Abstract
(9-Methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)hydrazine (1) was used as a precursor for preparation of some novel 1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-1H-pyrazoles 2–7, -1H-isoindole-1,3(2H)-dione 8, and -pyridazin-3(2H)-one 9. Moreover, the acyclic C-nucleosides 10 and 11 were prepared by treating compound 1 with D-glucose. The in vitro antimicrobial activity of the tested compounds was evaluated by measuring the zone diameters and some of the prepared products showed potent antimicrobial activity in compared with those of well known drugs (standard). In general, the non-acetylated sugar hydrazone derivative 10 showed the highest antibacterial and antifungal potency among the tested compounds and standard with IZ = 22, 21 and 22 mm and MIC = 62.5 and 31.25 μg/ml, respectively.
Keywords: Thieno[2,3-d]pyrimidine; Pyrazole; Pyridazine; C-Nucleosides; Antimicrobial activity
Introduction
Pyrimidine and fused heterocyclic pyrimidine derivatives have attracted a great deal of interest in particular 4-hydrazinopyrimidine derivatives, which were tested for their medicinal, bactericidal and fungicidal activity [1–4]. Pyrimidine and heterocyclic annulated pyrimidine derivatives attracted great interest due to the wide variety of interesting biological activities observed for these compounds, such as anticancer [4], antiviral [5], Anti-HIV-1 Activity [6], anti-inflammatory [7] and antimicrobial activities [8]. In addition, several substituted dihydronaphthothienopyrimidine derivatives showed antimicrobial activities against Bacillus subtilis, Escherichia coli, Aspergillus niger and Candida albicans [9], and their ester-containing derivatives demonstrated more antimicrobial activities than the corresponding cyano-containing analogs. In view of the above and in continuation of our research program concerned with structural modification of certain biologically active heterocyclic nuclei with the purpose of enhancing their biological activity [10–16], we aimed to incorporate a fused pyrimidine moiety with other heterocyclic ring system to obtain new functions in an attempt to improve the antimicrobial profile of compounds containing the dihydronaphthothienopyrimidine ring system.
Results and Discussion
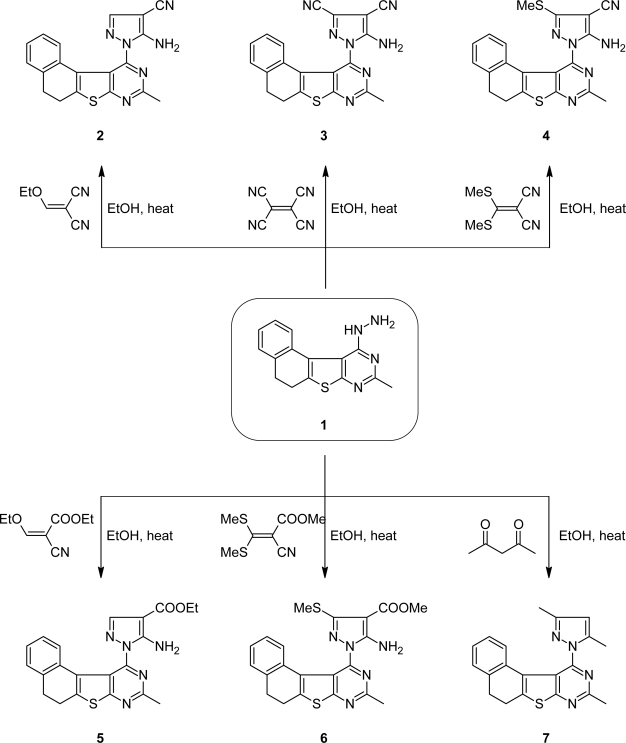
From the view of biological activity, polycondensed fused heteroaromatic systems are often of much greater interest than the constituent monocyclic compounds. The appearance of qualitatively new properties of an annelated molecule, enlargement of the possibility of varying pharmacophore groups in different positions of the molecule and the ability of the latter to interact with various receptors adopting several conformations are apparently of crucial importance [17]. In this investigation, compound 1 [18] was dissolved in ethanol and refluxed with (ethoxymethylene)malononitrile, tetracyanoethylene, [bis(methylthio)methylene]malononitrile, ethyl (ethoxymethylene)cyanoacetate or methyl [bis(methylthio)methylene]cyanoacetate to afford the corresponding substituted pyrazole derivatives 2–6, respectively (Sch. 1). The structures of the latter compounds were confirmed on the basis of their elemental analysis and spectral data (cf. Exp.). The IR spectra of compounds 2–4 showed absorption bands characteristic for NH2 and C≡N groups, while those of compounds 5 and 6 revealed absorption bands characteristic for NH2 and C=O. Also, the 1H NMR spectra showed signals at δ = 7.10, 6.55, 6.60, 6.81 and 7.00 ppm due to NH2 (exchangeable with D2O) for compounds 2–6, respectively. The MS gave the molecular ion peaks at m/z (%) = 358 (100), 383 (89), 404 (100), 405 (100), and 437 (100) for compounds 2–6, respectively. Similarly, when compound 1 was refluxed with acetyl acetone the pyrazole derivative 7 was obtained (Sch. 1). The 1H NMR and 13C NMR spectra of the latter compound showed signals at δ = 2.05, 2.19 ppm and 11.97, 13.29 ppm for (2CH3), while its IR spectrum revealed the absence of NH, NH2 groups. The MS, gave the molecular ion peak at m/z (%) = 346 (78).
