Abstract
Murine knock-out models and blastocyst co-culture studies have identified prostaglandin-endoperoxide synthase (PTGS) 2, prostaglandin (PG) E receptor 2 (PTGER2) and the chemokine receptor CXCR4 as important regulators of early pregnancy events. In vitro studies and studies in non-human primates have shown that these proteins are regulated in the endometrium by the early embryonic signal, chorionic gonadotrophin (CG). Here we show that expressions of PTGER2 and CXCR4 are elevated during the mid-secretory phase of the menstrual cycle and decidua of early pregnancy in humans. Using first trimester decidua explants, we show that CG induces expression of PTGS2 and biosynthesis of PGE2, and expression of PTGER2. Subsequently, PGE2via PTGER2 induces expression of CXCR4. Using an in vitro model system of Ishikawa endometrial epithelial cells stably expressing PTGER2 and human first trimester decidua explants, we demonstrate that CXCR4 expression is regulated by PTGER2 via the epidermal growth factor receptor (EGFR)-phosphatidylinositol-3-kinase (PI3K)-extracellular signal-regulated kinase (ERK1/2) pathway.Taken together, our data suggest that early embryonic signals may regulate fetal–maternal crosstalk in the human endometrium by inducing CXCR4 expression via the PGE2–PTGER2-mediated induction of the EGFR, PI3K and ERK1/2 pathways.
Keywords: chorionic gonadotrophin, prostaglandin, PTGER2, implantation, signalling
Introduction
Prostaglandins (PGs) have been recognized for many years as key regulators of female reproductive tract function, including ovulation, implantation, menstruation and myometrial contractility (Baird et al., 1996; Dong et al., 1996; Kniss, 1999; Jabbour et al., 2006). In the human endometrium, PGE2 is abundantly biosynthesized from arachidonic acid by prostaglandin-endoperoxide synthase (PTGS; also called cyclooxygenase or COX) and terminal PG E synthase enzymes. Following biosynthesis, PGE2 is transported out of the cell and functions by binding to and activating G protein-coupled receptors of which there are 4 subtypes (PTGER1-4) (Sales and Jabbour, 2003; Jabbour and Sales, 2004).
Although all PG receptors are widely distributed throughout the human body, expression of PTGER2 is most abundant in the uterus and placenta (Smock et al., 1999), suggesting an important role for this receptor in uterine function. Indeed, knock-out mouse studies have shown that PGE2 and PTGER2 play important roles in uterine implantation. These studies have highlighted that inhibition of PTGS2 activity and PGE2 biosynthesis results in delayed implantation and ablation of PTGER2 signalling results in implantation failure (Hizaki et al., 1999; Kennedy et al., 1999; Tilley et al., 1999; Narumiya and FitzGerald, 2001; Shah and Catt, 2005).
Successful implantation is reliant on fetal–maternal dialogue during the window of uterine receptivity, which commences around 6 days after the luteinizing hormone peak, when the uterus is responsive to embryonic signals (Tabibzadeh, 1998). One of the earliest embryonic signals in primates is chorionic gonadotrophin (CG), which is biosynthesized by the syncitiotrophoblast cells of the blastocyst (Spencer and Bazer, 2004). Little is known of the precise molecular mechanism regulating fetal–maternal dialogue to facilitate successful pregnancy outcomes.
Endometrial receptivity is associated with the induction of expression of a host of adhesive molecules, growth factors, cytokines, chemokines and chemokine receptors. One such receptor, CXCR4, increases in the endometrium of the baboon during the window of implantation in response to CG treatment (Sherwin et al., 2007), is elevated in human decidualized stromal cells in response to conditioned medium from trophoblasts (Hess et al., 2007) and is induced in endometrial epithelial cells at the site of blastocyst apposition in vitro (Dominguez et al., 2003). Furthermore, its expression is reduced in the receptive period of women who were refractory to implantation (Tapia et al., 2008). These data highlight the potential importance of this receptor for early pregnancy events, however, the molecular mechanisms regulating its expression in the human endometrium remain to be fully elucidated.
We have previously shown that PGs can regulate expression of inflammatory mediators and angiogenic growth factors in endometrial and cervical epithelial cells via the PTGER2-mediated transphosphorylation of the epidermal growth factor receptor (EGFR) and extracellular signal-regulated kinase pathways (ERK1/2) (Sales et al., 2002b; Jabbour and Boddy, 2003; Battersby et al., 2007). The EGFR, ERK1/2, and more recently, the phosphatidylinositol-3-kinase (PI3K) pathways are thought to play a role in regulating genes involved in growth, differentiation and uterine receptivity (Evans et al., 2008, 2009; Banerjee et al., 2009).
The aim of this study was to determine the mechanism of regulation of CXCR4 expression in the human endometrium and first trimester human decidua by CG. We found that CXCR4 was elevated in the epithelial and stromal compartments during the mid-secretory phase of the menstrual cycle coincident with PTGER2 and was further elevated in the decidua of early pregnancy. We investigated the molecular mechanism whereby CG regulates CXCR4 expression in first trimester decidua. We found that CG induces expression of PTGS2 and PTGER2 and release of PGE2 in first trimester decidua explants. In turn PGE2 via PTGER2 induces the expression of CXCR4. Using an in vitro model system of Ishikawa endometrial epithelial cells stably expressing PTGER2, we found that CXCR4 expression was regulated by PTGER2 via the EGFR-PI3K-ERK1/2 pathway.
