Abstract
Aims: Previous studies show that alcohol exposure can affect the differentiation of progenitor B cells. Before final commitment to a B lineage, progenitor B cells usually undergo several important stages. However, it is still unclear whether alcohol alters B cell differentiation at which stages. The aim of this study was to determine which stage(s) of progenitor cell differentiation are affected by alcohol and to elucidate the mechanism(s) responsible for the effect of alcohol on B cell differentiation. Methods: Oligoclonal-neonatal-progenitor (ONP) cells from bone marrow cells of 2-week-old mice were cultured under different conditions in vitro with or without the exposure of 100 mM alcohol. Phenotype analysis was performed at different time points and expression levels of transcription factors (TFs) and cytokine receptors were measured quantitatively and kinetically. Results: After 3 days in vitro culture, ONP cells differentiated into two populations: B220−CD11b− and B220−CD11b+ cells. B220−CD11b− cells can further differentiate into B lineage cells only with the support of B220−CD11b+ cells. Cells exposed to 100 mM of alcohol during the first 3 days of culture showed no statistically significant difference in B cell formation after 12 days compared with the control group. However, cells exposed to alcohol from Day 4 till the end of culture yield very few B cells. Expression levels of TFs and cytokine receptors were down-regulated kinetically among ONP cells co-cultured with the addition of 100 mM alcohol. Conclusions: Alcohol affects the ONP cell differentiation into B lineage at a late stage. Alcohol also down-regulates the expression level of TFs and cytokine receptors resulting in the impairment of B cell differentiation.
INTRODUCTION
Alcohol abuse has a tremendous impact on human health and is associated with increased morbidity and mortality (Anonymous, 2000; Blot, 1992; Doll et al., 1994; Driver and Swann, 1987; Fuchs et al., 1995; MacGregor and Louria, 1997; Ringborg, 1998; Szabo, 1999). Chronic alcohol abuse adversely affects the homeostasis of the hematopoietic system causing deregulation of many of the developmental pathways and in some cases lead to cytopenia (Chanarin, 1979, 1982; Cowan, 1980; Dai et al., 2000; Hillman, 1975; Michot and Gut, 1987; Seppa et al., 1991). The deleterious effects of alcohol abuse on hematopoiesis are apparent in both peripheral blood and bone marrow (BM). Chronic abuse of alcohol also leads to alterations in immune regulation that can be manifested as immunodeficiency or autoimmunity (Cook, 1998), with the consequence of increased susceptibility to disease and damage to organ systems, respectively.
The immunologic effects of alcohol abuse tend to be long lived and are not readily reversible by short periods of abstinence. There are many clinical and experimental studies that support the hypothesis that alcohol abuse leads to immunodeficiency in both the innate and adaptive immune systems (Baker and Jerrells, 1993; Cook, 1998; MacGregor, 1986; MacGregor and Louria, 1997; Szabo, 1999). Alcohol can result in the loss of lymphocytes from both primary and secondary lymphoid organs (Sibley et al., 1995). There is a growing awareness, from studies of alcoholic patients, that the regulation of B lymphocytes is particularly sensitive to chronic alcohol exposure (Cook, 1998). The capacity of the bone marrow to supply B lymphocytes far exceeds the number of mature B cells transported to the periphery (Forster and Rajewsky, 1990; Opstelten and Osmond, 1983). Furthermore, suppression of bone marrow B-cell development may not always be immediately apparent in the periphery. For example, in pregnant animals, the increased estrogen causes >90% decline in bone marrow B-cell precursors with no apparent decline in the number of mature peripheral B lymphocytes (Kincade et al., 1994; Kouro et al., 2001; Medina and Kincade, 1994; Medina et al., 2000). Therefore, a possible explanation, among others, for the B lymphopenia observed in alcoholics is, over a period of time alcohol could affect bone marrow B-cell development to an extent that is, eventually, manifested as a decrease in the number of peripheral B lymphocytes.
Recent work from this laboratory has shown that fetal alcohol exposure can affect bone marrow B-cell development (Biber et al., 1998; Moscatello et al., 1999; Reimold et al., 1996; Wolcott et al., 1995). In these studies, we showed that fetal exposure to alcohol impeded neonatal B-cell development in both the spleen and the bone marrow through the first 5–6 weeks of life. These studies have led to the identification of a progenitor cell, the oligoclonal-neonatal-progenitor (ONP), which has the capacity in in vitro cultures to differentiate to both B lymphocytes and myeloid linage cells depending on the growth conditions and cytokines present. When ONP cells are isolated from neonates that were exposed to alcohol in utero, they demonstrate a greatly reduced capacity to respond to interleukin (IL)-7 and commit to the B lineage but have no diminution in the response to the granulocyte–monocyte colony-stimulating factor (GM-CSF) growth factor and commit to the myeloid lineage. Recent studies also showed that ONP cells isolated from normal neonatal mice and cultured in vitro in the presence of alcohol also failed to respond to IL-7 and commit to the B lineage. The results of these studies confirmed that alcohol affected the cell fate decisions of this progenitor cell to commit to the B lineage but not to the myeloid lineage.
