Abstract
Aims
The ex vivo expansion of cardiac stem cells from minimally invasive human heart biopsies yields tens of millions of cells within 3–4 weeks, but chromosomal abnormalities were frequently detected in preliminary production runs. Here we attempt to avoid aneuploidy and improve cell quality by expanding human cardiac stem cells in physiological low-oxygen (5% O2) conditions, rather than in traditional culture in a general CO2 incubator (20% O2).
Methods and results
Human heart biopsies (n = 16) were divided and processed in parallel to expand cardiac stem cells under 5% or 20% O2. Compared with 20% O2, 5% O2 culture doubled the cell production and markedly diminished the frequency of aneuploidy. Cells expanded in 5% O2 showed lower intracellular levels of reactive oxygen species, less cell senescence, and higher resistance to oxidative stress than those grown in 20% O2, although the expression of stem cell antigens and adhesion molecules was comparable between groups, as was the paracrine secretion of growth factors into conditioned media. In vivo, the implantation of 5% O2 cells into infarcted hearts of mice resulted in greater cell engraftment and better functional recovery than with conventionally cultured cells.
Conclusion
The expansion of human adult cardiac stem cells in low oxygen increased cell yield, and the resulting cells were superior by various key in vitro and in vivo metrics of cell quality. Physiological oxygen tensions in culture facilitate the ex vivo expansion of healthy, biologically potent stem cells.
Keywords: Physiological oxygen, Ex vivo expansion, Cardiac stem cells
1. Introduction
Expansion in culture is the foundation for the vast majority of applications of adult stem cells and embryonic stem (ES) cells, including regenerative medicine and drug development. Chromosomal abnormalities are frequently found in stem cells after long-term culture,1–3 and chromosomal abnormalities may enhance carcinogenesis and impair functional potency,4,5 complicating the therapeutic application of stem cells. Therefore, it is critically important to expand better-quality stem cells with fewer chromosomal abnormalities for clinical applications.
Recently discovered resident cardiac stem cells are considered to be particularly promising for myocardial regeneration, as they inherently mediate cardiogenesis and angiogenesis.6–16 We have developed techniques to expand tens of millions of cardiac stem cells and supporting cells (collectively termed cardiac-derived cells, CDCs) from minimally invasive human heart biopsies using ex vivo culture.13–16 Clinical application of CDCs is already under way in the CADUCEUS (CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction, ClinicalTrials.gov. Identifier NCT00893360) trial. Unfortunately, karyotyping revealed that approximately one-third of preliminary CDC production runs included cytogenetically abnormal cells, most often due to changes in chromosome number (aneuploidy). Given these findings, we included genetic screening as a product release criterion in CADUCEUS, while initiating process improvement efforts to minimize chromosomal abnormalities.
Ex vivo expansion of stem cells, including our CDCs, is generally performed by incubating cells in incubators equilibrated with 95% air and 5% CO2 (∼20% O2). However, the oxygen concentration of the in vivo microenvironment of stem cells in biological tissues is much lower (1–7%, depending on the tissue).17,18 Stem cells used for a low-oxygen microenvironment in vivo may be subjected to oxidative stress under conventional culture conditions. Recent studies have shown that stem cells cultured in low oxygen (2–3% O2) exhibit fewer chromosomal abnormalities and less cell senescence.19,20 Given that oxidative stress figures prominently in DNA damage and genomic instability,21 here we test the hypothesis that ex vivo expansion of human cardiac stem cells in ‘hypoxic’ conditions (mimicking the low oxygen tensions operative in vivo) may produce better-quality cells with fewer chromosomal abnormalities. Such technical developments would greatly facilitate safe clinical applications.
2. Methods
2.1. Ex vivo expansion of human cardiac stem cells under low oxygen
Adult human cardiac stem cells were expanded using similar methods as described previously.13,14 Briefly, endomyocardial heart tissue biopsies (∼10 mg) were obtained from patients during clinically indicated procedures after informed consent, in an Institutional Review Board-approved protocol. All investigations conform to the Declaration of Helsinki. Biopsies were minced into small fragments and digested with 0.2 mg/mL of collagenase for 30 min. The digested tissue fragments were then equally moved to two 6 cm diameter culture dishes coated with 20 µg/mL of fibronectin (BD Biosciences) and randomly selected to culture as ‘explants’ in a typical CO2 incubator (20% O2) or a incubator with physiological low oxygen (5% O2). Following the step of cardiosphere formation, the CDCs were grown from cardiospheres and expanded by passaging under 20% O2 (20% O2 CDCs) or 5% O2 (5% O2 CDCs), respectively. IMDM basic medium (Gibco) supplemented with 10% FBS (Hyclone) and 20 mg/mL gentamycin was used for all cultures. Twice-passaged CDCs (1–2 months culture from the date of tissue biopsy) were used for experiments except as indicated.
