Abstract
Aims
Prolonged P2Y-receptor signalling can cause vasoconstriction leading to hypertension, vascular smooth muscle hypertrophy, and hyperplasia. G protein-coupled receptor signalling is negatively regulated by G protein-coupled receptor kinases (GRKs) and arrestin proteins, preventing prolonged or inappropriate signalling. This study investigates whether GRKs and arrestins regulate uridine 5′-triphosphate (UTP)-stimulated contractile signalling in adult Wistar rat mesenteric arterial smooth muscle cells (MSMCs).
Methods and results
Mesenteric arteries contracted in response to UTP challenge: When an EC50 UTP concentration (30 µM, 5 min) was added 5 min before (R1) and after (R2) the addition of a maximal UTP concentration (Rmax: 100 µM, 5 min), R2 responses were decreased relative to R1, indicating desensitization. UTP-induced P2Y-receptor desensitization of phospholipase C signalling was studied in isolated MSMCs transfected with an inositol 1,4,5-trisphosphate biosensor and/or loaded with Ca2+-sensitive dyes. A similar protocol (R1/R2 = 10 µM; Rmax = 100 µM, applied for 30 s) revealed markedly reduced R2 when compared with R1 responses. MSMCs were transfected with dominant-negative GRKs or siRNAs targeting specific GRK/arrestins to probe their respective roles in P2Y-receptor desensitization. GRK2 inhibition, but not GRK3, GRK5, or GRK6, attenuated P2Y-receptor desensitization. siRNA-mediated knockdown of arrestin2 attenuated UTP-stimulated P2Y-receptor desensitization, whereas arrestin3 depletion did not. Specific siRNA knockdown of the P2Y2-receptor almost completely abolished UTP-stimulated IP3/Ca2+ signalling, strongly suggesting that our study is specifically characterizing this purinoceptor subtype.
Conclusion
These new data highlight roles of GRK2 and arrestin2 as important regulators of UTP-stimulated P2Y2-receptor responsiveness in resistance arteries, emphasizing their potential importance in regulating vasoconstrictor signalling pathways implicated in vascular disease.
Keywords: UTP, G protein-coupled receptor kinase, Arrestin, P2Y-purinoceptor, Resistance artery
1. Introduction
Extracellular nucleotides play important roles in cardiovascular physiology, and in vascular tissues prolonged nucleotide signalling has been implicated in the pathophysiology of restenosis, diabetic microvascular disease, and hypertension.1 Purine/pyrimidine nucleotide release can have different effects on vascular tone depending on the site of release and cell type(s) with which the nucleotide interacts.1 Nucleotides released from endothelial cells after shear stress and/or hypoxia can cause smooth muscle relaxation;1,2 contrastingly, nucleotide release from sympathetic neuronal inputs act directly on vascular smooth muscle causing contraction.1,3,4
Nucleotide signalling is mediated through different classes of receptor, including the ionotropic P2X and metabotropic P2Y receptor families.5 Vascular smooth muscle cell contractile responses are mediated through the P2X1 subtype and several P2Y subtypes (i.e. P2Y2,4,6). P2X1-receptors cause a rapid influx of Ca2+ to initiate smooth muscle contraction. The G protein-coupled receptors (GPCRs) P2Y2, P2Y4, and P2Y6 couple with Gαq/11 proteins to activate phospholipase C (PLC), generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. This initiates the mobilization of intracellular Ca2+ stores, activation of protein kinase C, and induces contraction.1,5 Both P2X- and P2Y-mediated pathways are known to contribute significantly to the control of vascular tone.3,6,7
Considering the potentially important roles that P2Y-receptors may play in the aetiology of vascular disease, very little is currently known regarding the regulation of their signalling in vascular smooth muscle. P2Y-receptors, like most GPCRs, are known to undergo desensitization,8,9 a process which can prevent prolonged/inappropriate receptor-mediated signalling.10 Desensitization of agonist-occupied GPCRs is usually initiated by the recruitment of G protein-coupled receptor kinases (GRKs), which phosphorylate key serine/threonine residues within the third intracellular (i3) loop and/or C-terminal tail of the GPCR.10 GRK-mediated phosphorylation promotes the binding of non-visual arrestins (arrestin2 and/or arrestin3), which sterically inhibit GPCR-G protein interactions, and promote receptor internalization.10,11 Of the seven GRK isoenzymes (GRK1–7) and four arrestin isoforms (arrestin1–4) expressed in mammals, GRK2, GRK3, GRK5, and GRK6, and arrestin2 (β-arrestin-1) and arrestin3 (β-arrestin-2) are commonly reported to be ubiquitously expressed.10 Here, we have characterized the desensitization and resensitization of P2Y-receptor responses in mesenteric arterial smooth muscle cells (MSMCs), a widely documented model of systemic resistance arteries.12 Furthermore, we have combined confocal imaging with biochemical and molecular biological manipulation of individual GRK and arrestin subtypes to identify their roles in the regulation of endogenous P2Y-receptor-stimulated PLC signalling and contraction of MSMCs.
2. Methods
2.1. Isolation and culture of MSMCs
Adult male Wistar rats were killed by cervical dislocation, a method approved under the United Kingdom Animals (Scientific Procedures) Act 1986. The investigation also conforms to the Guide for Care and Use of Laboratory Animals US (NIH Publication No. 85-23, revised 1996). Smooth muscle cells were isolated from small branches of mesenteric artery by enzymatic dissociation as previously described.12 Smooth muscle cells were separated by gentle trituration in 231 medium (Cascade Biologics, Nottingham, UK), supplemented with smooth muscle growth supplement, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 2.5 µg/mL amphotericin B. Cells plated onto coverslips were maintained at 37°C and 5% CO2 in humidified conditions.