Hierarchical expression of transcription factors (TFs) and growth factor receptors serve as important developmental checkpoints in B-cell differentiation. The sequential expression of the TFs PU.1, early B-cell factor (EBF) and the B cell regulator protein (Pax5) and signaling through growth factor receptors, including the tyrosine kinase Flk2/Flt3 and IL-7R, are important steps in the differentiation of progenitor cells to the B lineage (Adams et al., 1992; Busslinger, 2004; DeKoter et al., 2002; Greenbaum and Zhuang, 2002; Medina and Singh, 2005). TFs activate or repress target genes and signaling receptors induce or modify the activities of gene regulatory proteins. Previous studies in this laboratory showed that ONP cells sorted from alcohol-exposed animals failed to up-regulate IL-7Rα and had decreased expression levels of down-stream EBF and Pax5 during in vitro culture under conditions that favored differentiation to the B lineage (Wang et al., 2009). This indicated the impaired commitment of progenitors to the B lineage.
Despite evidence that alcohol affects B-cell differentiation from an ONP, it is unclear whether it alters B-cell development at an early stage, late stage or in both stages The aim of this study was to determine which stage or stages of progenitor cell differentiation are affected by alcohol and to elucidate the mechanism(s) responsible for the effect of alcohol on B-cell differentiation.
MATERIAL AND METHODS
Mice
Six- to 8-week-old male and female C57BL/6J mice were purchased from the Jackson Laboratories (Bar Harbor, ME, USA) and allowed to acclimate for 1 week prior to experimental use. Mice were bred in house and 2-week-old neonatal mice were used in all experimental protocols. All procedures utilizing animals have been reviewed and approved by the Animal Care and Use Committee (Protocol No. H07-008).
Antibodies
The following monoclonal antibodies (mAbs) were purchased from either eBioScience (San Diego, CA, USA) or BD-PharMingen (San Diego, CA, USA): Allophycocyanin (APC)-conjugated anti-B220, Phycoerythrin (PE)-conjugated anti-B220 (Clone RA3-6B2), APC-conjugated anti-CD19 (Clone 6D5), APC-conjugated anti-Gr-1 (Clone RB6-8C5), APC-conjugated anti-CD11b (Mac-1, Clone M1/70), APC-conjugated anti-CD3 (Clone 17A2), APC-conjugated anti-CD4 (Clone GK1.5), APC-conjugated CD8(Clone 53-6.7), APC-conjugated and FITC-conjugated anti-CD11b (Mac-1, Clone M1/70), APC-conjugated anti-Ter-119 (clone Ter-119), APC-conjugated anti-Sca-1 (Ly-6A/E, Clone D7), APC-Cy7-conjugated anti-CD117 (c-Kit, Clone 2B8) and FITC-conjugated anti-CD24 (HSA, clone M1/69). Two isotype controls were purchased from eBioScience: FITC-conjugated Rat IgG2b κ, control for rat IgG2b antibodies and PE-conjugated Rat IgG2a κ, control for rat IgG2a antibodies. Two other isotype controls, APC-Cy7-conjugated Rat IgG2a κ and APC-conjugated Rat IgG 2b κ, were purchased from CalTag (Burlingame, CA, USA). PE-conjugated anti-CD43 (Clone S7) and APC-Cy7-conjugated anti-CD19 (Clone 1D3) were purchased from PharMingen (San Diego, CA, USA). Anti-Fc receptor mAb [anti-crystallizable fragment receptor (FcR), clone 2.4G2] was produced in house from cells purchased from American Type Culture Collection (Rockville, MD, USA).
Cytokines
Recombinant mouse stem cell factor (SCF), mouse Flt-3/Flk-2 ligand (FL), mouse IL-3, mouse granulocyte monocyte colony-stimulating factor (GM-CSF) and mouse IL-7 were purchased from R&D Systems (Minneapolis, MN, USA).
Flow cytometry and cell-sorting strategy
ONP cells were sorted from bone marrow of 2-week-old C57BL/6J mice as previously described (Wang et al., 2006). Analyses of cells were performed on a FACS Vantage SE Turbo Sorter flow cytometer/sorter (Becton Dickinson, San Jose, CA, USA) or a FacsCalibur cytometer (Becton Dickinson), made available through the University Research Core Facility. A lineage cocktail was prepared from a mixture of APC-conjugated antibodies specific for CD-11b(Mac-1), Gr-1, Ter-119, B220, CD19, CD3, CD4, CD8 and Sca-1. Lin−HSAloCD43loSca-1−c-Kit+-stained cells were sorted on FACS Vantage SE Turbo Sorter. Cultured cells were stained using the appropriate mAbs and isotype-matched antibodies were used as negative controls. All samples were treated with an unlabeled anti-FcR (crystallizable fragment receptor) to prevent inappropriate binding of antibodies. Data analysis was accomplished using FlowJo software (TreeStar, San Carlos, CA, USA).
Cell culture
A two-step culture strategy was used to grow the hematopoietic progenitors. Sorted ONP cells were placed into 24-well culture plates Costar, Cambridge, MA, USA containing complete IMDM (Iscove's Modified Dulbecco's Media) supplemented with 10% fetal bovine serum, 10 ng/ml recombinant mouse SCF, 10 ng/ml recombinant mouse FL and 10 ng/ml recombinant mouse IL-3 and cultured for the first 3 days. After 3 days, cells were harvested and stained with B cell surface marker (B220) and myeloid cell surface marker (CD11b). These cultured cells were sorted into different wells based on the positive or negative expression of these surface markers. Cells were then cultured either with or without OP9 stromal cells. An additional 10 ng/ml exogenous IL-7 was added to the culture media, and cells were cultured with OP9 stromal cells for another 9 days.
