Abstract
Variants in transcription factor 7-like 2 (266096218TCF7L2266096218USuser266096218Gene names have been italicized per house style. Please check and confirm whether there are other instances that need to be italicized or instances where italics have been inappropriately applied.) gene have been found strongly associated with an increased risk of type 2 diabetes, as well as with an impairment of glucagon-like peptide-1 (GLP-1) signalling chain. In rats, stimulation of central GLP-1 receptors increases heart rate and activates autonomic regulatory neurons. We aimed to evaluate the potential role of TCF7L2 gene polymorphisms on sympathovagal response in relation to changes in plasma insulin and/or GLP-1 concentration after glucose ingestion. Genotyping was performed for rs12255372 and rs7903146 TCF7L2 gene variants in 250 non-related healthy volunteers (mean age 27±3 years). Consistent with previous reports, both single-nucleotide polymorphisms were in strong linkage disequilibrium (D′=0.87, r2=0.76). A subset of 167 patients underwent an oral glucose tolerance test while a continuous recording of heart rate variability was performed. At baseline, no differences in fasting plasma insulin, in GLP-1 levels and in LF/HF (low frequency/high frequency) ratio between the three genotypes were found. Along with glucose ingestion TT subjects had lower INSAUC (insulin area under curve), as well as higher LF/HFAUC (LF/HF area under curve) values. No difference in GLP-1AUC (GLP-1 area under curve) between TCF7L2 gene variants was found. A multivariate analysis including multiple covariates showed that only INSAUC, GLP-1AUC and TCF7L2 gene variants were independently associated with LF/HFAUC. In conclusion, TT genotype of rs12255372 and rs7903146 TCF7L2 gene variants is associated with lower insulin secretion and higher cardiosympathetic activity. Moreover, such effect is independent of GLP-1 and insulin plasma concentrations suggesting a potential role of such gene variants in increasing cardiovascular risk through enhanced sympathetic nervous system activity.
Keywords: gene polymorphism, glucose metabolism, cardiac electrophisiology
Introduction
Transcription factor 7-like 2 (TCF7L2) has a role in the wingless-type MMTV integration site family, member 1 (WNT) signalling pathway, which influence the synthesis, and possibly secretion from intestinal L cells of GLP-1, an incretin hormone with insulinotropic and insulino-mimetic actions regulating glucose and energy homeostasis, food intake and cardiovascular functions.1, 2 Variants in the TCF7L2 gene have been found strongly associated with an impaired insulin secretion and with an increased risk of type 2 diabetes (T2D).3, 4, 5, 6, 7, 8, 9, 10, 11
Shafer et al11 have recently showed that the presence of T allele of rs7903146 and rs12255372 TCF7L2 gene single-nucleotide polymorphisms (SNPs) was associated with reduced GLP-1-induced insulin secretion probably because of an impairment of GLP-1 signalling chain.2, 11
Interestingly, previous animal data have revealed beneficial effects of GLP-1 and its analogs on cardiovascular system by improving endothelial function and increasing myocardial contractility.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Moreover, GLP-1 is a neuropeptide synthesized by neurons in the caudal regions of the nucleus of the solitary tract, and GLP-1 receptors are also found in the heart and in areas of the central nervous system that govern autonomic control.14, 15
In rats, stimulation of central GLP-1 receptors increases blood pressure and heart rate, and activates autonomic regulatory neurons.16, 17 In humans, only few data have been provided, showing that GLP-1 increases muscle sympathetic nerve traffic without affecting heart rate, plasma catecholamines or cardiovagal and cardiosympathetic activity.18
The potential role of TCF7L2 gene polymorphisms on sympathovagal response in relation to changes in plasma insulin and/or GLP-1 concentration after glucose ingestion is still unknown. In healthy young subjects, glucose ingestion and insulin infusion increases low frequency/high frequency (LF/HF) ratio, an index of cardiac sympathovagal balance,19, 20, 21 producing a shift in cardiac autonomic nervous system activity toward sympathetic predominance.21, 22
Indeed, an alteration in the WNT signalling pathway related to TCF7L2 gene variant might result in an altered GLP-1 response and/or insulin secretion, which in turn could lead to an impaired cardiac sympathovagal balance, an thus to an increased cardiovascular risk.