2.2. Karyotype analysis
CDCs were seeded onto fibronectin-coated 25 cm2 tissue culture flasks (104 cells/cm2) and continuously cultured under 5% O2 or 20% O2, respectively. The karyotyping data here are extended from our recent report.22 After ∼24 h of incubation, cells were treated with 0.1 µg/mL of colcemid (Invitrogen) for 4 h. Then the cells were trypsinized, treated with hypotonic solution, and fixed. Metaphases were spread on microscope slides, and karyotype analysis was done using standard G banding technique. The chromosomes were classified according to the International System for Human Cytogenetic Nomenclature. At least 20 metaphases were analysed per cell sample. Notably, all but one of the patients whose samples underwent karyotypic analysis were immunosuppressed heart transplant recipients.
2.3. Flow cytometry
To investigate how physiological low-oxygen culture affects the subpopulation of c-kit+ stem cells and the expression of p16INK4A, a marker for cell senescence, CDCs expanded in 5% O2 and 20% O2 were harvested as single-cell suspensions using trypsin digestion. Cells were then incubated with PE-conjugated mouse anti-human c-kit antibody (eBioscience) or mouse anti-human p16INK4A antibody (BD Biosciences) for 60 min. The expressions of c-kit and p16INK4A were quantitatively measured using a FACS Calibur flow cytometer with CellQuest software (BD Biosciences).13,23–25
2.4. Immunostaining
To determine the myogenic differentiation in vitro, CDCs were seeded on fibronectin-coated four-chamber culture slides and continuously cultured in 5% O2 or 20% O2. After 3 days of culture, cells were fixed, blocked with goat serum for 30 min, and then incubated with mouse anti-human troponin T antibody (R&D Systems Inc.) for 1 h at room temperature. Culture slides were washed and then incubated with a PE-conjugated secondary antibody. Cell nuclei were stained with DAPI.23–25 Myogenic differentiation was quantified by calculating the positive-stained area using the Image-Pro Plus software (version 5.1.2, Media Cybernetics Inc., Carlsbad, CA, USA).
The telomerase activity and DNA damage in CDCs were also estimated by immunostaining with mouse anti-human telomerase catalytic subunit (TERT) antibody (Lifespan Bioscience) or rabbit polyclonal antibody against γ-H2AX (phosphor S139, Abcam Inc.), as described above. Positively stained cells were counted by fluorescence microscopy.
2.5. Senescence-associated β-galactosidase staining
Third-passage CDCs were seeded on fibronectin-coated four-chamber culture slides and continuously cultured in 5% O2 or 20% O2. After 3 days of culture, senescence-associated β-galactosidase (SA-β-Gal) was performed as described previously.24 The SA-β-Gal-positive cells were counted under a microscope.
2.6. TUNEL assay
To quantify the resistance to oxidative stress in vitro, CDCs were seeded on fibronectin-coated four-chamber culture slides and continuously cultured in 5% O2 or 20% O2. After 2 days of culture, cells were moved into a general incubator (20% O2), and cultured with or without the addition of 100 µM H2O2 in the medium for another 24 h. Cells were fixed, and apoptotic cells were detected by TUNEL assay (Roche Diagnostics, Mannheim, Germany), according to the manufacturer's instructions. Cell nuclei were stained with DAPI, and TUNEL-positive cells were counted under fluorescence microscopy.
2.7. Measurement of intracellular reactive oxygen species
To measure intracellular reactive oxygen species (ROS), CDCs were seeded in 6- or 96-well plates coated with 20 µg/mL of fibronectin and continuously cultured in 5% O2 or 20% O2. At ∼90% confluence, cells were incubated with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen) for 60 min. The fluorescence of 2′,7′-dichlorodihydrofluorescein (DCF) in cells cultured in 96-well plates is directly determined using SpectraMax M5 (Molecular Devices Corp.) with an excitation wavelength of 495 nm and an emission wavelength of 520 nm. Cells cultured in six-well plates were trypsin-treated and fixed. The DCF fluorescence intensity in cells was analysed by flow cytometry as described above.
2.8. Western blotting
Western blot analysis was performed to compare the expressions of integrin-α2, laminin-β1, and c-Myc in CDCs cultured under 5% O2 and 20% O2, as described previously.26 The equivalent of 30 µg of total protein was loaded onto SDS–PAGE gels, and then transferred to PVDF membranes. After overnight blocking, membranes were incubated with mouse anti-human integrin-α2 antibody, mouse anti-human laminin-β1 antibody, rabbit anti-β-actin monoclonal antibody (Lifespan Bioscience), or mouse anti-human c-Myc antibody (BD Biosciences). The appropriate horseradish peroxidase-conjugated secondary antibodies were used, and then the blots were visualized by using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific) and exposed to Gel Doc™ XR System (Bio-Rad Lab. Inc.). Quantitative analysis for blots was done by Quantity One software, and expressions were normalized by β-actin.