2.2. Myography
Contractile force recordings were made from 2 to 4 mm ring segments of third-order mesenteric arteries mounted in a Mulvany-Halpern wire myograph (JP Trading, Denmark). The bath solution contained (mM): NaCl 134, KCl 6, MgCl2 1, CaCl2 1.8, glucose 4, mannitol 6, HEPES 10, pH 7.4. NaCl was reduced to 81 mM and replaced with 60 mM K+ for the high K+ solution. All bathing solutions contained l-NAME (20 µM) to prevent endogenous nitric oxide production. Solutions and drugs were added directly to the organ bath, maintained at 37°C.
2.3. Relative contributions of P2Y receptors to MSMC Ca2+ signalling
MSMCs were plated into 96-well multiplates. Monolayers (∼90% confluency) were loaded with the Ca2+-sensitive dye Fluo4-AM (3 µM) at room temperature for 60 min. Selective P2Y2/4/6-receptor agonists (Tocris, Bristol, UK) were added and changes in intracellular Ca2+ levels ([Ca2+]i) were determined as the relative change in fluorescence using a NovoStar imaging system (BMG Labtech, Aylesbury, UK).
2.4. Single cell confocal imaging
UTP-stimulated PLC activity was determined in MSMCs transfected (24–48 h after isolation) with the pleckstrin-homology domain of phospholipase Cδ1 tagged with enhanced green fluorescent protein (eGFP-PH, 0.5 µg an extensively characterized IP3 biosensor13) using confocal imaging exactly as previously described14 (see Supplementary material online). Changes in cytosolic eGFP-PH fluorescence are displayed as the fluorescence emission (F)/initial basal fluorescence (F0) (F/F0). Additional Ca2+ experiments in the absence of eGFP-PH were conducted using Fluo-4-AM (3 µM, 60 min) and imaged as for eGFP.
2.5. Manipulating cellular GRK and arrestin levels
To assess the role that GRKs play in the regulation of P2Y-receptor signalling, MSMCs were co-transfected with eGFP-PH (0.5 µg), and the following dominant-negative, catalytically inactive GRK mutants: D110A,K220RGRK2, D110A,K220RGRK3, K215RGRK5, K215RGRK6 (0.5 µg),14 or negative control plasmid (pcDNA3). Additionally, GRK2 expression was depleted using anti-GRK2 small interfering (si)RNA (10 nM) [with non-targeting siRNA as negative-control (NC)].14 Our previous data demonstrated that co-transfection rates are >90%,14 therefore we have assumed that eGFP-expressing cells are successfully co-transfected. To examine the role that arrestins play in the regulation of P2Y-receptor signalling, MSMCs were transfected with anti-arrestin2 (5′-GCCACUGACUCGGCUACAAtt-3′; 100 nM), anti-arrestin3 (5′-GCCUUCUGUGCCAAAUCUAtt-3′; 100 nM), or NC siRNA (100 nM) (Applied Biosystems, UK). Arrestin depletion was assessed 48 h after nucleofection (Lonza AG, Cologne, Germany) by immunoblotting using an anti-arrestin2 antibody (A1CT). Arrestin expression was quantified using the GeneGnome image analysis system (Syngene, Cambridge, UK). For signalling studies, MSMCs were transfected using Lipofectamine2000 (Invitrogen, Paisley, UK) according to manufacturer's instructions and PLC activity assessed as described above.
2.6. Manipulating cellular P2Y receptor levels
An siRNA approach was also applied to delineate the respective roles of P2Y2- and P2Y4-receptors in UTP-mediated signalling. MSMCs were transfected with differing concentrations of anti-P2Y2 (5′-GAACUGACACUGUGAGGAAtt-3′), or anti-P2Y4 (5′-CGUCUACUUCAGUUCGGCAtt-3′) siRNAs. To validate the siRNAs and to quantify P2Y2/P2Y4 knockdown, rat aortic smooth muscle cells were nucleofected and after 48 h lysed, mRNA isolated and transcribed into cDNA, prior to PCR amplification using a LightCycler instrument (Roche Applied Science, Burgess Hill, UK). Data are presented as ΔCT values (calculated as CT (scrambled siRNA-transfected cells)−CT (anti-P2Y siRNA-transfected cells)). A more detailed method is provided in the accompanying Supplementary Material.
2.7. Data and statistical analysis
Data presented are from cells obtained from at least three separate preparations, expressed as means ± SEM. Data were analysed using one-way ANOVA as indicated, with appropriate post hoc testing (GraphPad Prism, San Diego, CA, USA).
3. Results
3.1. Characterization of UTP-mediated contractions in mesenteric arteries
Concentration-dependent contractions of third-order mesenteric arteries (Figure 1A and B) were induced by UTP addition, with maximal contraction seen at UTP concentrations ≥50 µM (EC50 28 µM). Repeated addition of maximal UTP (100 µM, 5 min) concentrations, interspersed by 5 min wash periods produced robust arterial contractions with little evidence of tachyphylaxis (data not shown). These findings are consistent with those previously obtained for H1 histamine receptor-mediated Ca2+ responses,15 where desensitization is masked by the presence of significant cell/tissue receptor reserve with respect to the observed response. To uncover any potential loss of UTP-induced contractile responsiveness, an amended protocol was applied where arteries were challenged with an approximate EC50 concentration of UTP (30 µM) for 5 min before (R1) and after (R2) the addition of a maximal UTP concentration (Rmax = 100 µM, 5 min). Arteries were washed for 5 min between R1 and Rmax, and for 2–15 min between Rmax and R2 agonist challenges. Reduced R2/R1 ratios are interpreted as an index of receptor/tissue desensitization.14,15 R2 contractile responses compared with R1 were dramatically reduced (by ∼80%) following Rmax exposure when the inter-Rmax/R2 delay was 2 min (Figure 1E); extending the inter-Rmax/R2 wash period revealed a time-dependent resensitization of UTP-induced contractions with the R1/R2 ratio returning to ∼1 after 12–15 min (Figure 1C–E).