Thus, our study aims to: (a) verify the effects of TCF7L2 gene polymorphisms on baseline and glucose ingestion stimulated plasma insulin and GLP-1 levels; (b) evaluate the potential role of TCF7L2 gene variants in regulating cardiac sympathetic activity at baseline and after glucose ingestion.
Materials and methods
Subjects
A total of 250 Caucasians non-related non-diabetic subjects (115 men and 135 women), from 21–35 years of age (mean age 28±3), living in Southern Italy, volunteered for the study. Clinical information was obtained by routine laboratory analyses, history and physical examination. All subjects were not hypertensive, had no evidence of metabolic or cardiovascular diseases and had liver, kidney, and thyroid function tests within the normal range. According to American Diabetes Association criteria, no subject was diabetic or affected by impaired fasting glucose.23 The participants did not take any drugs known to affect glucose tolerance. After a clear explanation of the potential risk of the study, all subjects provided written informed consent to participate in the study, which was approved by the ethics committee of our institutions.
Analytical methods
Anthropometric determinations (weight, height and body mass index (BMI)) were measured by standard technique. BMI was calculated as weight in kilograms divided by square of height expresses in meters (kg/m2). Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest and hip circumference at the level of the grater trochanter. Waist/hip ratio (WHR) was calculated. Plasma glucose was determined immediately by glucose oxidase method (Glucose Autoanalayzer, Beckman Coulter, Inc., Fullerton, CA, USA). Plasma fasting cholesterol and triglycerides were determined by routine laboratory methods (Roche Diagnostics, GmbH, Mannheim, Germany).
For the estimation of circulating intact GLP-1, plasma immunologic active forms of GLP-1 were determined using a specific ELISA kit (LINCO Research USA). Active isoforms of GLP-1 include GLP-1(7–36) amide and glycine-extended GLP-1(7–37). After secretion from enteroendocrine L cells, GLP-1(7–36) amide is rapidly degraded by dipeptidyl peptidase-4 (DPP-4) to its N-terminally truncated metabolite GLP-1(9–36), which does not interact with the known GLP-1 receptor. Blood samples were collected in ice-cooled EDTA-plasma tubes and immediately, within 30 s, an appropriate amount of DPP-IV inhibitor was added according to the manufacturer's instructions. Tubes were centrifuged at 2500 r.p.m. for 10 min in refrigerated centrifuge. Samples were stored at −80°C. Plasma insulin quantitative determinations were obtained using Mercodia Insulin ELISA kit (Mercodia, Uppsala, Sweden).
Genotyping
A total of 250 individuals were genotypized for rs12255372 (intron 3) G/T and rs7903146 (intron 3 C/T) allelic variants of TCF7L2 gene on chromosome 10q25.3 (Genbankgi: 89161187). DNA was isolated from whole blood collected into EDTA-containing tubes using DNA extraction kits (Illustra Blood GenomicPrep Mini Spin Kit, GE Healthcare UK Limited, Buckinghamshire, UK). The rs12255372 polymorphism was identified using the following primers: upstream, 5′-GGCTTGCAGGTCAGATTTTC-3′, downstream 5′-ATTTGGCATTCAAATGGAGG-3′. Polymerase chain reaction was carried out under the following conditions: 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, 62°C for 30 s, 72°C for 30 s, with a final extension of 72°C for 10 min. Restriction fragment length polymorphism was carried out using Tsp509I enzyme. The resulting products were electrophoresed on a 4.5% agarose gel. The rs7903146 was identified using the following primers: forward 5′-AAGAGAAGATTCCTTTTTAAATGGTG-3′, reverse 5′-CCTCATACGGCAATTAAATTATACA-3′. Polymerase chain reaction was carried out under the following conditions: 95°C for 5 min, followed by 34 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, with a final extension of 72°C for 9 min. Restriction fragment length polymorphisms were carried out using Hpy-CH4III enzyme. The resulting products were electrophoresed on a 4.5% agarose gel.