2.9. ELISA
To compare the potency of the productions of growth factors, CDCs expanded under 5% O2 or 20% O2 were seeded in 24-well culture plates at a density of 1 × 105 mL−1 and incubated for 3 days with hypoxic stimulation (under 1% O2 to mimic the microenvironment of the ischaemic heart). The supernatants were collected and the concentrations of Angiopoietin-2, bFGF, HGF, IGF-1, SDF-1, and VEGF were measured with human ELISA kits (R&D Systems Inc.), according to the manufacturer's instructions.
2.10. In vitro angiogenesis assay
Angiogenic potency was estimated by tube formation using an in vitro angiogenesis assay kit (Chemicon Int.), according to the manufacturer's instructions. Briefly, CDCs expanded under 5% O2 or 20% O2 were seeded on ECMatrix™-coated 96-well plates at a density of 2 × 104 cells per well. After 6 h incubation with hypoxic stimulation (under 1% O2 to mimic the microenvironment of ischaemic heart), the tube formation were imaged. The total tube length was then measured with Image-Pro Plus software.
2.11. Myocardial infarction model and cell implantation
An acute myocardial infarction was created in adult male SCID-beige mice (10–12 weeks old), as described previously.13,24 The study was approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Briefly, after general anaesthesia and tracheal intubation, mice were artificially ventilated with room air. A left thoracotomy was performed through the fourth intercostal space and the left anterior descending artery was ligated with 9-0 prolene under direct vision. The mice were then randomized and subjected to intramyocardial injections with a 30 G needle at four points in the infarct border zone with 40 µL of PBS (PBS group, n = 10), 1 × 105 CDCs expanded under 20% O2 (20% O2 CDCs, n = 10), or 1 × 105 CDCs expanded under 5% O2 (5% O2 CDCs, n = 12).
2.12. Quantification of engraftment by real-time PCR
Quantitative PCR was performed 24 h and 7 days after cell injection in six to seven animals from each cell-injected group to quantify cell retention/engraftment. We injected male human CDCs to enable detection of the SRY gene located on the Y chromosome. The whole mouse heart was harvested, weighed, and homogenized. The TaqMan® assay was used to quantify the number of transplanted cells with the human SRY gene as template (Applied Biosystems, CA, USA). A standard curve was generated with multiple dilutions of genomic DNA isolated from the injected CDCs to quantify the absolute gene copy numbers. All samples were spiked with equal amounts of genomic DNA from non-injected mouse hearts as control. For each reaction, 50 ng of genomic DNA was used. Real-time PCR was performed with an Applied Biosystems 7900 HT Fast real-time PCR machine. Experiments were performed in triplicate. The number of engrafted cells per heart was quantified by calculating the copy number of SRY gene in the total amount of DNA based on the standard curve.
2.13. Echocardiography
Mice underwent echocardiography at 3 h (baseline) and 3 weeks after surgery using Vevo 770™ Imaging System (VISUALSONICS™, Toronto, Canada).13,24 After the induction of light general anaesthesia, the hearts were imaged two-dimensionally (2D) in long-axis views at the level of the greatest LV diameter. LV end diastolic volume, LV end systolic volume, and LV ejection fraction (LVEF) were measured with VisualSonics V1.3.8 software from 2D long-axis views taken through the infarcted area.
2.14. Histology
Mice were sacrificed 3 weeks after treatment. Hearts were sectioned in 5 µm slices and fixed with 4% paraformaldehyde. The engraftment of implanted human CDCs was identified by immunostaining with anti-human nuclear antigen (HNA) antibody (Chemicon). To measure cell engraftment, 10 images of the infarction and border zones were selected randomly from each animal (three sections/animal; 1 mm separation between sections; 20× magnification, Eclipse TE2000-U). The percentages of human nuclei per total nuclei were quantified using Image-Pro Plus software (version 5.1.2, Media Cybernetics Inc.), and the average value from each heart was used for statistical analysis.24
The differentiation of cardiac stem cells into myocytes, smooth muscle cells, and endothelial cells was identified by immunostaining with monoclonal antibodies against human-specific α-sarcomeric actin, smooth muscle actin, and von Willebrand factor (vWF) (Sigma), respectively, as described above.