In a liquid medium culture system, ONP cells cultured for 3 days as mentioned above, without OP9 stromal cells support, were washed and divided into two wells under two different culture conditions. The first set of cultures contained SCF, FL, IL-3 and IL-7 (10 ng/ml) to facilitate cell differentiation to B lineage (thereafter referred to as culture condition 1; Ito et al., 1996), and the second set of cultures contained SCF, FL, IL-3 and GM-CSF (10 ng/ml) to facilitate cell differentiation along the myeloid pathway (referred to as culture condition 2; Kondo et al., 2000). Cells were then grown for another 9 days and fed every 2–3 days.
For single ONP cell culture, a single ONP cell was sorted under 96-well culture plate (Costar) and cultured for 5 days with the presence of SCF, FL and IL-3. After 5 days, cells were splint into two wells and continuously cultured under either culture condition 1 or 2 for another 12 days.
To determine whether alcohol affects the ONP cell at a certain stage during cell differentiation, 100 mM alcohol was added to the growth medium at different time points either temporarily or permanently during the culture.
Quantitative real-time polymerase chain reaction analysis of TFs (Pax5 and EBF) and the cytokine receptor (IL-7Rα)
RNA was prepared from ONP cells using an RNA extraction kit (Qiagen Inc., Valencia, CA, USA). cDNA was generated with the ThermoScript RT-PCR kit (GibcoBRL). Quantitative real-time polymerase chain reaction (RT-PCR) was performed using the Applied Biosystems Model 7700 sequence detection system (Foster City, CA, USA). Primers and probes were designed using Primer Express Software (Applied Biosystems) and were as previously described (Wang et al., 2006). mRNA for the TFs Pax5, EBF and cytokine receptor IL-7Rα was quantitatively measured using Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. The relative expression level of EBF, Pax5 and IL-7Rα versus GAPDH in ONP cells was calculated by determining the ΔCt value for each sample. The ΔCt value = (threshold cycle for the transcriptional factor−the threshold cycle for GAPDH); i.e. the number of additional cycles at which the sample reached the threshold value after the GAPDH threshold value. Therefore, the amount of input RNA for a given sample relative to GAPDH is given by 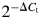 .
.
Statistics
Student t-test and ANOVA of mean values were used in comparison of differences in cell populations between the alcohol and normal control groups and analyzed by a statistical software package (Instat, Graphpad Software, San Diego, CA, USA). Differences between means were considered significant when P < 0.05.
RESULTS
Bipotential characteristic of ONP cells when cultured in vitro
ONP cells can be differentiated to either B cells or myeloid cells with different culture conditions in vitro. As mentioned in MATERIAL AND METHOD section, with two-step culture conditions, after 12 days in culture, ONP cells differentiated into B cells in liquid culture with the presence of SCF, FL, IL-3 and IL-7. However, with the culture condition more favored to myeloid cells, ONP cells generated into a myeloid lineage (Fig. 1A and B).
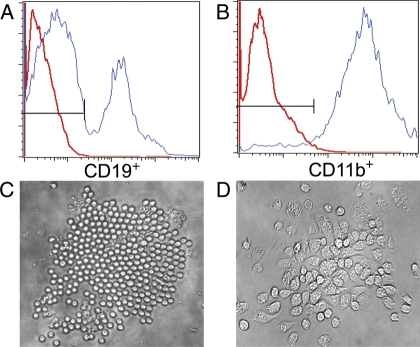
Fig. 1.
ONP cells can differentiate into different lineages in vitro. ONP cells can differentiate into both myeloid and lymphoid lineage. (A) showed ONP cells yielded CD19+ cells after 12 days bulk culture in liquid medium containing SCF, FL, IL-3 and IL-7. However, when cultured in above medium with SCF, FL, IL-3 and GM-CSF, ONP cells yielded only CD11b+ cells (B). (C) and (D) showed the different morphologies of clones derived from a single ONP cell after cultured for 17 days with different culture condition.
To avoid any possible contamination, single ONP cells were also sorted into 96-well culture plates and cultured for 5 days initially and then split into two wells. Cells in one well were cultured under the condition 1 and the other well under the condition 2. Cells were harvested after another 12 days in culture and observed under the microscope (Fig. 1C and D).
Due to the ONP cell bipotential characteristics, it is important to further investigate whether; (a) an intermediate stage exists during the ONP cell differentiation that acts as a branch point on B/myeloid cell divergence or (b) two different cell populations developed during the ONP cell differentiation and each cell population will eventually generate into a certain lineage. To answer this question, cells were cultured under the stem cell reagents (SCF, FL and IL-3) for 3 days. After 3 days in culture, cells were stained with both B cell surface marker B220 and myeloid cell surface marker CD11b. Results showed after 3 days in culture, ONP cells differentiated into two subpopulations. The majority of cultured cells became B220−CD11b+, however, very few cultured cells were still B220−CD11b− (Fig. 2A).
Fig. 2.
