]. Outcome measurements were not detailed. Garvey and colleagues used IVIG 1 g/kg/day for 2 days. The severity of the chorea was assessed using a rating scale which graded the frequency, amplitude and intensity of the chorea, as well as assessing the ability to walk, eat, dress, drink and write. A trend to improved outcome with IVIG exists but larger controlled trials with similar objective outcome measurements are needed to draw definitive conclusions.
Supportive measures
It is the authors’ opinion that management of SC requires a multidisciplinary approach. Medical interventions have been discussed above. Management of comorbid psychopathologies are important, as the condition is very distressing for the child and the family. Supportive psychotherapy and family therapy is recommended. Educational interventions are needed. It is necessary to engage the support of the educators to ensure that patients are given time to attend hospital follow-up visits and to receive penilente injections. Empowering educators to understand the condition will promote tolerance and understanding and educators can help by ensuring that children with SC are exposed to minimal teasing and bullying. Increased insight will facilitate prevention and better outcomes as educators will become advocates for early diagnosis and treatment of sore throats and SC.
In South Africa there is increasing surveillance and intent to prevent rheumatic fever and rheumatic heart disease. Every child with SC must be notified and must undergo echocardiography [Robertson et al. 2006].
Analysis of treatment-related studies
Aims of treatment are to improve symptoms, shorten the course of the illness and to prevent recurrences whilst doing no harm. Table 1 summarizes the 20 larger published studies. Based on the review of the literature and clinical study search engines, studies pertaining to pharmacotherapy are uncommon, control groups are small and objective outcome measurements are limited. As a result it is difficult to grade the level of evidence for each intervention. Combining the case studies reviewed in Table 1, 33 patients received haloperidol, 30 pimozide, 51 sodium valproate/valproic acid and 33 carbamazepine. Grading the quality of evidence and providing convincing recommendations was attempted looking at the study design, study quality, consistency and directness [GRADE Working Group, 2004]. The study design comprised nine case reports, two retrospective, five observational, three comparative and one randomized, double-blind study. There was little consistency regarding time of chorea symptoms prior to treatment, details of adverse events, specification of outcome measurement tools, protocols for discontinuation of medication and documentation of recurrent chorea. The variable natural history of both severity and time to resolution make it difficult to draw meaningful conclusions as spontaneous resolution may occur [Garvey and Asbahr, 2002]. Graded quality of evidence and recommendations were not achieved. A large cohort of patients in a multicentre randomly selected comparative trial using consistent methods and outcome measurement is required to obtain statistical and clinical certainty regarding protocols which reduce the symptom burden, shorten the course and prevent complications whilst causing no harm.
Conclusion
SC is a poststreptococcal, autoimmune, neuropsychiatric movement disorder and is a major criterion for the diagnosis of acute rheumatic fever. Establishment of universal management guidelines is difficult. General management consists of treating with penicillin, reducing symptom stress by careful use of medications to decrease the burden of abnormal movements and emotional problems and educating the patient regarding the prevention of rheumatic heart disease by the use of long-term penicillin. Immunomodulatory interventions which target the underlying autoimmune response should be considered.
As SC is synonymous with acute rheumatic fever the main goal of treatment should be primary prevention, which encompasses eradication of poverty and poor living conditions. Until this millennium goal is achieved, strategies to optimize management which reduce the burden of disease in children living with SC should be targeted.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The National Bioproducts Institute provided intravenous gamma globulin for trial number NCT 00615797. KG Walker was the principal investigator.
