Abstract
Aims
Glucagon-like peptide 1 (GLP-1) is an incretin hormone released from the gut in response to food intake. Whereas GLP-1 acts in the periphery to inhibit glucagon secretion and stimulate insulin release, it also acts in the central nervous system to mediate autonomic control of feeding, body temperature, and cardiovascular function. Because of its role as an incretin hormone, GLP-1 receptor analogs are used as a treatment for type 2 diabetes. Central or peripheral administration of GLP-1 increases blood pressure and heart rate, possibly by activating brainstem autonomic nuclei and increasing vagus nerve activity. However, the mechanism(s) by which GLP-1 receptor stimulation affects cardiovascular function are unknown. We used the long-lasting GLP-1 receptor agonist Exendin-4 (Ex-4) to test the hypothesis that GLP-1 signalling modulates central parasympathetic control of heart rate.
Methods and results
Using a telemetry system, we assessed heart rate in mice during central Ex-4 administration. Heart rate was increased by both acute and chronic central Ex-4 administration. Spectral analysis indicated that the high frequency and low frequency powers of heart rate variability were diminished by Ex-4 treatment. Finally, Ex-4 decreased both excitatory glutamatergic and inhibitory glycinergic neurotransmission to preganglionic parasympathetic cardiac vagal neurons.
Conclusion
These data suggest that central GLP-1 receptor stimulation diminishes parasympathetic modulation of the heart thereby increasing heart rate.
Keywords: Nucleus ambiguus, Parasympathetic, Glucagon-like peptide 1, Medulla, Vagus
1. Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin hormone derived from proglucagon and released from the small intestine following food ingestion.1 Under hyperglycaemic conditions, GLP-1 inhibits glucagon secretion, stimulates insulin release, and increases glucose uptake in muscle and liver cells to lower blood glucose levels.2,3 GLP-1 effectively improves glucose regulation in humans,4 and the long-lasting GLP-1 receptor agonist, Exendin-4 (Ex-4) is an approved treatment for diabetes.5
In addition to its insulinotropic effects, GLP-1 acts in the central nervous system (CNS) to regulate energy metabolism and autonomic function.6–9 In some studies peripheral or central GLP-1 receptor stimulation increases heart rate and blood pressure.10,11 In human subjects, peripheral administration of GLP-1 or the long-lasting GLP-1 receptor agonist Ex-4 is reported to increase12 or have no effect on heart rate.13,14 However, GLP-1 may modulate CNS centres that regulate heart rate. As evidence, GLP-1 receptor agonists activate cells in the adrenal medulla and sympathetic regulatory nuclei of the brainstem,10 and recent findings suggest that central GLP-1 receptor activation increases vagal nerve activity.15 However, the effects of central GLP-1 receptor stimulation on the autonomic modulation of heart rate are unknown.
The predominant negative chronotropic control of heart rate is mediated by preganglionic parasympathetic cardiac vagal neurons in the nucleus ambiguus. Cardiac vagal neurons provide tonic parasympathetic activity to the heart.16 However, cardiac vagal neurons do not exhibit spontaneous bursting properties; rather they require synaptic neurotransmission to direct their activity.17 Cardiac vagal neurons receive excitatory glutamatergic and inhibitory glycinergic and GABAergic (y-Aminobutyric acid) neurotransmission.18 This synaptic input arises from, among others, the nucleus tractus solitarius (NTS) and local neurons in close proximity to the NTS and nucleus ambiguus.19,20 GLP-1 peptide and receptors are expressed in the NTS21; however, whether brainstem GLP-1 receptor signalling affects synaptic neurotransmission to cardiac vagal neurons remains unclear.
Diabetes is associated with significant cardiovascular dysfunction; hypertension, heart failure, and coronary artery disease are prevalent in diabetic populations.22–25 Diabetes is also associated with autonomic imbalance, manifest as parasympathetic withdrawal, and sympathetic dominance,23 suggesting these patients may be particularly sensitive to drugs which alter autonomic control of heart rate. We used Ex-4 to investigate the mechanism(s) mediating changes in central parasympathetic modulation of heart rate upon GLP-1 receptor activation. We assessed parasympathetic modulation of the heart rate using spectral analysis of heart rate variability (HRV). Further, we examined the effects of GLP-1 receptor signalling on neurotransmission to cardiac vagal neurons in the nucleus ambiguus.
