Abstract
Members of the testis-specific serine/threonine kinases (Tssk) family may have a role in sperm differentiation in the testis and/or fertilization. To gain insight into the functional relevance of these kinases, their expression was examined both at the mRNA and protein levels. Quantitative PCR analysis confirmed that all five Tssk mRNAs are almost exclusively expressed postmeiotically in the testis. Recombinant mouse and human Tssks were cloned and used for validation of an array of commercial and custom-made antibodies against Tssks. Immunolocalization in mouse testis, and in mouse and human sperm, showed that Tssk1, Tssk2, Tssk4 and Tssk6, but not Tssk3, were present in mouse sperm and in germ cells from mouse testis. TSSK1, TSSK2 and TSSK6 were also detected in human sperm, while TSSK3 was absent. In both mouse and human sperm, Tssk1 was partially soluble, while Tssk2, Tssk4 and Tssk6 were insoluble in non-ionic detergents. In vitro recombinant TSSK2 activity assays showed maximum enzymatic activity at 5 mM Mg2+ and a Km for ATP of ∼10 µM. These, observations together with findings that the Tssk1/Tssk2 double knock-out as well as the Tssk6 null mice are sterile without presenting other detectable defects, suggest that these kinases could be used as targets for male contraception.
Keywords: Tssk kinases, testis-specific, signal transduction, contraception, sperm function
Introduction
Phosphorylation by protein kinases forms the basis of intracellular signaling networks including transduction of extracellular signals, intracellular transport and cell cycle progression (Johnson, 2009) and appears to play a role in signaling events that are involved in sperm differentiation and function. Although meiotic events are well characterized in the female germ cells, the regulation of male germ cell meiosis and differentiation into mature sperm is still not well understood. This process is known as spermatogenesis and can be divided in to three main phases: (i) the proliferative or spermatogonial phase, in which spermatogonia undergo mitotic division and generate a pool of spermatocytes; (ii) the meiotic phase that generates the haploid spermatids and (iii) spermiogenesis, whereby each round of spermatids differentiates into a spermatozoon (Sharpe, 1994). Spermiogenesis includes formation of the acrosome, chromatin condensation, cell elongation and the assembly of the flagellum. This remarkable morphological differentiation is the consequence of changes in both gene transcription (Hecht, 1988) and protein translation (Hake et al., 1990). Importantly, because spermatozoa are transcriptionally and translationally inactive, several sperm proteins synthesized in the haploid spermatid will remain in the morphologically mature sperm after it leaves the testis. Therefore, proteins that are synthesized during spermiogenesis might be necessary for spermatid differentiation and/or for sperm function during fertilization. Among these proteins, a family of protein kinases has been recently shown to be expressed almost exclusively during spermiogenesis (Kueng et al., 1997; Nayak et al., 1998; Zuercher et al., 2000; Visconti et al., 2001).
The testis-specific serine kinase (Tssk) protein family is composed of six members: Tssk1 through 6; however, it is not yet known whether Tssk5 has all the domains needed to be an active kinase. These protein kinases belong to the 5′ adenosine monophosphate-activated protein kinase (AMPK) family (Xu et al., 2007). Some of the AMPK family members are required to be phosphorylated at a conserved threonine residue in the subdomain VI of the kinase domain, also known as T-loop, in order to be active. While AMPKs as well as other related kinases are regulated by an upstream kinase that phosphorylates this residue, Tssk1, Tssk2 and Tssk6 do not seem to require a second kinase and appear to undergo autophosphorylation in this threonine residue (Jaleel et al., 2005; Spiridonov et al., 2005). With respect to the other members, experiments using recombinant Tssk3 suggest that this kinase would require an upstream kinase for its regulation (Bucko-Justyna et al., 2005). The mode of regulation of the Tssk4 T-loop has not been tested to date.
The restricted postmeiotic expression of Tssks as well as the importance of phosphorylation in signaling processes strongly suggests that Tssk(s) have an important role(s) in germ cell differentiation and/or sperm function. This prediction has been recently confirmed by the sterile phenotype of the Tssk6 (aka Sstk) knock-out (KO) mice (Spiridonov et al., 2005; Sosnik et al., 2009) and of the double Tssk1 and Tssk2 KO (Shang et al., 2007, 2010). Despite these advances, little is known about the presence and localization of Tssk proteins either in the testis or in sperm. Previous work from our laboratory has shown that antibodies directed against Tssk2 recognized a protein in mouse and human sperm. However, this antibody was not validated against other Tssks; therefore, the conclusion from this work was that at least one Tssk (not necessarily Tssk2) was present in mature sperm (Hao et al., 2004). In the present study, antibodies against five members of the Tssk family were validated against the respective recombinant proteins. Using these antibodies, we show that Tssk1, 2, 4 and 6 are present in mature sperm. Although Tssk3 mRNA is expressed in spermatids, we were unable to detect the Tssk3 protein either in testis extracts or in mature sperm. Immunolocalization experiments using mouse and human sperm indicate that these kinases distribute in different sperm regions, suggesting that each Tssk plays a distinct role in sperm function. Furthermore, initial characterization of recombinant human Tssk2 activity provides evidence on the autophosphorylation ability of this enzyme in vitro and on its dependence on magnesium ions when using myelin basic protein (MBP) and an MBP-derived peptide as a phosphorylation substrate.
Materials and Methods
Antibodies and other reagents
All chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise stated. Protein molecular weight standards and other reagents used for SDS–polyacrylamide gel electrophoresis (PAGE) were from Bio-Rad (Hercules, CA, USA). Protease inhibitors were from Roche Applied Science (Indianapolis, IN, USA). Enhanced chemiluminescence (ECL) and ECLplus chemiluminescence detection kits were from GE Healthcare (Piscataway, NJ, USA). Polyvinylidene difluoride (PVDF) membrane was from Millipore (Bedford, MA, USA). His-tagged, human recombinant proteins rhTssk1, rhTssk2 (Invitrogen Corporation, Carlsbad, CA, USA), and glutathione-S-transferase (GST)-tagged rhTssk3 and rhTssk6 (Abnova/Novus Biologicals, Littleton, CO, USA) were also used. Antigenic peptides used in Tssk4 competition assays were custom-designed and ordered from EZ BioLabs (Carmel, IN, USA). DNA oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IO, USA). DNA sequencing services were provided by Genewiz (South Plainville, NJ, USA). Radioisotopes were purchased from PerkinElmer (Waltham, MA, USA). Mouse RNA was from Applied Biosystems/Ambion (Austin, TX, USA).
Mouse monoclonal antibodies against Tssk1 (clone 4F12), Tssk2 (clone 1E12), Tssk3 (clone 6G3) and Tssk6 (clone 6F5) were obtained from Abnova Taiwan Corporation (Abnova/Novus Biologicals); a polyclonal anti-Tssk3 antibody (A02) (Abnova/Novus) was also tested. A custom-made rabbit polyclonal Tssk4 antibody was generated against a unique peptide (YQPTSSAKRHQSLEITT) in the C-terminus tail of mouse Tssk4 which is present in both Tssk4 and Tssk4b but not in Tssk4c (see below). Two mouse monoclonal antibodies against Tssk6 (clones 16 and 3 + 13) were produced using a synthetic peptide corresponding to the C-terminal region (aa 218-273) of mouse Tssk6, and purified with Protein G immobilized on agarose beads (Pierce, Rockford, IL, USA). Alexa-Fluor-555-conjugated secondary antibodies, Alexa-Fluor-488-conjugated peanut agglutinin lectin (PNA) and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Molecular Probes (Invitrogen Corporation). FITC-labeled prostate-specific antigen (PSA) was from Sigma. An anti-β-tubulin monoclonal antibody (Clone E7) was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa, IA, USA. Horse-radish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG; H+L), HRP-linked anti-mouse IgG (light chain) and HRP-conjugated anti-rabbit IgG (light chain) were from Jackson Immunoresearch Laboratories (West Grove, PA, USA). HRP-linked anti-rabbit IgG (H+L) (GE Healthcare) was also used.