References
- Al-Eissa A. (1993) Sydenham’s chorea: a new look at an old disease. Br J Clin Pract 47: 14–16 [PubMed] [Google Scholar]
- Alvarez L.A., Novak G. (1985) Valproic acid in the treatment of Sydenham’s chorea. Paediatr Neurol 1(5): 317–319 [DOI] [PubMed] [Google Scholar]
- Appleton R.E., Jan J.E. (1998) Efficacy of valproic acid in the treatment of Sydenham’s chorea. J Child Neurol 3: 147–147. [DOI] [PubMed] [Google Scholar]
- Aron A.M., Freeman J.M., Carter S. (1965) The natural history of Sydenham’s chorea. Am J Med 38: 83–93 [DOI] [PubMed] [Google Scholar]
- Asbahr F.R., Negrao A.B., Gentil V. (1998) Obsessive–compulsive and related symptoms in children and adolescents with rheumatic fever with and without chorea: a prospective 6-month study. Am J Psychiatry 155: 1122–1124 [DOI] [PubMed] [Google Scholar]
- Ayoub E.M. (1992) Resurgence of rheumatic fever in the United States. The changing picture of a preventable illness. Postgrad Med 92: 133–136, 139–142 [DOI] [PubMed] [Google Scholar]
- Barash J., Margalith D., Matitiau A. (2005) Corticosteroid treatment in patients with Sydenham’s chorea. Pediatr Neurol 32: 205–207 [DOI] [PubMed] [Google Scholar]
- Ben Hamida M., Hamza F. (1979) [Sydenham’s chorea in Tunisia: a report on 65 cases]. Ann Med Interne (Paris) 130(6–7): 359–364 [PubMed] [Google Scholar]
- Berrios X., Quesney F., Morales A., Blazquez J., Bisno A.L. (1985) Are all recurrences of ‘pure’ Sydenham’s chorea true recurrences of acute rheumatic fever. J Paediatr 107: 867–872 [DOI] [PubMed] [Google Scholar]
- Bimpong-Buta K., Froescher W. (1982) Carbamazepine-induced choreathetoid dyskinesias [letter]. J Neurol Neurosurg Psychiatry 45: 560–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis J.R., Currie B.J. (1999) Rheumatic chorea in northern Australia: a clinical and epidemiological study. Arch Dis Child 80: 355–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapetis J., McDonald M., Wilson N.J. (2005a) Letter. Lancet 366: 1355–1355. [DOI] [PubMed] [Google Scholar]
- Carapetis J.R., McDonald M., Wilson N. (2005b) Acute rheumatic fever. Lancet 366: 155–168 [DOI] [PubMed] [Google Scholar]
- Cardoso F. (2008) Sydenham’s chorea. Curr Treat Options Neurol 10: 230–235 [DOI] [PubMed] [Google Scholar]
- Cardoso F.(2010)Sydenham chorea, In: Dale R.C., Vincent A. (eds).Inflammatory and Autoimmune Disorders of the Nervous System in Children (Clinics in Developmental Medicine, No. 184–185), MacKeith Press: London [Google Scholar]
- Cilliers A.M. (2006) Rheumatic fever and its management. BMJ 333: 1153–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D.E. (1994)Tourette Syndrome and Human Behaviour, 3rd edn, Hope Press: Duarte, CA [Google Scholar]
- Daoud A.S., Zaki M., Shakir R., al-Saleh Q. (1990) Effectiveness of sodium valproate in the treatment of Sydenham’s chorea. Neurology 40: 1140–1141 [DOI] [PubMed] [Google Scholar]
- Davutoglu V., Kilinc M., Dinckal H., Soydinc S., Sezen Y. (2004) Sydenham’s chorea—clinical characteristics of nine patients. Int J Cardiol 96: 483–484 [DOI] [PubMed] [Google Scholar]
- Demiroren K., Yavuz H., Cam L., Oran B., Karaaslan S., Demiroren S. (2007) Sydenham’s chorea: a clinical follow-up of 65 patients. J Child Neurol 22: 550–554 [DOI] [PubMed] [Google Scholar]
- Dhanaraj M., Radhakrishnan A.R., Srinivas K., Sayeed Z.A. (1985) Sodium valproate in Sydenham’s chorea. Neurology 35: 114–115 [DOI] [PubMed] [Google Scholar]