2. Methods
2.1. Animals
Twenty male B6C3F1/J mice (Jackson Laboratories; Bar Harbor, ME, USA) were housed under a 12 h light/dark cycle and had access to food and water ad libitum. Body weight and blood glucose levels were recorded at the same time of the day (between 11:00 and 12:00) throughout the study. This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All procedures using these mice were approved by the Institutional Animal Care and Use Committees of the National Institute on Aging and George Washington University.
2.2. Telemetry and surgical implantation
A telemetry system was used to continuously monitor physiological and behavioural parameters of mice in their home cages as described previously.26 Briefly, a transmitter, TA10ETA-F20 (Data Sciences International, St Paul, MN, USA), which monitors electrocardiogram (ECG), core body temperature, and general activity, was surgically implanted in each of the mice. Two biopotential leads were routed subcutaneously lateral to midline of the chest and secured to chest muscles with silk sutures (Ethicon). Telemetry data were continuously recorded in 2.5 min bins, every 10 min. Mice were implanted with transmitters at 2 months of age and allowed to recover for a month before beginning recording.
2.3. Intracerebroventricular cannulation and exendin-4 infusion
A chronic indwelling cannula was implanted into the lateral ventricle of mice to allow intracerebroventricular (icv) application of Ex-4 or artificial cerebrospinal fluid (aCSF). Cannulae (Brain infusion kit 3) and micro-osmotic minipumps (model 1002) were purchased from Alzet (DURECT Corp., Cupertino, CA, USA). At 12 weeks, following recovery from transmitter implantation, mice were anaesthetized with isoflurane and a cannula was implanted and fixed on the surface of the skull using dental cement. The tip of the cannula was located in the lateral ventricle (AP −0.25 mm, L 1.0 mm, Depth 2.5 mm) for icv infusion. Ex-4 (AnaSpec, Freemont, CA, USA) was dissolved in aCSF. Preliminary studies determined the effective dose of Ex-4 to be 10 ng/h (1 pmol/kg/min). This dose is similar to that used in other studies examining central cardiovascular effects of Ex-4 in rodents and is well below the effective peripheral dose.10,15,27 Artificial CSF (n = 10) or Ex-4 (n = 10) was delivered using a subcutaneous micro-osmotic pump (model 1002) attached to the icv cannula. Mice were allowed to recover from the cannulation surgery for 24 h before infusion began. Acute infusions were calculated as the first 24 h cycle of aCSF or Ex-4 infusion.
2.4. Power spectral analysis
Twenty-four hour blocks of inter-beat interval were extracted using Dataquest A.R.T. 3.0 software (Data Sciences International). ECG data were extracted at basal levels (prior to aCSF or Ex-4 infusion) and on each day throughout infusion with either aCSF or Ex-4. All data were subsequently processed offline using MATLAB (Mathworks). For each 2.5 min recording, ectopic beats and outliers greater than 50 standard deviations from the mean were replaced by the means of the previous and next valid measures. Subsequently, the signal was resampled at 10 Hz using a spline function to ensure equidistant measures. A modified periodogram, which included a discrete fast Fourier transform and Hamming window to prevent spectral leakage, was used to calculate the power spectral density of each 2.5 min segment. Each spectrum was integrated between 0.4–1.5 and 1.5–5.0 Hz28 to calculate the power in the low frequency (LF) and high frequency (HF) bands, respectively. To ascertain the time course of these measures, plots of LF and HF power over 24 h blocks were constructed, and area under the curve (AUC) values were calculated using the linear trapezoidal rule.
2.5. Electrophysiology
Cardiac vagal neurons were retrogradely labelled from the heart as described previously29 in B6C3F1/J mouse pups at postnatal days 1–3. Briefly, a right thoracotomy was performed, and the retrograde fluorescent tracer X-rhodamine-5-(and-6)-isothiocyanate (XRITC, Molecular Probes, Eugene, OR, USA) was injected into the fat pads at the base of the heart. On the day of the experiment (2–4 days later), the mice were anaesthetized with isoflurane and killed by rapid cervical dislocation. The brain was submerged in cold (4°C) buffer (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM HEPES) and continually gassed with 100% O2.