Analysis of mouse Tssks expression by real-time PCR
The following real-time PCR oligonucleotides were used: Tssk1 (sense: 5′-AAA CTT GGG AGA GGG CTC AT-3′ and antisense: 5′-TGG CCA GAA TCT CAA TCT CC-3′); Tssk2 (sense: 5′-CCA CGC TCC AAG AAC CTA AC-3′ and antisense: GAA GGA GGC AGA AGA CAT GG-3′); Tssk3 (sense: 5′-GAT GCT GGA GTC AGC AGA TG-3′ and antisense: 5′-GGC AAT AGC GAA TAG CCT CA-3′); ‘Universal’ Tssk4 (sense: 5′-CTG TCA AGA TCA TCT CGA AG-3′ and antisense: 5′-GAG CCA CGT CCA AAA TGA TGT-3′); Tssk6 (sense: 5′-CGC TCA AGA TCA CGG ATT TC-3′ and antisense: 5′-AGG CTC CAC ACG TCG TAT TT-3′); actin (sense: 5′-GAC GAT GCT CCC CGG GCT GTA TTC-3′ and antisense: 5′-TCT CTT GCT CTG GGC CTC GTC ACC) and GAPDH (sense: 5′-TGA AGC AGG CAT CTG AGG G-3′ and antisense: 5′-CGA AGG TGG AAG AGT GGG AG-3′). First, primers were tested by regular RT–PCR to determine specificity for each Tssk, and by using plasmid constructs carrying each Tssk open reading frame (ORF; positive control) or the corresponding empty vector (negative control). Each real-time PCR primer set was designed so that the amplimer size ranged between 150 and 250 bp. Total murine tissue RNA was obtained either from Applied Biosystems/Ambion (liver, brain, thymus, heart, lung, spleen, testicle, ovary, kidney and embryo) or from OriGene (muscle). cDNA was synthesized from RNA of murine tissues using the SuperScript III kit (Invitrogen Corporation) and oligo(dT)20 according to the manufacturers' instructions, and cDNA tested with the Tssk1, 2, 3, 4 and 6 primers to first determine PCR product specificity. Real-time PCR was performed with cDNA from all 11 tissues using the SYBR Green I Premix Ex Taq (Takara Bio Inc.) plus 10 µM of the corresponding set of sense and antisense primer for each Tssk. Reactions were run on a Stratagene M × 3000P® instrument using the following parameters: 95° for 10 s; 95° for 5 s and 60° for 22 s for 40 cycles; 95° for 1 min, 55° for 30 s and 95° for 30 s. For each assay, duplicate reactions were run and CT values were averaged per sample; at least three separate assays were performed in duplicate on each tissue for each Tssk. ‘Normalizing’ (housekeeping) genes for each mouse tissue (actin or Gapdh) were also assayed in duplicate, and by running reactions in parallel to each Tssk. The CT values from each run were then averaged per tissue and per target (Tssk or normalizing gene). The  method was then applied for data analysis using the Stratagene M × 3000P QPCR Software®, where values of each Tssk expressed in each tissue were compared with those of housekeeping genes Gapdh or actin; and values of each Tssk in the testis were used as the ‘calibrator’ (Livak and Schmittgen, 2001). A similar procedure was used for analyses of Tssks during testis development, using mouse testis total RNA isolated from mice of 7, 14, 21, 24 and 28 days of age. All real-time PCR data are expressed as the means ± SEM using testis (calibrator) values as 1 arbitrary unit = 100%.
method was then applied for data analysis using the Stratagene M × 3000P QPCR Software®, where values of each Tssk expressed in each tissue were compared with those of housekeeping genes Gapdh or actin; and values of each Tssk in the testis were used as the ‘calibrator’ (Livak and Schmittgen, 2001). A similar procedure was used for analyses of Tssks during testis development, using mouse testis total RNA isolated from mice of 7, 14, 21, 24 and 28 days of age. All real-time PCR data are expressed as the means ± SEM using testis (calibrator) values as 1 arbitrary unit = 100%.
Molecular cloning of mouse Tssks
Total RNA from adult mouse testis was used, and first-strand cDNA synthesized using the SuperScript III kit and oligo(dT)20, following the manufacturer's instructions. NCBI accession numbers for mouse Tssk sequences are: NM_009435 for Tssk1, NM_009436 for Tssk2, NM_080442 for Tssk3 and NM_032004 for Tssk6. Full-length ORF of mouse Tssk1, 2, 3, 4, 4b, 4c and 6 were amplified by PCR using the following oligonucleotides: Tssk1 (sense 5′-CAG CCT CGG AGG CAC TGG-3′ and antisense 5′-CCC TGG GCT GCA AGA GGC-3′); Tssk2 (sense 5′-CCC ACA GTG GGA ATG AGG-3′ and antisense 5′-CTG CAC ACC ACG ATA CCC-3′); Tssk3 (sense 5′-TGG AGT AGA GCT GCC TCG-3′ antisense 5′-GGG AGG ACT TGC CAT TGC-3′) and Tssk6 (sense 5′-CCA TGT CGG GCG ACA AAC TC-3′ and antisense 5′-AGG CGC AAT GCT TCT CTC TC-3′). Based on in silico analysis of novel, putative testis-specific kinases, three variants of Tssk4, i.e. Tssk4, Tssk4b and Tssk4c were cloned using the following primers: (sense 5′-CTT CTC CGT AGA CAG AGG CT-3′ and antisense 5′-TCA GCC ACT TTC AGG TTG TG-3′), and sequences deposited at NCBI database with the following accession numbers: DQ397204 for Tssk4, DQ397205 for Tssk4b and DQ397206 for Tssk4c. According to these results, it is predicted that Tssk4b and Tssk4c would be translated into proteins that are likely to have no activity due to the 10-aminoacid insertion before exon 3, which disrupts the predicted catalytic site of these kinases. In addition, the missense insertion in the message of Tssk4c creates a premature stop codon further truncating part of the catalytic site of this protein.
Expression and purification of mouse GST-tagged recombinant Tssk proteins
For production of GST-fusion mouse Tssk proteins, each mouse Tssk ORF was subcloned into vector (GST gene fusion system)-KG2 vector using the following restriction sites: XbaI and SacI for Tssk1; BamHI and XbaI for Tssk2; XbaI and XhoI for Tssk3; XbaI and SalI for Tssk4, Tssk4b and Tssk4c splicing variants and EcoRI and NcoI for Tssk6. The GST-tagged Tssks were then expressed in Escherichia coli BL21 strain following a previously described procedure (Guan and Dixon, 1991) with minor modifications. After induction of protein expression with isopropyl-β-d-1-thiogalactopyranoside, bacteria pellets were resuspended in 10 ml phosphate-buffered saline (PBS; pH 8.0), and 2 mM phenyl methane sulfonylfluoride plus 5 mM EDTA were added. Sonication was carried out in ice for three times, 30 s each. Triton X-100 was then added to a final concentration of 1%, and the suspension was rocked at 4°C for 20 min. After centrifugation for 20 min at 4°C (11 000 rpm), the supernatant was transferred to 0.5 ml suspension of glutathione-immobilized beads (Pierce) and subsequent binding of GST-fusion proteins carried out at 4°C overnight. The beads were then washed twice with Triton 1% in PBS, and once more with PBS alone. Lastly, elution of purified proteins was performed on a filtration chromatography column with 0.5 ml elution buffer (10 mM glutathione, 50 mM Tris, pH 8.0).
Preparation of mouse and human sperm samples
Mouse sperm samples were collected from CD1 retired breeder males (Charles River Laboratories, Wilmington, MA, USA) sacrificed in accordance with IACUC guidelines. Cauda epididymis sperm was obtained by the ‘swim-out’ method using a Whitten's Hepes-buffered medium (WH) as previously described (Jha et al., 2006). After release into the medium during a 10-min time period, sperm were collected by centrifugation at 10 000g for 5 min at room temperature, after which cell viability and cell count were determined. Human semen specimens from normozoospermic volunteers were obtained according to a protocol approved by the University of Massachusetts IRB Committee for Protection of Human Subjects. Modified human tubal fluid (mHTF) (Irvine Scientific, Santa Ana, CA, USA) was the basic medium for all handling of human sperm samples. Individual semen samples were allowed to liquefy at room temperature (0.5–2 h), and mature sperm were then obtained by the ‘swim-up’ method into HTF medium. Sperm presenting 90% motility or higher were treated immediately for analysis by SDS–PAGE or for immunofluorescence analysis.
SDS–PAGE and western analysis
Whole cell protein extracts from sperm were obtained by resuspension in SDS-sample buffer (Laemmli, 1970) without 2-mercaptoethanol, followed by boiling for 10 min. After centrifugation, supernatants were saved, and 2-mercaptoethanol was added to a final concentration of 5%. Proteins were denatured by boiling for 5 min and subjected to 12% SDS–PAGE; protein extracts equivalent to 1–2 × 106 sperm were loaded per lane. Proteins were then transferred to PVDF membranes, and blockage of non-specific binding done by incubation in 5% non-fat dry milk/tris-buffered saline (TBS)–Tween20 for 1 h at room temperature, as described (Jha et al., 2006). Western blotting was carried out using the following dilutions of anti-Tssk antibodies: 1:500 for Tssk1,1:250 for Tssk2, 1:250 for Tssk3, 1:500 for Tssk4 and 1:5000 for Tssk6 in 1% non-fat dry milk/TBS–Tween20; incubation proceeded at 4°C overnight with rocking. For control experiments by Tssk4 peptide competition, polyclonal anti-Tssk4 antibody was incubated in the presence of Tssk4 antigenic (blocking) peptide (1:10 molar excess) in TBS–Tween20 for 2 h at room temperature before adding to membranes. After washes with TBS–Tween20, incubation with the corresponding HRP-conjugated secondary antibodies (1:10 000 in 1% non-fat dry milk/TBS-Tween20) was conducted for 1 h at room temperature. Equal protein loading was determined by stripping membranes post-incubation with anti-Tssk antibodies, and reprobing with either anti-PY for hexokinase detection in soluble fractions (not shown) or anti-tubulin antibody (1:5000) (insoluble fractions). Signals were developed by using regular ECL chemiluminescence reagents; in some cases, ECLplus was used instead.