Sch. 1.
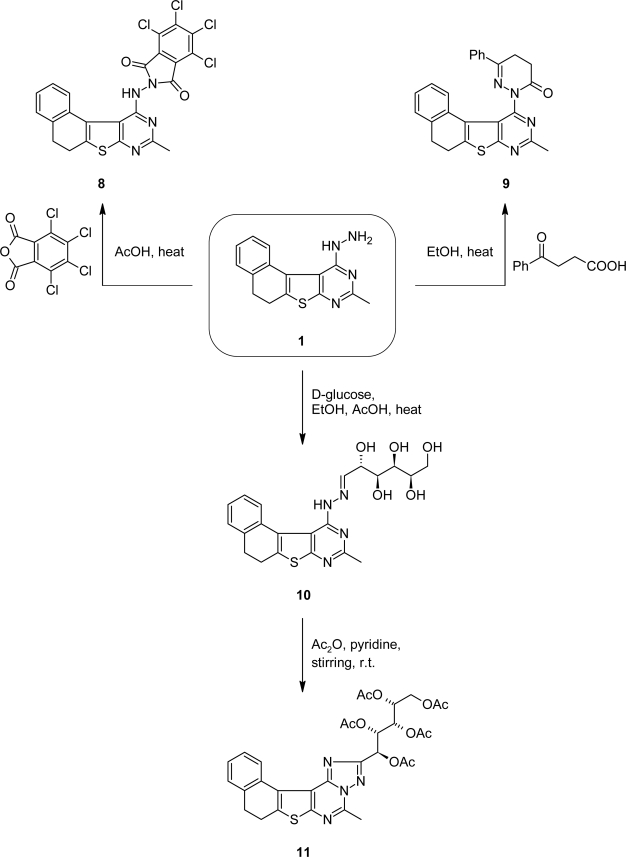
On the other hand, compound 1 was refluxed with tetrachlorophthalic anhydride in glacial acetic acid to afford N-imido derivative 8 (Sch. 2). The presence of NH and two C=O groups in the IR spectra and the NH-proton (D2O exchangeable) in the 1H-NMR spectrum confirmed its structure. Also, the MS of compound 8 gave fragments showing the isotopic pattern due to the presence of chlorine atoms (cf. Exp.).
Sch. 2.
2-(9-Methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-6-phenyl-4,5-dihydropyridazin-3(2H)-one (9) was prepared by heating the hydrazine derivative 1 with β-benzoylpropionic acid. Inspection of the IR spectrum of product 9 showed the presence of the C=O group and its 1H NMR spectrum revealed signals at δ 2.43, 3.61 ppm for 2CH2 of pyridazine ring. In addition, the 13C NMR spectrum showed signal at δ 173.80 accountable for the C=O group (cf. Exp.).
The hydrazone derivative 10 was prepared by reacting compound 1 with D-glucose in the presence of a catalytic amount of glacial acetic acid. The product 10 revealed absorption bands for OH and NH groups in IR spectra and its 1H NMR spectrum showed the presence of the sugar protons, NH, and azo-methine (CH=N) (cf. Exp.).
Acetylation of the hydrazone derivative 10 with acetic anhydride/pyridine at room temperature gave unexpected O-acetylated cyclic C-nucleosides 11 (Sch. 2). The absence of NH as well as the azo-methine (CH=N) in 1H NMR spectra confirmed its structure (cf. Exp.). Also, the 13C NMR spectrum showed signals accountable for the acetylated sugar residue (cf. Exp.).
The formation of compound 11 might have taken place via oxidative cyclization of the O-acetylated sugar. In general, the Dimroth type rearrangement of S-triazolopyrimidines was intensively discussed and verified with X-ray diffraction by Rashad et al [16]. So, the triazolo[1,5-c]pyrimidine derivative 11 was obtained directly via Dimroth type rearrangement of its triazolo[4,3-c]pyrimidine derivative.