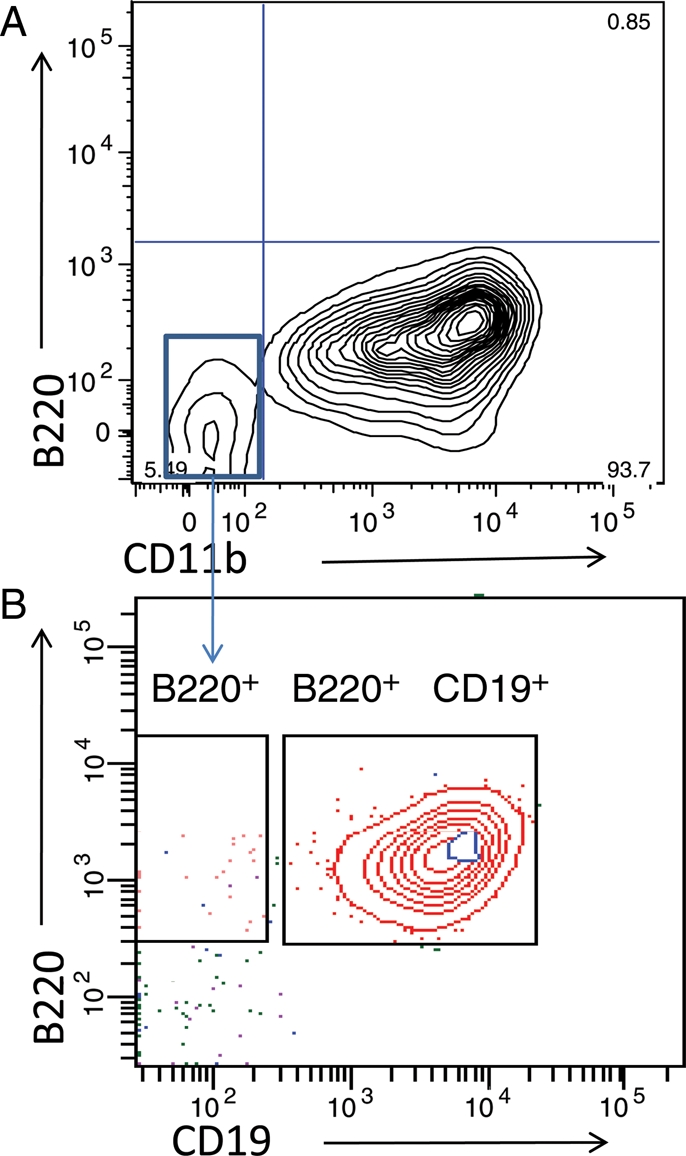
B220−CD11b− cells can differentiate into B cells with the support of OP9 stromal cells. ONP cells were sorted and cultured with SCF, FL and IL-3 for 3 days, phenotype analysis showed <5% cells differentiated into B220−CD11b− cells (A). When sorted B220−CD11b− cells separately and cultured with OP9 stromal cells for another 9 days, over 90% of these cells differentiated into B cells (B).
We then decided to sort these two cell populations separately and cultured each under the different liquid cell culture conditions (either under culture condition 1 or condition 2). Unfortunately, when sorted separately, only myeloid cells were developed by B220−CD11b+ cells. Both B220−CD11b+ and B220−CD11b− cells yielded no B cells no matter which culture conditions were present (data not shown). However, when B220−CD11b− and B220−CD11b+ cells were mixed together, and cultured under condition 1 in vitro, B cells were generated after 12 days (Fig. 1A).
It is understood that in vitro B cell culture requires stromal cell support. We further cultured B220−CD11b− and B220−CD11b+ cells under the support of OP9 stromal cells separately. After 9 days in culture, cells were harvested and stained with B220 and CD11b. Our results showed only B220−CD11b− cells generated B cells with the support of OP9 stromal cells (Fig. 2B). These above results indicated that ONP cells first differentiated into two cell populations, which then further grew into the different lineages. B220−CD11b− cells can be differentiated into B cells, and B220−CD11b+ cells can be developed into myeloid lineage. During in vitro cell culture, B220−CD11b+ cells actually act as stromal cells to support B220−CD11b− cells differentiation into a B lineage.
Alcohol affects the late stage of the ONP cells differentiation into a B lineage
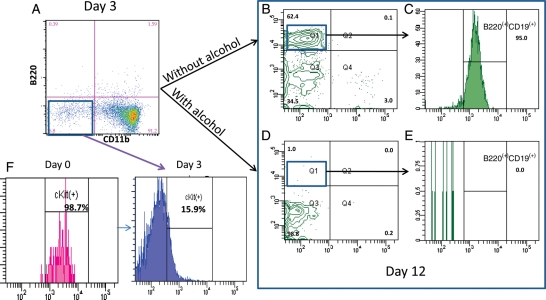
Previous laboratory results showed alcohol affects the ONP cell differentiation into a B cell lineage both in vivo and in vitro. Since ONP cells can further differentiated into either B220−CD11b+ or B220−CD11b− cells, and it is the B220−CD11b− cell that eventually generates into a B lineage. It is important to know whether alcohol affects the ONP cell in an early stage which occurs in the first 3 days or affects the B220−CD11b− cell, which represents the late stage of ONP cell differentiation to a B lineage. To address this question, ONP cells were treated with 100 mM alcohol for the first 3 days, then cultured cells were harvested and stained with both B and myeloid cell surface marker (B220 and CD11b). Our results showed that there was no statistically significant difference between the alcohol-treated group and normal control group on phenotype (Figs. 3A and 4A). After 3 days of exposure to 100 mM alcohol, B220−CD11b− cells were washed, stained and then sorted and cultured with OP9 stromal cells in another 9 days without the addition of alcohol, these cells yielded the same amount of B cells (Figs. 3B and C and 4B and C). However, with continued exposure to 100 mM alcohol during the whole course of in vitro culture, B220−CD11b− cells yield very few B lineage cells (Fig. 3D and E). This indicated that continued exposure to alcohol is required to affect the ONP cells further differentiation into a B lineage.