Figure 1.
Characterization of UTP-stimulated contractions of third-order mesenteric arteries. (A) Representative trace showing K+-induced contraction followed by washout and concentration-dependent UTP (0–100 µM)-stimulated contractions (B) Cumulative concentration-dependent UTP-stimulated arterial contraction data shown as means ± SEM for arterial preparations from at least four animals. (C–E) Desensitization protocol: arteries were exposed to 30 µM UTP (R1, for 1 min) challenge, followed by 5 min washout, maximal UTP (Rmax = 100 µM, 1 min) challenge, followed by a variable period of 2–15 min washout before a further 30 µM UTP (R2, 1 min) exposure. Representative traces from single arteries with either a 5 min (C) or 15 min (D) Rmax-to-R2 washout period are shown. (E) Time-course of resensitization of the contractile response to UTP expressed as a percent change in R2 relative to R1 (means ± SEM for arterial preparations from at least four animals).
3.2. Characterization of UTP-mediated Ca2+ signalling in isolated MSMC
Vascular smooth muscle cells are known to express several UTP-responsive (types 2/4/6) P2Y-receptors,1 although it has not been conclusively determined which receptors contribute to UTP-stimulated Ca2+ signals, particularly in mesenteric smooth muscle. Therefore, various P2Y2-, P2Y4- and P2Y6-selective agonists were utilized to characterize the P2Y-receptor subtypes present in our preparation. The P2Y2-selective agonist 2-thio-UTP was more potent [pEC50 (M) = 6.56], but less efficacious than UTP [pEC50 (M) = 5.23] in causing concentration-dependent [Ca2+]i increases in MSMCs. The P2Y4-selective agonists, inosine 5′-triphosphate, and UTPγS produced almost identical responses, again showing a slightly reduced efficacy relative to UTP (Supplementary material online, Figure S1B). The P2Y6-selective agonist PSB0474 failed to cause any Ca2+ signals, while the P2Y6-selective antagonist MRS2578 (10 µM) had no significant effect on the UTP-mediated [Ca2+]i response (Supplementary material online, Figure S1C).
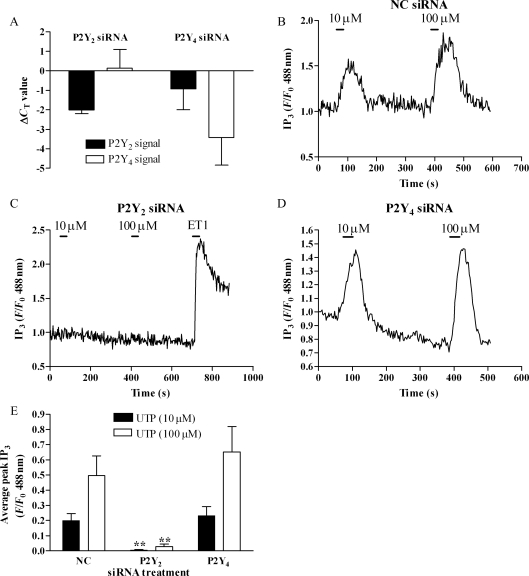
Since our data suggested that MSMCs express a mixed P2Y2/P2Y4-receptor population, we applied an siRNA approach specifically to deplete the expression of these P2Y-receptor subtypes individually. The effectiveness of siRNA constructs to suppress P2Y2- and P2Y4-receptor mRNAs was determined using real-time reverse transcription-polymerase chain reactions (RT-PCR) (see Section 2.6) in rat aortic smooth muscle cells 48 h after nucleofection with siRNA constructs targeting P2Y2- or P2Y4-receptor mRNAs or a NC (non-targeting) siRNA. Data show that nucleofection with NC siRNA had no effect on P2Y2 or P2Y4 signals (data not shown), while in the presence of anti-P2Y2 siRNA, mean P2Y2 cycle threshold values were suppressed (ΔCT = −2.0, relative to NC siRNA-transfected samples), with no effect on the P2Y4 signal (Figure 2A). Conversely, anti-P2Y4 siRNA suppressed mean cycle threshold values for P2Y4 (ΔCT = −3.4), but was without effect on P2Y2 signals. Neither P2Y-targeting siRNA altered β-actin mRNA levels significantly (data not shown). Collectively, these data suggest that ≥75% and ≥90% P2Y mRNA transcripts are selectively depleted following transfection of rat vascular smooth muscle with anti-P2Y2 or anti-P2Y4 siRNAs, respectively. The contributions P2Y2- and P2Y4-receptors play in UTP-mediated PLC signalling were determined in MSMCs co-transfected with eGFP-PH and NC, anti-P2Y2 or anti-P2Y4 siRNAs. NC siRNA nucleofection had no significant effect on the magnitude and profile of UTP-stimulated responses in MSMCs (data not shown). UTP-induced eGFP-PH translocation was virtually ablated in MSMCs treated with anti-P2Y2 siRNA, whereas anti-P2Y4 siRNA treatment was without effect (Figure 2C–E). Furthermore, responses to endothelin-1 were also unaltered following P2Y2-depletion (Figure 2C). Collectively, these data strongly suggest that in cultured MSMCs, UTP-mediated signalling via PLC is mediated primarily and perhaps exclusively by P2Y2-receptors.
Figure 2.