To better assess the potential role of TCF7L2 gene polymorphism on HRV and on glucose ingestion induced GLP-1 levels, 167 subjects underwent an oral glucose tolerance test (OGTT) while a continuous recording of HRV by the Holter technique was performed.
OGTT
A subset of 167 patients underwent an OGTT for stimulating secretion patterns of GLP-1. Tests were performed after an overnight fast of 12 h. At 08:00 h, participants ingested, within 5 min, a solution containing 75 g anhydrous glucose dissolved in 250 ml water. Venous blood samples were obtained at 0′, 30′, 60′, 120′ and 180′ min for determination of plasma glucose, insulin and GLP-1 concentrations. All subjects were studied in a comfortable supine position.
Cardiovascular determinations
All cardiovascular measurements were carried out under quiet conditions in a room maintained at 21°C, in a supine position for at least 30 min before starting the baseline Holter recording. Subjects with a respiratory rate <10 breaths/min (ie <0.15 Hz) were excluded from the study, to avoid the overlap of oscillations of low and high frequency at spectral analysis. Blood pressure and heart rate were recorded starting at 08.00 h for a 120 min period (30 min baseline and 90 min after glucose ingestion) using a standard mercury sphygmomanometer and a 2-channel 24-h Holter ECG recordings (Del Mar Medical Systems, Irvine, CA, USA), respectively. All recordings were visually examined and manually over read to verify beat classification. Abnormal beats and areas of artifact were automatically and manually identified and excluded from the analysis. The frequency domain analysis of HRV was performed.24 The mean heart rate was calculated, for a complete 10-min segment of RR interval, 10 min before and 10 min after glucose ingestion (T0), for the time 20 to the time 30 (T1), for the time 50 to the time 60 (T2), for the time 110 to the time 120 (T3). Spectral components were identified and estimated using the spectral decomposition algorithm proposed by Johnsen et al25 and were then assigned, on the basis of their central frequency, to 1 of the 3 bands: very-low-frequency (VLF; 0–0.03 Hz), LF (0.04–0.15 Hz) and HF (0.16–0.45 Hz).19 The low and high frequency components are normally considered19 and reported in normalized units,20 which represent the relative value of the power of each component in proportion to the total power minus the VLF component. Because LF power is modulated by baroreceptors and reflects both sympathetic and parasympathetic influences and HF power reflects parasympathetic (vagally mediated) respiratory variation, LF/HF ratio, rather than LF, reflect relative sympathetic–parasympathetic activity and were used for calculating and reporting our data.
Calculations and statistical analysis
All data are presented as means±SD. Areas under the curve for insulin (INSAUC), glucose (GLYAUC) and GLP-1 (GLP-1AUC) were calculated by trapezoidal method. To approximate normal distribution, plasma insulin and triglycerides were log-transformed for data analyses and back transformed for data presentation. Genotype frequencies were compared for investigating the Hardy–Weinberg equilibrium model by the χ2 test. One-way ANOVA followed by Bonferroni multiple testing correction was applied to assess differences in clinical, biochemical and cardiovascular data among the genotype groups. Simple correlation analyses allowed us to study the relationship between variables. Multivariate general linear model analyses were used to evaluate the difference in plasma glucose and triglycerides among different genotype, after adjustment for gender, BMI and WHR. A multivariate linear regression analyses allowed us to investigate the association of TCF7L2 gene variants with LF/HF ratio independently of plasma GLP-1 levels, BMI, gender, INSAUC and GLYAUC. A P<0.05 was chosen for levels of significance. Statistical analyses were performed using SPSS 15 software package (SPSS, Inc., Chicago, IL, USA).
To investigate the difference in cardiovascular and metabolic parameters between the two genotype groups, sample size calculation was estimated on an IBM PC computer by GPOWER software (Cristian-Albrechts University, Kiel, Germany). The resulting sample size, estimated according to a global effect size of 27% with type I error of 0.05 and a power of 80%, was 110 patients. Pairwise LD coefficients (D′) between SNPs were calculated using Haploview software version 4.0 (Daly Lab, Broad Institute Cambridge, USA).