Experimental procedures
Reagents
Dulbecco's modified Eagle's medium (DMEM) F-12 GLUTAMAX cell culture medium was purchased from Invitrogen Life Technologies (Paisley, UK). The protein kinase A (PKA) inhibitor [4-cyano-3-methylisoquinoline (4C3MQ), used at 1 µM], mitogen-activated protein kinase (MAPK kinase)/MEK inhibitor (PD98059, used at 50 µM), the phosphatidylinositol 3-kinase (PI3K/Akt) inhibitor (wortmannin, used at 20 nM) and the EGFR tyrosine kinase inhibitor (AG1478; used at 100 nM) were purchased from Calbiochem (Nottingham, UK). Recombinant hCG (used at a final concentration of 1 international unit; IU), the PTGER2 agonist (butaprost, used at 5 µM) and PTGER2 antagonist (AH6809, used at 10 µM) were purchased from Sigma (Dorset, UK). The anti-phospho-ERK1/2, anti-total ERK1/2, anti-phospho-FAKy397, anti-phospho-FAKy925 and anti-phospho-AKTthr308 were purchased from Cell Signaling Technologies (New England Biolabs, Ltd., Hertfordshire, UK).
Patients and tissue collection
Non-pregnant endometrial tissue (n = 30) at different stages of the menstrual cycle was collected from women undergoing surgery for minor gynaecological procedures using an endometrial suction curette (Pipelle, Laboratoire CCD, France). The women had no underlying endometrial pathology and had regular menstrual cycles of between 25 and 35 days. None of these women had received a hormonal preparation in the 3 months preceding biopsy collection. Biopsies were dated according to stated last menstrual period and confirmed by histological assessment by a pathologist according to criteria of Noyes et al. (1975). Furthermore, circulating estradiol and progesterone concentrations were consistent for both the stated last menstrual cycle and histological assessment of the stage of the menstrual cycle. First trimester decidua (6–10 weeks, n = 27) was collected from women undergoing elective first-trimester surgical termination of pregnancy. Ethical approval was obtained from Lothian Research Ethics Committee and written informed consent was obtained from all subjects before tissue collection.
Cell and tissue culture and treatment
Ishikawa endometrial epithelial cells were obtained from the European Collection of Cell Culture (Wiltshire, UK). Stable PTGER2-transfected cells were designed and characterized as described before (Abera et al., 2010). PTGER2 Ishikawa cells were cultured in DMEM/F-12 cultured medium supplemented with 10% fetal bovine serum (Sigma) and a maintenance dose of 200 µg/ml of G418 antibiotic (Sigma). Cells (∼2 × 105) were incubated in 2 ml serum-free DMEM overnight prior to agonist treatments either alone or in the presence of inhibitors for times indicated in figure legends. Inhibitors were added 30 min prior to agonist treatment. Vehicle/agonist treatments were administered once at time zero. Cells were harvested and RNA and protein was extracted for RT–PCR and western blot/phospho-array analysis, respectively. Cell experiments were performed in 6-well plates in duplicate.
Tissue explants were finely chopped with scissors and divided into equal portions of ∼20 mg and incubated in 1 ml of serum-free DMEM overnight. Tissues were then stimulated with vehicle or agonist in the presence of inhibitors in 1 ml of serum-free DMEM for the times indicated in figure legends. Inhibitors were added 30 min prior to vehicle or agonist treatment. Vehicle/agonist treatments were administered once at time zero. Tissues were removed from the culture medium and weighed. RNA was extracted from the tissues for RT–PCR analysis and the conditioned media was collected for PGE2 ELISA. Tissue experiments were performed in duplicate.
Taqman quantitative RT–PCR
Total RNA was extracted from cells using Total RNA Isolation Reagent (TRI reagent) from Sigma following the manufacture's instructions using phase lock tubes from Eppendorf (Cambridge, UK). Quantified RNA samples were reverse transcribed and quantitative RT–PCR was performed as described before (Sales et al., 2004) using the ABI7500 or ABI7900 real-time PCR platform (Applied Biosystems, Warrington, UK). Sequence-specific primers and probes were designed to span an intron. The sequences of the primers and probes for PTGER2, PTGS2 and ribosomal 18S have been described in our earlier studies (Sales et al., 2001, 2002a). Primer and probe sequences for CXCR4 are as follows: forward: 5′-CAG-TGG-CCGACC-TCC-TCT-3′: reverse: 5′-CAG-TTT-GCC-ACG-GCA-TCA-3′ and probe 5′-FAM-TCA-TCA-CGC-TTC-CCT-TCT-GGG-CA-3′. Analysis of all samples was performed using the comparative CT method (ΔΔCT) and results were expressed relative to a positive RNA standard (cDNA obtained from a single endometrial tissue) included in all reactions. The expression of all analysed genes was normalized for RNA loading using ribosomal 18S RNA as an internal standard in the same reaction. Where data are expressed as fold above control, the relative ΔΔCT value for the treatment group was divided by the ΔΔCT for the vehicle group. All data are expressed as mean ± SEM.
PGE2 ELISA
The PGE2 ELISA is an established in-house assay system validated and reported previously (Denison et al., 1999; Ledingham et al., 1999). All culture medium from tissue experiments for ELISA were treated 1:1 with methyloximating solution (0.1 M methoxyamine hydrochloride in 10% ethanol diluted in 1 M sodium acetate, pH 5.6) overnight at 4°C. Plates (Costar Amine-binding plates, Paisley, UK) were coated with donkey anti-rabbit (DAR serum; Scottish Antibody Production Unit, Carluke, UK) using the direct γ-globulin binding procedure. The assay used a PGE–biotin link as a pro-label as described previously (Denison et al., 1999; Ledingham et al., 1999). Samples and synthetic standards (Applied Therapeutics, Paisley, UK; highest concentration 5120 pg/ml) were analysed in duplicate. The intra- and inter-assay coefficients of variation were 7.8 and 15.0%, respectively and the ED50 was 195 pg/ml. The PGE2 concentration in all samples was normalized to the wet weight of the tissue. Fold increase was calculated by dividing the value obtained for the agonist treatment by the vehicle treatment for the same sample. Data are presented as mean ± SEM.