Masson's trichrome staining was also performed to examine the infarction size, LV wall thickness, LV chamber area, and viable myocardium, as described previously.27
2.15. Statistical analysis
All results are presented as mean ± SD. Statistical significance between two groups was determined using the two-tailed paired t-test and among groups by ANOVA followed by Bonferroni post hoc test (Dr SPSS II, Chicago, IL, USA), unless otherwise indicated. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. Cell production
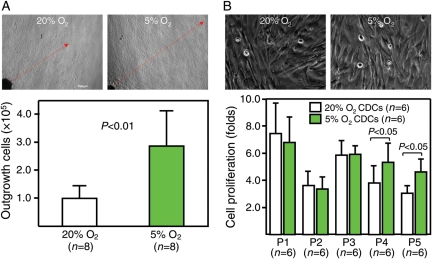
Compared with culture in 20% O2, the outgrowth of cells from the ‘explants’ was faster in 5% O2, resulting in more than two-fold higher yield of outgrowth cells (P < 0.001, Figure 1A). The proliferation of CDCs was similar at subsequent early passages,1–3 although proliferation at later passages4,5 was higher in CDCs expanded in 5% O2 (P < 0.05, Figure 1B). It is unclear why proliferation in 5% O2 slows down temporarily after replating, but overall the net result is a substantial increase in the yield of CDCs relative to conventional culture conditions.
Figure 1.
Growth and proliferation of cardiac stem cells in 5% O2 and 20% O2. (A) Representative images (upper) show that cell outgrowth from ‘explants’ (red arrow) was much faster in 5% O2 than in 20% O2. The number of cells harvested (lower bar graph) was more than two-fold higher in 5% O2 than in 20% O2, although the amount of starting material was equivalent. (B) CDCs at earlier passages show no differences in either morphology (upper images, passage #2) or proliferative activity (lower bar graph) under 5% O2 and 20% O2, although greater proliferation was observed in cells expanded under 5% O2 at later passages.
3.2. Chromosomal abnormalities
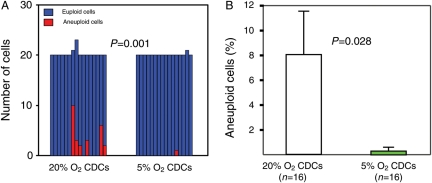
Consistent with our preliminary karyotyping data,22 6 of 16 (37.5%) CDC samples expanded in 20% O2 included aneuploid cells (26 of 323 examined cells, Figure 2A). The most common changes were trisomy 8 (Supplementary material online, Figure S1A) and Y chromosome loss. In contrast, in 16 CDC samples expanded under 5% O2, there was only 1 aneuploid cell (from a total of 321 examined cells; P < 0.01 by χ2 test, Figure 2A). The aneuploidy here reflected loss of the Y chromosome, an innocuous cytogenetic abnormality.28 These differences in genomic stability cannot be due to differences in the source tissue, as the same biopsies were subdivided and cultured in parallel to facilitate direct comparison. The percentages of aneuploid cells were also lower in CDCs expanded under 5% O2 than in 20% O2 (P = 0.028 by unpaired t-test, Figure 2B). Thus, ex vivo expansion of human cardiac stem cells under physiological low-oxygen conditions dramatically diminishes the incidence of chromosomal abnormalities.
Figure 2.
Chromosomal abnormalities. (A) Lower numbers of aneuploid cells were found in CDCs expanded in 5% O2 than in 20% O2. (B) The percentages of aneuploid cells were also decreased in 5% O2 culture.
3.3. Phenotype and in vitro myogenic differentiation
The subpopulation of c-kit+ stem cells was comparable in CDCs expanded in 5% O2 and in 20% O2 (P = 0.106, Supplementary material online, Figure S2A). Likewise, the propensity to cardiomyogenic differentiation, by immunostaining for human-specific cardiac troponin T, was equivalent in the two conditions (Supplementary material online, Figure S2B).
3.4. Cell senescence
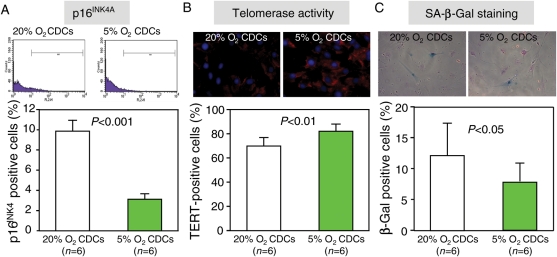
Cell senescence was evaluated by the expression of p16INK4A, telomerase activity, and SA-β-Gal staining. The expression of p16INK4A was lower (Figure 3A, P < 0.001), and the telomerase activity (evaluated by the expression of TERT) was higher (Figure 3B, P < 0.01) in CDCs expanded in 5% O2. The fraction of SA-β-Gal-positive cells was also lower in third-passage CDCs expanded in 5% O2 than in 20% O2 (Figure 3C, P < 0.05).
Figure 3.
Cell senescence. Compared with 20% O2 culture, cell senescence of CDCs was improved under 5% O2 culture, by flow cytometry for p16INK4A (A), immunostaining for telomerase activity (B), and senescence-associated β-galactosidase staining (C).