Dissecting the P2Y2/P2Y4-receptor dependency of UTP signalling. (A) Real-time PCR data showing changes in cycle threshold (ΔCT) values for P2Y2 or P2Y4 transcripts, relative to negative-control (NC) and untransfected cells, following transfection with 100 nM of anti-P2Y2 or anti-P2Y4 receptor siRNAs. Data shown are means ± SEM for n = 6 experiments (in triplicate) undertaken in cell preparations from six separate animals. Representative traces (B–D) and cumulative data (E) showing affects of NC (B), anti-P2Y2 (C), or anti-P2Y4 (D) siRNAs on UTP-stimulated (10 or 100 µM) IP3 signals. Data expressed as means ± SEM for n = 8–10 cells from cell preparations produced from four or more separate animals. Statistical significance is indicated as asterisk **P < 0.01 vs. NC siRNA (one-way ANOVA, Dunnett's post hoc test).
3.3. Desensitization and resensitization of UTP-signalling in isolated MSMC
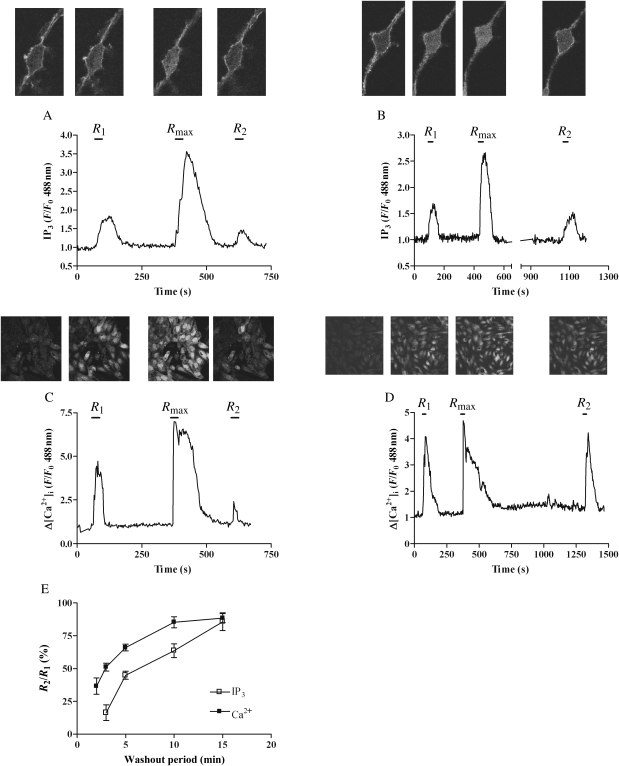
Time-courses of desensitization/resensitization of receptor–PLC signalling in response to UTP were assessed using similar protocols as those described above for myography experiments, however shorter agonist applications at lower concentrations (R1/R2 10 µM UTP, 30 s; Rmax 100 µM UTP, 30 s) were sufficient to induce significant desensitization (Figure 3). Comparison of UTP-induced changes in IP3 (eGFP-PH translocation) and [Ca2+]i (Fluo4), before (R1) and after (R2) the desensitizing UTP (Rmax) challenge resulted in a 64% decrease for [Ca2+]i and 84% decrease for IP3 in R2 vs. R1 measured 2 and 3 min after Rmax challenge, respectively (Figure 3A, C, and E). Prolonging the inter-Rmax/R2 washout period revealed a time-dependent resensitization of UTP-stimulated IP3 and [Ca2+]i responses (Figure 3B, D, and E), with essentially complete recovery of UTP-stimulated IP3 and [Ca2+]i responses after 15 and 10 min, respectively (Figure 3E).
Figure 3.
Time-course of UTP-induced P2Y2-receptor desensitization. MSMCs were transfected with eGFP-PH (0.5 µg), or loaded with Fluo4 and subjected to the following desensitization protocol: cells were challenged with UTP (R1, 10 µM, 30 s) 5 min before a desensitizing UTP concentration (Rmax = 100 µM for 30 s), and again after a variable washout period (2, 3, 5, 10, or 15 min) (R2, 10 µM, 30 s). Representative traces and images from single cells either expressing eGFP-PH (A and B) or loaded with Fluo4 (C and D) with either a 3 (A and C), 10 (B), or 15 min (D) wash period are shown. P2Y2 receptor desensitization was determined as the relative (%) change in R2 response compared with R1 for both eGFP-PH and Fluo4 signals. (E) Cumulative data are presented as means ± SEM for five to nine cells (eGFP-PH), or 21–45 cells (Fluo4) at each time-point. MSMCs used were generated from preparations from three or more different animals.
3.4. GRK isoenzymic dependency of P2Y-receptor desensitization in MSMCs
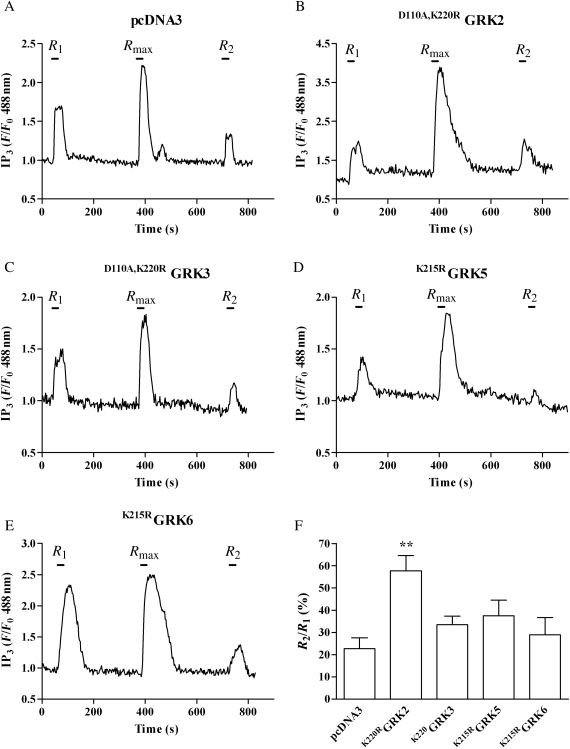
To determine whether endogenous GRKs regulate UTP signalling in MSMCs, individual GRK isoenzymes were inhibited using catalytically inactive, dominant-negative mutant GRKs. MSMCs were co-transfected with eGFP-PH (0.5 µg) together with pcDNA3 (control), D110A,K220R GRK2, D110A,K220RGRK3, K215RGRK5, or K215RGRK6 (0.5 µg).14 When subjected to the previously described desensitization protocol (with 5 min washes between R1/Rmax/R2 agonist additions), MSMCs transfected with pcDNA3, D110A,K220RGRK3, K215RGRK5, or K215RGRK6 displayed similar reductions in R2 responses (∼70%) compared with R1, comparable to responses observed in non-transfected cells (Figure 4). However, the presence of D110A,K220RGRK2 significantly attenuated reductions in the R2 response when compared with R1 (Figure 4B and F), suggesting that endogenous GRK2 activity plays a significant role in regulating UTP-stimulated P2Y-receptor-mediated PLC/Ca2+ signalling.