- Edgar T.S. (2003) Oral pharmacotherapy of childhood movement disorders. J Child Neurol 18: 840–849 [DOI] [PubMed] [Google Scholar]
- Feigin A., Keiburtz K., Shoulsen L. (1995) Treatment of Huntington’s disease and other choreic disorders, In: Kurlan R. (ed.).Treatment of Movement Disorders. Philadelphia, Lippincott: PA [Google Scholar]
- Garvey M.A., Asbahr F.R. (2002) Sydenham chorea, In: Guerrini R., Aicardi J., Andermann F., Hallett M. (eds). Epilepsy and Movement Disorders, Cambridge University Press: Cambridge [Google Scholar]
- Garvey M.A., Snider L.A., Leitman S.F., Werden R., Swedo S.E. (2005) Treatment of Sydenham’s chorea with intravenous immunoglobulins, plasma exchange, or prednisone. J Child Neurol 20: 424–429 [DOI] [PubMed] [Google Scholar]
- Garvey M.A., Swedo S.E. (1997) Sydenham’s chorea clinical and therapeutic update. Adv Exp Med Biol (2) 418: 115–120 [DOI] [PubMed] [Google Scholar]
- Genel F., Arslanoglu S., Uran N., Saylan B. (2002) Sydenham’s chorea: clinical findings and comparison of the efficacies of sodium valproate and carbamazepine regimens. Brain Develop 24: 73–76 [DOI] [PubMed] [Google Scholar]
- Gharagozloo R.A., Daneshpajooh M., Ghavamian P. (1976) Rheumatic fever and rheumatic heart disease among 568000 inhabitants in southeast Teheran from 1972–1974. Acta Trop 33: 215–222 [PubMed] [Google Scholar]
- Ghram N., Allani C., Oudail B., Fitouri Z., Ben Becher S. (1999) Sydenham’s chorea in children. Arch Pediatr 6: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Gordan N. (2009) Sydenham’s chorea, and its complications affecting the nervous system. Brain Develop 31: 11–14 [DOI] [PubMed] [Google Scholar]
- Gowers W.R. (1881) On paralytic chorea. Br Med J i: 636–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADE Working Group (2004) Education and debate. Grading quality of evidence and strength of recommendations. BMJ 328: 1490–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. (1978) Corticosteroids in the treatment of Sydenham’s chorea. Arch Neurol 35: 53–54 [DOI] [PubMed] [Google Scholar]
- Harel L., Zecharia A., Straussberg R., Volovitz B., Amir J. (2000) Successful treatment of rheumatic chorea with carbamazepine. Pediatr Neurol 23: 147–151 [DOI] [PubMed] [Google Scholar]
- Harries-Jones R. (1985) Successful treatment of refractory Sydenham’s chorea with pimozide. J Neurol Neurosurg Psychiatry 48: 390–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Van de Rinj I., Zabriskie J.B., Abdin A.H., Williams R.C. (1976) Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med 114: 1094–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M., Abbas K.A. (1989) Clinical profile of acute rheumatic fever in children. J Trop Pediatr 35: 10–13 [DOI] [PubMed] [Google Scholar]
- Kulkarni M.L. (1992) Sodium valproate in Sydenham’s chorea. Indian Pediatr 29: 385–386 [PubMed] [Google Scholar]
- Lancman M.E., Asconape J.J., Penry J.K. (1994) Choreiform movements associated with the use of valproate. Arch Neurol 51: 702–704 [DOI] [PubMed] [Google Scholar]
- Manyemba J., Mayosi B.M. (2003) Intramuscular penicillin is more effective than oral penicillin in secondary prevention of rheumatic fever- systematic review. SAMJ 93: 212–218 [PubMed] [Google Scholar]
- Marijon E., Celermajer D.S., Tafflet M., El-Haou S., Jani D.N., Ferreira B., et al. (2009) Rheumatic heart disease screening by echocardiography: the inadequacy of World Health Organisation criteria for optimizing the diagnosis of subclinical disease. Circulation 120: 663–668 [DOI] [PubMed] [Google Scholar]
