Abstract
Aims: The effect of transdermal nicotine on stress reactivity was investigated in currently smoking, detoxified, substance-dependent individuals (65% alcohol dependent, n = 51; 31 male) following a psychosocial stressor. Methods: Using a randomized, double-blind, placebo-controlled design, subjects were assigned to receive either active transdermal nicotine (low or high dose) or placebo. Six hours following nicotine administration, subjects performed a laboratory psychosocial stressor consisting of two 4-min public-speaking sessions. Results: Consistent with prior reports, substance-dependent individuals displayed a blunted stress response. However, a review of the cortisol distribution data encouraged additional analyses. Notably, a significant minority of the substance-dependent individuals (33%) demonstrated elevated poststress cortisol levels. This group of responders was more likely to be alcohol dependent and to have received the high dose of nicotine [χ2(2) = 32, P < 0.0001], [χ2(2) = 18.66, P < 0.0001]. Differences in salivary cortisol responses between responders and nonresponders could not be accounted for by the length of sobriety, nicotine withdrawal levels, anxiety or depressive symptomatology at the time of the psychosocial stressor. Conclusion: These results suggest that nicotine administration may support a normalization of the salivary cortisol response following psychosocial stress in subgroups of substance-dependent individuals, particularly those who are alcohol dependent. Given the association between blunted cortisol levels and relapse, and the complex actions of nicotine at central and peripheral sites, these findings support the systematic study of factors including nicotine, which may influence stress reactivity and the recovery process in alcohol-dependent individuals.
INTRODUCTION
Stress system dysregulation is one of the hallmark features of alcohol-dependent individuals (Lovallo, 2006). This dysregulation persists well into recovery following acute stress (Harris et al., 2005; Lovallo et al., 2000; Sinha et al., 2009), even though diurnal rhythmicity normalizes relatively early in recovery (Adinoff et al., 1998; Keedwell et al., 2001; Lovallo, 2006). The stress system dysregulation is characterized in part by peripheral markers of the stress system, cortisol and corticotropin, which are blunted following psychosocial stressors (Harris et al., 2005; Lovallo et al., 2000). Unique to psychosocial stress protocols is the capability to assess stress system function in an everyday, nondrug context. This approach differs from stress elicitation techniques that use drug imagery cues and are associated with heightened stress responses (Sinha et al., 2006). Understanding the factors that influence the stress response following psychosocial stressors may be critical to improving the recovery process characterized by psychosocial adaptation within a drug- and alcohol-free context.
Stress responsivity is known to be influenced by a host of individual variables including psychiatric disorders, age, personality traits, prior life experiences and genetics (Kudielka et al., 2009; Pruessner et al., 1997; Wust et al., 2004). Thus far, the addiction literature has focused primarily on group differences between community controls versus treatment-seeking individuals with limited attention to within group variability or potential sources of this variability (but Harris et al., 2005; Junghanns et al., 2003, 2005; Kudielka et al., 2009; Sinha et al., 2006). A particularly pertinent variable, given its comorbid use in treatment-seeking substance abusers and its reported effect on the stress response is the impact of nicotine use, generally in the form of cigarette smoking. Interestingly, despite a reduction in the number of smokers in the general population, current estimates suggest that ∼80% of treatment-seeking alcoholics are chronic smokers (Ceballos, 2006a; Littleton, et al., 2007).
The effects of nicotine administration on stress system responsivity are well characterized in community controls. Acute exposure to nicotine via smoking causes an immediate spike (within minutes) in cortisol and corticotropin levels even in habitual smokers (Ceballos and al'Absi 2006b; Kirschbaum et al., 1992; Mendelson et al., 2005; Pomerleau et al., 1983; Wilkins et al., 1982). These levels return to baseline within 2 h (Winternitz and Quillen, 1977; Wilkins et al., 1982). However, similar data are lacking for substance-dependent individuals, excluding a small study of alcoholics (Coiro and Vescovi, 1999). The findings of Coiro and Vescovi (1999) are inconclusive, given the small sample size and route of nicotine administration (cigarette smoking). The limitations of the existing literature concerning individual factors related to stress system reactivity such as nicotine use, and the importance of stress system responsivity to the recovery process provide the rationale for the current study.
To address the question regarding the effects of nicotine on stress reactivity, salivary cortisol, cardiovascular and subjective measures were assessed in inpatient treatment-seeking individuals, the majority of whom met alcohol dependence criteria (65%), following a psychosocial stressor. Given the previous literature showing, a blunted cortisol response in these treatment-seeking populations (Adinoff et al., 2005a, 2005b; Contoreggi et al., 2003), and suggestions that nicotine elevates cortisol levels (Ceballos and al'Absi 2006b; Kirschbaum et al., 1992; Mendelson et al., 2005; Pomerleau et al., 1983; Wilkins et al., 1982), we hypothesized that subjects who received nicotine would demonstrate normalized stress reactivity following the stressor (greater self-reported negative affect and salivary cortisol levels) when compared with placebo. This hypothesis at first appears counter-intuitive, given that smokers who receive the placebo patch are presumed to be in nicotine withdrawal, which increases cortisol levels in community controls (al'Absi, 2006). However, the question of interest addressed stress responsivity following a psychosocial stressor; that is, how stress reactivity changes over time in subjects who receive nicotine versus those who receive placebo. Thus, given the lack of a guiding literature, our hypothesis that nicotine would normalize the stress response (i.e. provide a greater salivary cortisol response) in substance-dependent individuals when compared with placebo, was examined.
METHOD
Subjects
Subjects were treatment-seeking, substance-dependent individuals recruited from inpatient drug and alcohol treatment facilities (ages 25–59, n = 51; 31 male). They had a minimum of 10 years of education and were between 21–60 days sober. Substance-dependent individuals met diagnostic and statistical manual of mental disorders-IV (DSM-IV) criteria (APA, 1994) for current alcohol (ALC; n = 15, 29.41%), stimulant (STIM; n = 17, 33.33%) or alcohol and stimulant (ALC/STIM; n = 19, 37.25%) dependence. Subjects listed their race as white (67%) or black (33%). All subjects met criteria for current DSM-IV nicotine dependence. Nicotine-dependent community controls (n = 7; 4 male) were included as a comparison group on follow-up analyses (see Data analysis for detail). Women who were pregnant or breast feeding as determined by self-report and urine analysis at the time of testing were not allowed to participate in the study. All procedures were approved by the University of Florida Medical Institutional Review Board, and subjects gave written informed consent for screening and laboratory procedures.