3.5. Intracellular ROS, DNA damage, and resistance to oxidative stress
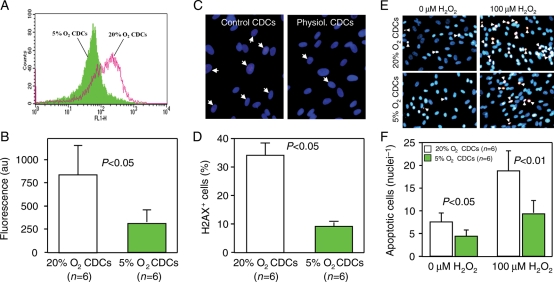
We measured intracellular ROS by DCF staining and found that the intracellular ROS level was lower in 5% O2-cultured CDCs than in 20% O2-cultured CDCs (Figure 4A, P < 0.01). The percentage of cells with γ-H2AX foci, a marker of DNA damage,29 was likewise lower in 5% O2-cultured CDCs (Figure 4B, P < 0.01). This finding agrees well with the karyotyping data showing a dramatic reduction of aneuploidy in CDCs expanded under 5% O2 (Figure 2). We also wondered whether cells cultured in physiological O2 might be more resistant, or more sensitive, to imposed oxidative stress. We addressed this question by exposing CDCs to H2O2, a powerful oxidant. After 24 h of exposure to 100 µM H2O2, the number of apoptotic cells was lower in 5% O2 CDCs than in those grown in 20% O2 (Figure 4C, P < 0.01). The basal frequency of apoptosis was also lower in the 5% O2 CDCs (P < 0.05).
Figure 4.
Intracellular ROS, DNA damage, and resistance to oxidative stress. (A) The levels of intracellular ROS are lower in CDCs expanded in 5% O2 when compared with those in 20% O2. (B) DNA damage evidenced by the formation of γ-H2AX foci (upper images) was lower in CDCs expanded in 5% O2 than in 20% O2 (lower bar graph). (C) Representative images (upper images) show TUNEL-positive (red) CDCs after 24 h exposure to 100 µM H2O2. The number of apoptotic cells (lower bar graph) was lower in CDCs expanded in 5% O2 than 20% O2.
3.6. Expression of adhesion molecules and c-Myc
The expression levels of integrin-α2, laminin-β1, and c-Myc were comparable in CDCs expanded in 5% O2 and in 20% O2 (see Supplementary material online, Figure S3).
3.7. In vitro production of growth factors and tube formation
CDCs are known to secrete a variety of growth factors.27,30 We analysed conditioned media to determine whether hypoxic culture influences the paracrine secretion of selected growth factors by CDCs. Cells cultured either in 5% O2 or 20% O2 were plated in fresh media and grown for 3 days with hypoxic stimulation (1% O2) to mimic the microenvironment of ischaemic heart. The production of the majority of growth factors under hypoxic stimulation, including angiopoietin-2, bFGF, HGF, IGF-1, SDF-1, and VEGF, was comparable in CDCs expanded under 5% O2 or 20% O2 (see Supplementary material online, Figure S4), although some factors tended to be higher in CDCs expanded under 5% O2.
By in vitro angiogenesis assay, CDCs expanded under both 5% O2 and 20% O2 could form capillary-like networks (tube formation) on matrigel in 6 h with hypoxic stimulation, and there was no significant difference between groups (see Supplementary material online, Figure S5).
3.8. In vivo cell engraftment and differentiations
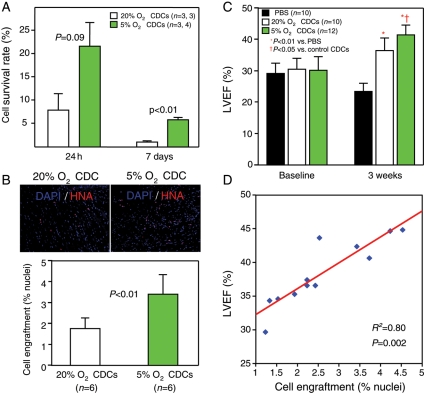
Any assessment of cell quality ultimately must include tests of efficacy in vivo. As a first step, we quantified cell retention (24 h) and engraftment (7 days) in hearts injected with CDCs cultured in 5% O2 or 20% O2. Quantitative PCR analysis showed that CDCs expanded in 5% O2 survived better than CDCs expanded in 20% O2, both at 24 h and at 7 days after implantation into the infarcted hearts of SCID mice (Figure 5A). Furthermore, in the scar and marginal regions of the infarcted mouse heart 3 weeks after treatment, we detected greater survival of CDCs expanded in 5% O2 than in 20% O2 (Figure 5A). Quantitative analysis of percentages of human nuclei revealed better cell engraftment after implantation of CDCs expanded under 5% O2 than under 20% O2 (3.40 ± 0.94 vs. 1.77 ± 0.52%, P < 0.01, Figure 5B). The improvements in retention and engraftment imply greater transplanted cell resilience, consistent with the observed increase in resistance to oxidative stress (Figure 4C) and the decrease in senescence (Figure 3) in 5% O2-cultured CDCs.