Fig. 3.
Alcohol affects the late stage of the ONP cell differentiation into a B lineage. ONP cells were sorted and first cultured with SCF, FL and IL-3 for 3 days. After 3 days in vitro culture, cells were differentiated into two populations: B220−CD11b− and B220−CD11b+ cells (A). These B220−CD11b− cells started to lose their surface expression of c-Kit (F). B220−CD11b− cells were sorted and ‘splint’ into two wells, cells in one well were cultured with OP9 stromal cells without alcohol for another 9 days and yielded B220+ cells (B). These B220+ cells were further stained with anti-CD19 (B-cell marker) showed over 90% of CD19 positive expression (C). Cells in the other well were cultured with OP9 stromal cells with the addition of 100 mM of alcohol for another 9 days. Very few cells differentiated into a B lineage (D) with minimal expression of CD19 (E).
Fig. 4.
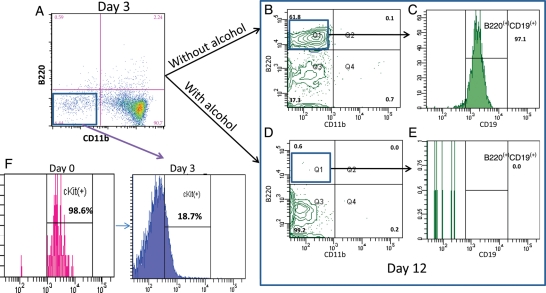
Alcohol does not affect the early stage of the ONP cell differentiation. ONP cells were sorted and cultured with SCF, FL, IL-3 and 100 mM of alcohol for the first 3 days. Cells were analyzed after 3 days and showed two cell populations: B220−CD11b− and B220−CD11b+ cells (A). There is no statistically significant difference between early alcohol-treated and normal control groups (P > 0.05, A and Fig. 3A). These B220−CD11b− cells also lost their surface expression of c-Kit (F). After 3 days of culture, B220−CD11b− cells were also sorted and ‘split’ into two wells, cells were all co-cultured with the support of OP9 stromal cells. Cells in one well without the exposure of alcohol were differentiated into B220+ cells (B) with strong CD19 expression (C) and reached no statistically significant difference (B, C versus Fig. 3B and 3C P > 0.05). Cells in the other well with the addition of 100 mM of alcohol yielded no B cells (D and E).
To demonstrate whether only exposure to alcohol at a late stage affects B-cell differentiation; ONP cells were cultured in vitro without the exposure of 100 mM alcohol for the first 3 days, then B220−CD11b− cells were sorted and cultured under the condition 1 with the presence of alcohol. After another 9 days in culture, cells were analyzed and the results showed significant impairment of B-cell formation (Fig. 4D and E), and there was no statistically significant difference between the early alcohol-treated group receiving continued alcohol (Fig. 3D and E) and late alcohol-treated group (Fig. 4D and E).
Taken together, this indicated that alcohol will not affect the early differentiation of ONP cells. Alcohol only affects the differentiation of ONP cells that have already developed into the B220−CD11b− stage.
B220−CD11b− cells were also further analyzed to confirm these cells are not undifferentiated ONP cells. Our results showed that these B220−CD11b− cells became c-Kit− cells instead of the ONP cells with the positive expression of c-kit (Figs. 3F and 4F). Negative expression of c-kit on cell surface indicates the cell underwent further differentiation.
Alcohol down-regulated transcription factor and cytokine receptor expression during ONP cell differentiation
One of the mechanisms by which progenitor B cells differentiate into B lineage is the appropriate up-regulation of several TFs and cytokine receptors. TF EBF, Pax5 and cytokine receptor IL-7Rα play critical roles in development of B cells. Failure to up-regulate EBF, Pax5 and IL-7Rα results in impairment of B-cell differentiation. EBF is one of the upstream TFs of Pax5, and inappropriate expression of EBF results in the poor expression of Pax5. IL-7Rα expression also can affect progenitor B cells’ expression of EBF and Pax5. Therefore, it is important to find out whether alcohol affects these TFs and/or cytokine receptor expression.
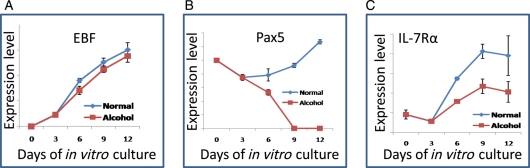
In this study, the expression level of TF EBF, Pax5 and cytokine receptor IL-7Rα were analyzed kinetically during the in vitro liquid culture. According to the results mentioned above, B220−CD11b+ cells are not progenitor B cells but acts as the supporting stromal cells, which are also essential during B cell culture in vitro. Therefore, ONP cells were sorted and cultured for 3 days. After 3 days, these cultured cells were split into two groups and grew for another 9 days, one group with the addition of 100 mM of alcohol under culture condition 1, the other without the exposure of alcohol under culture condition 1 as a normal control group. The relative expression level of EBF, Pax5 and IL-7 were measured on Day 0, 3, 6, 9 and 12 kinetically in the alcohol-treated group and compared with the normal control group. The results showed that alcohol affects the up-regulation of TF EBF, Pax5 and IL-7Rα (Fig. 5) leading to the poor differentiation of ONP cell into a B lineage. B220−CD11b+ cells were also cultured with or without the exposure of alcohol, with the expression level of TF EBF, Pax5 and IL-7Rα were also measured kinetically on Day 6, 9 and 12. Results showed no change in expression levels of EBF, Pax5 and IL-7Rα between the alcohol and non-alcohol groups (data not shown).