Subjects were screened for neurological, psychiatric or medical disorders, which could interfere with the study protocol. Exclusionary criteria included: lifetime history of schizophrenic disorders, bipolar disorder, current major depressive disorder, current posttraumatic stress disorder (PTSD) or current panic disorders. Due to the high prevalence of anxiety disorders (excluding panic disorder and PTSD), childhood conduct disorder, or antisocial personality disorder in substance using/ abusing populations (Bucholz et al., 2000), persons who were positive for lifetime diagnoses of these disorders were not excluded from the study. Persons with other serious medical conditions not limited to serious head injury, prolonged unconsciousness, epilepsy, hypertension, liver cirrhosis, hepatitis C or HIV were excluded.
Screening procedures
Subjects completed a multi-tiered screening protocol and were compensated for their time. The packet administered during the screening phase consisted of depression (Beck Depression Inventory (BDI)-II; Beck et al., 1996) and state anxiety (State Anxiety Inventory (STAI); Spielberger, 1983) inventories, measurement of abstracting and verbal skills (Zachary, 1986), a four-generation family tree of alcohol, nicotine and drug use/ abuse (adapted from Mann et al., 1985) and a detailed history of marijuana, alcohol, nicotine and other drug use. Subjects continuing to meet study inclusion criteria were scheduled for medical and psychiatric interviews. During the medical interview, subjects were asked questions about their medical history and current prescriptions. Blood pressure and expired carbon monoxide (CO) were also measured to establish baseline levels. Psychiatric history was also assessed using the computerized National Institute of Mental Health Diagnostic Interview Schedule for the DSM-IV (APA, 1994; Robins et al., 1995). Subjects meeting study eligibility criteria were scheduled for the laboratory study day.
Laboratory study
Subjects were instructed to refrain from smoking overnight prior to the study. Before arriving the laboratory (7 a.m.), all subjects provided a CO breath analysis (Vitalograph® Inc., Lenexa, KS, USA). Smoking abstinence was confirmed with CO levels of 12 p.p.m. or less, or a reading of 50% of screening baseline. Measures of depression (BDI-II; Beck et al., 1996), state anxiety (STAI; Spielberger, 1983) and nicotine withdrawal symptoms checklist (WSC; Hughes and Hatsukami, 1986) were also completed prior to transdermal patch administration. Subjects provided breath samples (Intoxilyzer® Model 400, CMI Inc., Owensboro, KY, USA) to confirm alcohol sobriety and urine samples (OnTrak TestCup 5®, Varian Inc., Cary, NC, USA) to confirm drug sobriety from tetrahydrocannabinol, cocaine, benzodiazepines, morphine and methamphetamine. Following transdermal patch administration (∼7:30 a.m.), the laboratory study day involved assessment of pre- and poststress measures including subjective reports (self-reported positive and negative affect, anxiety and nicotine withdrawal symptoms), cardiovascular measures (blood pressure, heart rate) and salivary cortisol levels. Smoking measures were also evaluated ((Fagerström Test for Nicotine Dependence (FTND); Heatherton et al., 1991), (Smoking Consequences Questionnaire (SCQ); Copeland et al., 1995)).
Transdermal nicotine patch administration
Transdermal patches were randomly assigned and consisted of either placebo, or nicotine (low dose (7 mg), or high dose (14 mg for women, 21 mg for men) (Equate®)). Previous experience in our laboratory showed that female subjects experienced intolerance to the 21 mg patch (extreme nausea and other side-effects), thus differential dosing was employed for men and women following discussion with the local Institutional Review Board (Nixon et al., 2007).
To maintain the double-blind design, placebo patches matched the size and shape of active patches. All patches were placed on the upper left shoulder by a research assistant not participating in laboratory testing. The psychosocial stressor, a part of a larger battery, occurred about 6 h after the nicotine patch was placed. However, nicotine levels stabilize within 1.5 h following patch administration (Gorsline et al., 1993). Following laboratory study completion, subjects were fully debriefed as to which transdermal patch they had received as well as the nuances of the psychosocial stress protocol described below.
Laboratory psychosocial stress protocol
Subjects completed a psychosocial stress protocol consisting of two 4-minute speeches (al'Absi et al., 1997). For each speech, subjects were informed of their topic and allowed 4 min of preparation time in room A. Following the preparation time, subjects were escorted by laboratory personnel into room B. Laboratory personnel were unknown to the subject, of opposite gender and wore white coats. Laboratory personnel listened to and rated the subjects’ speech. Laboratory personnel were instructed to maintain an expressionless demeanor in all interactions with the subject including while rating the subject's speech. A video camera was used to simulate recording of the subject's performance, and a timer, set on the table in front of the subject, measured the 4 min. Subjects who finished speaking prior to 4 min were told by the laboratory personnel that there was time remaining and to please continue. The subjects were told that their speech would be rated for how ‘convincing, organized, articulate and enthusiastic’ they were during the speech (al'Absi et al., 1997) and that they would be financially compensated based on their rating. The speeches consisted of the hypothetical defense of two crimes (a) committing a hit and run accident and (b) shoplifting (al'Absi et al., 2003). The psychosocial stress manipulation, including transition time, took 20 min to complete.
Following the stress manipulation, subjects were debriefed regarding the sham video recording. Subjects were told that they were not taped and that the purpose of the deception was to activate their stress system. Subjects were also offered an opportunity to view the videocassette recorder tape deck to ensure no recording was made. All subjects received the financial bonus.
Subjective reports
Baseline measurements of nicotine withdrawal and anxiety were assessed by the WSC (Hughes and Hatsukami, 1986) and the STAI (Spielberger, 1983) prior to the psychosocial stress manipulation to ensure that groups were equated for nicotine withdrawal and anxiety symptoms. Self-reported positive and negative affect were also assessed at baseline (−10 min), after delivery of each speech (+10 and +20 min) and at the end of the testing day following 30 min of relaxation (+60 min) (al'Absi et al., 2003). Positive affect items included cheerfulness, contentment, calmness, controllability and interest. Negative affect items included anxiety, irritability, impatience and restlessness. Items were assessed using a seven-point Likert scale (Not at All (0) to Very Strong (7)).
Smoking Characteristics
The FTND (Heatherton et al., 1991) and the SCQ (Copeland et al., 1995) are two widely used pencil/paper measures in nicotine research. The FTND measures nicotine dependence severity using items such as number of cigarettes smoked per day, and latency between waking up and smoking (Heatherton et al., 1991). The SCQ measures smoking expectancies, organizing items into 10 different subscales including craving/addiction and negative affect reduction (Copeland et al., 1995).
Cardiovascular measures
A ReliOn® blood pressure monitor (Omron Healthcare, Inc. Bannockburn, IL, USA) was used to assess blood pressure and heart rate at baseline (−10 min), after delivery of each speech (+10 and +20 min) and at the end of the testing day following 30 min of relaxation (+60 min).