Antimicrobial activity
The in vitro antimicrobial activity of the tested compounds was evaluated by measuring the zone diameters and the results were compared with those of well known drugs (standard). Among the tested compounds, for gram-positive and gram-negative bacteria, it was noticed that ß-enaminonitriles of pyrazole ring system 2–4 (IZ = 16–18 mm and MIC = 65 μg/ml) demonstrated inhibitory activities more than β-enaminoesters 5 and 6 (IZ = 10–12 mm and MIC = 125 μg/ml). However, the pyridazine derivative 9 and non-acetylated sugar 10 revealed the most significant antibacterial activities (IZ = 20–22 mm and MIC = 62.5 μg/ml). On the other hand, the non-acetylated sugar 10 and the acetylated cyclic C-nucleoside 11 revealed more effective antifungal activity than the other tested compounds showing IZ = 22 mm and MIC = 31.25 μg/ml. However, replacement of the hydroxyl moiety in 10 by acetyl group in 11 led to decrease the antibacterial potency of 10 showing IZ = 11, 12 mm, MIC = 62.5 μg/ml and the antifungal activity was not affected. In general, the non-acetylated sugar hydrazone derivative 10 showed the highest antibacterial and antifungal potency among the tested compounds and standard with IZ = 22, 21 and 22 mm and MIC = 62.5 and 31.25 μg/ml, respectively.
Experimental
All melting points were uncorrected and measured using an Electro-thermal IA 9100 apparatus (Shimadzu, Japan). Microanalytical data were performed by Vario El-Mentar apparatus (Shimadzu, Japan), National Research Centre, Cairo, Egypt. The IR spectra (KBr) were recorded on a Perkin-Elmer 1650 spectrophotometer, National Research Centre, Cairo, Egypt. 1H NMR and 13C NMR spectra were determined on a Varian Mercury (300 MHz) spectrometer (Varian, UK) and the chemical shifts were expressed in ppm relative to TMS as internal reference, Faculty of science, Cairo University, Egypt. Mass spectra were recorded on 70 eV EI Ms-QP 1000 EX (Shimadzu, Japan), National Research Centre, Cairo, Egypt.
Compound 1 was prepared according to a reported method [18].
Preparation of compounds 2–6
General procedure
To a solution of compound 1 (2.82g, 1mmol) in (20 ml) dry ethanol, (ethoxymethylene)malononitrile, tetracyanoethylene, [bis(methylthio)methylene]malononitrile, ethyl (ethoxymethylene)cyanoacetate or methyl [bis(methylthio)methylene]cyanoacetate (1 mmol) was added and the reaction mixtures were refluxed for 2, 5, 4, 3 and 4 h, respectively. The products, which separated on cooling, were collected by filtration and recrystallized from ethanol to give compounds 2–6.
5-Amino-1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-1H-pyrazole-4-carbonitrile (2)
Yield (95%), M.p. 260–262°C; IR (KBr) ν = 3407, 3200 (NH2), 2209 (CN) cm−1; 1H NMR (DMSO-d6) δ = 2.77 (s, 3H, CH3), 2.95–3.00 (m, 4H, 2CH2), 6.34 (d, J = 8 Hz, 1H, Ar-H), 6.91–7.31 (m, 5H, 3Ar-H and NH2, D2O exchangeable), 7.55 (s, 1H, C3-H); 13C NMR (DMSO-d6) δ = 25.32 (CH3), 25.67 (C-5′), 29.62 (C-6′), 115.29 (C-4), 118.72 (CN), 125.21, 126.83, 127.29, 128.12, 128.47 (Ar-C), 131.89 (C-3), 135.48 (C-6a′), 141.92 (C-11c′), 143.35 (C-11b′), 150.45 (C-7a′), 153.64 (C-5), 162.16 (C-9′), 172.10 (C-11′); MS, m/z (%): 358 (M+, 100). Anal. calcd for C19H14N6S: C, 63.67; H, 3.94; N, 23.45; S, 8.95. Found: C, 63.62; H, 4.00; N, 23.39; S, 9.01.
5-Amino-1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-1H-pyrazole-3,4-dicarbonitrile (3)
Yield (65%), M.p. 166–168 °C; IR (KBr) ν = 3407, 3200 (NH2), 2219 (CN) cm−1; 1H NMR (DMSO-d6) δ = 2.80 (s, 3H, CH3), 3.00–3.15 (m, 4H, 2CH2), 6.25 (d, J = 8 Hz, 1H, Ar-H), 6.55 (s, 2H, NH2, D2O exchangeable), 7.00–7.26 (m, 3H, Ar-H); 13C NMR (DMSO-d6) δ = 25.14 (CH3), 25.23 (C-5′), 29.18 (C-6′), 110.68 (CN), 116.69 (CN), 124.34, 125.90, 127.25, 128.05 (Ar-C), 131.05 (C-3), 134.59 (C-6a′), 142.71 (C-11c′), 148.35 (C-11b′), 150.40 (C-7a′), 152.69 (C-5), 162.16 (C-9′), 173.10 (C-11′); MS, m/z (%): 383 (M+, 89). Anal. calcd for C20H13N7S: C, 62.65; H, 3.42; N, 25.57; S, 8.36. Found: C, 62.60; H, 3.48; N, 25.54; S, 8.40.