Results
Clinical data
Clinical and biochemical characteristics of the study subjects are reported in Table 1. All subjects (n=250) were young (mean age=28.1±3.2), not obese, with no difference in gender ratio (135F/115M, χ2=1.6 P=0.206). According to gender ratio, female had a significantly lower BMI (21.8±2.27 vs 25.5±3.2 kg/m2; P<0.001), WHR (0.75±0.08 vs 0.85±0.07; P<0.001), plasma triglycerides (79.1±12 vs 99.7±27.2 mg/dl; P<0.001) levels and higher HDL-cholesterol (48.8±2.2 vs 46.6±2.2 mg/dl; P<0.001) levels compared to male subjects.
Table 1. Clinical characteristics of study population according to genotype at the rs12255372.
| All | GG | GT | TT | ||
|---|---|---|---|---|---|
| (n=250) | (n=81) | (n=133) | (n=36) | P | |
| Age (years) | 28.1±3.2 | 27.2±3.4 | 27.6±3.3 | 28.2±2.7 | 0.737 |
| Gender | 135F/115M | 42F/39M | 73F/60M | 20F/16M | 0.573 |
| BMI (kg/m2) | 23.2±3.3 | 22.7±3.3 | 23.9±3.5 | 24.0±2.8 | 0.395 |
| WHR | 0.77±0.12 | 0.76±0.10 | 0.80±0.11 | 0.78±0.06 | 0.196 |
| FPG (mmol/l) | 5.1±0.7 | 4.8±0.4 | 4.7±0.4 | 5.3±0.4§ | 0.002 |
| Total cholesterol (mg/dl) | 158±17 | 161±17 | 156±17 | 158±21 | 0.567 |
| HDL cholesterol (mg/dl) | 48±2 | 48±2 | 47±3 | 48±2 | 0.455 |
| Triglycerides (mg/dl) | 87±21 | 87±14 | 91±23 | 98±17 | 0.321 |
| HR (beats/min) | 71±5 | 71±6 | 72±7 | 73±6 | 0.391 |
| SBP (mm Hg) | 113±9 | 114±10 | 111±10 | 115±10 | 0.550 |
| DBP (mm Hg) | 72±7 | 73±8 | 71±8 | 74±7 | 0.500 |
Data are means±SD.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HR, heart rate; SBP, systolic blood pressure; WHR, waist/hip ratio.
P values between groups, §P=0.01 TT vs GT; P=0.02 TT vs GG by one-way ANOVA followed by Bonferroni multiple testing correction.
TCF7L2 gene variant analysis
The observed genotypes and allele distributions were in Hardy–Weinberg equilibrium (P>0.05). The T allele frequencies were 0.41 for rs12255372 and 0.39 for rs7903146. The frequency of rs12255372 GG, GT and TT genotypes was 32.3, 53.2 and 14.5%, respectively. As far as rs7903146 gene variant is concerned the frequency of CC, CT and TT genotypes was 35.4, 48.8 and 15.8%, respectively. The genotypic frequencies observed were almost similar to that previously reported in European populations and consistent with previous reports, both SNPs were in strong linkage disequilibrium (D′=0.87, r2=0.76). As the two SNPs give the same information, results for SNP rs7903146 are shown in as Supplementary material.
Table 1 shows clinical and biochemical characteristics of the whole population (n=250) stratified according to rs12255372 (G/T) genotypes. No difference in genotype distribution between male and female subjects was found (χ2=1.115; P=0.573). Subjects carrying the TT genotype had significantly higher fasting plasma glucose levels compared with the GT and GG carriers, even after adjusting for gender, BMI and WHR (P=0.04). No differences in other clinical and biochemical parameters among the three genotypes were found.
Biochemical data and cardiovascular determinations over OGTT administration
A subset of 167 subjects underwent an OGTT while a continuous recording of HRV by the Holter technique was performed. No subjects resulted affected by glucose intolerance.