Immunohistochemistry
Localization of CXCR4 protein expression was investigated in endometrial (n = 5 proliferative, n = 5 early secretory and n = 5 mid secretory) and decidual tissues (n = 10 decidual tissues ranging from week 6 to week 10 of pregnancy) by immunohistochemistry using the Vision Biosystems Bond Immunostaining Robot under normal operating conditions (Leica Microsystems Wetzlar, Germany) as described (Sales et al., 2009). Immunostaining was performed following antigen retrieval in 0.01 M sodium citrate pH 6 using a specific primary antibody for CXCR4 (1:100; Santa Cruz Biotechnology, Wiltshire, UK). Control tissue was incubated with immunoglobulin (IgG) from the host species.
KinetworksTM phosphoprotein analysis
For phosphoprotein array analysis, approximately 3 × 106cells were seeded in 10 cm dishes. On the following day, the cells were washed with PBS and incubated in serum-free culture medium containing penicillin/streptomycin for at least 16 h. After stimulation with vehicle (ethanol v/v) or butaprost for 10 min, cells were washed with ice-cold PBS and proteins were extracted with protein lysis buffer [150 mM NaCl, 50 mM Tris–HCl (pH 7.4), 10 mM EDTA, 0.6% nonidet-P40 and 10% glycerol containing protease inhibitors (Protease inhibitor mini-cocktail, Roche, UK and 1 mM Na3VO4)]. Insoluble material was pelleted by centrifugation at 14 000 × rpm for 20 min at 4°C. The clarified lysate was removed to a new tube and protein content was quantified by the method of Lowry (Bio-Rad, Hemel Hempstead, UK). Detergent-solubilized extracts (500 µg) from PTGER2 cells were subjected to Kinetworks phospho-site screening (KPSS) as described on the Kinexus Bioinformatics Corporation Website (www.kinexus.ca). These screens use panels of highly validated commercial phosphosite-specific antibodies and 20-lane multichannel blotters. The intensities of the enhanced chemiluminescence signals for the target protein bands on the Kinetworks immunoblots were quantified with a fluorS Max imager and Quantity One software (Biorad) as described on the Kinexus Bioinformatics Corporation Website (www.kinexus.ca).
Western blot analysis
Immunoprecipitation and western blot analysis was carried out as described previously (Sales et al., 2004, 2005, 2008; Maldonado-Perez et al., 2009). After resolving and immunoblotting, membranes were incubated overnight at 4°C, with a specific primary antibodies as described in the figure legend and secondary antibodies conjugated to Alexafluor 680 (1:5000; Invitrogen) or IRDyeTM 800 (1:5000; Rockland, Gilbersville, PA, USA) for 60 min at room temperature. Blots were visualized and quantified using an Odyssey infrared imaging system (LI-COR, Cambridge, UK). Data are presented as mean ± SEM for three independent experiments and are calculated as fold above control unstimulated cells at time zero after normalizing to either total ERK or IgG as loading controls as described previously (Sales et al., 2004, 2005, 2008; Maldonado-Perez et al., 2009).
Statistical analysis
Data are represented as mean ± SEM and were analysed by t-test or ANOVA using Prism 5.0c (Graph Pad, San Diego, CA, USA).
Results
PTGER2 and CXCR4 expression are co-regulated during the menstrual cycle
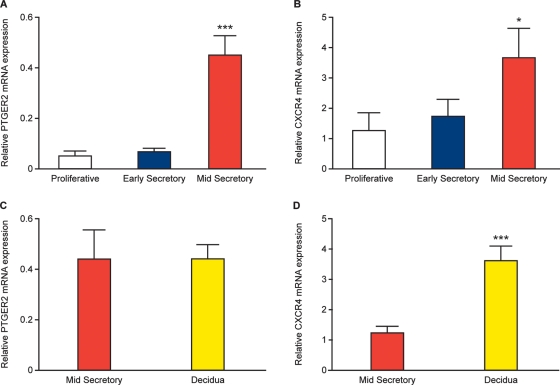
We investigated the temporal expression of PTGER2 and CXCR4 mRNA across the human menstrual cycle by quantitative RT–PCR. We found that PTGER2 (Fig. 1A) and CXCR4 (Fig. 1B) mRNA expression levels were significantly elevated in mid-secretory phase (n = 10), compared with proliferative phase (n = 10) and early secretory phase (n = 10) endometrium (P < 0.05). Furthermore, we found that PTGER2 mRNA expression was sustained (Fig. 1C), while CXCR4 mRNA expression was further increased (Fig. 1D) in the decidua of early pregnancy (n = 30) compared with mid-secretory phase endometrium (P < 0.01).
Figure 1.
Relative mRNA expression for PTGER2 (A and C) and CXCR4 (B and D) in proliferative phase (n = 10), early secretory phase (n = 10) and mid-secretory phase (n = 10) endometrium and first trimester decidua (n = 30) as determined by quantitative RT–PCR analysis. Data are presented as mean ± SEM. *and ***denotes significance at P < 0.05 and P < 0.001, respectively.
Localization of CXCR4 expression in the endometrium and first trimester decidua
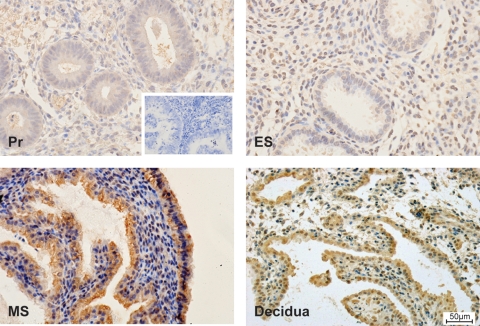
CXCR4 protein was immunolocalized to the glandular and stromal compartment of endometrial tissues across the menstrual cycle and decidua of early pregnancy, with greatest immunoreactivity observed in the mid-secretory phase and decidua, compared with proliferative phase and early secretory phase endometrium (Fig. 2). CXCR4 protein expression was localized to the same cellular compartment as PTGER2 in our previous study (Milne et al., 2001). The expression pattern and intensity of staining for CXCR4 were similar for all patients at different stages of the menstrual cycle and early pregnancy.
Figure 2.