5-Amino-1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-3-(methylsulfanyl)-1H-pyrazole-4-carbonitrile (4)
Yield (90%), M.p. 124–126 °C; IR (KBr) ν = 3460, 3346 (NH2), 2215 (CN) cm−1; 1H NMR (DMSO-d6) δ = 1.58 (s, 3H, SCH3), 2.80 (s, 3H, CH3), 2.90–3.05 (m, 4H, 2CH2), 6.50 (d, J = 8 Hz, 1H, Ar-H), 6.60 (s, 2H, NH2, D2O exchangeable), 7.00–7.25 (m, 3H, Ar-H); 13C NMR (DMSO-d6) δ = 11.64 (CH3), 25.15 (CH3), 25.34 (C-5′), 29.32 (C-6′), 113.04 (C-4), 115.59 (CN), 126.03, 126.16, 126.47, 127.13, 128.51 (Ar-C), 132.50 (C-3), 133.82 (C-6a′), 140.67 (C-11c′), 150.75 (C-11b′), 151.25 (C-7a′), 153.83 (C-5), 160.17 (C-9′), 172.31 (C-11′); MS, m/z (%): 404 (M+, 100). Anal. calcd for C20H16N6S2: C, 59.38; H, 3.99; N, 20.78; S, 15.85. Found: C, 59.29; H, 4.05; N, 20.71; S, 15.92.
Methyl 5-amino-1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-1H-pyrazole-4-carboxylate (5)
Yield (90%), M.p. 139–141 °C; IR (KBr) ν = 3464, 3354 (NH2), 1685 (C=O) cm−1; 1H NMR (DMSO-d6) δ = 1.34 (t, J = 6.9 Hz, 3H, CH3), 2.81 (s, 3H, CH3), 2.96–3.07 (m, 4H, 2CH2), 4.28 (q, J = 7.5 Hz, 2H, CH2), 6.43 (d, J = 8 Hz, 1H, Ar-H), 6.81 (s, 2H, NH2, D2O exchangeable), 6.90–7.35 (m, 4H, 3Ar-H and C3-H); 13C NMR (DMSO-d6) δ 14.45 (CH3), 25.17 (CH3), 25.29 (C-5′), 29.32 (C-6′), 59.82 (OCH2), 96.49 (C-4), 125.65, 126.03, 126.25, 127.33, 128.30 (Ar-C), 131.85 (C-3), 134.14 (C-6a′), 140.77 (C-11c′), 141.41 (C-11b′), 151.35 (C-7a′), 151.83 (C-5), 160.39 (C-9′), 164.31 (C-11′), 172.29 (C=O); MS, m/z (%): 405 (M+, 100). Anal. calcd for C21H19N5O2S: C, 62.20; H, 4.72; N, 17.27; S, 7.91. Found: C, 62.30; H, 4.65; N, 17.33; S, 7.81.
Ethyl 5-amino-1-(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-3-(methylsulfanyl)-1H-pyrazole-4-carboxylate (6)
Yield (90%), M.p. 250–252 °C; IR (KBr) ν = 3460, 3346 (NH2), 1685 (C=O) cm−1; 1H NMR (DMSO-d6) δ = 1.45 (s, 3H, SCH3), 2.77 (s, 3H, CH3), 2.97–3.10 (m, 4H, 2CH2), 3.83 (s, 3H, OCH3), 6.51 (d, J = 8 Hz, 1H, Ar-H), 6.97–7.23 (m, 5H, 3Ar-H and NH2, D2O exchangeable); 13C NMR (DMSO-d6) δ = 11.16 (SCH3), 25.12 (CH3), 25.34 (C-5′), 29.37 (C-6′), 51.03 (OCH3), 93.95 (C-4), 125.92, 126.27, 126.53, 126.85, 128.78 (Ar-C), 132.76 (C-3), 133.59 (C-6a′), 139.92 (C-11c′), 150.86 (C-11b′), 151.46 (C-7a′), 153.56 (C-5), 160.06 (C-9′), 164.52 (C-11′), 172.04 (C=O); MS, m/z (%): 437 (M+, 100). Anal. calcd for C21H19N5O2S2: C, 57.65; H, 4.38; N, 16.01; S, 14.66. Found: C, 57.71; H, 4.33; N, 16.09; S, 14.60.
11-(3,5-Dimethyl-1H-pyrazol-1-yl)-9-methyl-5,6-dihydronaphtho[1′,2′:4,5]-thieno[2,3-d]pyrimidine (7)
To a solution of compound 1 (2.82 g, 1 mmol) in ethanol (20 ml), acetyl acetone (1 mmol) was added and the reaction mixture was refluxed for 10 h. The solvent was then removed under reduced pressure and the residue was recrystallized from ethanol to give compound 7. Yield (70%), M.p. 129–131 °C; 1H NMR (DMSO-d6) δ = 2.05 (s, 3H, CH3), 2.19 (s, 3H, CH3), 2.86 (s, 3H, CH3), 2.95–3.03 (m, 4H, 2CH2), 5.91 (s, 1H, C4′-H), 6.10 (d, J = 8 Hz, 1H, Ar-H), 6.79–7.19 (m, 3H, Ar-H); 13C NMR (DMSO-d6) δ = 11.97 (CH3), 13.29 (CH3), 25.04 (CH3), 25.36 (C-5), 29.41 (C-6), 108.56 (C-4′), 119.21, 124.89, 126.19, 126.33, 127.15, 127.82 (Ar-C), 131.06 (C-3′), 134.49 (C-6a), 140.49 (C-11c), 141.58 (C-11b), 151.03 (C-7a), 161.85 (C-9), 172.04 (C-11); MS, m/z (%): 346 (M+, 78). Anal. calcd for C20H18N4S: C, 69.34; H, 5.24; N, 16.17; S, 9.26. Found: C, 69.30; H, 5.29; N, 16.09; S, 9.33.