Figure 5.
Cell engraftment, cardiac functional recovery, and their relationships. (A) Quantitative data on the survival rate of human CDCs 24 h and 7 days after implantation into mice-infarcted heart. (B) Cells positively stained by HNA are more frequently observed in mice 3 weeks after implantation with CDCs expanded in 5% O2 than in 20% O2 (upper images). Lower bar graph shows quantitative data for cell engraftment (% nuclei) in the infarcted heart. (C) LVEF at baseline does not differ among groups, indicating a similar infarct size to begin with. After 3 weeks, the LVEF was higher in mice implanted with CDCs expanded in 5% O2 than in 20% O2, although the LVEF was also higher in mice implanted with CDCs expanded in 20% O2 than in controls with PBS injection only. (D) The engraftment of human CDCs (% nuclei) within the infarcted hearts of mice is strongly correlated with the absolute values of LVEF at 3 weeks.
One distinctive feature of CDCs is the ability to detect consistent cardiomyogenic and angiogenic differentiation of injected cells,12–15,27,31 even though the mechanism of functional benefit is largely paracrine.27,31 We therefore confirmed that CDCs can differentiate into cardiomyocytes, endothelial and vascular smooth muscle cells, irrespective of the level of oxygen in which they were cultured. Histology of mouse hearts that had been injected with CDCs 3 weeks earlier revealed expression of α-sarcomeric actin (see Supplementary material online, Figure S6), smooth muscle actin (see Supplementary material online, Figure S7), and vWF (see Supplementary material online, Figure S8) in some of the surviving progeny of human CDCs expanded in either 5% O2 or 20% O2, indicative of multilineage differentiation. Although CDCs expanded in 5% O2 exhibited greater survival, their differentiation potential was not noticeably different than that of 20% O2-cultured CDCs.
3.9. Cardiac function and infarct size
To assess efficacy in myocardial repair, we quantified left ventricular function in hearts injected with vehicle or with CDCs cultured in 5% O2 or 20% O2. Although LVEF at baseline did not differ between groups, LEVF measured 3 weeks after treatment was higher in mice implanted with 5% O2-cultured CDCs that with those expanded in 20% O2 (41.5 ± 3.2 vs. 36.5 ± 4.1%, P = 0.043, Figure 5B). Both cell groups outperformed vehicle (Figure 5C). In addition, the absolute values of LVEF correlate strongly with the degree of cell engraftment (r2 = 0.80, P = 0.002, Figure 5D), as seen before in our work on magnetic targeting.31
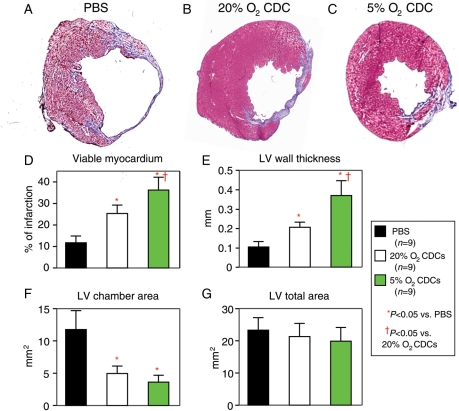
Compared with the control treatment with PBS injection only, the implantation of CDCs expanded under either 5% O2 or 20% O2 results in obviously smaller infarct size, more viable myocardium, increased infarct wall thickness, and lower LV chamber area 3 weeks after treatment (P < 0.05 vs. PBS, Figure 6). These parameters were even better with 5% O2-cultured CDCs that with those expanded in 20% O2 (P < 0.05).
Figure 6.
Histological assessments of infarct size and ventricular morphology. Representative images of Masson's staining show that, compared with an infarcted heart receiving PBS injection only (A), the infarct size was much smaller in a heart that had received CDCs expanded in both 5% O2 (C) and 20% O2 (B). Quantitative analyses of viable myocardium (D), LV wall thickness (E), LV chamber area (F), and LV total area (G) show that better therapeutic efficiency was achieved by the implantation of CDCs expanded in 5% O2 than in 20% O2, although a significant improvement was also observed by the implantation of CDCs expanded under 20% O2 when compared with the control treatment with PBS injection only.