Fig. 5.
Alcohol down-regulated the transcription factor expression during the ONP cell differentiation. ONP cells were harvested at day 0, 3, 6, 9 and 12 during in vitro culture in both normal control and alcohol groups. The expression level of EBF, Pax-5 and IL-7Rα were analyzed using real-time PCR. Results showed the relative expression levels of TFs EBF (A), Pax5 (B) and cytokine receptor IL-7Rα (C) were increased kinetically in the control group but not in the alcohol group.
DISCUSSION
Although myeloid and lymphoid lineage cells have shown to be derived from different hematopoietic precursors, some previous studies have identified the existence of biphenotypic myeloid/lymphoid cells (Ford et al., 1992; Graf et al., 1999; Zeisig et al., 2003). The data present here demonstrate that ONP cells can differentiate into both myeloid and lymphoid lineages under different culture conditions. In this study, cells with multipotential properties were analyzed phenotypically by anti-mouse B220 and CD11b after culture in a timely and ordered manner. The observations obtained from our experiments illustrated that these cells first differentiated into two cell populations; B220−CD11b− precursors, which differentiated into a lymphoid lineage and B220−CD11b+ precursors, which differentiate into myeloid cells.
Moreover, in vitro culturing of progenitor B cells requires stromal cell support (Rolink et al., 2000). Cells without stromal cell support will either undergo apoptosis or failure to further differentiate into a B lineage (Crooks et al., 2000; Gimble et al., 1993; Nutt et al., 1999; Rolink et al., 2000). Our results showed that ONP cells first differentiated into a stage which contained both B220−CD11b+ and B220−CD11b− cells. It is the B220−CD11b+ cells, which function as stromal cells, supporting the B220−CD11b− cells differentiation into B cells.
Previous studies showed that alcohol can affect the ONP cell differentiation into a B lineage both in vitro and in vivo (Wang et al., 2006, 2009). However, it is important to know; (a) whether alcohol affects cell differentiation at an early stage, late stage or on both stages, (b) does the effect of alcohol on B-cell differentiation temporary or permanent and (c) the mechanism by which alcohol affects the progenitor B-cell differentiation.
To determine whether alcohol affects cell differentiation at an early or late stage, 100 mM of alcohol was added at different time points during the cell culture. The100 mM concentration of alcohol was used in this study, because it has no direct cytotoxicity to ONP cells and will not affect the proliferation of ONP cell cultures in vitro (Wang et al., 2006, 2009). Results of this experiment showed that alcohol does not affect the early differentiation of ONP cells until the B220−CD11b− stage. Alcohol-exposed B220−CD11b− cells were not the same as undifferentiated ONP cells since they started to lose the expression of SCF receptor (c-kit) on their surface indicating cells underwent further differentiation. However, our results showed that the exposure of 100 mM alcohol affects the ONP cell differentiation to a B lineage. Significant reduction in B lymphocytes after 12 days indicates that alcohol affects the ONP cell differentiation in the late stage only. The effect of alcohol on the late stage ONP cell differentiation was permanent.
Further investigation on the molecular basis of B lineage commitment was performed by measuring the transcription factor EBF and Pax5 and cytokine receptor IL-7Rα among these ONP cells. EBF and Pax5 are believed to be the important TFs in the development of B lymphocytes (Busslinger et al., 2000; Medina and Singh, 2005). Previous studies showed that maturation of B cells was severely blocked in B progenitors of transgenic mice lacking of EBF or Pax5 (Kee and Murre, 1998; Nutt et al., 1999). It is still uncertain whether cells will strictly differentiate into B lineage with the Pax5 gene turned on. Current experimental evidence indicates that Pax5 expression within cells does not block the early myeloid lineage, or natural killer cell development (Cotta et al., 2003). Moreover, Pax5 can be reversibly switched in immature hematopoietic progenitors (Okubo et al., 2002). Based on the results of these studies, it is possible that the expression of TFs can be dynamically modulated within multipotential progenitors by environmental factors in favor of a specific lineage commitment. Our findings seem to support this hypothesis. Among ONP cells, directly sorted from mouse bone marrow, EBF and Pax5 message RNA were measured by using quantitative real-time RT-PCR. After 3 days, culture with the addition of stem cell reagent including SCF, FL and IL-3, which favors the myeloid lineage in vitro, both EBF and Pax5 are down-regulated. Compared with their phenotypes, the majority of cultured cells showed CD11b positive as well. With exposure to IL-7, which favors B lineage commitment, EBF and Pax5 were up-regulated rapidly. Meanwhile, without the addition of IL-7, Pax5 was undetectable. This dynamic switch of the expression level of EBF and Pax5 is likely to address, at least partially, the molecular basis of B lineage differentiation in ONP cells. However, the addition of 100 mM of alcohol to an in vitro culture down-regulates the expression level of TF EBF, causing minimal expression of Pax5. Low Pax5 expression results in the paucity of B-cell commitment.