4,5,6,7-Tetrachloro-2-[(9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)amino]-1H-isoindole-1,3(2H)-dione (8)
A mixture of compound 1 (2.82 g, 1 mmol) and tetrachlorophthalic anhydride (2.85 g, 1 mmol) in glacial acetic acid (50 ml) was refluxed for 2 h. The formed precipitate was filtered on hot, dried and recrystallized from dioxane to give compound 8. Yield (73%), M.p. 285–287 °C; IR (KBr) ν = 3326 (NH) and 1791, 1724 (anhydride CO) cm−1; 1H NMR (DMSO-d6) δ = 2.35 (s, 3H, CH3), 2.83–3.28 (m, 4H, 2CH2), 7.08–7.35 (m, 2H, Ar-H), 7.85 (d, J = 7.2 Hz, 1H, Ar-H), 8.37 (d, J = 7.6 Hz, 1H, Ar-H), 9.60 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6) δ = 25.10 (CH3), 26.02 (C-5′), 30.13 (C-6′), 98.95 (C-2a), 125.90, 126.27, 126.71, 126.85, 128.78 (Ar-C), 132.35 (C-3), 134.82 (C-6a′), 139.92 (C-11c′), 150.68 (C-11b′), 154.46 (C-7a′), 155.50 (C-6a), 160.06 (C-9′), 162.52 (C-11′), 164.10 (C=O), 168.50 (C=O); MS, m/z (%): 552 (M+, Cl37, 11.32), 550 (M+, Cl35, 4.72). Anal. calcd for C23H12Cl4N4O2S: C, 50.20; H, 2.20; Cl, 25.77; N, 5.82; S, 5.83. Found: C, 50.31; H, 2.12; Cl, 25.81; N, 5.78; S, 5.90.
2-(9-Methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)-6-phenyl-4,5-dihydropyridazin-3(2H)-one (9)
A mixture of compound 1 (2.82 g, 1 mmol) and β-benzoylpropionic acid (1.78 g, 1 mmol) in ethanol (50 ml) was refluxed for 4 h. The formed precipitate was filtered off hot, dried and recrystallized from dioxane to give compound 9. Yield (97%), M.p. 154–156 °C; IR (KBr) ν = 1691 (C=O) cm−1; 1H NMR (DMSO-d6) δ = 2.43 (m, 5H, CH2 and CH3), 2.81–2.95 (m, 4H, 2CH2), 3.61 (t, J = 7.5 Hz, 2H, CH2), 7.10–7.45 (m, 9H, Ar-H); 13C NMR (DMSO-d6) δ = 17.74 (CH3), 23.87 (C-5′), 29.35 (C-6′), 39.92 (C-5), 56.64 (C-4), 125.30, 125.85, 126.53, 127.62, 128.45 (Ar-C), 131.24 (C-3), 134.66 (C-6a′), 139.58 (C-11c′), 150.21 (C-11b′), 154.40 (C-7a′), 160.25 (C-9′), 162.51 (C-11′), 173.80 (C=O); MS, m/z (%): 424 (M+, 100). Anal. calcd for C25H20N4OS: C, 70.73; H, 4.75; N, 13.20; S, 7.55. Found: C, 70.66; H, 4.81; N, 13.12; S, 7.62.
D-Glucose (9-methyl-5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidin-11-yl)hydrazone (10)
A mixture of compound 1 (2.82 g, 1 mmol), D-glucose (1.80 g, 1 mmol), ethanol (30 ml), and a catalytic amount of glacial acetic acid (3 drops) was heated at 80 °C for 2 h. The formed precipitate was filtered off, dried and recrystallized from ethanol to give compound 10. Yield (60%), M.p. 142–144 °C; IR (KBr) ν = 3353–3220 (broad, OH+NH) cm−1; 1H NMR (DMSO-d6) δ = 2.40 (m, 3H, CH3), 2.80–3.02 (m, 4H, 2CH2), 3.20–3.60 (protons of the alditol congregated with the solvent absorption) [14], 3.70–3.80 (m, 2H, CH2OH), 4.40-5.11 (m, 5H, 5OH, D2O exchangeable), 7.05–7.42 (m, 5H, Ar-H and NH, D2O exchangeable), 8.30 (s, 1H, N=CH); 13C-NMR (DMSO-d6) δ = 18.82 (CH3), 25.60 (C-5), 29.63 (C-6), 61.26, 70.71, 72.78, 77.12, 92.65 (C-alditol), 125.91, 126.20, 126.75, 126.80, 128.74 (Ar-C), 134.62 (C-6a′), 139.91 (C-11c′), 150.52 (C-11b′), 153.42 (C-7a′), 160.06 (C-9′), 162.51 (C-11′), 162.90 (N=CH). Anal. calcd for C21H24N4O5S: C, 56.74; H, 5.44; N, 12.60; S, 7.21. Found: C, 56.84; H, 5.39; N, 12.69; S, 7.11.