Figure 4.
GRK2 inhibition decreases P2Y2-receptor desensitization induced by UTP. MSMCs were co-transfected with 0.5 µg eGFP-PH and either pcDNA3 (control), D110A,K220RGRK2, D110A,K220RGRK3, K215RGRK5, or K215RGRK6 (0.5 µg). Cells were subjected to the standard R1/Rmax/R2 protocol (see Section 2). Representative traces show IP3 changes in cells transfected with pcDNA3 (A), D110A,K220RGRK2 (B), D110A,K220RGRK3 (C), K215RGRK5 (D), or K215RGRK6 (E). P2Y receptor desensitization was determined as the relative (%) change in R2 response compared with R1. (F) Cumulative data are presented as means ± SEM from 13–19 cells from MSMC preparations from four or more different animals. Statistical significance is indicated as asterisk **P < 0.01 vs. vector-transfected MSMCs (one-way ANOVA; Dunnett's post hoc test).
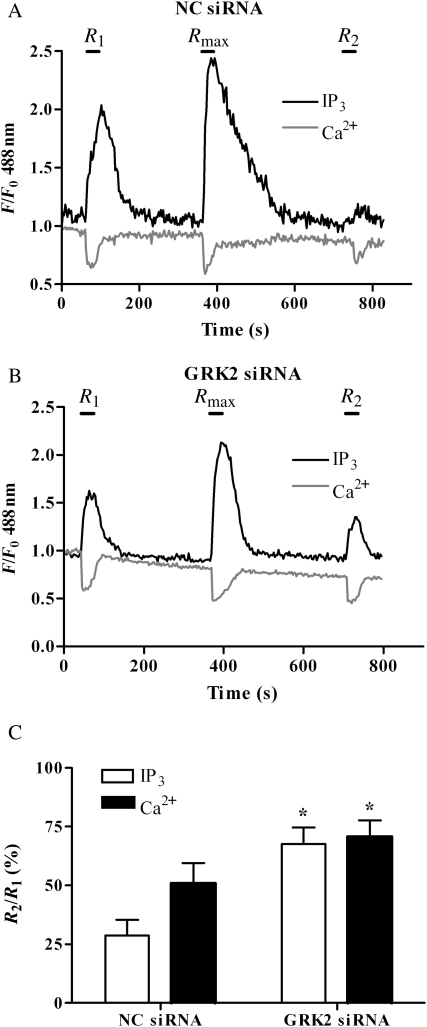
To corroborate these findings, we applied a previously validated siRNA that specifically depletes endogenous GRK2 (by ≥75%) without altering the expression of non-targeted GRKs.14 MSMCs were co-transfected with eGFP-PH (0.5 µg) and either 10 nM anti-GRK2 or NC siRNAs and 48 h later subjected to the standard R1/Rmax/R2 desensitization protocol (Figure 5A and B). In agreement with our data from D110A,K220RGRK2 co-transfections, siRNA-induced depletion of GRK2 attenuated the decrease in R2 (relative to R1) caused by Rmax UTP-addition (Figure 5B and C). This was most striking for the eGFP-PH/IP3 response (R2/R1 (%), negative control siRNA, 32 ± 8; anti-GRK2 siRNA, 71 ± 7; P < 0.05; data are means ± SEM). Taken together, these findings strongly suggest that GRK2 is a key mediator of UTP-induced P2Y2 receptor desensitization.
Figure 5.
Depletion of endogenous GRK2 attenuates P2Y2-receptor desensitization. MSMCs were nucleofected with 0.5 µg eGFP-PH and either negative-control (NC) or anti-GRK2 (10 nM) siRNAs. Cells were loaded with Fura-Red and subjected to the standard R1/Rmax/R2 desensitization protocol (see Section 2). Representative traces are shown for single cells transfected with NC siRNA (A) or anti-GRK2 siRNA (B). P2Y receptor desensitization was determined as the relative (%) change in R2 response compared with R1. Cumulative data (C) show means ± SEM from 8 to 15 cells from MSMCs prepared from more than four separate animals. Statistical significance is indicated as *P < 0.01 vs. NC siRNA-treated MSMCs (one-way ANOVA; Dunnett's post hoc test).