In conclusion, our data shows that development of myeloid or lymphoid cells from ONP cells diverges through B220−CD11b+/ B220−CD11b− branch points. Expression of transcription factor EBF and Pax5 does not block early myeloid lineage commitment. The microenvironment that favors the specific lineage differentiation seems to play a critical role. With the exposure of IL-7, EBF and Pax5 reversibly switch to a high level and progenitor cells generate a B lineage. However, the exposure to 100 mM of alcohol, at a late stage of ONP cell differentiation in vitro, severely blocks the expression of TFs, resulting in impairment of B-cell differentiation. Taken together, these results may indicate the common developmental pathway and the molecular basis of myeloid/lymphoid divergence among ONP cells.
Funding
This work was supported in part by grants from the National Institutes of Health R01 AA-14141 and Louisiana State University Health Science Center Intramural Grant.
REFERENCES
- Adams B, Dorfler A, Aguzzi A, et al. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–607. doi: 10.1101/gad.6.9.1589. doi:10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- Anonymous. Health risks and benefits of alcohol consumption. Alcohol Res Health. 2000;24:5–11. [PMC free article] [PubMed] [Google Scholar]
- Baker RC, Jerrells TR. Recent developments in alcoholism: immunological aspects. Recent Dev Alcohol. 1993;11:249–71. [PubMed] [Google Scholar]
- Biber KL, Moscatello KM, Dempsey DC, et al. Effects of in utero alcohol exposure on B-cell development in the murine fetal liver. Alcohol Clin Exp Res. 1998;22:1706–12. [PubMed] [Google Scholar]
- Blot WJ. Alcohol and cancer. Cancer Res. 1992;52:2119s–23. [PubMed] [Google Scholar]
- Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. doi:10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- Busslinger M, Nutt SL, Rolink AG. Lineage commitment in lymphopoiesis. Curr Opin Immunol. 2000;12:151–8. doi: 10.1016/s0952-7915(99)00065-5. doi:10.1016/S0952-7915(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Chanarin I. Alcohol and the blood. Br J Haematol. 1979;42:333–6. doi: 10.1111/j.1365-2141.1979.tb01140.x. doi:10.1111/j.1365-2141.1979.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Chanarin I. Haemopoiesis and alcohol. Br Med Bull. 1982;38:81–6. doi: 10.1093/oxfordjournals.bmb.a071739. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–42. [PubMed] [Google Scholar]
- Cotta CV, Zhang Z, Kim HG, et al. Pax5 determines B- versus T-cell fate and does not block early myeloid lineage development. Blood. 2003;101:4342–6. doi: 10.1182/blood-2002-10-3139. doi:10.1182/blood-2002-10-3139. [DOI] [PubMed] [Google Scholar]
- Cowan DH. Effect of alcoholism on hemostasis. Semin Hematol. 1980;17:137–47. [PubMed] [Google Scholar]
- Crooks GM, Hao QL, Petersen D, et al. IL-3 increases production of B lymphoid progenitors from human CD34+ J Immunol. 2000;165:2382–9. doi: 10.4049/jimmunol.165.5.2382. [DOI] [PubMed] [Google Scholar]
- Dai J, Lin D, Zhang J, et al. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J Clin Invest. 2000;106:887–95. doi: 10.1172/JCI10483. doi:10.1172/JCI10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16:297–309. doi: 10.1016/s1074-7613(02)00269-8. doi:10.1016/S1074-7613(02)00269-8. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Hall E, et al. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–8. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver HE, Swann PF. Alcohol and human cancer (review) Anticancer Res. 1987;7:309–20. [PubMed] [Google Scholar]
- Ford AM, Healy LE, Bennett CA, et al. Multilineage phenotypes of interleukin-3-dependent progenitor cells. Blood. 1992;79:1962–71. [PubMed] [Google Scholar]
- Forster I, Rajewsky K. The bulk of the peripheral B cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci USA. 1990;87:4781–4. doi: 10.1073/pnas.87.12.4781. doi:10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–50. doi: 10.1056/NEJM199505113321901. doi:10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Medina K, Hudson J, et al. Modulation of lymphohematopoiesis in long term cultures by gamma interferon: direct and indirect action on lymphoid and stromal cells. Exp Hematol. 1993;21:224–30. [PubMed] [Google Scholar]
- Graf BA, Nazarenko DA, Borrello MA, et al. Biphenotypic B/macrophage cells express COX-1 and up-regulate COX-2 expression and prostaglandin E(2) production in response to pro-inflammatory signals. Eur J Immunol. 1999;29:3793–803. doi: 10.1002/(SICI)1521-4141(199911)29:11<3793::AID-IMMU3793>3.0.CO;2-3. doi:10.1002/(SICI)1521-4141(199911)29:11<3793::AID-IMMU3793>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Greenbaum S, Zhuang Y. Regulation of early lymphocyte development by E2A family proteins. Semin Immunol. 2002;14:405–14. doi: 10.1016/s1044532302000751. doi:10.1016/S1044532302000751. [DOI] [PubMed] [Google Scholar]
- Hillman RS. Alcohol and hematopoiesis. Ann NY Acad Sci. 1975;252:297–306. doi: 10.1111/j.1749-6632.1975.tb19172.x. doi:10.1111/j.1749-6632.1975.tb19172.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Anan K, Misawa M, et al. In vitro differentiation of murine Sca-1+Lin- cells into myeloid, B cell, and T cell lineages. Stem Cells. 1996;14:412–8. doi: 10.1002/stem.140412. doi:10.1002/stem.140412. [DOI] [PubMed] [Google Scholar]
- Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. doi:10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade PW, Medina KL, Smithson G. Sex hormones as negative regulators of lymphopoiesis. Immunol Rev. 1994;137:119–34. doi: 10.1111/j.1600-065x.1994.tb00661.x. doi:10.1111/j.1600-065X.1994.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Kondo M, Scherer DC, Miyamoto T, et al. Cell fate conversion of lymphoid committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–6. doi: 10.1038/35030112. doi:10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- Kouro T, Medina KL, Oritani K, et al. Characteristics of early murine B lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001;97:2708–15. doi: 10.1182/blood.v97.9.2708. doi:10.1182/blood.V97.9.2708. [DOI] [PubMed] [Google Scholar]
- MacGregor RR. Alcohol and immune defense. JAMA. 1986;256:1474–9. doi:10.1001/jama.256.11.1474. [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Medina KL, Kincade PW. Pregnancy related steroids are potential negative regulators of B lymphopoiesis. Proc Natl Acad Sci USA. 1994;91:5382–6. doi: 10.1073/pnas.91.12.5382. doi:10.1073/pnas.91.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Singh H. Genetic networks that regulate B lymphopoiesis. Curr Opin Hematol. 2005;12:203–9. doi: 10.1097/01.moh.0000160735.67596.a0. doi:10.1097/01.moh.0000160735.67596.a0. [DOI] [PubMed] [Google Scholar]
- Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B lineage precursors. Blood. 2000;95:2059–67. [PubMed] [Google Scholar]
- Michot F, Gut J. Alcohol induced bone marrow damage. A bone marrow study in alcohol dependent individuals. Acta Haematol. 1987;78:252–7. doi: 10.1159/000205888. doi:10.1159/000205888. [DOI] [PubMed] [Google Scholar]
- Moscatello KM, Biber KL, Jennings SR, et al. Effects of in utero alcohol exposure on B cell development in neonatal spleen and bone marrow. Cell Immunol. 1999;191:124–30. doi: 10.1006/cimm.1998.1420. doi:10.1006/cimm.1998.1420. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, et al. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–62. doi: 10.1038/44076. doi:10.1038/44076. [DOI] [PubMed] [Google Scholar]
- Okubo T, Yanai N, Ikawas S, et al. Reversible switching of expression of c-kit and Pax-5 in immature hematopoietic progenitor cells by stromal cells. Exp Hematol. 2002;30:1193–201. doi: 10.1016/s0301-472x(02)00899-8. doi:10.1016/S0301-472X(02)00899-8. [DOI] [PubMed] [Google Scholar]
- Opstelten D, Osmond DG. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu-chain-bearing cells in normal mice. J Immunol. 1983;131:2635–40. [PubMed] [Google Scholar]
- Reimold AM, Ponath PD, Li YS, et al. Transcription factor B cell lineage specific activator protein regulates the gene for human X-box binding protein. J Exp Med. 1996;183:393–401. doi: 10.1084/jem.183.2.393. doi:10.1084/jem.183.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringborg U. Alcohol and risk of cancer. Alcohol Clin Exp Res. 1998;22:322s–28. doi: 10.1097/00000374-199807001-00008. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Schaniel C, Busslinger M, et al. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175:104–11. doi:10.1111/j.1600-065X.2000.imr017512.x. [PubMed] [Google Scholar]
- Seppa K, Laippala P, Saarni M. Macrocytosis as a consequence of alcohol abuse among patients in general practice. Alcohol Clin Exp Res. 1991;15:871–6. doi: 10.1111/j.1530-0277.1991.tb00615.x. doi:10.1111/j.1530-0277.1991.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Sibley DA, Fuseler J, Slukvin I, et al. Ethanol induced depletion of lymphocytes from the mesenteric lymph nodes of C57Bl/6 mice is associated with RNA but not DNA degradation. Alcohol Clin Exp Res. 1995;19:324–31. doi: 10.1111/j.1530-0277.1995.tb01510.x. doi:10.1111/j.1530-0277.1995.tb01510.x. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–41. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou H, Moscatello KM, et al. In utero exposure to alcohol alters cell fate decisions by hematopoietic progenitors in the bone marrow of offspring mice during neonatal development. Cell Immunol. 2006;239:75–85. doi: 10.1016/j.cellimm.2006.04.002. doi:10.1016/j.cellimm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou H, Chervenak R, et al. Ethanol exhibits specificity in its effects on differentiation of hematopoietic progenitors. Cell Immunol. 2009;255:1–7. doi: 10.1016/j.cellimm.2008.08.008. doi:10.1016/j.cellimm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott RM, Jennings SR, Chervenak R. In utero exposure to ethanol affects postnatal development of T- and B- lymphocytes, but not natural killer cells. Alcohol Clin Exp Res. 1995;19:170–6. doi: 10.1111/j.1530-0277.1995.tb01487.x. doi:10.1111/j.1530-0277.1995.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Zeisig BB, Garcia-Cuellar MP, Winkler TH, et al. The oncoprotein MLL-ENL disturbs hematopoietic lineage determination and transforms a biphenotypic lymphoid/myeloid cell. Oncogene. 2003;22:1629–37. doi: 10.1038/sj.onc.1206104. doi:10.1038/sj.onc.1206104. [DOI] [PubMed] [Google Scholar]