(1S)-1,2,3,4,5-Penta-O-acetyl-1-C-(5-methyl-8,9-dihydronaphtho[1′,2′:4,5]-thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-D-arabinitol (11)
A solution of compounds 10 (1 mmol) in a mixture of acetic anhydride (10 mL) and anhydrous pyridine (10 ml) was stirred at room temperature for 24 h. The reaction mixture was poured into ice-water with stirring and the solids that precipitated were collected by filtration, washed with water, dried and recrystallized from ethanol to give compounds 11. Yield (70%), M.p. 109–111 °C; IR (KBr) ν = 1748 (OAc), 1602 (C=N); 1H NMR (DMSO-d6) δ = 1.80–2.10 (m, 15H, 5OAc), 2.40 (m, 3H, CH3), 2.80–3.10 (m, 6H, 3CH2), 4.20–5.50 (m, 4H, 4CHOAc), 7.10–7.40 (m, 4H, Ar-H); 13C-NMR (DMSO-d6) δ = 20.50, 20.62, 20.66, 20.74, 20.88 (5CH3), 22.70 (C-5′), 29.86 (C-6′), 61.41, 65.90, 67.08, 67.52, 70.52, 73.99 (C-alditol), 109.91 (C-4), 126.81, 127.24, 127.38, 127.53 (Ar-C), 135.13 (C-3), 136.62 (C-6a′), 142.12 (C-11c′), 149.52 (C-11b′), 153.40 (C-7a′), 160.10 (C-9′), 162.51 (C-11′), 169.56, 170.18, 170.59, 170.62, 170.83 (5CO). Anal. calcd for C32H34N4O10S: C, 57.65; H, 5.14; N, 8.40; S, 4.81. Found: C, 57.55; H, 5.20; N, 8.45; S, 4.80.
Antimicrobial activity
In vitro antimicrobial screening
The newly synthesized compounds were screened for their antibacterial activity against one gram positive bacteria, Bacillus subtilis NRRL B-543 and one gram negative bacteria, Escherichia coli NRRL B-210 and yeast Candida albicans NRRL Y-477. These microorganisms were obtained from Northern Utilisation Research and Development Division, U.S. Departement of Agricultural Peoria, Illinois, USA. Chloramphenicol and Fluconazole were purchased (pure form) from Egyptian market and used in a concentration of 2 mg/ml as references for antibacterial and antifungal activities. These compounds were assayed by the agar diffusion method [19]. The assay medium flasks containing 50 ml of nutrient agar medium for bacteria and Czapek’s-Dox agar media for yeast. The holes each of 9 mm diameter were made by scooping out medium with a sterilized cork borer in a petri dish which was seeded with the organisms. The solutions of each test compound (0.10 ml) were added separately in the holes and Petri dishes were subsequently incubated. The incubation was carried out at 30°C for 24h. Simultaneously, controls were maintained by employing 0.10 ml of dimethylsulfoxide (DMSO) which did not reveal any inhibition and zones of inhibition produced by each compound was measured in mm. The results of antimicrobial studies are given in Table 1.
Tab. 1.
The inhibition zones diameter (IZ) in mm.
| Compound No. | Escherichia coli | Bacillus subtilis | Candida albicans |
|---|---|---|---|
| 2 | 16 | 16 | 11 |
| 3 | 16.5 | 16 | 12 |
| 4 | 16 | 18 | 16 |
| 5 | 12 | 11 | 11 |
| 6 | 11 | 10 | 10 |
| 7 | 11 | 12 | 10 |
| 8 | 12 | 12 | 10 |
| 9 | 20 | 20 | 15 |
| 10 | 22 | 21 | 22 |
| 11 | 12 | 11 | 22 |
| Chloramphenicol | 24 | 24 | – |
| fluconazole | – | – | 26 |
Highly active (inhibition zone > 20 mm); Moderately active (inhibition zone16–19 mm); Slightly active (inhibition zone 11–15 mm); (–) no inhibition zone
Minimal inhibitory concentration (MIC) measurement
The bacteriostatic activity of the active compounds (having inhibition zones (IZ) ≥ 16 mm) was then evaluated using the two fold serial dilution technique [20]. Two fold serial dilutions of the test compounds and reference drugs solutions were prepared using the proper nutrient broth. The final concentration of the solutions varied between 500 and 7.81 μg/ml with the concentration of DMF not exceeding 2.5%. Each 0.10 ml from the tested compounds in DMF was mixed with 1 ml, 2 ml, and 3 ml sterilized distilled water and 0.10 ml from each diluted samples was added to test tubes. The tubes were then inoculated with the test organisms, grown in their suitable broth at 37 °C for 24 hours for bacteria and 48 hours for fungi (about 1×106 cells/ml), each 5 ml received 0.10 ml of the above inoculum and were incubated at 37 °C for 48 hours. The lowest concentration showing no growth was taken as the minimum inhibitory concentration (MIC) (Table 2).