4. Discussion
We have found that long-term ex vivo expansion of human CDCs under physiological oxygen (5% O2) increased cell yield, decreased aneuploidy and cell senescence, and increased resistance to oxidative stress. Furthermore, human CDCs expanded in 5% O2 showed greater engraftment and functional benefit after implantation into infarcted hearts of SCID mice. These findings indicate that CDCs grown in ‘hypoxic’ conditions are functionally superior to those grown conventionally in 20% O2.
Mammalian cells, including stem cells, are generally cultured in room air or in incubators equilibrated with 95% air and 5% CO2 (net O2 ∼20%).32 According to measurements of the partial oxygen pressure in animal tissues, the physiological oxygen tensions in vivo are much lower (∼1–7% O2, depending on the tissue).17,18 This means that the culture of stem cells in a general CO2 incubator actually places the cells in a hyperoxic state relative to their in vivo environment. We find that CDCs expanded in 5% O2 have lower levels of intracellular ROS and less γ-H2AX foci, consistent with the notion that 20% O2 creates oxidative stress.
As oxidative stress is well known to potentiate DNA damage and cell senescence,21,33,34 it is not surprising that CDCs expanded in 5% O2 exhibit fewer chromosomal alterations and less cell senescence. While cytogenetic abnormalities in stem cells have been acknowledged to occur with some frequency,1,2,22 the problem has been largely ignored in clinical trials. Our findings highlight the magnitude of this complication even for primary adult stem cells. However, it seems likely that cytogenetic abnormalities may have been more frequent in the immunosuppressed heart transplant patient population which predominated in the samples studied here. Owing to the immunosuppression, post-transplant patients are predisposed to lymphomas with clonal chromosomal abnormalities.22 Notably, in the immunocompetent population studied in CADUCEUS, only 1 of 13 clinical-production samples to date has been abnormal by cytogenetic screening (unpublished results). In any case, the paucity of chromosomal abnormalities in 5% O2 culture means that cytogenetic screening may no longer be required as a product release criterion.
Another notable finding of this study is the accelerated cell production, especially within the ‘explant’ culture stage. As hypoxia can induce the expression of HIF-1α,35 we considered the possibility that 5% O2 culture may increase cell migration out from the ‘explants’ by such a mechanism. However, the degree of hypoxia in 5% O2 culture was insufficient to increase the concentrations of chemokines and cytokines, including VEGF and SDF-1, in the culture medium (data not shown), making it unlikely that hypoxia-induced cell migration accounts for the increased yields.
In contrast to previous reports in which physiological low-oxygen culture increases the ‘stemness’ of ES cells,36 the c-kit+ subpopulation and differentiation potency were not augmented in these twice-passaged CDCs expanded in 5% O2 relative to 20% O2. Furthermore, the expression levels of integrin-α2, laminin-β1, and c-Myc were also comparable in the two conditions, although previous studies have reported that hypoxia can enhance the expression of some adhesion molecules.26,37,38 These differences between studies may reflect genuine differences in the responses of specific cell types to hypoxia.39
When implanted into infarcted hearts of SCID mice, we found that 5% O2-cultured human CDCs engrafted better and improved function more than did 20% O2-cultured CDCs. The observed decrease in cell senescence and increased resistance to oxidative stress in vitro are in the right direction to help explain the superior effects in vivo. We know that the implantation of CDCs into infarcted heart leads to functional improvement via a combination of direct regeneration and paracrine effects.27 In this study, we confirm in vivo multilineage differentiation from human CDCs expanded under either 5% O2 or 20% O2,12–15,27,31 as well as the robust production of various angiogenic and anti-apoptotic growth factors in vitro.27,30 Here we did not attempt to quantify the relative roles of direct regeneration vs. indirect paracrine effects, but we believe that both are operative. The higher cell engraftment seen with 5% O2-cultured CDCs appears sufficient to rationalize their greater functional benefit, given the strong linear correlation between cell engraftment and functional improvement.
Although cardiac stem cell niches have been identified in the mammalian heart,40,41 the exact physiological oxygen tension within the niche is still unknown. Although 2–5% O2 has been used successfully for long-term culture of ES cells, bone marrow-derived mesenchymal stem cells, and adipose-derived stem cells,19,20,42 we chose 5% O2 in this study reasoning that heart tissue may be better oxygenated than bone marrow (1–2% O2) or adipose tissue (3% O2).17,18 In addition, we changed the medium in a laminar flow hood under room air; ∼10 min is required before the cells re-equilibrate with 5% O2 in the incubator. The fluctuation in O2 during these procedures may affect the quality of cells. Further experiments will be required to determine whether the quality of CDCs might be further improved with stricter control of oxygen tension (e.g. by using a closed hypoxia workstation).