Saliva collection
Four saliva samples were taken across the laboratory study day. Thus, saliva samples were taken to: measure cortisol prior to (a) transdermal nicotine patch administration following 30 min of relaxation (∼7:30 a.m.), (b) following lunch (baseline; −10 min from stress onset), (c) after speech delivery (+20 min from stress onset) and (d) at the conclusion of the testing day following 30 min of relaxation (+60 min from stress onset). The stressor was administered at ∼1:00 p.m., 2 h following lunch. Saliva collection at these time points was used in prior studies of substance-dependent individuals and is sensitive to stress system response changes (Lovallo et al., 2000). Although psychosocial stress protocols are typically administered in the morning, Kudielka and colleagues reported that sufficiency elevated salivary cortisol levels could be detected following either morning or afternoon administration of the Trier Social Stress Test, which includes a public-speaking component similar to the current protocol (Kudielka et al., 2004, 2009). Because the stress system is sensitive to metabolic changes, lunch, controlled for sugar, fat and calories (Healthy Choice Meals™), was provided. Saliva was collected using a commercially available kit containing an oral swab (Salimetrics, LLC). Following saliva collection, samples were placed on ice. Samples were centrifuged for 10 min at 3000 r.p.m. and aliquots were pipetted and frozen at −70°C. The concentration of cortisol in saliva was determined by liquid chromatography tandem mass spectrometry using positive electrospray ionization in the selected reaction monitoring mode. The liquid chromatography, tandem mass spectrometry system consisted of a Surveyor HPLC autosampler, Surveyor MS quaternary pump and a TSQ Quantum Discovery triple quadrupole mass spectrometer (ThermoFinnigan, San Jose, CA, USA).
Data analysis
Statistical analyses were completed using SAS Version 9.1 (SAS Institute, 2006).
Data analyses were designed to address the questions of interest concerning gender and nicotine effects on stress responsivity. Gender was retained as a grouping variable due to potential differences in stress reactivity (Kudielka and Kirschbaum, 2005) and response to transdermal nicotine (Perkins, 1996). Demographic data were assessed by a three transdermal patch (placebo, low dose and high dose) by two gender (male, female) analysis of variance (ANOVA). Baseline and stress reactivity data including subjective report, salivary cortisol and cardiovascular measures were analyzed by a three transdermal patch (placebo, low dose and high dose) by two gender (male, female) ANOVAs. Additionally, to assess change across time, stress reactivity data including subjective report and cardiovascular measures were analyzed by a three transdermal patch (placebo, low dose and high dose) by two gender (male, female) repeated measures ANOVA for −10, +10, +20 and +60 min time points. Salivary cortisol data were log transformed to achieve normality and subjected to a three transdermal patch (placebo, low dose and high dose) by two gender (male, female) repeated measures ANOVA for the −10, +20 and +60 min time points. As initial analyses indicated that subgroup differences were not significant, data were collapsed across substance-dependent subgroup. However, for comparison, significance values from critical analyses are presented in text.
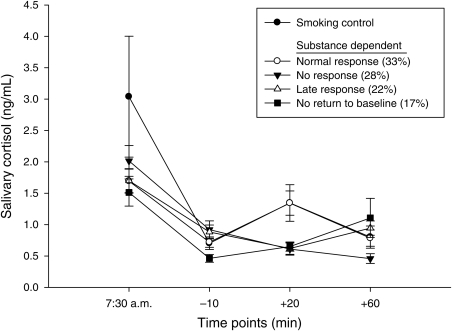
Because analyses showed variability in the salivary cortisol level response to the psychosocial stressor (Fig. 2), a follow-up analysis was conducted to separate subjects who responded to the psychosocial stressor from those who were nonresponsive (Buchanan, 2006; Buchanan and Tranel, 2008; Pruessner et al., 1997; Wust et al., 2000; Schoofs and Wolf, 2009). This data analysis technique is used more commonly in community controls, as though it was applied to one study in substance-dependent individuals (Sinha et al., 2006). Four groups were defined according to the following rules: (a) ‘Normal’ response: −10 < +20 > +60; (b) No response: −10 > +20 > +60; (c) Late response −10 > +20 < +60; and (d) No return to baseline −10 < +20 < +60. A multivariate ANOVA (MANOVA) was used to demonstrate that the four profile groups created described statistically distinct patterns (see Results). A comparison group of smoking community controls all of whom received nicotine was also included as part of the profile analysis (n = 7). Using the same rules stated above, all subjects in the control group were identified as ‘normal’ responders. The controls were originally included to ensure that the psychosocial stressor was effective.
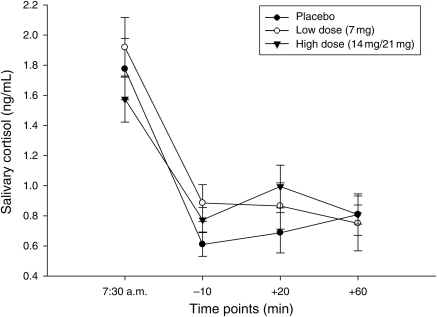
Fig. 2.
Salivary cortisol levels following the psychosocial stressor in substance-dependent individuals. Participants who received the high dose of the transdermal nicotine patch had a trend toward higher salivary cortisol levels following the psychosocial stressor than those who received the placebo transdermal patch (P = 0.08).
For all ANOVA analyses, simple effects analyses were used to determine significant differences when they occurred. Type III Sum of Squares F statistics are reported to account for unequal sample sizes. Differences in degrees of freedom indicate missing data.
RESULTS
Demographics
Demographic and tobacco-smoking characteristics for transdermal patch dose and gender are presented for substance-dependent individuals in Tables 1 and 2. Results showed that transdermal patch groups were equated on age, years of education and verbal and abstracting ages for men and women. Analyses also showed that substance-dependent subgroups did not differ in demographic characteristics (P > 0.28), except the quantity and frequency of alcohol use, as expected (P < 0.001). However, depressive symptoms assessed prior to patch application on the laboratory study day were different between transdermal patch groups ([F(2,44) = 6.44, P = 0.004]. For depressive symptoms, posthoc tests showed that individuals in the low-dose group had higher scores than the other groups (P < 0.05). Importantly, depressive symptoms did not correlate with salivary cortisol levels at any time point assessed (P > 0.19), and scores were in the mild, nonclinically significant range (Beck et al., 1996). Depressive symptoms also differed between substance-dependent subgroups ([F(2,41) = 8.00, P < 0.001]. Posthoc analyses showed that ALCs had greater depressive symptomatology than all other substance-dependent subgroups (P's < 0.05). Transdermal patch groups and substance-dependent subgroups were similar in all smoking characteristics assessed including smoking chronicity, number of cigarettes smoked per day, baseline CO readings and nicotine dependence (as measured by the FTND; P > 0.13).