Tab. 2.
MIC in μg/ml of the most active compounds.
| Compound No. | Escherichia coli | Candida albicans | Bacillus subtilis |
|---|---|---|---|
| 4 | 65 | 31.25 | 65 |
| 5 | 125 | 31.25 | 125 |
| 9 | 62.5 | 15 | 62.5 |
| 10 | 62.5 | 31.25 | 62.5 |
| 11 | 12 | 31.25 | 11 |
| Chloramphenicol | 10 | – | 10 |
| Fluconazole | – | 0.5 | – |
Conclusions
The overall results indicated that, the tested compounds showed promising antimicrobial activity against bacteria and Fungi. Among the tested compounds, for gram-positive and gram-negative bacteria, it was noticed that ß-enaminonitriles of pyrazole ring system 2, 3 and 4 demonstrated inhibitory activities more than ß-enaminoesters 5 and 6. However, the pyridazine derivative 9 and non-acetylated sugar 10 revealed the most significant antibacterial activities. On the other hand, the non-acetylated sugar 9 and the acetylated cyclic C-nucleoside 10 revealed more effective activity against yeast than the other tested compounds.
Supporting Information
The scanned 1H NMR spectra of compounds 2 and 4, and the scanned 13C NMR spectra of compounds 2, 4 and 7 are available in the online version (Format: PDF, Seize: ca. 0.3 MB): http://dx.doi.org/10.3797/scipharm.0910-11.
Acknowledgments
We are grateful to Dr. Klaus Banert, Chemistry Department, Technical University Chemnitz, Germany for facilities and support.
Authors’ Statements
Competing Interests
The authors declare no conflict of interest.
References
- [1].Rashad AE, Mohamed MS, Zaki MEA, Fatahala SS. Synthesis and Biological Evaluation of Some Pyrrolo[2,3-d]pyrimidines. Arch Pharm. 2006;339:664–669. doi: 10.1002/ardp.200600055. doi:10.1002/ardp.200600055. [DOI] [PubMed] [Google Scholar]
- [2].Jyh-Haur C, Kak-Shan S, Tsu-An H, Chia-Liang T, Chung-Chi L, Yen-Chun L, Chih-Shiang C, Sung-Nien T, Shin-Ru S. Design, synthesis, and structure–activity relationships of pyrazolo[3,4-d]pyrimidines: a novel class of potent enterovirus inhibitor. Bioorg Med Chem Lett. 2004;14:2519–2525. doi: 10.1016/j.bmcl.2004.02.092. doi:10.1016/j.bmcl.2004.02.092. [DOI] [PubMed] [Google Scholar]
- [3].Dolakova P, Dracinsky M, Masojidkova M, Solinova V, Kasicka V, Holy A. Acyclic nucleoside bisphosphonates: Synthesis and properties of chiral 2-amino-4,6-bis[(phosphonomethoxy)alkoxy]pyrimidines. Eur J Med Chem. 2009;44:2408–2424. doi: 10.1016/j.ejmech.2008.09.031. doi:10.1016/j.ejmech.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baraldi PG, Pavani MG, Nunez MC, Vitali PBB, Gambari R, Romagnoli R. Antimicrobial and antitumor activity of N-heteroimine-1,2,3-diathiazoles and their transformation in triazolo-, imidazo- and pyrazolopyrimidines. Bioorg Med Chem. 2002;10:449–456. doi: 10.1016/s0968-0896(01)00294-2. doi:10.1016/S0968-0896(01)00294-2. [DOI] [PubMed] [Google Scholar]
- [5].Nasr MN, Gineinah MM. Pyrido[2,3-d]pyrimidines and pyrimido[5’,4’:5,6]-pyrido[2,3-d] pyrimidines as new antiviral agents: Synthesis and biological activity. Arch Pharm. 2002;335:289–295. doi: 10.1002/1521-4184(200208)335:6<289::AID-ARDP289>3.0.CO;2-Z. doi:10.1002/1521-4184(200208)335:6<289::AID-ARDP289>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [6].Loksha YM, Pedersen EB, Loddo R, Colla P. Synthesis and Anti-HIV-1 Activity of 1-Substiuted 6-(3-Cyanobenzoyl) and [(3-Cyanophenyl)fluoromethyl]-5-ethyluracils. Arch Pharm. 2009;342:501–506. doi: 10.1002/ardp.200900058. doi:10.1002/ardp.200900058. [DOI] [PubMed] [Google Scholar]