In summary, we found that long-term culture under 5% O2 increased the production of human cardiac stem cells, and those cells were superior for myocardial regeneration. Our results are presumably generalizable to many other types of adult and pluripotent stem cells.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: E.M. is a founder and equity holder in Capricor, Inc. L.M. is a founder, equity holder, and consultant for Capricor, Inc. R.R.S. is partially employed by Capricor, Inc. Capricor provided no funding for the present study. The remaining authors report no conflicts.
Funding
This work was supported by National Institutes of Health (R01 HL083109 to E.M.).
Supplementary Material
References
- 1.Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. doi:10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 2.Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. doi:10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 3.Sareen D, McMillan E, Ebert AD, Shelley BC, Johnson JA, Meisner LF, et al. Chromosome 7 and 19 trisomy in cultured human neural progenitor cells. PLoS ONE. 2009;4:e7630. doi: 10.1371/journal.pone.0007630. doi:10.1371/journal.pone.0007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 5.Furlani D, Li W, Pittermann E, Klopsch C, Wang L, Knopp A, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2009;18:319–331. doi: 10.3727/096368909788534906. doi:10.3727/096368909788534906. [DOI] [PubMed] [Google Scholar]
- 6.Leri A. Human cardiac stem cells: the heart of a truth. Circulation. 2009;120:2515–2518. doi: 10.1161/CIRCULATIONAHA.109.911107. doi:10.1161/CIRCULATIONAHA.109.911107. [DOI] [PubMed] [Google Scholar]
- 7.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. doi:10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. doi:10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 9.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. doi:10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. doi:10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, et al. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. doi:10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 12.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. doi:10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 13.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. doi:10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 14.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. doi:10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston P, Sasano T, Mills K, Evers R, Lee ST, Smith RR, et al. Engraftment, differentiation and functional benefit of autologous cardiosphere-derived cells in a porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. doi:10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis DR, Kizana E, Terrovitis J, Barth AS, Zhang Y, Smith RR, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. doi:10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. doi:10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 18.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. doi:10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Thirumangalathu S, Loeken MR. Establishment of new mouse embryonic stem cell lines is improved by physiological glucose and oxygen. Cloning Stem Cells. 2006;8:108–116. doi: 10.1089/clo.2006.8.108. doi:10.1089/clo.2006.8.108. [DOI] [PubMed] [Google Scholar]
- 20.Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. doi:10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 21.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. doi:10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li TS, Marbán E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li TS, Suzuki R, Ueda K, Murata T, Hamano K. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells. 2007;25:911–917. doi: 10.1634/stemcells.2006-0497. doi:10.1634/stemcells.2006-0497. [DOI] [PubMed] [Google Scholar]
- 24.Li TS, Hayashi M, Liu ZL, Ito H, Mikamo A, Furutani A, et al. Low angiogenic potency induced by the implantation of ex vivo expanded CD117-positive stem cells. Am J Physiol Heart Circ Physiol. 2004;286:H1236–H1241. doi: 10.1152/ajpheart.00950.2003. doi:10.1152/ajpheart.00950.2003. [DOI] [PubMed] [Google Scholar]
- 25.Li TS, Hayashi M, Ito H, Furutani A, Murata T, Matsuzaki M, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. doi:10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 26.Li TS, Ito H, Hayashi M, Furutani A, Matsuzaki M, Hamano K. Cellular expression of integrin-beta 1 is of critical importance for inducing therapeutic angiogenesis by cell implantation. Cardiovasc Res. 2005;65:64–72. doi: 10.1016/j.cardiores.2004.08.019. doi:10.1016/j.cardiores.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. doi:10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo I. Chromosome abnormalities and cancer cytogenetics. Nat Educ. 2010;1:1. [Google Scholar]
- 29.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. doi:10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 30.Stastna M, Chimenti I, Marbán E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. doi:10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. doi:10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csete M. Oxygen in the cultivation of stem cells. Ann NY Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. doi:10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 33.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. doi:10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 34.Lee AC, Fenster BE, Ito H, Takeda K, Bae NS, Hirai T, et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J Biol Chem. 1999;274:7936–7940. doi: 10.1074/jbc.274.12.7936. doi:10.1074/jbc.274.12.7936. [DOI] [PubMed] [Google Scholar]
- 35.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. doi:10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. doi:10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubo M, Li TS, Kamota T, Ohshima M, Qin SL, Hamano K. Increased expression of CXCR4 and integrin alphaM in hypoxia-preconditioned cells contributes to improved cell retention and angiogenic potency. J Cell Physiol. 2009;220:508–514. doi: 10.1002/jcp.21803. doi:10.1002/jcp.21803. [DOI] [PubMed] [Google Scholar]
- 38.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. doi:10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–832. doi: 10.1038/nrg2665. doi:10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 40.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. doi:10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popescu LM, Gherghiceanu M, Manole CG, Faussone-Pellegrini MS. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–886. doi: 10.1111/j.1582-4934.2009.00758.x. doi:10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. doi:10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.