- McLachlan, R. (1981) Valproic acid in Sydenham’s chorea. Brit Med J 283: 275. [DOI] [PMC free article] [PubMed]
- Micro-Medics (2010) Micro-Medics Health Care Series Volume 144, 2nd Quarter 2010 [Google Scholar]
- Moore D.P. (1996) Neuropsychiatric aspects of Sydenham’s chorea: a comprehensive review. J Clin Psychiatry 57: 407–414 [PubMed] [Google Scholar]
- Nausieda P.A., Bieliauskas L.A., Bacon L.D., Hagerty M., Koller W.C., Glantz R.N. (1983) Chronic dopamanergic sensitivity after Sydenham’s chorea. Neurology 33: 750–754 [DOI] [PubMed] [Google Scholar]
- Nausieda P.A., Grossman B.J., Koller W.C., Weiner W.J., Klawans H.L. (1980) Sydenham chorea: an update. Neurology 30: 331–334 [DOI] [PubMed] [Google Scholar]
- Paz J.A., Silva C.A.A., Marques-Dias M.J. (2006) Randomized double-blind study with prednisone in Sydenham’s chorea. Pediatr Neurol 34: 264–269 [DOI] [PubMed] [Google Scholar]
- Pena J., Mora E., Cardozo J., Molina O., Montiel C. (2002) Comparison of the efficacy of carbamazepine, haloperidol and valproic acid in the treatment of children with Sydenham’s chorea: clinical follow-up of 18 patients. Arq Neuropsiquiatr 60: 374–377 [DOI] [PubMed] [Google Scholar]
- Ridel K.R., Lipps T.D., Gilbert D.L. (2010) The prevalence of neuropsychiatric disorders in Sydenham’s chorea. Pediatr Neurol 42: 243–248 [DOI] [PubMed] [Google Scholar]
- Robertson K.A., Volmink J.A., Mayosi B.M. (2006) Towards a uniform plan for the control of rheumatic fever and rheumatic heart disease in Africa—the Awareness Surveillance Advocacy Prevention (A.S.A.P.) Programme. SAMJ 96: 241–245 [PubMed] [Google Scholar]
- Sabui T.K., Pant P. (2010) Sodium valproate in the treatment of Sydenham’s chorea. Indian J Paedr, (E-pub ahead of print) PMID: 20454934 [DOI] [PubMed] [Google Scholar]
- Sandyk R. (1983) Sodium valproate and baclofen for Sydenham’s chorea. SAMJ 64: 6–6. [PubMed] [Google Scholar]
- Shannon, K. and Fernichel, G.M. (1990) Pimozide treatment of Sydenham’s chorea. Neurology 40: 186. [DOI] [PubMed]
- Shenker D.M., Grossman H.J., Klawans H.L. (1973) Treatment of Sydenham’s chorea with haloperidol. Develop Med Child Neurol 15: 19–24 [DOI] [PubMed] [Google Scholar]
- Swedo S.E. (1994) Sydenham’s chorea. A model for childhood autoimmune neuropsychiatric disorders. JAMA 272: 1788–1791 [DOI] [PubMed] [Google Scholar]
- Teixeira A.L., Cardoso F., Maia D.R., Cunningham M.C. (2003) Sydenham’s chorea may be a risk factor for drug induced Parkinsonism. J Neurol Neurosurg Psychiatry 74: 1350–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A.L., Maia D.P., Cardoso F. (2005) Treatment of acute Sydenham’s chorea with methyl-prednisolone pulse-therapy. Parkinsonism Rel Disord 11: 327–330 [DOI] [PubMed] [Google Scholar]
- Van Immerzeel T.D., van Gilst R.M., Hartwig N.G. (2010) Beneficial use of immunoglobulins in the treatment of Sydenham’s chorea. Eur J Pediatr, PMID: 20349351 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K., Lawrenson J., Wilmshurst J.M. (2006) Sydenham’s chorea- clinical and therapeutic update 320 years down the line. SAMJ 96: 906–912 [PubMed] [Google Scholar]
- Walker K.G., Lawrenson J., Wilmshurst J.M. (2005) Neuropsychiatric movement disorders following streptococcal infection. Dev Med Child Neurol 47: 771–775 [DOI] [PubMed] [Google Scholar]
- Wolf D.S., Singer H.S. (2008) Pediatric movement disorders: an update. Curr Opin Neurol 21: 491–498 [DOI] [PubMed] [Google Scholar]