Table 1.
Demographic characteristics of substance-dependent individuals by nicotine dose and gender
| Placebo |
Low dose |
High dose |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Variable | (n = 9) | (n = 6) | (n = 11) | (n = 5) | (n = 11) | (n = 9) |
| Age (years) | 39.89 (8.36) | 38.00 (11.05) | 41.64 (8.95) | 42.60 (8.91) | 35.91 (6.52) | 38.00 (7.57) |
| Education (years) | 11.44 (1.01) | 12.83 (1.47) | 12.64 (2.38) | 13.00 (2.45) | 12.55 (1.58) | 13.11 (1.90) |
| Depression symptoms (test)a | 3.38 (2.77) | 8.00 (10.06) | 10.55 (5.20) | 15.80 (6.14) | 7.73 (4.43) | 6.78 (6.36) |
| Anxiety symptoms (prior to task)b | 43.00 (6.41) | 49.00 (3.52) | 51.91 (6.59) | 55.80 (10.76) | 51.00 (7.99) | 49.00 (7.63) |
| Verbal age | 15.26 (0.82) | 16.57 (2.35) | 19.83 (6.88) | 16.42 (2.03) | 16.73 (2.11) | 16.11 (1.59) |
| Abstraction age | 13.48 (1.97) | 14.47 (2.19) | 14.77 (2.82) | 14.14 (1.96) | 15.39 (1.79) | 15.38 (2.56) |
| Quantity frequency index (alcohol) | 8.81 (9.88) | 3.12 (5.02) | 10.42 (8.40) | 9.23 (4.74) | 9.26 (6.78) | 9.61 (9.24) |
| Sobriety (days) | 51.00 (22.28) | 35.83 (17.97) | 37.45 (13.29) | 26.00 (5.10) | 37.63 (15.53) | 42.89 (14.84) |
All values are mean (SD).
Depression symptoms, Beck Depression Inventory (Beck et al., 1996); Anxiety symptoms, Spielberger State Anxiety Inventory (Spielberger, 1983); Verbal and abstracting age, Shipley Institute of Living Scale (SILS-V, SILS-A: Zachary, 1986); Quantity frequency index (QFI; Cahalan, 1969).
aDifferences between low dose and other groups significant [F(2,44) = 6.44, P = 0.004].
bDifferences between placebo and low dose significant [F(2,47) = 3.96, P = 0.03].
Table 2.
Tobacco smoking characteristics of substance dependent individuals by nicotine dose
| Placebo |
Low dose |
High dose |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Variable | (n = 9) | (n = 6) | (n = 11) | (n = 5) | (n = 11) | (n = 9) |
| Smoking Chronicity (years) | 24.11 (9.08) | 20.00 (12.41) | 24.18 (11.45) | 22.60 (7.50) | 19.82 (6.56) | 22.00 (9.30) |
| Average cigarettes (per day) | 18.56 (9.36) | 17.00 (11.92) | 19.09 (5.84) | 21.60 (13.52) | 17.55 (4.48) | 17.33 (8.53) |
| Carbon monoxide (screen) | 17.44 (7.11) | 13.33 (8.73) | 21.36 (10.13) | 21.20 (15.74) | 18.18 (6.69) | 22.89 (10.55) |
| Carbon monoxide (test) | 8.22 (2.99) | 6.33 (3.50) | 9.30 (3.74) | 7.00 (3.61) | 8.91 (3.05) | 9.33 (5.48) |
| Fagerström (total score) | 3.44 (2.60) | 4.67 (1.21) | 5.00 (1.73) | 4.50 (2.38) | 4.18 (1.72) | 4.44 (2.24) |
| Smoking consequences (total score) | 43.82 (14.65) | 43.19 (9.28) | 52.59 (11.82) | 47.39 (2.57) | 45.43 (8.20) | 52.70 (9.86) |
| Withdrawal symptoms (prior to task) | 5.11 (3.66) | 3.33 (2.25) | 7.55 (5.30) | 6.75 (4.11) | 5.55 (2.94) | 4.78 (4.27) |
All values are mean (SD).
Fagerström, Fagerström Test for Nicotine Dependence (Heatherton et al., 1991); Smoking consequences, Smoking Consequences Questionnaire (Copeland et al., 1995); Withdrawal symptoms, Withdrawal Symptoms Checklist (Hughes and Hatsukami, 1986).
*Differences between doses nonsignificant.
Baseline measures
Subjective reports
Baseline (−10 min) measures of self-reported positive and negative affect, nicotine withdrawal symptoms and anxiety were assessed for transdermal patch (placebo, low dose or high dose), Gender or the Transdermal patch × Gender interaction. These results showed that measures of baseline positive and negative affect did not differ by transdermal patch, Gender or the Gender × Transdermal interaction (P's > 0.13). Similarly, nicotine withdrawal baseline scores also did not differ by transdermal patch, Gender or the Gender × Transdermal interaction (P > 0.10). However, subjects who received the low dose reported greater baseline anxiety symptoms when compared with those who received placebo (transdermal patch [F(2,44) = 3.96, P = 0.03]), posthoc tests (P < 0.05). Baseline anxiety symptoms for those who received the high dose did not differ from placebo (P > 0.05; see Table 1 for the means). Baseline measures of self-reported positive and negative affect, nicotine withdrawal symptoms and anxious or depressive symptomatology did not correlate with baseline or poststress salivary cortisol levels (P's > 0.09).
Salivary cortisol
Following randomized transdermal patch administration (placebo, low dose or high dose), baseline salivary cortisol levels were assessed in subjects for Transdermal patch, Gender and the Gender × Transdermal patch interaction effects. Results showed that log transformed baseline cortisol levels did not differ by Transdermal patch (P > 0.22), Gender (P > 0.96) or the interaction of Transdermal patch × Gender (P > 0.97).
Cardiovascular measures
Following randomized transdermal patch administration (placebo, low dose or high dose), baseline cardiovascular measures (systolic and diastolic blood pressure, heart rate) were assessed in subjects for Transdermal patch, Gender or the Transdermal patch × Gender interaction. A main effect of gender was noted for systolic blood pressure (gender [F(1,51) = 9.63, P = 0.003]), where male subjects had significantly higher values than female subjects. Diastolic blood pressure or heart rate was not significant for Transdermal patch, Gender or the Transdermal patch × Gender interaction in subjects (P > 0.10).