Immunolocalization of CXCR4 protein expression. CXCR4 protein (brown staining) was immunolocalized to the glandular and stromal compartment of endometrial tissues across the menstrual cycle and first trimester decidua. Images are shown for proliferative phase endometrium (Pr; n = 5), early secretory phase endometrium (ES; n = 5), mid-secretory phase endometrium (MS; n = 5) and decidua of early pregnancy (n = 10 from weeks 6 to 10), with greatest immunoreactivity observed in the mid-secretory phase endometrium and decidua, compared with proliferative phase and early secretory phase endometrium. A representative section at the same magnification is shown for each tissue. Scale bar shown in decidua sample =50 µm.
HCG induces PTGS2 and PTGER2 mRNA expression and PGE2 secretion in first trimester decidua tissue
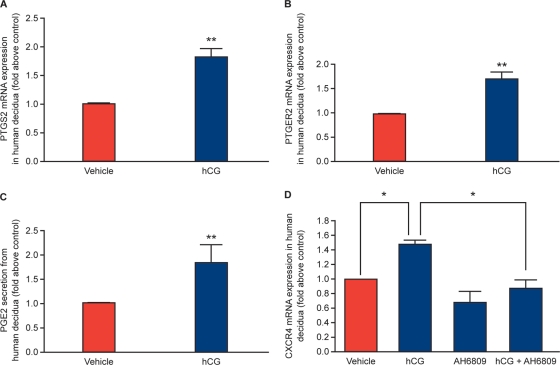
A recent study by Banerjee et al. demonstrated that CG regulates PG E synthase via a phosphatidylinositol 3-kinase (PI3K)–ERK1/2 pathway in human endometrial epithelial cells to potentially regulate endometrial responses for embryo implantation (Banerjee et al., 2009). We investigated whether hCG could regulate the PTGS2-PG pathway in human first trimester decidua tissue. We found that treatment of first trimester decidua tissue with 1 IU hCG for 8 h significantly elevated the mRNA expression of PTGS2 (Fig. 3A; n = 8; P < 0.05) and PTGER2 (Fig. 3B; n = 8; P < 0.05) and promoted the secretion of PGE2 (Fig. 3C; n = 8; P < 0.05) compared with vehicle (phosphate buffered saline; PBS v/v) treated tissue.
Figure 3.
(A) Expression of PTGS2 (n = 8) and (B) PTGER2 (n = 8) mRNA expression and secretion of PGE2 (C; n = 8) in first trimester decidua explants as determined by quantitative RT–PCR analysis and ELISA, respectively. Tissues were treated with vehicle (PBS) or 1 IU recombinant hCG for 8 h. (D) Tissues were treated with vehicle (PBS) or 1 IU recombinant hCG for 8 h, in the absence/presence of the specific PTGER2 antagonist AH6809 and subjected to quantitative RT–PCR analysis. Data are presented as mean ± SEM. *and **denotes significance at P < 0.05 and P < 0.01, respectively.
CXCR4 expression in first trimester decidua tissue is regulated by HCG via the PTGS2-PGE2-PTGER2 pathway
CG is known to regulate CXCR4 expression in the baboon endometrium during the window of implantation (Sherwin et al., 2007). We therefore investigated whether hCG regulates CXCR4 expression in human first trimester decidua tissue. We found that treatment of first trimester decidua tissue with 1 IU hCG for 8 h significantly elevated expression of CXCR4 mRNA (Fig. 3D; n = 8; P < 0.05) compared with vehicle (PBS v/v)-treated tissue. We next investigated whether hCG mediates expression of CXCR4 via PGE2–PTGER2 interaction since PGE2 has recently been shown to regulate CXCR4 expression in hematopoietic stem cells (Hoggatt et al., 2009) and stromal cells (Katoh et al., 2010). We found that co-treatment of first trimester decidua tissue with the specific PTGER2 antagonist AH6809 abrogated the hCG-mediated induction of CXCR4 (Fig. 3D; n = 8; P < 0.01), indicating that the hCG-mediated induction of CXCR4 occurred by the induction of the PTGS2–PTGER2 pathway via the biosynthesis of PGE2.
PTGER2 mediates CXCR4 expression in endometrial epithelial cells via the EGFR-PI3K-ERK pathway
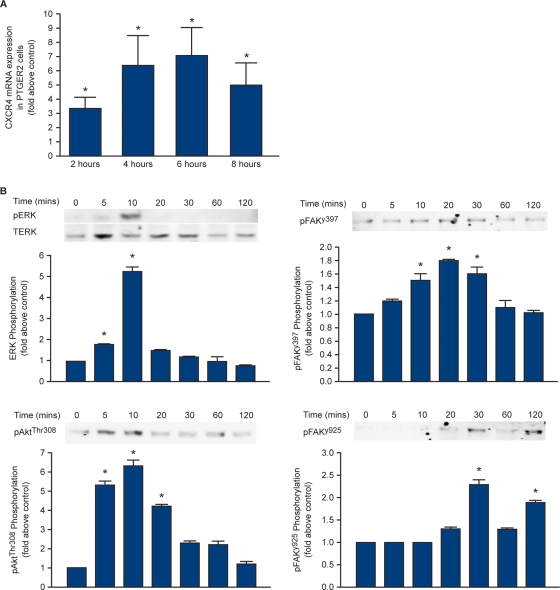
To investigate PTGER2 signalling to CXCR4, we used an Ishikawa endometrial epithelial cell line stably expressing PTGER2 (Abera et al., 2010). Treatment of PTGER2 cells with 5 µM butaprost, a specific PTGER2 agonist, resulted in a time-dependent increase in CXCR4 expression peaking at 6 h [7.1 ± 1.9-fold above vehicle (ethanol v/v) treated control; P < 0.001] (Fig. 4A). To investigate changes in cell signalling potential in PTGER2 cells after butaprost treatment, a Kinexus pathway microarray was used to determine target proteins which could mediate the PTGER2 induction of CXCR4. For this, PTGER2 cells were treated with vehicle (ethanol v/v) or 5 µM butaprost for 10 min and the detergent-solubilized cell lysates were subjected to Kinexus phosphosite-phosphoprotein array (Table I).