Immunolocalization of Tssks in mouse and human sperm
Sperm obtained in WH medium as described above were washed once, resuspended in PBS at a concentration of 1–2 × 105 sperm/ml and seeded on 8-well glass slides. After being air-dried, sperm were fixed with 3.7% formaldehyde in PBS for 20 min at room temperature, washed with PBS-Tween20 (5 washes) and permeabilized with 0.5% Triton X-100 for 5 min, then washed again. Sperm were then treated with 1% bovine serum albumin (BSA)/PBS-Tween20 for 2 h at room temperature, and then incubated either with the respective primary antibody (1:50–1:250) diluted in 1% BSA/PBS-Tween20, or with the same concentration of the corresponding normal purified IgG; incubations were carried out at 4°C overnight. For control experiments by Tssk4 peptide competition, polyclonal anti-Tssk4 antibody was incubated in the presence of Tssk4 blocking peptide in PBS-Tween20 for 2 h at room temperature before adding to slides. After incubation, sperm were washed thoroughly (10 times) with PBS-Tween20 and incubated with the corresponding Alexa 555-conjugated secondary antibody (1:200) diluted in 1% BSA/PBS for 1 h at room temperature; these solutions also contained either Alexa 488-conjugated PNA (1:200) for staining mouse sperm acrosomes, or FITC-conjugated PSA (1:200) as a marker for human sperm acrosomes. Incubation with the secondary antibody was followed by 10 washes in PBS-Tween20, mounting using Slow-Fade Light reagents (Molecular Probes, Eugene, OR, USA) and observation by epifluorescence microscopy using a Zeiss Axiophot microscope (Carl Zeiss, Inc., Thornwood, NY, USA) equipped with OpenLab imaging software. Differential interference contrast (DIC) images were taken in parallel, and served as control for sperm morphology. Negative controls run by parallel incubation in either peptide-preabsorbed antibodies or HRP-labeled secondary IgG alone were used to check for antibody specificity.
Immunohistochemistry of Tssks in mouse testis
Mouse testes were dissected and fixed overnight by rocking at 4°C in 3.7% formaldehyde/PBS. After progressive dehydration by incubation in ethanol (70, 80, 90% and anhydrous), testes were incubated in xylene for 1 h at RT followed by incubations at 65°C with 1:1 xylene:paraffin (1 h), paraffin (1 h) and then an overnight incubation in paraffin. Blocks of paraffin-embedded tissue were then prepared, and left to harden at room temperature before storage at 4°C. Tissue sectioning (4–6 µm-thick slices) was carried out with an Olympus microtome (Center Valley, PA, USA) equipped with disposable blades; tissue strips were floated on water and lifted onto Superfrost Plus positively charged glass slides (Erie Scientific, Portsmouth, NH, USA) and left to dry at 37°C overnight. Prior to staining, tissue sections were treated with 1% BSA/Tween20-PBS blocking solution at 4°C overnight, followed by incubation with primary and secondary antibodies for 3 h each at RT. To control for specificity, polyclonal anti-Tssk4 antibody was incubated in the presence of Tssk4 blocking peptide in PBS-Tween20 for 2 h at room temperature before adding to slides. Secondary antibody solutions also contained Alexa 488-conjugated PNA (1:200) for staining acrosomes, and DAPI for nuclear staining. Antibodies dilutions as well as washing and mounting were performed under the same conditions as those indicated above for sperm immunofluorescence.
Analysis of Tssks in mouse spermatogenic cell populations
Enriched populations of mouse testis spermatocytes and spermatids were obtained by sedimentation velocity at unit gravity, using Sta-Put gradient chambers. Briefly, seminiferous cells suspensions were prepared using sequential incubations in collagenase and trypsin as described previously (Romrell et al., 1976; Bellvé et al., 1977; Gerton and Millette, 1984), and purified cells populations were obtained in linear 2–4% wt/vol gradients of BSA, fraction V. After collecting fractions, different cell types were analyzed using Nomarski DIC microscopy, and purified spermatogenic cells populations (>90% pure) pooled accordingly. Protein extracts from pachytene spermatocytes (P), round spermatids (R) and condensed spermatids/residual bodies (C/Rb) were prepared in cell lysis buffer containing 1% SDS.
Analysis of Tssks solubility using different protein extraction conditions
An initial comparison of Tssks solubility between mouse and human sperm kinases was carried out using 1% Triton X-100, a non-ionic detergent. After swim-out in WH media, sperm samples were pelleted and resuspended in 1% Triton X-100/PBS buffer containing protease and phosphatase inhibitors. Cell lysis proceeded by incubation on ice for 30 min. Samples were then spun at 10 000g for 2 min, and both supernatant (Triton X-100-soluble fraction) as well as remaining pellets (Triton X-100-insoluble fraction) were saved for analysis by SDS–PAGE. Next, solubility of mouse sperm Tssk2 and Tssk6 was analyzed further using a variety of conditions, including detergents and high-salt buffers. Sperm samples were pelleted and resuspended in PBS buffer containing protease and phosphatase inhibitors, supplemented with one of the following components: NACl (500 mM), 1% Triton X-100 (non-ionic detergent, with a critical micelle concentration (CMC) of 0.2–0.9 mM), 1% n-octyl-β-d-glucopyranoside (non-ionic, CMC 20–25 mM), 1% CHAPS (zwitterionic detergent), 1% CTAB (cationic detergent), 1% SDS (anionic detergent) or RIPA buffer, containing all three detergents: Triton X-100 (1%), SDS (0.1%) and sodium deoxycolate (anionic, 0.5%). Cell lysis proceeded by incubation on ice for 30 min, except when using 1% SDS, which proceeded by boiling for 5 min. Samples were then spun at 10 000g for 2 min, and both supernatant as well as the remaining insoluble fractions were saved for analysis by SDS–PAGE. In all cases, protein fractions (soluble or insoluble) were prepared in sample buffer so that each lane contained equivalent amounts of sperm (∼2 × 106 cells) proteins.
Kinase activity assay of recombinant human TSSK2
Activity of recombinant TSSK2 was assayed following a method previously described with slight modifications (Visconti et al., 1997; Krapf et al., 2010). All assays were run at pH 7.2 and at a constant temperature of 30°C, and incubation lasted for 15 min unless indicated otherwise. For qualitative analysis of kinase activity, MBP (Sigma) was chosen as TSSK2 substrate. Increasing amounts of TSSK2 dissolved in 25 mM HEPES buffer (pH 7.2) were incubated with an equal volume of 2× kinase cocktail, so that the final concentration of components per reaction were [32P-ATP] (3000 Ci/mmol)(2 µCi/ ∼ 106 cpm/assay), 10 mM MgCl2, 10 mM MnCl2, 5 µg MBP, 1 mg/ml BSA, 40 mM β-glycerophosphate, 5 mM p-nitrophenylphosphate, 10 µM aprotinin and 10 µM leupeptin in a 20-µl reaction volume. Reactions proceeded at 30°C for 15 min, and were stopped by addition of 2× Laemmli sample buffer and boiling for 5 min. After separation by SDS–PAGE, proteins were transferred to PVDF membranes and subjected to autoradiography, followed by western analysis with anti-Tssk2 antibody. Recombinant TSSK2 activity was also determined quantitatively, using MBP fragment (Sigma) as substrate. In this case, the 2× kinase cocktail also contained 40 µM non-radioactive ATP and 100 µM MBP 104/118 peptide. On analysis of dose-dependence response to different parameters, the 2× kinase cocktail did not contain the corresponding reagent, which was added in increasing concentrations, as indicated in the appropriate figure legend. After incubation at 30°C for the indicated time period, reaction was stopped by addition of 20 µl of 20% TCA, cooled on ice for 20 min, centrifuged at 10 000g at RT for 5 min. The resultant supernatant was then spotted onto phosphocellulose paper (Whatman P81), and paper pieces were washed five times for 15 min each in 5 mM phosphoric acid. After drying, paper pieces were placed in glass vials containing 2.5 ml of Biosafe II scintillation solution, and subjected to liquid scintillation counting. Reactions were run in triplicate, and each set of experiments repeated at least three times. In each case, control (blank) reactions with no rhTssk2 were run in parallel. Dependence of rTSSK2 kinase activity on ATP, divalent cations or substrate was also analyzed, with either magnesium, manganese, cold ATP or MBP peptide present in increasing amounts in the 2× kinase cocktail, as indicated in the appropriate figure legend.