Stress responsivity
Subjective reports
Measures of self-reported positive and negative affect were assessed following each speech topic (+10, +20 min.), and the conclusion of the testing day following 30 min of relaxation (+60 min). Separate ANOVAs for the +10 and +20 min time points were used to assess differences in subjective reporting for transdermal patch (placebo, low dose or high dose), Gender or the Transdermal patch × Gender interaction. For positive affect, results showed an effect of the transdermal patch for both the +10 and +20 min time points ([F(2,36) = 6.73, P = 0.003]; [F(2,39) = 4.39, P = 0.019]; Fig. 1A). Posthoc tests showed that those in the placebo group had greater positive affect at the +10 and +20 min time points than either group that received nicotine ([F(1,25) = 13.29, P = 0.001]; [F(1,27) = 4.26, P = 0.049]; [F(1,23) = 4.74, P = 0.040]; [F(1,26) = 9.29, P = 0.005]). Further, paired t-tests conducted for each transdermal patch separately showed that while those who received the high dose of nicotine had significant changes from baseline for both the +10 and +20 min time points demonstrating less positive affect ([t(18) = 3.74, P = 0.002]; [t(18) = 2.04, P = 0.05]), those who received the low dose or placebo did not show a change from baseline following the psychosocial stressor (P > 0.25). Further, the transdermal patch × Gender interaction at the +10 min time point was at a trend level [F(2,36) = 2.96, P = 0.065] and posthoc analyses showed that the men carried this effect [F(2,29) = 5.16, P = 0.01, follow-up P's < 0.05].
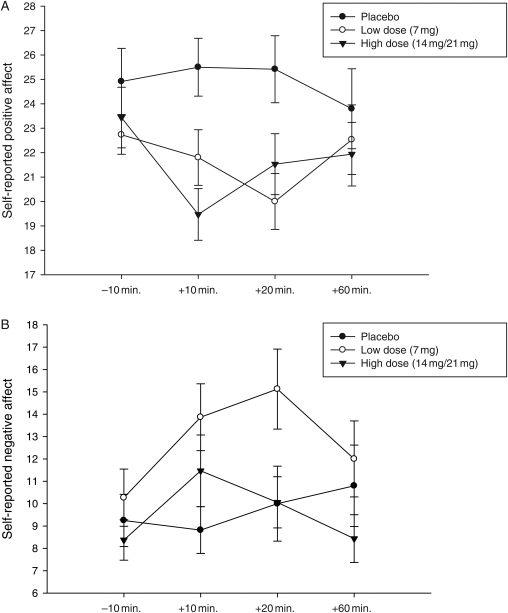
Fig. 1.
(A) Self-reported positive affect following the psychosocial stressor in substance-dependent individuals. Participants who received the placebo transdermal patch reported greater positive affect at the +10 and +20 min time points than either group that received nicotine (P < 0.049). (B) Self-reported negative affect following the psychosocial stressor in substance-dependent individuals. Participants who received the low dose of the transdermal nicotine patch reported greater negative affect at the +20 min time point than all other transdermal groups (P < 0.05).
For negative affect, results also showed an effect of the transdermal patch at the +20 min time point ([F(2,39) = 5.05, P = 0.011]), with posthoc tests showing that those in the low-dose group had greater negative affect than all other groups [F(1,31) = 5.84, P = 0.020]; [F(1,26) = 4.11, P = 0.050; Fig. 1B). Further, paired t-tests conducted for each transdermal patch separately showed that while those who received nicotine had significant changes from baseline for both the +10 and +20 min time points demonstrating greater negative affect (low dose [t(17) = 2.89, P = 0.01]; [t(18) = 2.86, P = 0.01]; high dose [t(19) = 3.11, P = 0.006]; [t(18) = 2.60, P = 0.01]), those who received placebo did not show a change from baseline following the psychosocial stressor (P > 0.71). The Gender Transdermal patch × Gender interactions were nonsignificant (P > 0.10).
To assess change over time, positive and negative affect were compared across baseline (−10 min.), and +10, +20 and +60 time points. Consistent within task objectives, the repeated measures ANOVA revealed that positive and negative affect changed over time ([F(3,87) = 2.90, P = 0.040]; [F(3,87) = 3.65, P = 0.021]) with subjects reporting more positive and fewer negative responses at the baseline (−10 min) than at the +10 and +20 time points, respectively (Positive [t(39) = 2.37, P = 0.022]; [t(41) = 2.39, P = 0.022]) (Negative [t(39) = −3.35, P = 0.0018]; [t(41) = −4.26, P = 0.0001]).
Nicotine withdrawal symptoms were assessed at the +60 time point and did not differ between transdermal patch doses [F(2,47) = 1.22, P = 0.31]. Anxiety symptoms were not measured at this time point.
Salivary cortisol
Salivary cortisol levels were assessed following the psychosocial stressor (+20 min.) and the conclusion of the testing day following 30 min of relaxation (+60 min). Log transformed data subjected to repeated measures ANOVA did not differ across time by Gender, Transdermal patch or the Gender × Transdermal patch interaction (P > 0.184). A comparison of the salivary cortisol levels in individuals who received the high dose and placebo was conducted at the +20 min time point. This analysis was at a trend level [t(30), = −1.81, P = 0.08], with salivary cortisol levels slightly higher in the high-dose group than in the placebo group (Fig. 2). Similar data analysis techniques produced nonsignificant findings for the Gender and Gender × Transdermal patch interactions (P > 0.21). To assess change over time, salivary cortisol levels were compared across baseline (−10 min.), and +20 and +60 time points. Overall, these analyses revealed that salivary cortisol levels did not change over time, demonstrating the blunted salivary cortisol effect [F(2,84) = 0.32, P = 0.73].
Analyses were also conducted to assess differences in salivary cortisol levels between substance-dependent subgroups and potential interaction with the transdermal patch following the psychosocial stressor (+20 min.), and the conclusion of the testing day following 30 min of relaxation (+60 min). Comparisons were conducted with a repeated measures ANOVA. Analyses showed that salivary cortisol levels did not differ across time by Substance-Dependent Subgroup, Transdermal patch or the Substance-Dependent Subgroup × Transdermal patch interaction (P's > 0.112). Additionally, a comparison of the salivary cortisol levels in individuals who received the high dose and placebo was conducted at the +20 min time point to assess the Substance-Dependent Subgroup × Transdermal patch interaction which was nonsignificant (P > 0.54).