Figure 4.
(A) Expression of CXCR4 mRNA in PTGER2 cells. Cells were treated with vehicle (ethanol v/v) or 5 µM butaprost for 2, 4, 6 or 8 h and subjected to quantitative RT–PCR analysis. (B) PTGER2 cells were treated with 5 µM butaprost for 5, 10, 20, 30, 60 and 120 min or left unstimulated (time = 0) and subjected to western blot analysis. Representative immunoblots with densitometric quantification are shown for phosphoERK1/2 (top left-hand panel), phosphoAktthr308 (bottom left-hand panel), phosphoFAKy397 (top right-hand panel) and phosphoFAKy925 (bottom right-hand panel). Data are presented as mean ± SEM from at least three independent experiments. *denotes significance at P < 0.05.
Table I.
Target proteins phosphorylated in response to treatment with butaprost.
| Target protein | Fold increase |
|---|---|
| Extracellular regulated protein-serine kinase 1 (p44 MAP kinase) [T202 + Y204] | 2.199204413 |
| Extracellular regulated protein-serine kinase 2 (p42 MAP kinase) [T185 + Y187] | 1.509530949 |
| Glycogen synthase-serine kinase 3 alpha [S21] | 1.174561456 |
| Glycogen synthase-serine kinase 3 alpha [Y279] | 1.105722953 |
| Glycogen synthase-serine kinase 3 beta [Y216] | 1.047696379 |
| Jun N-terminus protein-serine kinase [stress-activated protein kinase (SAPK)] [T183 + Y185] | 0.829448891 |
| Jun proto-oncogene-encoded AP1 transcription factor [S73] | 1.282139734 |
| Jun proto-oncogene-encoded AP1 transcription factor [S73] | 1.763621955 |
| MAPK/ERK protein-serine kinase 1/2 (MKK1/2) [S218 + S222] | 1.964825861 |
| MAPK/ERK protein-serine kinase 3/6 (MKK3/6) [S189/S207] | 0.980927973 |
| Mitogen-activated protein-serine kinase p38 alpha [T180 + Y182] | 1.391426114 |
| p70 ribosomal protein-serine S6 kinase alpha [T389] | 0.495045106 |
| Protein-serine kinase B alpha [S473] | 0.977595521 |
| Protein-serine kinase B alpha [T308] | 1.391434041 |
| Protein-serine kinase C alpha [S657] | 0.909071756 |
| Protein-serine kinase C alpha/beta 2 [T638/T641] | 0.906168084 |
| Protein-serine kinase A alpha/beta | 0.48832985 |
| Raf1 proto-oncogene-encoded protein-serine kinase [S259] | 1.381611395 |
| Raf1 proto-oncogene-encoded protein-serine kinase [S259] | 1.586721643 |
| 3-phosphoinositide-dependent protein-serine kinase 1 [S244] | 1.332741421 |
| Signal transducer and activator of transcription 3 [S727] | 0.505564594 |
| Src proto-oncogene-encoded protein-tyrosine kinase [Y529] | 0.68232502 |
| Src proto-oncogene-encoded protein-tyrosine kinase [Y529] | 0.59734941 |
| Calcium/calmodulin-dependent protein-serine kinase 2 alpha [T286] | 0.750772375 |
| Catenin (cadherin-associated protein) beta 1 [S45] | 0.989877993 |
| cAMP response element binding protein 1 [S133] | 1.12843861 |
| Epidermal growth factor receptor-tyrosine kinase [Y1148] | 1.559698077 |
| ErbB2 (Neu) receptor-tyrosine kinase [Y1248] | 0.681088989 |
| Focal adhesion protein-tyrosine kinase [Y397] | 1.495268512 |
A Kinexus antibody pathway array was used to detect changes in cell signalling pathways after treatment of PTGER2 cells with vehicle (ethanol v/v) or 5 µM butaprost for 10 min. The results are reported as fold change above vehicle control. Selected proteins that increased more than 25% over the empirically verified limit (indicated in bold by a fold increase of 1.25 or more) were validated by western blot (Fig. 4B).
The Kinexus phosphosite-phosphoprotein array identified the EGFR, PI3K/protein kinase B (also called Akt), extracellular-signal-regulated kinase (ERK1/2) and focal adhesion kinase (FAK) pathways as significantly elevated more than 25% over the empirically verified limit by butaprost stimulation (as highlighted in bold on Table I). To verify the results of the phosphosite array, PTGER2 cells were treated with 5 µM butaprost for 5, 10, 20, 30, 60 and 120 min or left unstimulated (time = 0) and the detergent-solubilized lysates were subjected to western blot analysis. As shown in Fig. 4B, butaprost rapidly phosphorylated ERK1/2 and Akt with maximal response after 10 min of agonist treatment (top and bottom left-hand panel for ERK1/2 and Akt, respectively; P < 0.05), whereas FAK at tyrosine residues 397 and 925 (top and bottom right-hand panels, respectively; P < 0.05) were phosphorylated maximally at 20 and 30 min of agonist treatment.