Statistical analysis
In order to assume normal distribution, real-time PCR data in percentages were converted to ratios and all data subjected to the arcsine square root transformation (Sokal and Rolf, 1995). Statistical analysis was then performed with the aid of the Graph Pad software, using a parametric one-way analysis of variance (ANOVA) followed by Tukey's post-test. Analysis of enzyme kinetics data was performed by one-way ANOVA followed by Tukey's test.
Results
All Tssks are expressed postmeiotically in spermatids
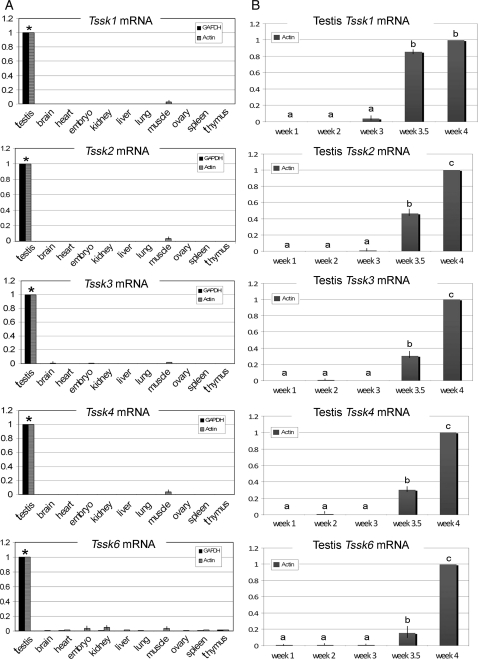
Using northern blot analysis, previous reports have shown that Tssks are expressed almost exclusively in the testis. To further analyze the tissue distribution of Tssks mRNAs using a more sensitive method, commercially available total mRNA from a variety of mouse tissues was assayed by real-time PCR (Fig. 1A). For this purpose, specific primers were designed for detection of Tssk1, 2, 3, 4 (all 3 variants) and 6 and validated using the respective recombinant clone. Real-time PCR analysis revealed that all Tssk mRNAs are expressed at least 1000 times more in the testis than in all the other assayed tissues, such as brain, heart, kidney, liver and others, when values were normalized against either actin or Gapdh (Fig. 1A). In addition to the tissue expression pattern analysis, total testis mRNA from mice sacrificed at different days after birth was obtained and analyzed by real-time PCR. All Tssks RNA transcripts remained almost undetectable, and appeared to initiate expression not earlier than 21 days (3 weeks) after birth, strongly suggesting a postmeiotic expression of the respective mRNAs in mouse testes. Mouse Tssks transcript reached significantly high levels by 24 days (3.5 weeks) post-birth, comparable to those detected in adult mice testes. Values were normalized against actin (Fig. 1B); normalization of data by Gapdh provided equivalent results (data not shown).
Figure 1.
Quantitative analysis of mouse Tssks mRNA expression in mouse tissues (A) and in mouse testis during development (B). (A) Mouse Tssk mRNA in various mouse tissues (testis, brain, heart, whole embryo, kidney, liver, lung, muscle, ovary, spleen and thymus) was determined by real-time PCR, and relative quantities expressed using the corresponding testis Tssk as calibrator. Shown are pooled results of three separate experiments, run in duplicate. Average expression levels were normalized against Gapdh (black bars) or actin (grey bars) and the mean (± SEM) fold-change expressed relative to testis values (scaled to 1; *P<0.001). (B) Relative expression of mouse Tssk mRNA was determined by real-time PCR during testis development in 1-, 2-, 3-, 3.5- and 4-week-old mice. Relative quantity of each testicular Tssk at different developmental stages was obtained by calibration against the corresponding value of Tssk in 4-week-old mice (scaled to 1). Gene expression was normalized against actin (grey bars). Shown are pooled results of three separate experiments. Mean values in developmental stage groups indicated with different letters (a, b and c) differ significantly (P < 0.05).
Validation of Tssk antibodies in mouse and human sperm
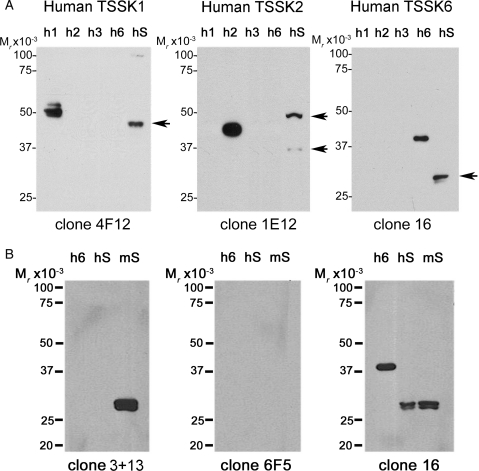
Because of the sequence homology between different members of the Tssk family, it has been difficult to demonstrate the presence and localization of a particular Tssk member in sperm, testis or other tissues. An array of anti-Tssk antibodies was evaluated; while some of the antibodies are commercially available, others were prepared in our laboratories. Five of these antibodies are monoclonal, i.e. anti-Tssk1, anti-Tssk2, anti-Tssk3 and two antibodies against Tssk6. On the other hand, anti-Tssk4 is a custom-made, anti-peptide antibody specifically designed for mouse Tssk4. Of all these antibodies, four mAbs (anti-Tssk1, anti-Tssk2, anti-Tssk3 and anti-Tssk6 clone 16) were able to recognize both mouse and human enzymes. Of the other two antibodies, clone 3 + 13 anti-Tssk6 and polyclonal anti-Tssk4 antibody only detected the respective ortholog in mouse. Validation of these antibodies was conducted using western blot analysis of all the mouse recombinant Tssks (Fig. 2) and four human recombinant proteins (Fig. 3). The predicted molecular mass of Tssk1, Tssk2, Tssk3, Tssk4, Tssk4b, Tssk4c and Tssk6 are ∼ 41, 40, 30, 37, 38, 32 and 30 KDa, respectively. As shown in Fig. 2, all antibodies are specific for the respective targeted mouse Tssk, with the exception of the anti-Tssk1 monoclonal antibody that appears to cross-react weakly with mouse Tssk2. The last lane in each western blot represents total mouse or human sperm protein extracts (mS or hS, respectively). Western blot analyses using anti-Tssk1, anti-Tssk2 and anti-Tssk6 demonstrated that these kinases are present in mature mouse (Fig. 2) and human sperm (Fig. 3A). Tssk2 antibodies detected two protein species, one of which might represent a posttranslational modification of this kinase, in both mouse and human sperm. Although anti-Tssk3 antibodies recognized recombinant mouse and human Tssk3, this antibody did not detect Tssk3 in either mouse or human sperm, strongly suggesting that this kinase is not present in these cells. Tssk4 has at least three splicing variants (see ‘Materials and Methods’ section) which were also cloned and expressed in bacteria (Fig. 2). Because the peptide sequence used to generate the anti-Tssk4 antibody is not present in either Tssk4c or hTssk4, these proteins were not detected, providing further evidence on the specificity of this antibody. Figure 3B shows data validating three anti-Tssk6 monoclonal antibodies targeted against the human kinases. Clone 16 anti-Tssk6 proved to be the most informative of the three anti-Tssk6 antibodies, and was able to detect both mouse and human Tssk6 even at high dilutions (1:5000). Under our experimental conditions, monoclonal anti-Tssk6-clone 6F5 (Abnova) did not recognize the hTssk6 control, while anti-Tssk6 clone 3+13 recognized mouse sperm Tssk6 only.
Figure 2.
Validation of antibodies against mouse Tssks by immunoblot analysis. A battery of antibodies against mouse Tssks was validated using the corresponding purified recombinant mouse kinase (100 ng/lane), as follows: Tssk1 (m1), Tssk2 (m2), Tssk3 (m3), Tssk4 and splicing variants (m4, m4b, m4c) and Tssk6 (m6). Protein extracts from mouse sperm (mS, equivalent to 106 cells per lane) were prepared in Laemmli buffer under reducing conditions. After separation on 12% PAGE, immunoblotting was performed with anti-Tssk antibodies as indicated in ‘Materials and Methods’ section. Regular-strength ECL reagent was used, except for Tssk2 analysis, where ECLplus was used instead. Numbers on the left-hand side of the gel denote the relative molecular mass standards. The predicted molecular masses of Tssk1 (41 KDa), Tssk2 (40 KDa), Tssk3 (30 KDa), Tssk4 (37 kDa), Tssk4b (38 kDa), Tssk4c (32 kDa) and Tssk6 (30 kDa) are indicated by an arrow; GST-tagged recombinant mouse Tssks are indicated by arrowheads. Result shown for each kinase is representative of at least three independent experiments.