Cardiovascular measures
Diastolic blood pressure and heart rate were assessed following both speech topics (+10, +20 min.), and the conclusion of the testing day following 30 min of relaxation (+60 min). To assess change over time, cardiovascular measures were compared at baseline (−10 min), +10, +20 and +60 time points. These analyses showed that systolic blood pressure changed over time [F(3,111) = 17.94, P < 0.0001]. Subsequent analyses showed that systolic blood pressure was higher following both the first and second speeches for all subjects than at baseline (−10 min) or at the conclusion of the testing day (+60 min) ([t(46) = −6.15, P < 0.0001; t(47) = −4.90, P < 0.0001]; t(45) = 4.55, P < 0.0001; t(45) = 4.17, P < 0.0001]; Fig. 3A).
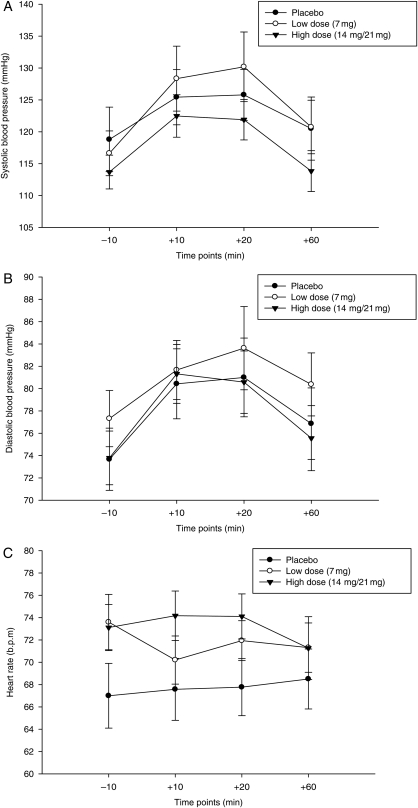
Fig. 3.
(A) Systolic blood pressure following the psychosocial stressor in substance-dependent individuals. Systolic blood pressure was higher following the delivery of the first and second speeches for all substance-dependent individuals than at baseline, or at the conclusion of the testing day (P < 0.0001). (B) Diastolic blood pressure following the psychosocial stressor in substance-dependent individuals. Diastolic blood pressure was higher following the delivery of the first and second speeches for all substance-dependent individuals than at baseline, or at the conclusion of the testing day (P < 0.01). (C) Heart rate following the psychosocial stressor in substance-dependent individuals. Participants who received the high dose of the transdermal nicotine patch had higher heart rates at baseline and following the delivery of both speeches than all other transdermal patch groups (P < 0.05).
Diastolic blood pressure also changed over time [F(3,108) = 9.80, P < 0.0001] with levels being higher following both the first and second speeches than that at baseline (−10 min.) and the conclusion of the testing day following 30 min of relaxation (+60 min) [t(46) = −7.49, P < 0.0001; t(46) = −4.45, P < 0.0001]; t(44) = 2.67, P < 0.01; t(44) = 2.92, P < 0.005]; Fig. 3B).
For heart rate, repeated measures analyses revealed a significant time × transdermal patch interaction [F(6,99) = 3.08, P = 0.008]. Separate ANOVAs conducted for each time point revealed that the high dose had higher heart rates than placebo at baseline (−10 min.), +10 and +20 time points ([F(2,45) = 16.39, P < 0.0001]; [F(2,44) = 13.98, P < 0.0001]; [F(2,45) = 12.29, P < 0.0001]), posthoc tests (P < 0.05). This difference was nonsignificant at the +60 min. time point [P > 0.69; Fig. 3C).
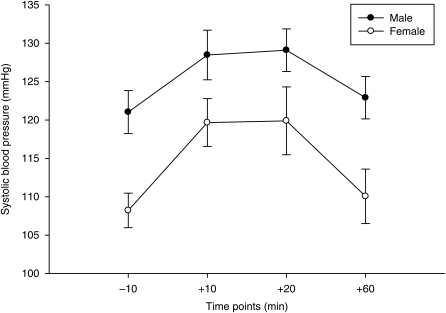
A separate ANOVA for the +60 min time point was also used to assess differences in cardiovascular measures for transdermal patch (placebo, low dose or high dose), Gender or the Transdermal patch × Gender interaction. This data analysis strategy was deemed appropriate due to the obvious differences in the plotted data following visual inspection at the +60 min time point. These analyses revealed that men had higher systolic blood pressure at baseline (see baseline analyses above) and at the +60 min time point [F(1,42) = 6.96, P = 0.011], but that these normal differences between genders disappeared during the psychosocial stressor (P > 0.116; Fig. 4). Similar data analysis techniques produced nonsignificant results for diastolic blood pressure and heart rate.
Fig. 4.
Gender similarities in systolic blood pressure following the psychosocial stressor in substance-dependent individuals. Although men displayed the normal gender difference of higher systolic blood pressure at baseline and at the +60 (P < 0.015), these differences were non-significant following the psychosocial stressor (P > 0.116).
Salivary cortisol profile analysis
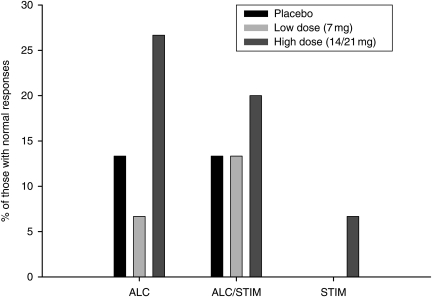
A profile analysis was conducted to describe the heterogeneity in salivary cortisol response to the psychosocial stressor (Fig. 5). Data were collapsed across gender and transdermal patch for these analyses, as differences between groups were nonsignificant for salivary cortisol levels (see salivary cortisol section above). Following group profiling, MANOVA results indicated that the four salivary cortisol response patterns identified were statistically distinct [F(9,95) = 15.20, P < 0.0001]. Frequencies of profile analysis groups showed that 33% of subjects had a ‘Normal’ response to the psychosocial stressor, followed by No response (28%), Late response (22%) and No return to baseline (17%). Participants in the ‘normal’ response group did not differ from smoking controls at the −10, +20 or +60 time points (P > 0.96) and displayed higher salivary cortisol levels than any other profile group following the +20 time point [F(3,42) = 7.26, P = 0.0005], posthoc (P < 0.05). Participants in the ‘normal’ response group were also more likely to be alcohol or alcohol and stimulant dependent [χ2(2) = 32, P < 0.0001], have received an active nicotine transdermal patch versus placebo [χ2(2) = 18.66, P < 0.0001], (see Fig. 6) and be of either gender [χ2(1) = 0.02, P > 0.89]. Profile groups did not differ in the number of days of sobriety, nicotine withdrawal levels or anxiety symptoms at the time of the psychosocial stressor, or depressive symptoms on the laboratory study day (P > 0.25).