At baseline, no significant correlations between LF/HF values and fasting plasma glucose (r=0.08, P=0.397), insulin (r=0.12, P=0.398) and GLP-1 (r=0.12, P=0.691) levels were found.
In all subjects studied, oral glucose ingestion evoked a significant increase in plasma glucose (from 4.9±0.5 to 7.1±0.7 mmol/l P=0.038) and insulin (from 49.6±8 to 118.3±10.2 pmol/l P=0.01) levels at 60 min and in plasma GLP-1 (from 8.4±1 to 17.6±1 pmol/l P=0.02) concentrations at 30 min compared with fasting values. The plasma peak of GLP-1 was transient and levels returned to near fasting within 90–120 min. Basal LF/HF ratio also increased significantly after 30 min (from 2.07±0.3 to 2.24±0.5 P=0.03) reaching the maximum peak at 60 min and later declined without significant difference vs baseline.
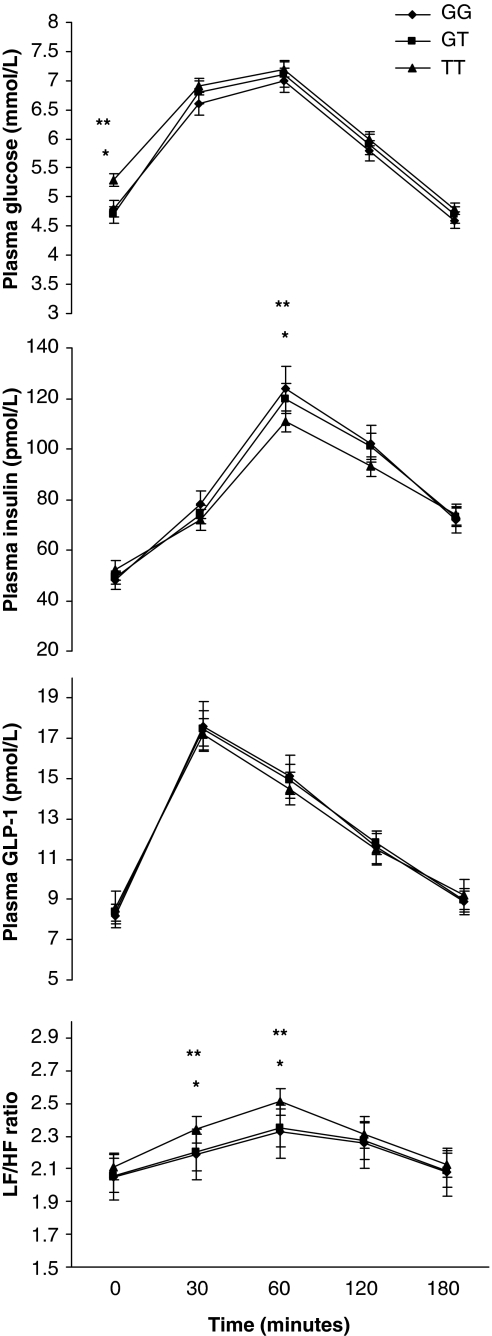
Categorizing subjects in different genotype groups, no differences in baseline plasma insulin, in GLP-1 levels and in LF/HF ratio between genotypes for either SNP were found, whereas fasting plasma glucose levels were significantly higher in TT carriers (Figure 1; Supplementary Figure 1).
Figure 1.
Change in plasma glucose, insulin, GLP-1 and LF/HF during oral glucose tolerance test (n=167) stratified according to GG (n=81) GT (n=50) and TT (n=36) genotypes of rs12255372 (G/T) TCF7L2 gene variant. Data are means±SD. *P=0.02 TT vs GT; **P=0.01 TT vs GG by Bonferroni multiple testing correction.