Figure 3.
Validation of antibodies against human Tssks by immunoblot analysis. Antibodies recognizing human Tssks were validated using commercially available recombinant human kinases (100 ng/lane), as follows: His-tagged rhTssk1 (h1), His-tagged rhTssk2 (h2), GST-tagged rhTssk3 (h3) and GST-tagged rhTssk6 (aa 174-274) (h6). Protein extracts, either from mouse (mS) or human (hS) sperm (equivalent to 106 cells per lane), were prepared in Laemmli buffer under reducing conditions. (A) After separation on 12% PAGE, immunoblotting was performed with anti-Tssk antibodies: clone 4F12 for anti-Tssk1, clone 1E12 for anti-Tssk2 and clone 16 for anti-Tssk6. The predicted molecular masses of TSSK1 (41 KDa), TSSK2 (40 KDa), TSSK3 (30 KDa) and TSSK6 (30 KDa) is indicated by an arrow. In (B), three different anti-human Tssk6 antibodies were tested in parallel: anti-Tssk6 clone 3 + 13 (1:1000), anti-Tssk6 clone 6F5 from Abnova (1:250) and anti-Tssk6 clone 16 (1:5000). Regular-strength ECL reagent was used, except for hTssk2 analysis, where ECLplus was used instead. Numbers on the left-hand side of the gel denotes the relative molecular mass standards. Data shown for each kinase are representative of at least three independent experiments with similar results.
Immunolocalization of Tssks in sperm and testis
Once validated, the antibodies against each Tssk were used in immunofluorescence experiments to investigate their cellular localization in mouse testis (Fig. 4). Consistent with real-time PCR which showed that developmental expression of these kinases is starting at day 24, immunofluorescence studies revealed protein expression of these kinases in condensed spermatids which also are stained for the acrosomal marker PNA. Both Tssk2 and Tssk6 appear to be expressed exclusively in condensed spermatids, while it is possible to see staining of Tssk1 and Tssk4 close to the basal membrane, suggesting that Tssk1 and Tssk4 might be expressed in other spermatogenic germ cells that have not completed meiosis. Alternatively, antibodies against Tssk1 and Tssk4 might be cross-reacting with some other protein when used for immunostaining. To further analyze protein expression, different germ cell populations were prepared using the Sta-put technique (Romrell et al., 1976; Bellvé et al., 1977). These data confirmed that Tssk1, Tssk2 and Tssk6 were expressed late in spermiogenesis and were mainly present in condensed spermatids. On the other hand, Tssk4 was found in round and condensed spermatids; however, Tssk4 appears to have a different molecular weight in each germ cell type. Although not clear at present, this could be due either to posttranslational modifications or to the presence of different Tssk4 splicing variants. In agreement with western blotting results (Figs. 2 and 7), Tssk3 was undetectable in mouse testis.
Figure 4.
Analysis of Tssks in mouse testis and in purified spermatogenic cells. Testes from adult mice were fixed, dehydrated, paraffin-embedded and cut into 4–6 µm-thick sections. After treatment with blocking solution, tissue sections were incubated with anti-Tssk1 (clone 4F12, 1:50), anti-Tssk2 (clone 1E12, 1:50), anti-Tssk3 (clone 6G3, 1:25), anti-Tssk6 (clone 16, 1:100) monoclonal antibodies or anti-Tssk4 (custom-made, polyclonal antibody, 1:125). Specificity of polyclonal anti-Tssk4 was tested by pre-absorption with Tssk4 (blocking) peptide prior to sperm treatment. Alexa 555-conjugated secondary antibodies (1:200) were used for detection of mouse Tssks; secondary antibody solution also contained Alexa 488-conjugated PNA (1:200) and DAPI to stain acrosomes and nuclei, respectively. Pseudo-colored images were then merged. Images shown are representative results of at least three independent experiments. Testicular cells populations were obtained in linear 2–4% wt/vol gradients of BSA by the Sta-put method. Different cell types were analyzed by DIC microscopy, and purified spermatogenic cells populations (>90% pure) pooled accordingly, extracted in 1% SDS-containing lysis buffer, and analyzed by western blotting. P, pachytene spermatocytes; R, round spermatids; C/Rb, condensed spermatids plus residual bodies. Whole cell protein extracts from mature mouse sperm (Sp) were used as control. Arrows indicate expected size of each kinase in mature sperm. The presence of a lower molecular weight Tssk4 variant, detected in C/Rb cells (see ‘Results’ section), is noted by an asterisk.
Figure 7.
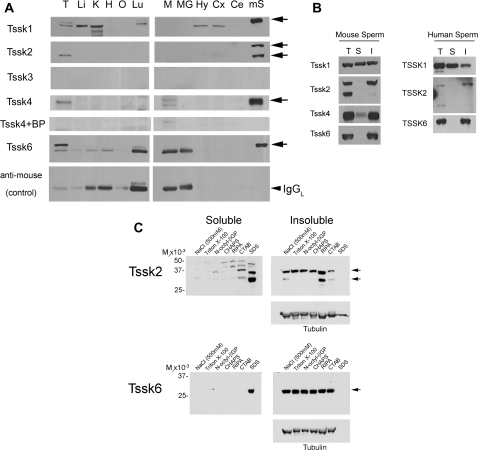
Analysis of mouse Tssks tissue expression by western blotting (A), and Tssks solubility under different extraction conditions, in human (B) and mouse sperm (B,C). (A) Protein extracts (50 µg per lane) from an array of 11 mouse tissues were analyzed by 10% PAGE and immunoblotting for the presence of mouse Tssks as explained in Fig. 2 legend. T (testis); M (muscle); MG (mammary gland); H (heart); O (ovary); Li (liver); K (kidney); Lu (lung); Hy (hypothalamus); Cx (brain cortex); Ce (cerebellum). Mouse sperm (mS) extracts were included as control. Arrows indicate the presence of each Tssk member in mouse sperm. Control for antibody specificity (anti-mouse-HRP alone) is also shown (IgGL: IgG light chain). (B) After swim-up, sperm were centrifuged and resuspended in buffer containing 1% Triton X-100 (see ‘Materials and Methods’ section). Whole cell protein extracts (T) were saved, or separated by centrifugation, and detergent-soluble (S) and detergent-insoluble (I) fractions analyzed by western blotting using anti-Tssks antibodies. Regular-strength ECL reagent was used, except for Tssk2 analysis in mouse and human sperm, where ECLplus was used instead. Shown are representative results of at least three independent experiments using mouse (left) or human (right) sperm. (C) Solubility of mouse sperm Tssk2 and Tssk6 was analyzed using cell lysis buffers supplemented with either NaCl (500 mM) or the following detergents: 1% Triton X-100, 1% n-octyl-beta-D-glucopyranoside, 1% CHAPS (zwitterionic detergent), RIPA(buffer containing three detergents), 1% CTAB (cetrimonium bromide, cationic surfactant) or 1% SDS . Soluble and insoluble fractions were analyzed by SDS–PAGE as indicated in ‘Materials and Methods’ section. Equal protein loading (insoluble fraction) was determined by stripping and reprobing membranes with anti-tubulin antibody.
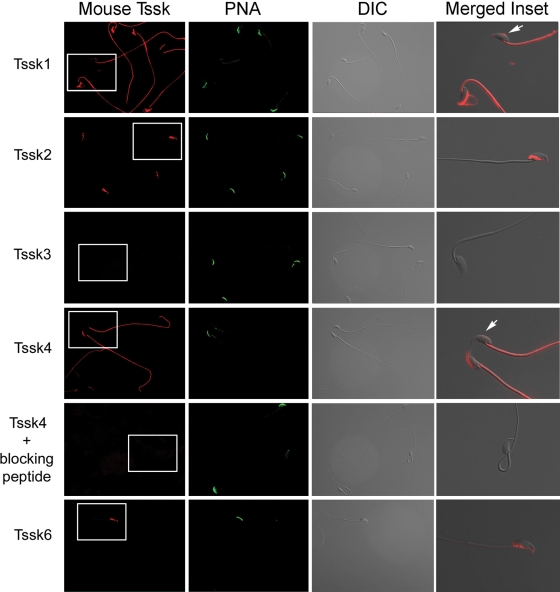
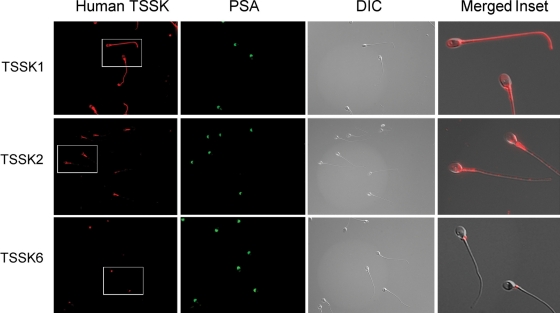
The same antibodies were then used to evaluate the subcellular localization of Tssks in mouse and human sperm (Figs. 5 and 6). In mouse sperm, Tssk1 and Tssk4 appear to be present in the anterior head as well as in the sperm flagellum (Fig. 5). Interestingly, the signal in the anterior head is lost in sperm that have undergone the acrosome reaction (see inset), suggesting that Tssk1 and Tssk4 have an acrosomal localization. Mouse Tssk2 and Tssk6 were found only in a specific sperm head compartment which is not strictly the postacrosomal or the equatorial region of the sperm head. This region is rich in polymerized actin (F-actin; Sosnik et al., 2009). Consistent with the lack of signal in western blots, results using monoclonal antibodies against Tssk3 were negative. As shown in Fig. 6, immunolocalization data of the respective Tssk in human sperm are comparable to our findings in mouse sperm. Interestingly, TSSK6 appears to localize to the neck of human sperm. Whether this localization corresponds to the one observed in mouse sperm remains to be investigated.