3.5. Arrestin dependency of P2Y2-receptor desensitization in MSMCs
To examine the potential role that arrestin proteins play in regulating P2Y2-receptor signalling, we utilized an siRNA approach to selectively deplete endogenous arrestin2/3 expression. MSMCs were transfected with anti-arrestin2, anti-arrestin3, or NC siRNAs (100 nM) 48 h prior to cell lysis and immunoblotting. Substantial arrestin depletion (>70% for arrestin2 and arrestin3) was observed at this time-point, and both arrestin2- and arrestin3-targeted siRNAs appeared to be highly selective for their respective targets (Figure 6A and B). To determine the effects of arrestin depletion on the desensitization of UTP-stimulated PLC signalling, MSMCs were co-transfected with eGFP-PH (0.5 µg) and anti-arrestin2 or anti-arrestin3, before being subjected to the standard R1/Rmax/R2 desensitization protocol. In the absence or presence of NC siRNA reductions in R2 relative to R1 responses of ≥80% for eGFP-PH and ≥70% for [Ca2+]i signals were observed (Figure 6C and E). Selective depletion of arrestin2 markedly reversed UTP-induced decreases in R2 relative to R1 IP3/Ca2+ responses (Figure 6E). Contrastingly, while arrestin3 depletion had a small effect on R2/R1 ratios (Figure 6E), this did not achieve statistical significance, suggesting that attenuation of the UTP signal is primarily effected by the arrestin2 isoform.
Figure 6.
Depletion of endogenous arrestin2 attenuates P2Y2-receptor desensitization. MSMCs were nucleofected with NC, anti-arrestin2 or anti-arrestin3 siRNAs (100 nM) (see Section 2). After 48 h, cells were lysed and 40 µg of protein loaded for SDS–PAGE separation and immunoblotting. (A) Representative immunoblot showing arrestin2 depletion (upper panel), and the same blot is shown after longer exposure to highlight arrestin3 (lower panel, lower band) depletion, shown in: non-transfected cells (lane 1), or cells treated with 100 nM of anti-arrestin2 (lane 2), anti-arrestin3 (lane 3), or NC (lane 4) siRNAs. (B) Cumulative densitometric data show mean arrestin expression ± SEM from four animal cell preparations, **P<0.01 vs. NC siRNA (one-way ANOVA; Dunnett's post hoc test). To assess the effects of arrestin depletion on UTP- or ET1-stimulated PLC signalling, MSMCs were nucleofected with 0.5 µg eGFP-PH and with 100 nM of either NC, anti-arrestin2 or anti-arrestin3 siRNAs. Cells were loaded with Fura-Red and subjected to the standard R1/Rmax/R2 (for UTP) or R1/R1 (for ET-1) desensitization protocols (see Section 2). Representative traces from single cells transfected with control siRNA (C and F), anti-arrestin2 (D), or anti-arrestin3 (G). Traces (C) and (D) show UTP responses, while (F) and (G) show ET-1 responses. Cumulative data are shown as means ± SEM from 5 to 13 cells from preparations from three to four separate animals for UTP- (E) and ET1-stimulated cells (H). Statistical significance is indicated as *P < 0.05 or **P < 0.01 vs. NC siRNA-treated MSMCs (one-way ANOVA; Dunnett's post hoc test).
Previously we showed that GRK2 regulates endothelin (ETA) receptor desensitization,14 suggesting that ETARs are also likely substrates for arrestin recruitment in MSMCs. Consequently, the potential involvement of arrestin proteins in the regulation of ETA receptor signalling was assessed in MSMCs co-transfected with eGFP-PH and either anti-arrestin2 or anti-arrestin3 siRNAs. Here, ETA receptor desensitization was assessed by exposing cells to a short desensitizing pulse of endothelin-1 (50 nM, 30 s, termed R1) followed by a 10 min wash period and second endothelin-1 challenge (50 nM, 30 s, termed R2). The extent of receptor desensitization, assessed at the level of IP3 or Ca2+, was similar in the absence or presence of NC, or anti-arrestin2 siRNAs (Figure 6F and H), whereas, in anti-arrestin3 siRNA-transfected MSMCs endothelin-1-induced receptor desensitization was significantly attenuated (Figure 6G and H). These data suggest while both P2Y2- and ETA-receptors are desensitized via a GRK2-dependent mechanism in MSMCs, it is likely that different arrestins are recruited to the desensitized receptor, with P2Y2- and ETA-receptors preferentially recruiting arrestin2 and arrestin3, respectively.
4. Discussion
The relative importance of different P2 receptor subtypes present in smooth muscle for contraction is complex and delineation of the contributions of specific subtypes complicated by the lack of highly selective agonists/antagonists. Available data suggest that G protein-coupled P2Y2, P2Y4, and P2Y6 receptors, and ionotropic P2X1 receptors contribute to nucleotide-mediated contraction.4,16 Furthermore, evidence from aortic smooth muscle cells suggests that P2Y-receptor mRNA abundance differs between contractile and synthetic phenotypes, with decreases in P2Y4/P2Y6, and increases in P2Y2 mRNA being observed in the synthetic phenotype.17 Our pharmacological and siRNA knockdown data indicate that UTP/PLC responses in isolated MSMCs are primarily mediated by P2Y2 receptors, which concurs with previous findings that UTP-mediated contractions in mesenteric arteries (>180 µm) are sensitive to blockade by the P2Y2-selective antagonist suramin.16 Furthermore, our UTP-stimulated arterial contractile (EC50 28 µM) and MSMC Ca2+ (EC50 6 µM) responses are comparable to those reported in small (>100 µm) mesenteric arteries.16
Previous studies have described desensitization of P2Y2-receptor responses in several cell backgrounds,8,9,18,19 however, few studies have addressed the regulation of native P2Y2-receptor responsiveness and none to our knowledge have done so in mesenteric arteries. In human promonocytic U937, astrocytoma 132N1 and epithelial HT-29 cells P2Y2-receptor desensitization has been reported to occur rapidly and is enhanced following phorbol ester addition, however in all cases inclusion of PKC inhibitors failed to prevent receptor desensitization.9,18 Indeed, the observed attenuations of signalling may have been the result of PKC-mediated inhibition of PLC activity.20 Interestingly, protein phosphatase inhibitors can attenuate P2Y2-receptor resensitization.18 Collectively, these findings suggest that P2Y2-receptor phosphorylation, by a kinase(s) other than PKC is required for desensitization.18 Our preliminary experiments demonstrated that a protocol similar to that utilized to study H1 histamine-receptor desensitization allows quantitative assessment of the desensitization of MSMC responses to UTP.15 By employing a protocol where a sub-maximally effective (∼EC50) concentration of agonist is added to cells before (R1) and after (R2) a maximal, desensitizing agonist exposure (Rmax), attenuations of responsiveness are detected irrespective of P2Y2-receptor reserve. Moreover, we have extended this approach and utilized a similar R1/Rmax/R2 protocol in small, intact mesenteric vessels (∼150 µm diameter) to assess receptor desensitization/resensitization at the level of the UTP-evoked contractile response. Interestingly, our data indicate that UTP-stimulated intact vessel contractions are relatively resistant to desensitization when compared with PLC/Ca2+ responses in primary MSMCs. These differences might relate to the differing capacities of intact mesenteric smooth muscle and cultured MSMC preparations to metabolize UTP, and/or ex vivo changes in receptor populations and/or post-receptor components. Nevertheless, using comparable protocols, it is possible to assess the time-course of receptor desensitization/resensitization with respect to both UTP-stimulated contractile and signalling responses in tissue/cell preparations.