After glucose ingestion, no significant difference in plasma glucose and GLP-1 levels between the three genotype groups for each SNP was found. Subjects with TT genotype for both rs12255372 and rs7903146 variants had a significantly lower plasma insulin levels at 60′and higher LF/HF ratio at 30′ and 60 min. No difference in LF/HF ratio at baseline and after 120 min of glucose ingestion between the three genotype groups for either SNP was found (Figure 1; Supplementary Figure 1).
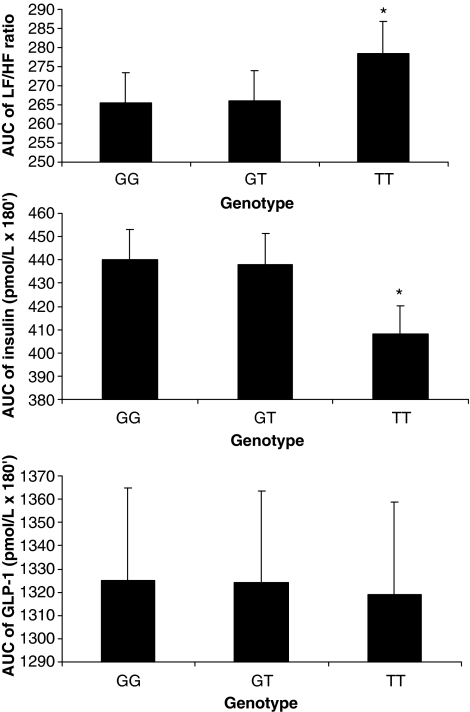
AUC for insulin (INSAUC), GLP-1 (GLP-1AUC) and LF/HF (LF/HFAUC) were also calculated. LF/HFAUC positively correlated with GLP-1AUC (r=0.39, P=0.03) and INSAUC (r=0.48, P=0.03). A positive association between GLP-1AUC and INSAUC (r=0.52, P=0.02) was also found. All such associations were still significant after adjusting for BMI (data not shown).
No difference in GLP-1AUC among TCF7L2 gene variants was found, whereas subjects carrying the TT genotype for both SNP had lower INSAUC, as well as higher LF/HFAUC values compared with the other genotype. No difference in INSAUC and LF/HFAUC between GG (or CC) and GT (or CT) genotype was also found. (Figure 2; Supplementary Figure 2)
Figure 2.
LF/HFAUC, INSAUC and GLP-1AUC values according to rs12233572 TCF7L2 gene polymorphism. Data are means±standard errors. *P=0.01 TT vs GT and TT vs GG by Bonferroni multiple testing correction.
The independent association of TCF7L2 gene variant with LF/HF ratio was tested in a multivariate analysis (Table 2). A model including LF/HFAUC, as the dependent variable and BMI, gender, GLP-1AUC, GLYAUC, INSAUC, TCF7L2 (G/T) gene polymorphism as covariates, showed that only INSAUC, GLP-1AUC and TCF7L2 (G/T) gene variant were independently associated with LF/HFAUC.
Table 2. Linear multiple regression analyses with AUC of LF/HF ratio as the dependent variable (n=167).
| β | t | P | |
|---|---|---|---|
| Model 1 | |||
| BMI | −0.65 | −0.621 | 0.558 R2=0.554 |
| Sex | 0.03 | 0.027 | 0.979 |
| AUC glucose (mmol/l × 180′) | 0.11 | 1.218 | 0.259 |
| AUC insulin (pmol/l × 180′) | 0.32 | 1.041 | 0.029 |
| AUC GLP-1 (pmol/l × 180′) | 0.18 | 1.953 | 0.036 |
| TCF7L2 polymorphisma | 0.78 | 9.948 | 0.022 |
Abbreviations: AUC, area under curve; BMI, body mass index; GLP-1, glucagon-like peptide-1; HF, high frequency; LF, low frequency; TCF7L2, transcription factor 7-like 2.
Calculated as GG carrier (=1) vs GT carrier (=2) vs TT carrier (=3). β=standardized regression coefficient; t=‘t' statistics computed by dividing the estimated value of the β-coefficient by its standard error.