Figure 5.
Immunolocalization of Tssks in mouse sperm. To determine subcellular localization of Tssks, mouse sperm were air-dried, fixed, permeabilized, treated with 1% BSA and probed with anti-Tssk1 (clone 4F12, 1:50), anti-Tssk2 (clone 1E12, 1:50), anti-Tssk3 (clone 6G3, 1:25), anti-Tssk6 (clone 16, 1:100) monoclonal antibodies or anti-Tssk4 (custom-made, polyclonal antibody, 1:125). Specificity of polyclonal anti-Tssk4 was tested by pre-absorption with Tssk4 (blocking) peptide prior to sperm treatment. Alexa 555-conjugated secondary antibodies (1:200) were used for detection of mouse Tssks; secondary antibody solution also contained Alexa 488-conjugated PNA (1:200) to follow the mouse acrosome status. DIC images were taken in parallel, and served as control for sperm morphology. A pseudo-colored, higher magnification image of the corresponding inset, merged to DIC image, has been included for each human kinase. Arrowheads indicate acrosome-reacted sperm. Images shown are representative of at least three independent experiments with similar results.
Figure 6.
Immunolocalization of TSSKs in human sperm. Human sperm were air-dried, fixed, permeabilized, treated with 1% BSA and probed with anti-TSSK1 (clone 4F12, 1:50), anti-TSSK2 (clone 1E12, 1:50) or anti-TSSK6 (clone 16, 1:100) monoclonal antibodies. Alexa 555-conjugated anti-mouse antibody (1:200) was used for detection of human TSSKs; this antibody solution also contained FITC-conjugated PSA (1:200) to follow the human acrosome status. A pseudo-colored, higher magnification image of the corresponding inset, merged to DIC image, has been included for each mouse kinase. Images shown are representative results of at least three independent experiments with similar results.
Based on evidence obtained by northern analyses, all Tssks are testis-specific proteins. Nevertheless, we have observed low expression of Tssks in other tissues by using the more sensitive quantitative PCR approach. To further evaluate the tissue specificity of Tssks, protein extracts from an array of 11 mouse tissues were prepared and analyzed by western blotting (Fig. 7A); mouse sperm extracts were included as controls. Of note, in the case of Tssk1, bands of slightly different molecular mass were found in other tissues such as kidney. At present, it is not clear whether these bands are Tssk1 or not. Consistent with the quantitative PCR analysis, Tssk2, Tssk4 and Tssk6 expression was restricted to the testis. As expected, Tssk3 tested negative in all tissues examined.
Subcellular fractionation studies reveal that Tssks are highly insoluble proteins
As shown in Fig. 5, both Tssk2 and Tssk6 localized to a postacrosomal compartment that is rich in polymerized actin (Sosnik et al., 2009). Several proteins in this sperm region are insoluble in non-ionic detergents because of their association with sperm internal structures. To evaluate the solubility properties of the different Tssks, mouse sperm were extracted with the non-ionic detergent Triton X-100 at 1% concentration (Fig. 7B). After centrifugation for 10 min at 10 000g, only Tssk1 was found in soluble fractions of mouse sperm, while Tssk4 and Tssk6 were mostly insoluble. As shown in Figs. 2 and 3A, Tssk2 antibodies detected two protein species in whole cell extracts; Tssk2 was recovered in the insoluble fractions, being the higher molecular mass species the predominant one. In addition, and similarly to our findings in mouse sperm, human TSSK1 was partially soluble in Triton X-100, while TSSK2 and TSSK6 were found only in the particulate fraction (Fig. 7B). The solubility of Tssk2 and Tssk6 was further examined using a variety of detergents as well as a high-salt lysis buffer (Fig. 7C). While Tssk6 remained in the pellet under all conditions tested, Tssk2 was slightly soluble in the cationic detergent CTAB and fully soluble in the anionic detergent SDS.
Biochemical characterization of human TSSK2 kinase activity in vitro
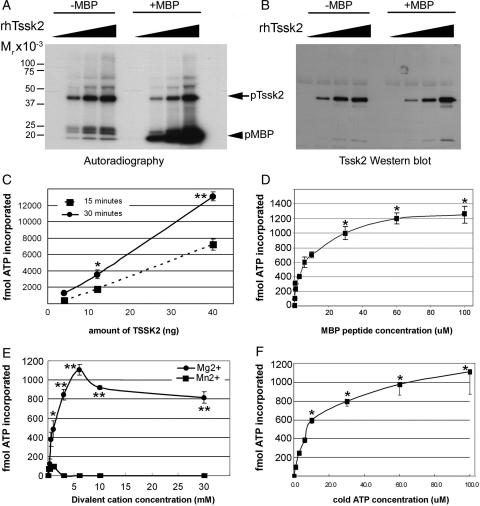
Next, recombinant human TSSK2 was used to evaluate its kinetic parameters using an in vitro kinase activity assay (Fig. 8). All assays were run at pH 7.2 and at a constant temperature of 30°C, with incubation lasting for 15 min unless indicated otherwise. According to autoradiography PAGE-based analysis, TSSK2 is able to phosphorylate MBP in a dose-dependent manner (Fig. 8A). Supporting our previous observations (Hao et al., 2004), autophosphorylation of TSSK2 occurred in parallel, and was confirmed by probing the same membrane with anti-TSSK2 specific antibody (Fig. 8B). To gain further insight into the enzymatic properties of these testis-specific kinases, recombinant TSSK2 activity was also determined using a MBP-derived peptide (MBP104/118) as substrate. ATP incorporation by TSSK2 (Fig. 8C) occurred in a time- and concentration-dependent fashion. Phosphorylation of MBP peptide was already significant at ∼5 µM of MBP peptide (Fig. 8D), with a maximum response at ∼50 µM. Dependence of TSSK2 kinase activity on divalent cations, either Mg2+ or Mn2+ was also assessed. Recombinant TSSK2 showed a clear preference for Mg2+, with a maximum enzymatic activity at ∼5 mM (Fig. 8E). Our results demonstrate that TSSK2-dependent ATP incorporation into substrate is almost undetectable when Mn2+ is used, even at high concentration (0–30 mM), as opposed to previous reports on enzymatic requirements for Tssk3 (Bucko-Justyna et al., 2005). Finally, dependence of this kinase on ATP concentration was analyzed. Our experiments indicate that human TSSK2 has a Km for ATP of ∼10 µM (Fig. 8F). These results indicate that recombinant TSSK2 is active and that high-throughput screening (HTS) assays can be designed using relatively low ATP concentrations and a small peptide as substrate.
Figure 8.
Enzymatic properties of recombinant TSSK2. Increasing amounts (0–40 ng) of rTSSK2 were assayed for kinase activity with MBP (5 µg per reaction) as substrate, and analyzed by PAGE, followed by autoradiography (A) and by immmunoblotting with anti-Tssk2 antibody (B). (C–F) Enzyme kinetics of rhTssk2 (10 ng per reaction) were examined in terms of its dependence on incubation time (C), MBP 104/118 peptide as substrate (0–100 µM) (D), divalent cations (0–30 mM) (E) or non-radioactive ATP (0–100 µM) (F) and quantified as explained in ‘Materials and Methods’ section. In each assay, control (blank) reactions with no rTSSK2 were run in parallel. Data represent mean ± SEM of three independent experiments, run in triplicate. In (D) and (F), significant differences versus control (0 µM) (*P < 0.001) are indicated. In (C) and (E), asterisks indicate significant differences between 15- versus 30-min incubation time, or either divalent cation tested, respectively (*P < 0.05, **P < 0.001).