Since GRK proteins are known to regulate the signalling of other PLC-coupled GPCRs expressed in MSMCs,14,21,22 we initially utilized dominant-negative (kinase-dead) GRK mutants to disrupt P2Y2-receptor/GRK isoenzyme-specific interactions in an attempt to attenuate or prevent the reduction in receptor responsiveness observed on re-addition of UTP subsequent to a desensitizing pulse of this agonist. The D110A,K220RGRK2 construct, which has been mutated to prevent both kinase activity and Gαq/11-binding,20 markedly attenuated P2Y2-receptor desensitization. Conversely, over-expression of D110A,K220RGRK3, K215RGRK5, or K215RGRK6 mutants affected neither the extent of desensitization, nor the time-course of recovery of P2Y2-receptor responsiveness to UTP. To complement our findings (and address any potential criticisms associated with the recombinant over-expression of GRK mutants), we also depleted (>75%) endogenous GRK2 expression in MSMCs using isoenzyme-specific siRNAs, producing near-identical data to those obtained using the D110A,K220RGRK2 construct.
Together these findings indicate that GRK2 is a key endogenous GRK isoenzyme initiating P2Y2-receptor desensitization in MSMCs, with either GRK2 knockdown or disruption of the normal GRK2-receptor interaction causing an ∼60% attenuation of agonist-stimulated P2Y2-receptor desensitization; a figure only ∼15% less than that achievable after full receptor resensitization. It is possible that GRK2 is the only kinase involved in initiating P2Y2-receptor desensitization and that the observed partial effects arise because the experimental ablations of GRK2 activity are incompletely effective. On the other hand, while a predominant GRK isoenzyme can often be identified as being responsible for initiating receptor desensitization it is rare for this to be the only protein kinase involved.23,24 Therefore, other (minor) mechanisms may yet be shown to be involved in regulating P2Y2-receptor responsiveness in MSMCs.
GRK2 has previously been reported to be the key GRK isoenzyme regulating angiotensin II type 1 (AT1),25 α1D-adrenergic,22 and ETA14 receptor-mediated contractile responses. The finding that GRK2 is also key to the regulation of P2Y2-receptor signalling further emphasizes the importance of this GRK isoenzyme in Gαq/11/PLC-coupled receptor regulation in arterial smooth muscle.
GRK-mediated phosphorylation often leads to arrestin recruitment to the receptor, promoting internalization, receptor recycling, and/or downregulation.10,11 When expressed in HEK293 cells, P2Y2-receptors recruit both exogenous arrestin2 and arrestin3,26 however, while suggestive that P2Y2 receptors can interact with arrestin proteins, these studies are not necessarily predictive of how, or if, these receptors are regulated in native tissues. Here, we utilized an RNAi strategy to suppress individual arrestin isoform expression to delineate for the first time their role in P2Y2 receptor regulation in resistance artery smooth muscle. Our data indicate a high degree of isoform-selective arrestin interaction with P2Y2, with siRNA-mediated depletion of arrestin2 almost completely attenuating UTP-stimulated P2Y2-receptor desensitization. Conversely, knockdown of arrestin3 was without effect on P2Y2 signalling. We also assessed whether another important vasoconstrictor GPCR also differentially recruits arrestin2/3 isoforms, and showed that ETA-receptor responses are attenuated by arrestin3, but not arrestin2 knockdown. Moreover, we recently showed that GRK2 is also a critical kinase involved in ETA-receptor desensitization.14 Consequently, our findings clearly demonstrate that phosphorylation by a common GRK isoenzyme can lead to contrasting arrestin isoform recruitment. A likely explanation that deserves future investigation is that the pattern of GRK2-mediated P2Y2- and ETA-receptor phosphorylation differs sufficiently to promote differential recruitment of arrestins.
Finally, the potential importance of arrestin recruitment in vascular disease has been highlighted by two recent studies. Data from arrestin knockout mice reveal a role for arrestins in the regulation of vascular smooth muscle migration and proliferation during atherosclerosis and neointimal hyperplasia,27 while arrestin3 regulates angiotensin II-stimulated, ERK-mediated aortic smooth muscle proliferation.28 Since our new findings highlight a selective arrestin regulation of ETA- and P2Y2-receptors, it is likely that differential recruitment of arrestins to phosphorylated GPCRs has the potential to determine physiological and pathophysiological signalling outcomes activated by UTP and ET-1.