Fig. 5.
Salivary cortisol levels in substance-dependent individuals and smoking controls following profile analysis. Following group profiling, results indicated that the four salivary cortisol response patterns identified were statistically distinct (P < 0.0001). Substance-dependent individuals in the ‘normal’ response group (33% of the sample) did not differ from smoking controls at any time point (P > 0.96). Further, those in the ‘normal’ response group differed from all other groups at the +20 min time point (P = 0.0005).
Fig. 6.
Percentage of substance-dependent individuals in the ‘normal’ response group by group and transdermal nicotine dose. Responders were more likely to be alcohol dependent and to have received the high dose of nicotine [χ2(2) = 32, P < 0.0001], [χ2(2) = 18.66, P < 0.0001].
DISCUSSION
Prior research indicates that alcohol-dependent individuals demonstrate a blunted stress system response following an acute psychosocial stressor (Adinoff et al., 2005a; Lovallo et al., 2000). However, variability in stress reactivity in this population and individual factors, such as nicotine use, that may contribute to this variability have not been systematically examined. The current study was designed to assess the role of nicotine on subjective, cardiovascular and salivary cortisol response to a psychosocial stressor. To that end, subjects received either a placebo, low dose (7 mg) or high dose (14 mg for women, 21 mg for men) and performed a psychosocial stress protocol consisting of two 4-minute speeches (al'Absi et al., 1997). Self-reported measures of nicotine dependence and withdrawal were also completed concurrently with the stressor.
Consistent with previous work (Bernardy et al., 1996; Junghanns et al., 2003; Lovallo et al., 2000), substance-dependent individuals failed to demonstrate an increase in salivary cortisol to the psychosocial stressor although they reported elevated cardiovascular measures and self-reported distress. This disconnection between ‘blunted’ cortisol levels and ‘elevated’ self-reported distress and cardiovascular measures following a psychosocial stressor are similar to those of Errico et al. (1993). The study of Errico et al. (1993) showed that while alcoholics displayed blunted cortisol levels following a psychosocial stressor, their self-reported distress and cardiovascular measures were equivalent to those of community controls.
Comparisons in the current study also extended these findings of Errico et al. (1993). Specifically, substance-dependent individuals who received nicotine reported less positive affect (high dose) and more negative affect (high and low doses) following the psychosocial stressor when compared with those who received placebo. While an explanation for these findings is not immediately apparent, it could be suggested that higher level brain areas involved in appraisal and emotional response to the stressor may be activated by acute nicotine administration (Panknin et al., 2002). Thus, greater negative affect following the psychosocial stressor may reflect a normalization of the poststress emotional response as negative emotional responses are blunted in alcohol-dependent individuals (Salloum et al., 2007). Why nicotine had similar normalization in salivary cortisol levels for only a minority of substance-dependent individuals, as discussed below, remains an empirical question.
Also intriguing is the finding that substance-dependent individuals who received placebo demonstrated few signs of acute nicotine withdrawal. For instance, self-reported withdrawal symptoms scores ranged from 3 to 5 (out of a possible score of 33) in those who received placebo and did not differ from those who received the active dose either at baseline, or following the psychosocial stressor. Similarly, baseline physiological measures taken just prior to the stressor did not differ between groups. Self-reported anxiety symptoms were also below the range of clinical significance, although slight group differences did exist.
Several explanations exist for the absence of nicotine withdrawal symptomatology in these nicotine-dependent subjects. First, the time course may not have been sufficient to allow for the development of a nicotine withdrawal syndrome (Hughes, 2007; Shiffman et al., 2006). Subjects in the current study abstained from nicotine for less than 24 h, which may be too limited a time period for subjects to experience nicotine withdrawal symptoms, which peak in 2–5 days following cessation (Hughes, 2007; Shiffman et al., 2006). Second, the laboratory setting may be devoid of environmental contexts that prompt cigarette craving (Conklin, 2006; Van Gucht et al., 2010). Although the association between environmental contexts, cigarette craving and onset of nicotine withdrawal symptoms is unclear, the literature supports that these constructs may be related (Conklin, 2006; Shiffman and Paty, 2006; Van Gucht et al., 2010). Third, the task demands may have cognitively distracted subjects from physiological withdrawal symptoms. Although untested, this phenomenon has much anecdotal support. Arguably distinct, physical exercise also promotes reduced withdrawal symptoms, suggesting that withdrawal symptoms are not ubiquitous following smoking cessation (Taylor et al., 2007). It is possible that cognitive distraction produces a similar outcome, as may be the case in the current study. Regardless of the underlying mechanism, the similarity of nicotine withdrawal symptomatology in active and placebo groups suggests that the nicotine results discussed are not dependent upon this factor.
Using standard statistical techniques, it appears that nicotine had no effect on cortisol activation following a psychosocial stressor. However, distribution data suggested the need for focused analyses. First, the individuals who received a high dose of nicotine were compared with those who received placebo following the psychosocial stressor (+20 min). Differences between these two doses produced a statistical trend at P = 0.08, where salivary cortisol levels were higher in those who received nicotine compared with placebo. Second, a profile analysis was used to characterize distribution data (Tabachnick and Fidell, 1989, p.453). Through this technique, four groups of salivary cortisol responses were identified. In contrast to the overall findings based on mean comparisons, profile analyses showed that 33% of the substance-dependent individuals responded to the stressor, having significantly higher cortisol levels than at baseline. This group of responders displayed similar salivary cortisol level responses as nicotine-dependent community controls, were more likely to be alcohol dependent and had received the high dose of nicotine in the current study. Thus, in spite of the many drug, subgroup similarities in baseline and stress responsivity measures, it is notable that the alcohol-dependent subjects are most sensitive to nicotine effects in the current study, albeit in the substantial minority of subjects who responded to the psychosocial stressor. Alcohol-dependent individuals have also been shown to be differentially sensitive to the neurocognitive enhancing properties of transdermal nicotine administration (see Ceballos et al., 2005 and Nixon et al., 2007 for further discussion).
The mechanism of action of nicotine's effects on salivary cortisol levels in alcohol-dependent individuals could be attributed to either peripheral or centralized factors. While nicotinic receptors are abundant in the adrenal gland suggesting a peripheral mechanism (Bornstein and Chrousos, 1999; Tsigos and Chrousos, 2002), the cognitive appraisal of the psychological threat during the psychosocial stress protocol also suggests centralized mechanisms. The concept of allostasis, or ‘maintenance of stability outside the normal homeostatic range’ (Koob and Le Moal, 2001), may describe the stress system dysregulation demonstrated by blunted salivary cortisol levels and subsequent possible amelioration by nicotine. These findings strengthen the conclusion regarding individual variability in the integrity of the stress system and its potential susceptibility to adaptive deficits in homeostatic load accommodation (allostatis).