- [7].Raffa D, Maggio B, Plescia F, Cascioferro S, Raimondi MV, Plescia S, Cusimano MG. Pyrazolo[3,4-d]pyrimidine Derivatives as COX-2 Selective Inhibitors: Synthesis and Molecular Modelling Studies. Arch Pharm. 2009;342:321–326. doi: 10.1002/ardp.200800140. doi:10.1002/ardp.200800140. [DOI] [PubMed] [Google Scholar]
- [8].Aly AA. Synthesis and pharmacological activity of annelated pyrimidine derivatives. Phosphorus, Sulfur Silicon Relat Elem. 2006;181:1285–1298. doi:10.1080/10426500500326792. [Google Scholar]
- [9].Rashed AE, Shamroukh AH, Hegab MI, Awad HM. Synthesis of some biologically active pyrazoles and C-nucleosides. Acta Chim Slov. 2005;52:429–434. [Google Scholar]
- [10].Rashad AE, Hegab MI, Abdel-Megeid RE, Fatahala NA, Abdel-Megeid FME. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Eur J Med Chem. 2009;182:1535–1556. doi: 10.1016/j.bmc.2008.06.054. doi:10.1016/j.ejmech.2009.02.012. [DOI] [PubMed] [Google Scholar]
- [11].Shamroukh AH, Rashad AE, Sayed HH. Synthesis of some pyrazolo[3,4-d]pyrimidine derivatives for biological evaluation. Phosphorus, Sulfur Silicon Relat Elem. 2005;180:2347–2360. doi:10.1080/104265090921074. [Google Scholar]
- [12].Rashad AE, Hegab MI, Abdel-Megeid RE, Micky JA, Abdel-Megeid FME. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorg Med Chem. 2008;16:7102–7106. doi: 10.1016/j.bmc.2008.06.054. doi:10.1016/j.bmc.2008.06.054. [DOI] [PubMed] [Google Scholar]
- [13].Shamroukh AH, Zaki MEA, Morsy EMH, Abdel-Motti FM, Abdel-Megeid FME. Synthesis of pyrazolo[4′,3′:5,6]pyrano[2,3-d]pyrimidine derivatives for antiviral evaluation. Arch Pharm. 2007;340:236–243. doi: 10.1002/ardp.200700005. doi:10.1002/ardp.200700005. [DOI] [PubMed] [Google Scholar]
- [14].Rashad AE, Shamroukh AH, Ali MA, Abdel-Motti FM. Synthesis and antiviral screening of some novel pyridazines and triazolopyridazine nucleosides. Heteroatom Chem. 2007;18:274–282. doi:10.1002/hc.20296. [Google Scholar]
- [15].Hegab MI, Hassan NA, Rashad AE, Fahmy AA, Abdel-Megeid FME. Synthesis, reactions, and antimicrobial activity of some fused thieno[2,3-d]pyrimidine derivatives. Phosphorus, Sulfur Silicon Relat Elem. 2007;182:1535–1556. doi:10.1080/10426500701247151. [Google Scholar]
- [16].Rashad AE, Heikal OA, El-Nezhawy AOH, Abdel-Megeid FME. Synthesis and isomerization of thienotriazolopyrimidine and thienotetrazolo pyrimidine derivatives with potential anti-inflammatory activity. Heteroatom Chem. 2005;16:226–234. doi:10.1002/hc.20114. [Google Scholar]
- [17].Litvinov VP. Thienopyrimidines: synthesis, properties, and biological activity. Russ Chem Bull. 2004;53:487–516. doi:10.1023/B:RUCB.0000035630.75564.2b. [Google Scholar]
- [18].Abdel-Megeid FME, Hassan NA, Zahran MA, Rashad AE. Synthesis of 5,6-dihydronaphtho[1′,2′:4,5]thieno[2,3-d]pyrimidines, 5,6-dihydronaphtho [1′,2′:4,5]thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines and some of their nucleosides. Sulfur Lett. 1998;21:269–284. [Google Scholar]
- [19].Jain SR, Kar A. The antibacterial activity of some essential oils and their combinations. Planta Med. 1971;20:118–123. doi: 10.1055/s-0028-1099675. doi:10.1055/s-0028-1099675. [DOI] [PubMed] [Google Scholar]
- [20].Scott AC. Laboratory Control of Antimicrobial Therapy. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, editors. Mackie and MacCartney Practical Medical Microbiology. 13th Ed. Vol. 2. Churchill Livingstone: 1989. pp. 161–181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The scanned 1H NMR spectra of compounds 2 and 4, and the scanned 13C NMR spectra of compounds 2, 4 and 7 are available in the online version (Format: PDF, Seize: ca. 0.3 MB): http://dx.doi.org/10.3797/scipharm.0910-11.