Discussion
Members of the Tssk family were discovered as part of a search for novel kinases. The first member of this family, Tssk1, was cloned in 1994 using a PCR approach with degenerate oligonucleotide primers targeting two highly conserved motifs within the protein kinase catalytic domain (Bielke et al., 1994). Using a low stringency screening, the same research group described Tssk2, a closely related family member (Kueng et al., 1997), and showed that both proteins are expressed postmeiotically in testicular germ cells. Later, other investigators used degenerate primers as well as bioinformatics analysis to clone and characterize Tssk3 (Zuercher et al., 2000; Visconti et al., 2001), Tssk4 (Chen et al., 2005) and Tssk6 (Hao et al., 2004). Some confusion in the field has arisen due to the different names given to the same proteins by different authors. For example, Tssk1, 2 and 3 were initially annotated as Stk22a, b, c/d; Tssk4 has been named Tssk5 (Chen et al., 2005), and Tssk6 is also known as either Tssk4 (Hao et al., 2004) or Sstk (Spiridonov et al., 2005). In the present work, we have used the new curated nomenclature given by NCBI.
By northern blot analysis, the Tssk family has been shown to be expressed exclusively in the testis (Kueng et al., 1997; Visconti et al., 2001; Spiridonov et al., 2005). However, using quantitative RT–PCR analysis, low expression of these kinases in human tissues other than testis has been reported (Hao et al., 2004). In the present study, further analysis of mRNA as well as protein expression revealed that all these kinases are expressed postmeiotically in spermatids, suggesting that they might have a role in spermiogenesis, the process leading to mature sperm. Alternatively, these kinases could function in the sperm to regulate their ability to fertilize the oocyte. As part of this analysis, we have used a battery of antibodies to investigate whether these kinases remain in the sperm after epididymal maturation and ejaculation. In previous studies, our group (Hao et al., 2004) and others (Xu et al., 2008) used the same polyclonal antibody against Tssk2 to assess the presence of this protein in human and mouse sperm; this antibody was generated using the whole Tssk2 recombinant protein as antigen. Because of sequence similarities to other Tssks, it was not possible to conclude that the antibody did not recognize other Tssks in the sperm (Hao et al., 2004). To avoid this potential problem, the antibodies used in the present work were first evaluated against all mouse Tssks and four of the recombinant human counterparts. Using these antibodies, the presence of Tssk1, Tssk2 and Tssk6 was demonstrated in mouse and human sperm. Tssk4 was found in mouse sperm; however, because the antigenic peptide used to generate the anti-mouse Tssk4 is not conserved in humans, it was not possible to determine whether this kinase is also present in human sperm. On the other hand, although Tssk3 mRNA is present in mouse and human testis in amounts compatible with northern blot detection (Visconti et al., 2001), we have not been able to find Tssk3 protein in either the sperm or the testis by western blot analysis. It is possible that this kinase is translated transiently in certain cell populations and not at enough levels to be detected by western analysis. It has been suggested that Tssk3 is present in Leydig cells (Zuercher et al., 2000); however, this is unlikely because we were able to detect Tssk3 mRNA in purified round and condensed spermatids, and its mRNA appears at 24 days after birth, suggesting germ cell postmeiotic expression (Visconti et al., 2001). Furthermore, analysis of protein extracts obtained from different-age mouse whole testis, which includes proteins from Leydig cells, did not detect any protein by western blotting with anti-Tssk3 antibodies.
Sperm are highly polarized cells with distinct cellular compartments. In intact sperm, the head contains a complex array of membranes in which the plasma membrane surrounds the anterior acrosome, the equatorial segment and the postacrosomal region. In the sperm tail, the anterior part of the flagellum (midpiece) contains the mitochondria, and the posterior part of the tail, known as principal piece, includes the fibrous sheath surrounding the outer dense fibers (Eddy and O'Brien, 1994). These sperm compartments should be taken into account when the role of Tssks is analyzed. Therefore, the aforementioned array of specific antibodies was used to investigate the localization of Tssks in mouse and human sperm. Interestingly, all Tssks displayed a different cellular localization suggesting that their role is not redundant. Tssk1 and Tssk4 localized to the sperm anterior head as well as to the whole flagellum. In addition, the signals of both Tssk1 and Tssk4 were lost in sperm that had undergone the acrosome reaction, suggesting that these kinases are present in the sperm acrosome. Tssk2 and Tssk6 are present mainly in the head, in a compartment that is posterior to the equatorial segment and includes sections of the postacrosomal region as well as the acrosome tip. Although little is known about the role of this compartment in sperm function, we have recently shown that Izumo, a protein involved in sperm-oocyte fusion, migrates to this region after the acrosome reaction (Miranda et al., 2009; Sosnik et al., 2009). In addition, in capacitated sperm, this compartment is enriched in polymerized actin.
The role of Tssks has recently been explored using genetic models, which have established that either the combined absence of Tssk1 and Tssk2 (Shang et al., 2007, 2010; Xu et al., 2008) or the absence of Tssk6 (Spiridonov et al., 2005) render mouse infertile phenotypes. Based on current evidence, it is still unclear whether these kinases play a role in mature sperm, or the infertility phenotype is due to defects in spermiogenesis. Two Tssk1/2 double KO mice models have been made recently. On one hand, Xu et al. (2007) did not observe transmission of the null genotype and concluded that these mice were haploinsufficient. Contrary to this finding, Shang et al. (2007) reported that null mice for Tssk1 and Tssk2 show a male sterility phenotype. More recently, studies carried out by the same group (Shang et al., 2010) demonstrated that targeted deletion of both Tssk1/2 genes is accompanied by a loss of the chromatoid body in mouse spermatids indicating a role of Tssk1 and Tssk2 in postmeiotic cytodifferentiation of spermatids. Despite this controversy, analysis of these mice models points out an essential role of these kinases in reproduction. In the present study, the finding that Tssk1, Tssk2, Tssk4 and Tssk6 remain in the sperm after epididymal maturation suggests that in addition to a role in spermiogenesis they may have an essential role in sperm function.
In the case of the Tssk6 null mice, a significant fraction of their sperm population display morphological defects, which has been proposed to result from aberrant chromatin condensation. When expressed in vitro, Tssk6 interacts with heat shock proteins such as HSP90-1β, HSC70 and HSP70; in addition, it has been shown that this kinase is able to phosphorylate histones in vitro (Spiridonov et al., 2005). Recently, we have shown that sperm from the Tssk6 null mice are able to attach to the oocyte oolema but are not capable of fusing with zona pellucida-free oocyte in vitro (Sosnik et al., 2009). At present, Izumo is the only sperm protein with a confirmed role in sperm-oocyte fusion (Inoue et al., 2005). Izumo is localized to the anterior acrosome in both wild type and Tssk6 null sperm. After the acrosome reaction takes place, Izumo changes its immunofluorescent pattern and distributes to the postacrosomal region in wild type mice; this change is not observed in Tssk6 null sperm. In addition to these findings, Tssk6 KO mouse sperm presents defects in actin polymerization as observed using phalloidin staining. These data are consistent with the presence of Tssk6 in the same region in which actin polymerization was observed. Altogether, these findings suggest that the Tssk6 role in sperm-oocyte interaction is dependent on changes in Izumo localization. Whether this effect is due to a direct phosphorylation of Izumo by Tssk6, or it is grounded in more profound defects taking place during spermiogenesis, remains to be investigated.
The observation that Tssks are mostly testis-specific, together with findings that the Tssk1/Tssk2 double KO mice as well as the Tssk6 null mice are sterile without presenting other detectable defects, suggest that these kinases could be used as targets for male contraception. Protein kinases have become important targets for the development of novel drugs. Identification of Tssks-specific inhibitors could be achieved using a combination of high-throughput assays and elucidation of these kinases' crystal structure. Recently, Xu et al. (2008) have found a series of small molecules capable of inhibiting Tssk1 and Tssk2. Whether these inhibitors are specific for these kinases is not known yet. In the present study, we described conditions to measure TSSK2 activity and analyzed different kinetic parameters. The TSSK2 protein kinase assay could be adapted to HTS assays of organic inhibitors. Because of the complexity in untying the function of Tssks in spermiogenesis from their role in mature sperm, identification of specific inhibitors will be essential for a further understanding of the relevance of Tssks in reproduction.
Authors' roles
Each author's contribution towards this article is as follows: Y.L.: first author, experimental design, performed most of the experiments, writing and revision of manuscript. J.S.: experimental design, data acquisition, revision of manuscript. L.B.: samples preparation and data acquisition. M.R.: samples preparation and data acquisition. N.A.S.: generation and purification of Tssk6 monoclonal antibodies, revision of manuscript. T.C.B.: experimental design, samples preparation, revision of manuscript. G.R.J.: generation and purification of Tssk6 monoclonal antibodies, revision of manuscript. J.A.: provided reagents, experimental design and revision of manuscript. P.E.V.: experimental design, writing and revision of manuscript, provided most of funding. A.M.S.: corresponding author, experimental design, directing and performing experiments, writing and revising manuscript, providing funding.