The limitations of the current study leave several issues to be addressed in future research. First, logistical considerations limited the study design to a between-subjects investigation; that is, subjects who received placebo patches were compared with a second group who received nicotine. Results of the current study would be strengthened if they were replicated by future studies including a within-subjects design. Additionally, although saliva collection is a widely accepted methodology for measurement of cortisol levels, future studies addressing the heterogeneity of cortisol levels in substance-dependent individuals should include measures of both plasma and salivary cortisol. Lastly, while the community controls provided an important comparison group in the current study, future studies would be strengthened by inclusion of a greater number of subjects in this group.
CONCLUSIONS
In conclusion, these results indicate that while overall analyses may reveal blunted cortisol responses in treatment-seeking individuals following a psychosocial stressor, individual differences in stress reactivity in this population, particularly in alcohol-dependent subjects, may be an important area of future research. Given the role of stress reactivity in relapse and recovery, these findings have important implications not only for basic science but also have a clinical application for relapse prevention training in drug and alcohol treatment facilities. They further have implications for the benefit of nicotine replacement therapy in substance-dependent individuals enrolled in smoking cessation programs.
Funding
Funding for this study was provided by the National Institutes of Health NIDA and the McKnight Brain Institute at the University of Florida. These funding bodies had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or the decision to submit the paper for publication. Support was provided in part by grants R01 DA-13677 (Nixon) and a dissertation award from the McKnight Brain Institute (Gilbertson).
Acknowledgements
We wish to thank the staff at the Department of Pharmacy Practice, Center for Pharmacogenomics and Department of Psychiatry Neurocognitive Laboratory at the University of Florida for their assistance in completing this study.
Conflict of interest statement. None declared.
REFERENCES
- Adinoff B, Iranmanesh A, Veldhuis J, et al. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, et al. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005a;29:517–27. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, et al. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005b;29:528–37. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Bongard S, Buchanan T, et al. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, et al. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory, 2nd edn. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Bernardy NC, King AC, Parsons OA, et al. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13:493–8. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Chrousos GP. Clinical review 104: adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–36. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol Learn Mem. 2008;89:134–41. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learn Mem. 2006;13:382–7. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Hesselbrock VM, Heath AC, et al. A latent class analysis of antisocial personality disorder symptom data from a multi-centre family study of alcoholism. Addiction. 2000;95:553–67. doi: 10.1046/j.1360-0443.2000.9545537.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cissin L, Crossley H. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. American Drinking Practices: A National Study of Drinking Behaviors and Attitudes (Monograph No. 6) [Google Scholar]
- Ceballos NA. Tobacco use, alcohol dependence, and cognitive performance. J Gen Psychol. 2006a;133:375–88. doi: 10.3200/GENP.133.4.375-388. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, al'Absi M. Dehydroepiandrosterone sulfate, cortisol, mood state and smoking cessation: relationship to relapse status at 4-week follow-up. Pharmacol Biochem Behav. 2006b;85:23–8. doi: 10.1016/j.pbb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Tivis R, Lawton-Craddock A, et al. Visual-spatial attention in alcoholics and illicit stimulant abusers: effects of nicotine replacement. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:97–107. doi: 10.1016/j.pnpbp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Coiro V, Vescovi PP. Effect of cigarette smoking on ACTH/cortisol secretion in alcoholic after short- and medium-term abstinence. Alcohol Clin Exp Res. 1999;23:1515–8. [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–9. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Na P, et al. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiatry. 2003;54:873–8. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire—Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol Assess. 1995;7:484–94. [Google Scholar]
- Errico AL, Parsons OA, King AC, et al. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–8. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Gorsline J, Gupta SK, Dye D, et al. Steady-state pharmacokinetics and dose relationship of nicotine delivered from Nicoderm (Nicotine Transdermal System) J Clin Pharmacol. 1993;33:161–8. doi: 10.1002/j.1552-4604.1993.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Harris DS, Reus VI, Wolkowitz OM, et al. Repeated psychological stress testing in stimulant-dependent patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:669–77. doi: 10.1016/j.pnpbp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–27. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–5. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, et al. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol. 2001;6:247–56. doi: 10.1080/13556210120056580. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, et al. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–92. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, et al. Smoking kills (alcoholics)! shouldn't we do something about it? Alcohol Alcohol. 2007;42:167–73. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, et al. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–8. [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, et al. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–7. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, et al. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–63. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Lawton-Craddock A, Tivis R, et al. Nicotine's effects on attentional efficiency in alcoholics. Alcohol Clin Exp Res. 2007;31:2083–91. doi: 10.1111/j.1530-0277.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- Panknin TL, Dickensheets SL, Nixon SJ, et al. Attenuated heart rate responses to public speaking in individuals with alcohol dependence. Alcohol Clin Exp Res. 2002;26:841–7. [PubMed] [Google Scholar]
- Perkins KA. Sex differences in nicotine versus nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996;4:166–77. [Google Scholar]
- Pomerleau OF, Fertig JB, Shanahan SO. Nicotine dependence in cigarette smoking: an empirically-based, multivariate model. Pharmacol Biochem Behav. 1983;19:291–9. doi: 10.1016/0091-3057(83)90055-2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, et al. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22:615–25. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Bucholz KK, et al. The Diagnostic Interview Schedule, Version IV. St. Louis: Washington University; 1995. [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, et al. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res. 2007;31:1490–504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Wolf OT. Stress and memory retrieval in women: No strong impairing effect during the luteal phase. Behav Neurosci. 2009;123:547–54. doi: 10.1037/a0015625. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychol. 2006;115:509–23. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten C, Gwaltney C, et al. Natural history of nicotine withdrawal. Addiction. 2006;101:1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, et al. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, et al. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 2nd edn. New York: HarperCollins Publishers, Inc; 1989. [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–43. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Van den Bergh O, Beckers T, et al. Smoking behavior in context: where and when do people smoke? J Behav Ther Exp Psychiatry. 2010;41:172–7. doi: 10.1016/j.jbtep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, et al. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 1982;78:305–8. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- Winternitz WW, Quillen D. Acute hormonal response to cigarette smoking. J Clin Pharmacol. 1977;17:389–97. doi: 10.1002/j.1552-4604.1977.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, et al. The cortisol awakening response—normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Wust S, Van Rossum EF, Federenko IS, et al. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565–73. doi: 10.1210/jc.2003-031148. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised. Los Angeles: Western Psychological Services; 1986. [Google Scholar]