Discussion
The major findings of our investigation are: (i) TT genotype of TCF7L2 variants is associated with lower insulin secretion and higher cardiosympathetic activity (ii) TCF7L2 genotype effect on cardiosympathetic activity is independent of GLP-1 and insulin plasma concentrations.
TCF7L2 encodes a transcription factor having an important role in the WNT signalling pathway,26 required for the normal development of the pancreas and pancreatic islets and it is also involved in GLP-1 production.27 Variants in TCF7L2 gene are strongly associated with an increased risk of T2D.1 Shafer et al11 have recently shown that the presence of the allele T of the rs12255372 TCF7L2 gene polymorphism is associated with reduced GLP-1-induced insulin secretion.
Accordingly, in our study subjects carrying the TT genotype of TCF7L2 (G/T) gene had reduced insulin secretion. Interestingly, a predominant cardiosympathetic activity (higher LF/HF ratio) in response to glucose ingestion in TT subjects was also found. The higher LF/HF ratio found in TT carriers might contribute to explain the association of TCF7L2 gene polymorphism with an increased risk ofT2D. In fact, it is generally accepted that impaired autonomic function is not only the consequence, but also a precursor of hyperglycemia.18, 28 Chronically elevated levels of sympathetic activity may lead to gradual downregulation of the β-adrenoceptor-mediated thermogenic and metabolic responses, which may be involved in the development of obesity and insulin resistance as seen in new onset T2D mellitus.18, 28, 29
Moreover, our results might also indicate TCF7L2 polymorphism as a new potential cardiovascular risk factor. In fact, an unbalanced sympathetic/parasympathetic tone, with a prevalence of sympathetic activity, is associated with higher cardiovascular mortality in type 2 diabetic patients.30, 31
Sympathetic nervous system is an important regulatory mechanism of metabolic and cardiovascular functions.32 In healthy young subjects, glucose ingestion enhances LF/HF ratio, which is related to the insulin sensitivity, and negatively correlated with body fat content.21 It is well accepted that insulin stimulates sympathetic activity independently of changes in plasma glucose concentration33, 34 increasing the LF/HF ratio.35, 36 Furthermore, in response to food intake (as well as glucose ingestion) intestinal enteroendocrine cells secrete multiple hormones, including GLP-1. GLP-1 given peripherally or centrally increases heart rate and blood pressure, as well as activates autonomic regulatory neurons in rats, thus suggesting that central GLP-1 regulates cardiovascular function by increasing the sympathetic system and inhibiting vagal activity.16, 17
Accordingly, in our study, the glucose induced increase in cardiosympathetic activity, was positively associated with increased insulin and GLP-1 plasma levels. Furthermore, a multivariate analysis with LF/HFAUC as the dependent variable, confirmed an independent role of both insulin and GLP-1 levels in stimulating the cardiosympathetic activity.
Indeed, subjects carrying TT genotype had lower plasma insulin secretion and similar GLP-1 levels compared with heterozygote and GG carriers. Furthermore, the multivariate analysis showed that TCF7L2 gene variant was significantly associated with AUC of LF/HF ratio independently of both insulin and GLP-1 plasma levels, thus suggesting that an impaired GLP-1 secretion is not likely to explain the increased sympathetic activity in carriers risk allele. An altered GLP-1 signalling, rather than different GLP-1 plasma levels, might explain the effect of TCF7L2 variant on cardiosympathetic activity. Previous results, obtained by Shafer et al,11 showing that, despite similar basal and glucose induced total plasma GLP-1 levels, the presence of the allele T was associated with reduced insulin secretion after GLP-1 infusion compared with allele G, seem to support our hypothesis.
In our study the active form of GLP-1 (peptide 7–36 and 7–37), instead of the total GLP-1 (peptide 9–36) was evaluated. Indeed, systemic GLP-1 levels may not adequately reflect the level of the active hormone at tissue level. In fact, impaired TCF7L2 activity, through the TT genotype, might tissue specifically reduce or increase the GLP-1 levels. Thus, the reduced efficiency of GLP-1 in stimulating insulin secretion in pancreatic β-cell and the increased activity on cardiac autonomic system, found in our study, might both reflect a functional defect in GLP signal (ie, an impaired GLP-1 receptor function).