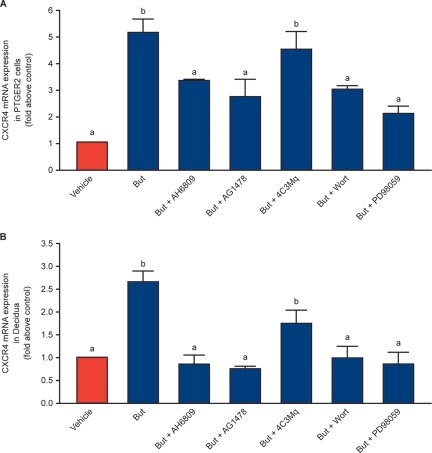
Based on the signalling pathways identified in Table I, we investigated PTGER2 signalling to CXCR4 by quantitative RT–PCR analysis using small molecule chemical inhibitors. PTGER2 cells (Fig. 5A) and first trimester decidua explants (Fig. 5B; n = 8) were treated with vehicle (ethanol v/v) or 5 µM butaprost for 6 h in the absence or presence of the specific PTGER2 antagonist AH6809 or inhibitors of adenosine 3′,5′-cyclicmonophosphate-mediated (PKA; 4C3MQ), EGFR tyrosine kinase activity (AG1478), PI3K/Akt (wortmannin) or ERK1/2 (MEK, PD98059). We found that butaprost-mediated CXCR4 induction in PTGER2 cells and first trimester decidua tissues was inhibited by the PTGER2 antagonist, EGFR, PI3K/Akt and ERK1/2 inhibitors, but not by the PKA inhibitor (P < 0.05).
Figure 5.
Expression of CXCR4 mRNA in PTGER2 cells and first trimester decidua explants. Cells (A) and tissues (B; n = 8) were treated with vehicle (ethanol v/v) or 5 µM butaprost for 6 h in the absence/presence of the specific PTGER2 antagonist AH6809, MEK inhibitor PD98 059, cAMP-PKA inhibitor 4C3MQ, PI3K/Akt inhibitor wortmannin or EGFR tyrosine kinase AG1478 and subjected to quantitative RT–PCR analysis. Data are presented as mean ± SEM. b denotes significance from a; P < 0.05.
Discussion
Fetal–maternal crosstalk between the blastocyst and endometrium is essential for successful implantation and pregnancy. The process of implantation may be divided into three stages: apposition, adhesion and invasion (Enders, 1976). During apposition, fetal–maternal dialogue often occurs in the absence of physical contact between the blastocyst and endometrium via soluble factors. This is followed by adhesion and anchorage of the trophoblast cells to the receptive endometrial epithelium and invasive penetration of the blastocyst through the luminal epithelium (Enders, 1976). Although it is known that the blastocyst can implant in different human tissues, in the endometrium it can only implant during the window of uterine receptivity, which commences around 6 days after the luteinizing hormone peak when the uterus is primed for blastocyst attachment (Psychoyos, 1973).
The blastocyst is known to secrete various factors during early embryogenesis in addition to CG, which influences endometrial receptivity. For example, the chemokine CXCL12, secreted from the blastocyst, has been shown to promote crosstalk between trophoblast cells and decidua during early pregnancy (Zhou et al., 2008). Furthermore the receptor for CXCL12, CXCR4 is regulated in the endometrium by embryonic signals (Sherwin et al., 2007). However, its regulation in the human endometrium during early pregnancy remains to be fully described.
Since ablation of PTGER2 expression in the mouse results in implantation failure and since PGE2 has been shown recently to regulate CXCR4 expression in hematopoietic stem cells (Hoggatt et al., 2009) and stromal cells (Katoh et al., 2010), we examined the expression of PTGER2 and expression and localization of CXCR4 in the human endometrium across the menstrual cycle and in first trimester human decidua. We found that CXCR4 was elevated in the epithelial and stromal compartments during the mid-secretory phase of the menstrual cycle. This is an agreement with previous observations for up-regulation of this chemokine receptor during the receptive phase of the menstrual cycle, where its expression is regulated endogenously by estradiol and progesterone (Dominguez et al., 2003).
We found that CXCR4 expression was coincident with PTGER2 expression in endometrial tissues in the mid-secretory phase of the cycle, and was further elevated in the decidua of early pregnancy. The spacio-temporal pattern of expression and localization of CXCR4 to epithelial cells and stromal cells in the endometrium was similar to that of PTGER2, reported in our previous study (Milne et al., 2001), suggesting a potential crosstalk between these two receptors.
In an in vitro model, blastocyst apposition can polarize CXCR4 expression in cultured endometrial epithelial cells (Dominguez et al., 2003). These findings, together with data from the baboon, showing that CG induces CXCR4 expression in the endometrium (Sherwin et al., 2007), and co-culture studies showing elevated CXCR4 expression in human decidualized stromal cells in response to conditioned medium from trophoblasts (Hess et al., 2007) suggest that embryonic signals in primates can induce, polarize and maintain expression of CXCR4 in the endometrial epithelium to ensure successful implantation. Indeed, women who exhibit recurrent implantation failure have lower levels of CXCR4 compared with fertile women, despite having normal levels of estradiol and progesterone (Tapia et al., 2008). These findings indicate that hormonal regulation alone may not be sufficient to regulate CXCR4 expression for successful implantation.
We therefore investigated the regulation of CXCR4 in the decidua of early pregnancy in response to the embryonic signal CG. We found that CG induces expression of PTGS2 and PTGER2 and release of PGE2 in first trimester decidua explants. These observations are similar to those of Zhou et al. (1999) and Banerjee et al. (2009) who have shown that CG can increase the expression of PTGS2 and PGE synthase in endometrial epithelial cells. Once biosynthesized and released, PGE2 via PTGER2 induces the expression of CXCR4, since treatment of human first trimester decidua tissue explants with CG and the specific PTGER2 antagonist, AH6809, abrogated the induction of CXCR4.