Funding
This study was supported by grants NIH HD038082, NIH HD044044 and NIH HD050341 (to P.E.V.), by the Collaborative Biomedical Research (CBR) Program between the University of Massachusetts and Baystate Medical Center (to A.M.S.), and by NIH F31HD049324 (to J.S.). Characterization of Tssks in human subjects was supported in part by the Gustavus and Louise Pfeiffer Research Foundation.
Acknowledgements
The authors wish to thank Dr George Gerton and Dr Mariano Buffone for their advice and help in the preparation of purified germ cells by the Sta-put method. We would also like to thank Dr Dominique Alfandari for reagents and advice on molecular biology techniques, and Dr Kimberly Tremblay lab for helping with tissue preparation for immunohistochemistry.
References
- Bellvé AR, Millette CF, Bhatnagar YM, O'Brien DA. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977;25:480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Bielke W, Blaschke RJ, Miescher GC, Zurcher G, Andres AC, Ziemiecki A. Characterization of a novel murine testis-specific serine/threonine kinase. Gene. 1994;139:235–239. doi: 10.1016/0378-1119(94)90762-5. doi:10.1016/0378-1119(94)90762-5. [DOI] [PubMed] [Google Scholar]
- Bucko-Justyna M, Lipinski L, Burgering BM, Trzeciak L. Characterization of testis-specific serine-threonine kinase 3 and its activation by phosphoinositide-dependent kinase-1-dependent signalling. FEBS J. 2005;272:6310–6323. doi: 10.1111/j.1742-4658.2005.05018.x. doi:10.1111/j.1742-4658.2005.05018.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Lin G, Wei Y, Hexige S, Niu Y, Liu L, Yang C, Yu L. TSSK5, a novel member of the testis-specific serine/threonine kinase family, phosphorylates CREB at Ser-133, and stimulates the CRE/CREB responsive pathway. Biochem Biophys Res Commun. 2005;333:742–749. doi: 10.1016/j.bbrc.2005.05.157. doi:10.1016/j.bbrc.2005.05.157. [DOI] [PubMed] [Google Scholar]
- Eddy EM, O'Brien DA. The spermatozoon. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1994. pp. 29–77. [Google Scholar]
- Gerton LG, Millette CF. Generation of flagella by cultured mouse spermatids. J Cell Biol. 1984;98:619–628. doi: 10.1083/jcb.98.2.619. doi:10.1083/jcb.98.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. doi:10.1016/0003-2697(91)90534-Z. [DOI] [PubMed] [Google Scholar]
- Hake LE, Alcivar AA, Hecht NB. Changes in mRNA length accompany translational regulation of the somatic and testis-specific cytochrome c genes during spermatogenesis in the mouse. Development. 1990;110:249–257. doi: 10.1242/dev.110.1.249. [DOI] [PubMed] [Google Scholar]
- Hao Z, Jha KN, Kim YH, Vemuganti S, Westbrook VA, Chertihin O, Markgraf K, Flickinger CJ, Coppola M, Herr JC, et al. Expression analysis of the human testis-specific serine/threonine kinase (TSSK) homologues. A TSSK member is present in the equatorial segment of human sperm. Mol Hum Reprod. 2004;10:433–444. doi: 10.1093/molehr/gah052. doi:10.1093/molehr/gah052. [DOI] [PubMed] [Google Scholar]
- Hecht NB. Post-meiotic gene expression during spermatogenesis. Prog Clin Biol Res. 1988;267:291–313. [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. doi:10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Jaleel M, McBride A, Lizcano JM, Deak M, Toth R, Morrice NA, Alessi DR. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–1423. doi: 10.1016/j.febslet.2005.01.042. doi:10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Jha KN, Salicioni AM, Arcelay E, Chertihin O, Kumari S, Herr JC, Visconti PE. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol Hum Reprod. 2006;12:781–789. doi: 10.1093/molehr/gal085. doi:10.1093/molehr/gal085. [DOI] [PubMed] [Google Scholar]
- Johnson LN. The regulation of protein phosphorylation. Biochem Soc Trans. 2009;37:627–641. doi: 10.1042/BST0370627. doi:10.1042/BST0370627. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of ser/thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. doi:10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng P, Nikolova Z, Djonov V, Hemphill A, Rohrbach V, Boehlen D, Zuercher G, Andres AC, Ziemiecki A. A novel family of serine/threonine kinases participating in spermiogenesis. J Cell Biol. 1997;139:1851–1859. doi: 10.1083/jcb.139.7.1851. doi:10.1083/jcb.139.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. doi:10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miranda PV, Allaire A, Sosnik J, Visconti PE. Localization of low-density detergent-resistant membrane proteins in intact and acrosome-reacted mouse sperm. Biol Reprod. 2009;80:897–904. doi: 10.1095/biolreprod.108.075242. doi:10.1095/biolreprod.108.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Galili N, Buck CA. Immunohistochemical analysis of the expression of two serine-threonine kinases in the maturing mouse testis. Mech Dev. 1998;74:171–174. doi: 10.1016/s0925-4773(98)00060-4. doi:10.1016/S0925-4773(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Romrell LJ, Bellvé AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976;49:119–131. doi: 10.1016/0012-1606(76)90262-1. doi:10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Shang P, Baarends WM, Dooijes D, Elfferich P, Wijgerde M, Ooms MP, Looijenga LH, Dohle GR, Van Eenennaam H, Gossen JA, et al. Testis-specific serine/threonine kinases encoded by the retrogenes Tssk1 and Tssk2 are essential for spermatogenesis in mouse. In: Andrology ASo., editor. XIX North American Testis Workshop: “Chromosome Structure and Gene Expression”. Tampa: Hyatt Regency; 2007. p. 115. [Google Scholar]
- Shang P, Baarends WM, Hoogerbrugge J, Ooms MP, van Cappellen WA, de Jong AA, Dohle GR, van Eenennaam H, Gossen JA, Grootegoed JA. Functional transformation of the chromatoid body in mouse spermatids requires testis-specific serine/threonine kinases. J Cell Sci. 2010;123:331–339. doi: 10.1242/jcs.059949. doi:10.1242/jcs.059949. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press, Ltd; 1994. pp. 1363–1434. [Google Scholar]
- Sokal RR, Rolf JF. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd edn. New York: Freeman, W.H; 1995. [Google Scholar]
- Sosnik J, Miranda PV, Spiridonov NA, Yoon SY, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–2749. doi: 10.1242/jcs.047225. doi:10.1242/jcs.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiridonov NA, Wong L, Zerfas PM, Starost MF, Pack SD, Paweletz CP, Johnson GR. Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol Cell Biol. 2005;25:4250–4261. doi: 10.1128/MCB.25.10.4250-4261.2005. doi:10.1128/MCB.25.10.4250-4261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Johnson LR, Oyaski M, Fornes M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol. 1997;192:351–363. doi: 10.1006/dbio.1997.8768. doi:10.1006/dbio.1997.8768. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Hao Z, Purdon MA, Stein P, Balsara BR, Testa JR, Herr JC, Moss SB, Kopf GS. Cloning and chromosomal localization of a gene encoding a novel serine/threonine kinase belonging to the subfamily of testis-specific kinases. Genomics. 2001;77:163–170. doi: 10.1006/geno.2001.6628. doi:10.1006/geno.2001.6628. [DOI] [PubMed] [Google Scholar]
- Xu B, Jha KN, Hao Z, Urekar C, Flickinger CJ, Herr JC. Functional studies of TSSK1 and 2 and their substrate TSKS by targeted gene deletion and immunobiochemistry, haploinsufficiency of TSSK 1 and 2 causes male infertility. In: Andrology ASo., editor. XIX North American Testis Workshop: “Chromosome Structure and Gene Expression”. Tampa: Hyatt Regency; 2007. p. 114. [Google Scholar]
- Xu B, Hao Z, Jha KN, Zhang Z, Urekar C, Digilio L, Pulido S, Strauss JF, III, Flickinger CJ, Herr JC. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev Biol. 2008;319:211–222. doi: 10.1016/j.ydbio.2008.03.047. doi:10.1016/j.ydbio.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuercher G, Rohrbach V, Andres AC, Ziemiecki A. A novel member of the testis specific serine kinase family, tssk-3, expressed in the Leydig cells of sexually mature mice. Mech Dev. 2000;93:175–177. doi: 10.1016/s0925-4773(00)00255-0. doi:10.1016/S0925-4773(00)00255-0. [DOI] [PubMed] [Google Scholar]