In conclusion, our study show that TT genotype of rs12255372 and rs7903146 TCF7L2 gene variants is associated with reduced insulin secretion and with increased cardiosympathetic activity. Such results might contribute to explain the association of TCF7L2 gene polymorphisms with an increased risk of T2D and suggest a potential role of TCF7L2 gene variants in increasing cardiovascular risk through enhanced sympathetic nervous system activity.
The limited number of subjects and the lack of replication study are potential limitations of this study. Thus, further studies will be necessary for replicating our findings in a larger population group, with ethnical diversity to better elucidate molecular mechanisms linking TCF7L2 gene and cardiac sympathetic/parasympathetic activity.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Jin T, Liu L. The Wnt signalling pathway effector TCF7L2 and type II diabetes mellitus. Mol Endocrinol. 2008;22:2383–2392. doi: 10.1210/me.2008-0135. [DOI] [PubMed] [Google Scholar]
- Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Groves CJ, Zeggini E, Minton J, et al. Association analysis of 6736 U.K. subjects provides replication and confirms TCF7L2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes. 2006;55:2640–2644. doi: 10.2337/db06-0355. [DOI] [PubMed] [Google Scholar]
- Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damcott CM, Pollin TI, Reinhart LJ, et al. Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for a role in both insulin secretion and insulin resistance. Diabetes. 2006;55:2654–2659. doi: 10.2337/db06-0338. [DOI] [PubMed] [Google Scholar]
- Saxena R, Gianniny L, Burtt NP, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55:2890–2895. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- Zhang C, Qi L, Hunter DJ, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes. 2006;55:2645–2648. doi: 10.2337/db06-0643. [DOI] [PubMed] [Google Scholar]
- Cauchi S, Meyre D, Dina C, et al. Transcription factor TCF7L2 genetic study in the French population: expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes. 2006;55:2903–2908. doi: 10.2337/db06-0474. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Bonnycastle LL, Willer CJ, et al. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006;55:2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- Schafer SA, Tscritter O, Machicao F, et al. Impaired glucagons-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia. 2007;50:2443–2450. doi: 10.1007/s00125-007-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:1209–1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha AE, Charkoudian N, Andrews CN, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:874–880. doi: 10.1152/ajpregu.00153.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability Standards of measurements, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Malliani A, Lombardi F, Pagani M, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:1482–1492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Manzella D, Ferrara N, et al. Glucose ingestion affects cardiac ANS in healthy subjects with different amounts of body fat. Am J Physiol. 1997;273:471–478. doi: 10.1152/ajpendo.1997.273.3.E471. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Manzella D, Tagliamonte MR, Rizzo MR, Gambardella A, Varricchio M. Effects of different insulin infusion rates on heart rate variability in lean and obese subjects. Metabolism. 1999;48:755–762. doi: 10.1016/s0026-0495(99)90176-2. [DOI] [PubMed] [Google Scholar]
- Aberti KGMM, Zimmet PZ, for the WHO Consultation Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of WHO consultation. Diab Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsen SJ, Andersen N. On power estimation in maximum entropy spectral analysis. Geophysics. 1978;43:681–690. [Google Scholar]
- Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem. 2005;280:1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity. Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- van Baak Marlen A. Meal-induced activation of sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol Behav. 2007;94:178–186. doi: 10.1016/j.physbeh.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- Berne C, Fagius J, Pollare T, Hjemdahl P. The sympathetic response to euglycaemic hyperinsulinaemia. Evidence from microelectrode nerve recordings in healthy subjects. Diabetologia. 1992;35:873–879. doi: 10.1007/BF00399935. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder EB, Chambless LE, Liao D, et al. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–674. doi: 10.2337/diacare.28.3.668. [DOI] [PubMed] [Google Scholar]
- Manzella D, Paolisso G. Cardiac autonomic activity and type II diabetes mellitus. Clinical Science. 2005;108:93–99. doi: 10.1042/CS20040223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.