Using an in vitro model system of endometrial epithelial cells stably expressing PTGER2 and first trimester decidua tissue explants, we investigated the molecular mechanism whereby PTGER2 signalling regulates CXCR4. We found that CXCR4 expression was regulated by PTGER2 via the EGFR-PI3K-ERK1/2 pathway. In a previous study, we found that PTGER2-mediated activation of ERK1/2 in endometrial epithelial cells is mediated via sequential transactivation of the EGFR and activation of the MAPK kinase kinase Raf, which results in the sequential phosphorylation of the MAPK/ERK protein serine kinase 1/2 (MEK), which in turn activates ERK1/2 (Sales et al., 2004). These findings have been confirmed by our phosphosite array in our present study. Furthermore, the phosphosite array has highlighted the potential importance of the PI3K/Akt pathway in PTGER2-mediated cell signalling. This is in accordance with a recent study by Banerjee et al. who showed that CG-mediated ERK1/2 phosphorylation in endometrial epithelial cells is mediated via the Raf-mediated activation of the PI3K/Akt pathway (Banerjee et al., 2009). It is therefore likely that the PI3K/Akt pathway is an intermediate step between the PTGER2-mediated activation of Raf-ERK1/2 phosphorylation in PTGER2 cells. The PI3K/Akt and ERK1/2 pathways are both pro-survival pathways which support cellular growth, proliferation, differentiation and survival: critical responses that occur in the endometrium during implantation (Gentilini et al., 2007). Interestingly we found that PTGER2 signalling to CXCR4 was independent of the cAMP-mediated PKA pathway since the phosphosite array showed that cAMP response element binding protein was not phosphorylated and the PKA inhibitor, 4C3MQ, failed to reduce the agonist-mediated induction of CXCR4.
Moreover, our phosphosite array has highlighted the activation of FAK at tyrosine residue 397. FAK is a non-receptor tyrosine kinase that forms part of the plasma membrane focal adhesion complex, which assembles on integrin heterodimers following integrin engagement of extracellular matrix proteins (Wozniak et al., 2004). FAK is activated by autophosphorylation at tyrosine 397 that is initiated by integrin engagement with its ligand. When phosphorylated, tyrosine 397 becomes a docking site for various adaptor proteins which in turn phosphorylate FAK at tyrosine 925 to further activate the FAK kinase activity (Wozniak et al., 2004). In our study, we found that FAK is phosphorylated at both tyrosine residues 397 and 925. In the reproductive tract, integrins undergo dramatic alterations in expression during the normal menstrual cycle, with integrin αvβ3 and its ligand expressed in the endometrium during implantation (Lessey et al., 1994). Indeed, FAK neutralization studies conducted in a blastocyst-endometrium co-culture model have shown that FAK is important during embryonic implantation by regulating blastocyst cellular outgrowth (Hanashi et al., 2003). Therefore in addition to regulating cell proliferation/differentiation/survival signals via activation of PI3K-Akt signalling during the window of implantation and early pregnancy, PTGER2 signalling may also prepare the endometrium for implantation by up-regulating expression of proteins involved in cell–matrix interaction and attachment, such as FAK.
Conclusion
Our data show that CXCR4 and PTGER2 receptors are coincidently up-regulated during the window of implantation and the decidua of early pregnancy. In first trimester decidua explants, CG induces the expression of PTGS2 and biosynthesis of PGE2, which in turn induces expression of CXCR4 via PTGER2 interaction. Taken together our data suggest that embryonic signals can prime the endometrium for implantation and early pregnancy by regulating expression of chemokine receptors, such as CXCR4 via the EGFR, PI3K/Akt and ERK1/2 pathways.
Authors' roles
K.J.S. study design, conception, final revision and final approval; V.G. data acquisition, analysis and final approval; R.D.C. data acquisition, analysis, interpretation and final approval; H.N.J. study design, conception, final revision and final approval.
Funding
This study was supported by MRC core funding to H.N.J. (U1276.00.004.00002.01). Funding to pay the Open Access Change was provided by the Medical Research Council.
Acknowledgements
We thank Sharon Donaldson and Catherine Murray for consenting patients and tissue collection and Prof. HOD Critchley for helpful discussions.
References
- Abera AB, Sales KJ, Catalano RD, Katz AA, Jabbour HN. EP2 receptor mediated cAMP release is augmented by PGF 2 alpha activation of the FP receptor via the calcium-calmodulin pathway. Cell Signal. 2010;22:71–79. doi: 10.1016/j.cellsig.2009.09.012. doi:10.1016/j.cellsig.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DT, Cameron ST, Critchley HO, Drudy TA, Howe A, Jones RL, Lea RG, Kelly RW. Prostaglandins and menstruation. Eur J Obstet Gynecol Reprod Biol. 1996;70:15–17. doi: 10.1016/s0301-2115(96)02568-7. doi:10.1016/S0301-2115(96)02568-7. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Sapru K, Strakova Z, Fazleabas AT. Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epithelial cell line: implications for endometrial responses for embryo implantation. Endocrinology. 2009;150:4326–4337. doi: 10.1210/en.2009-0394. doi:10.1210/en.2009-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby S, Sales KJ, Williams AR, Anderson RA, Gardner S, Jabbour HN. Seminal plasma and prostaglandin E2 up-regulate fibroblast growth factor 2 expression in endometrial adenocarcinoma cells via E-series prostanoid-2 receptor-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase pathway. Hum Reprod. 2007;22:36–44. doi: 10.1093/humrep/del328. doi:10.1093/humrep/del328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol. 1999;180:614–620. doi: 10.1016/s0002-9378(99)70263-2. doi:10.1016/S0002-9378(99)70263-2. [DOI] [PubMed] [Google Scholar]
- Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod. 2003;9:189–198. doi: 10.1093/molehr/gag024. doi:10.1093/molehr/gag024. [DOI] [PubMed] [Google Scholar]
- Dong YL, Gangula PR, Fang L, Yallampalli C. Differential expression of cyclooxygenase-1 and -2 proteins in rat uterus and cervix during the estrous cycle, pregnancy, labor and in myometrial cells. Prostaglandins. 1996;52:13–34. doi: 10.1016/0090-6980(96)00059-7. doi:10.1016/0090-6980(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Enders AC. Anatomical aspects of implantation. J Reprod Fertil Suppl. 1976;25:1–15. [PubMed] [Google Scholar]
- Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. doi:10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal–maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. doi:10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini D, Busacca M, Di Francesco S, Vignali M, Vigano P, Di Blasio AM. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol Hum Reprod. 2007;13:317–322. doi: 10.1093/molehr/gam001. doi:10.1093/molehr/gam001. [DOI] [PubMed] [Google Scholar]
- Hanashi H, Shiokawa S, Akimoto Y, Sakai K, Suzuki N, Kabir-Salmani M, Nagamatsu S, Iwashita M, Nakamura Y. Physiologic role of decidual beta1 integrin and focal adhesion kinase in embryonic implantation. Endocr J. 2003;50:189–198. doi: 10.1507/endocrj.50.189. doi:10.1507/endocrj.50.189. [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. doi:10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. doi:10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. doi:10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Boddy SC. Prostaglandin E2 induces proliferation of glandular epithelial cells of the human endometrium via extracellular regulated kinase 1/2-mediated pathway. J Clin Endocrinol Metab. 2003;88:4481–4487. doi: 10.1210/jc.2003-030297. doi:10.1210/jc.2003-030297. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab. 2004;15:398–404. doi: 10.1016/j.tem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. doi:10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, Kamata H, Mishima T, Tamaki H, Sakagami H, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol. 2010;176:1469–1483. doi: 10.2353/ajpath.2010.090607. doi:10.2353/ajpath.2010.090607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Kniss DA. Cyclooxygenases in reproductive medicine and biology. J Soc Gynaecol Investig. 1999;6:285–292. doi: 10.1016/s1071-5576(99)00034-9. doi:10.1016/S1071-5576(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Ledingham MA, Denison FC, Kelly RW, Young A, Norman JE. Nitric oxide donors stimulate prostaglandin F2alpha and inhibit thromboxane B2 production in the human cervix during the first trimester of pregnancy. Mol Hum Reprod. 1999;5:973–982. doi: 10.1093/molehr/5.10.973. doi:10.1093/molehr/5.10.973. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–649. doi: 10.1210/jcem.79.2.7519194. doi:10.1210/jc.79.2.643. [DOI] [PubMed] [Google Scholar]
- Maldonado-Perez D, Brown P, Morgan K, Millar RP, Thompson EA, Jabbour HN. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;179:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne SA, Perchick GB, Boddy SC, Jabbour HN. Expression, localization, and signaling of PGE2 and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2001;86:4453–4459. doi: 10.1210/jcem.86.9.7856. doi:10.1210/jc.86.9.4453. [DOI] [PubMed] [Google Scholar]
- Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. doi:10.1016/S0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in reproductive tract physiology and pathology. Prostaglandins Other Lipid Mediat. 2003;71:97–117. doi: 10.1016/s1098-8823(03)00050-9. doi:10.1016/S1098-8823(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Katz AA, Davis M, Hinz S, Soeters RP, Hofmeyr MD, Millar RP, Jabbour HN. Cyclooxygenase-2 expression and prostaglandin E2 synthesis are up- regulated in carcinomas of the cervix: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J Clin Endocrinol Metab. 2001;86:2243–2249. doi: 10.1210/jcem.86.5.7442. doi:10.1210/jc.86.5.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin e receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002a;62:424–432. [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Katz AA, Millar RP, Jabbour HN. Seminal plasma activates cyclooxygenase-2 and prostaglandin E2 receptor expression and signalling in cervical adenocarcinoma cells. Mol Hum Reprod. 2002b;8:1065–1070. doi: 10.1093/molehr/8.12.1065. doi:10.1093/molehr/8.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Maudsley S, Jabbour HN. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic 3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004;18:1533–1545. doi: 10.1210/me.2004-0022. doi:10.1210/me.2004-0022. [DOI] [PubMed] [Google Scholar]
- Sales KJ, List T, Boddy SC, Williams AR, Anderson RA, Naor Z, Jabbour HN. A novel angiogenic role for prostaglandin F2alpha-FP receptor interaction in human endometrial adenocarcinomas. Cancer Res. 2005;65:7707–7716. doi: 10.1158/0008-5472.CAN-05-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Boddy SC, Jabbour HN. F-prostanoid receptor alters adhesion, morphology and migration of endometrial adenocarcinoma cells. Oncogene. 2008;27:2466–2477. doi: 10.1038/sj.onc.1210883. doi:10.1038/sj.onc.1210883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Maldonado-Perez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. Prostaglandin F2alpha-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim Biophys Acta. 2009;1793:1917–1928. doi: 10.1016/j.bbamcr.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BH, Catt KJ. Roles of LPA3 and COX-2 in implantation. Trends Endocrinol Metab. 2005;16:397–399. doi: 10.1016/j.tem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148:618–626. doi: 10.1210/en.2006-0832. doi:10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- Smock SL, Pan LC, Castleberry TA, Lu B, Mather RJ, Owen TA. Cloning, structural characterization, and chromosomal localization of the gene encoding the human prostaglandin E2 receptor EP2 subtype. Gene. 1999;237:393–402. doi: 10.1016/s0378-1119(99)00323-6. doi:10.1016/S0378-1119(99)00323-6. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol. 2004;2:49. doi: 10.1186/1477-7827-2-49. doi:10.1186/1477-7827-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4:465–471. doi: 10.1093/humupd/4.5.465. doi:10.1093/humupd/4.5.465. [DOI] [PubMed] [Google Scholar]
- Tapia A, Gangi LM, Zegers-Hochschild F, Balmaceda J, Pommer R, Trejo L, Pacheco IM, Salvatierra AM, Henriquez S, Quezada M, et al. Differences in the endometrial transcript profile during the receptive period between women who were refractory to implantation and those who achieved pregnancy. Hum Reprod. 2008;23:340–351. doi: 10.1093/humrep/dem319. doi:10.1093/humrep/dem319. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. doi:10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004;1692:103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Zhou XL, Lei ZM, Rao CV. Treatment of human endometrial gland epithelial cells with chorionic gonadotropin/luteinizing hormone increases the expression of the cyclooxygenase-2 gene. J Clin Endocrinol Metab. 1999;84:3364–3377. doi: 10.1210/jcem.84.9.5943. doi:10.1210/jc.84.9.3364. [DOI] [PubMed] [Google Scholar]
- Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. doi:10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]