Funding
This work is supported by Project and Programme funding from the British Heart Foundation (grant nos. PG06/161/22136; RG06/008/22062). Funding to pay the Open Access publication charge was provided by the British Heart Foundation.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We thank Tobias Meyer (Stanford University, USA) for generously providing the eGFP-PH biosensor, and Robert J. Lefkowitz (Duke University, USA) for kindly providing the arrestin (A1CT) antibody.
Conflict of interest: none declared.
References
- 1.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. doi:10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter P, Dora KA. Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol. 2007;582:335–347. doi: 10.1113/jphysiol.2007.135202. doi:10.1113/jphysiol.2007.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Kugelgen I, Haussinger D, Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedeberg's Arch Pharmacol. 1987;336:556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]
- 4.Malmsjo M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. doi:10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. doi:10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumari R, Goh G, Ng LL, Boarder MR. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. doi:10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br J Pharmacol. 1996;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Gutierrez AM, Ring A, Persson AE. Nitric oxide induces resensitization of P2Y nucleotide receptors in cultured rat mesangial cells. J Am Soc Nephrol. 2002;13:313–321. doi: 10.1681/ASN.V132313. [DOI] [PubMed] [Google Scholar]
- 9.Otero M, Garrad RC, Velazquez B, Hernandez-Perez MG, Camden JM, Erb L, et al. Mechanisms of agonist-dependent and -independent desensitization of a recombinant P2Y2 nucleotide receptor. Mol Cell Biochem. 2000;205:115–123. doi: 10.1023/a:1007018001735. doi:10.1023/A:1007018001735. [DOI] [PubMed] [Google Scholar]
- 10.Willets JM, Challiss RAJ, Nahorski SR. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci. 2003;24:626–633. doi: 10.1016/j.tips.2003.10.003. doi:10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Luttrell LM, Lefkowitz RJ. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 12.Hayabuchi Y, Dart C, Standen NB. Evidence for involvement of A-kinase anchoring protein in activation of rat arterial KATP channels by protein kinase-A. J Physiol. 2001;536:421–427. doi: 10.1111/j.1469-7793.2001.0421c.xd. doi:10.1111/j.1469-7793.2001.0421c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson CP, Nahorski SR, Challiss RAJ. Temporal profiling of changes in phosphatidylinositol 4,5-bisphosphate, inositol 1,4,5-trisphosphate and diacylglycerol allows comprehensive analysis of phospholipase C-initiated signalling in single neurons. J Neurochem. 2008;107:602–615. doi: 10.1111/j.1471-4159.2008.05587.x. doi:10.1111/j.1471-4159.2008.05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris GE, Nelson CP, Standen NB, Challiss RAJ, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85:424–433. doi: 10.1093/cvr/cvp310. doi:10.1093/cvr/cvp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willets JM, Taylor AH, Shaw H, Konje JC, Challiss RAJ. Selective regulation of H1 histamine receptor signaling by G protein-coupled receptor kinase 2 in uterine smooth muscle cells. Mol Endocrinol. 2008;22:1893–1907. doi: 10.1210/me.2007-0463. doi:10.1210/me.2007-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gitterman DP, Evans RJ. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. doi:10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlinge D, Hou M, Webb TE, Barnard EA, Moller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. doi:10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- 18.Santiago-Perez LI, Flores RV, Santos-Berrios C, Chorna NE, Krugh B, Garrad RC, et al. P2Y2 nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases. J Cell Physiol. 2001;187:196–208. doi: 10.1002/jcp.1063. doi:10.1002/jcp.1063. [DOI] [PubMed] [Google Scholar]
- 19.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Willets JM, Nahorski SR, Challiss RAJ. Roles of phosphorylation-dependent and -independent mechanisms in the regulation of M1 muscarinic acetylcholine receptors by G protein-coupled receptor kinase 2 in hippocampal neurons. J Biol Chem. 2005;280:18950–18958. doi: 10.1074/jbc.M412682200. doi:10.1074/jbc.M412682200. [DOI] [PubMed] [Google Scholar]
- 21.Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle Gq signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol. 2007;293:H3072–H3079. doi: 10.1152/ajpheart.00880.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cohn HI, Harris DM, Pesant S, Pfeiffer M, Zhou RH, Koch WJ, et al. Inhibition of vascular smooth muscle G protein-coupled receptor kinase 2 enhances α1D-adrenergic receptor constriction. Am J Physiol. 2008;295:H1695–H1704. doi: 10.1152/ajpheart.00564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willets JM, Mistry R, Nahorski SR, Challiss RAJ. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell m3 muscarinic acetylcholine receptor signaling. Mol Pharmacol. 2003;64:1059–1068. doi: 10.1124/mol.64.5.1059. doi:10.1124/mol.64.5.1059. [DOI] [PubMed] [Google Scholar]
- 24.Willets JM, Brighton PJ, Mistry R, Morris GE, Konje JC, Challiss RAJ. Regulation of oxytocin receptor responsiveness by G protein-coupled receptor kinase 6 in human myometrial smooth muscle. Mol Endocrinol. 2009;23:1272–1280. doi: 10.1210/me.2009-0047. doi:10.1210/me.2009-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckhart AD, Ozaki T, Tevaearai H, Rockman HA, Koch WJ. Vascular-targeted overexpression of G protein-coupled receptor kinase-2 in transgenic mice attenuates β-adrenergic receptor signaling and increases resting blood pressure. Mol Pharmacol. 2002;61:749–758. doi: 10.1124/mol.61.4.749. doi:10.1124/mol.61.4.749. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. J Biol Chem. 2008;283:30933–30941. doi: 10.1074/jbc.M801472200. doi:10.1074/jbc.M801472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, et al. β-Arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. doi:10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent β-arrestin2 and Gq/protein kinase Cζ pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284:11953–11962. doi: 10.1074/jbc.M808176200. doi:10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.