The medulla was carefully removed and mounted on a cutting block and placed into a vibrating blade microtome (Leica, Nussloch, Germany). Serial transverse sections (250–500 µm) were sliced that included parasympathetic cardiac vagal neurons. Slices were perfused (4 mL/min) in a recording chamber with room temperature aCSF (125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 5 mM glucose, 5 mM HEPES) equilibrated with 95% O2 and 5% CO2 (pH 7.35–7.4).
Individual cardiac vagal neurons in the nucleus ambiguus were identified by the presence of the fluorescent tracer using a Zeiss Axioskop upright microscope (Carl Zeiss, Inc., Thornwood, NY, USA) using a ×40 water immersion objective. These identified cardiac vagal neurons were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 megaohms) were visually guided to the surface of individual cardiac vagal neurons (Zeiss, Oberkochen, Germany). For examination of excitatory postsynaptic currents (EPSCs), patch pipettes were filled with a solution consisting of 135 mM K-gluconic acid, 10 mM HEPES, 10 mM EGTA, 1 mM CaCl2, 2 mM Na-ATP, and 1 mM MgCl2 (pH 7.35–7.4). For recording inhibitory postsynaptic currents (IPSCs), patch pipettes were filled with a solution consisting of 150 mM KCl, 4 mM MgCl2, 2 mM EGTA, 2 mM Na-ATP, and 10 mM HEPES (pH 7.4). Voltage clamp whole-cell recordings were made with an Axopatch 200B, and pClamp 8 software (Axon Instruments, Union City, CA, USA) at a holding potential of –80 mV.
Drugs were continuously applied throughout the experiments by inclusion in the bath perfusate. Glutamatergic postsynaptic currents were isolated by application of gabazine (25 µM), a GABAA receptor antagonist, and strychnine (1 µM), a glycinergic receptor antagonist. Similarly, the non-NMDA (N-methyl-D-aspartic acid) and NMDA receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 50 µM) and D-2-Amino-5 phosphonovalerate (AP5; 50 µM), respectively, as well as strychnine (1 µM),were applied to isolate GABAergic neurotransmission. Gabazine (25 µM), CNQX (50 µM), and AP5 (50 µM) were applied to isolate glycinergic postsynaptic currents. Analysis of spontaneous events was performed using MiniAnalysis (Synaptosoft, version 4.3.1). The minimal acceptable amplitude of synaptic events was set by determining the lowest threshold that elicited no events in the presence of the appropriate antagonist at the end of each experiment (AP5 and CNQX for glutamatergic, gabazine for GABAergic, and strychnine for glycinergic events, respectively). Ex-4 (100 nM) was added to the perfusate. The concentrations chosen for the in vitro experiments were based on well-established studies examining electrophysiological responses to Ex-4 in vagal neurons in brainstem slices.8,30 Therefore, these studies are well matched to our experiments in both design and purpose. These studies extensively characterized the effective doses for GLP-1 receptor activation in brainstem slices and established that 100 nM Ex-4 mimics the effects of a submaximal dose of GLP-1. Other studies examining vagal neurons in the nodose ganglion also use 100 nM Ex-4 to mimic the effects of GLP-1.31 In addition, studies examining the electrophysiological properties of hypothalamic neurons upon GLP-1 receptor activation also applied 100 nM Ex-432 or the effective submaximal dose of 1 µM Ex-4, a concentration that is 10-fold greater than we used in our experiments.33 Only one experiment was performed per slice.
2.6. Data and statistical analysis
Telemetry data were divided into light and dark cycles and expressed as mean ± standard error (SE). Data were analysed by two-way ANOVA with Bonferroni's post-hoc test and Student's t-test for individual comparisons between groups using GraphPad (GraphPad software, Inc., San Diego, CA, USA); significance was set at P < 0.05. For electrophysiology, each cardiac vagal neuron served as its own control; neuronal responses were assessed before and after drug addition and compared using Student's t-test. Significance was set at P < 0.05. Results are expressed as mean ± SE.
3. Results
3.1. Acute Ex-4 treatment
We first evaluated physiological variables during the first 24 h of icv infusion of aCSF or Ex-4. Ex-4 (10 ng/h; icv) acutely lowered blood glucose levels (aCSF, 137 ± 6 mg/dL; Ex-4, 116 ± 2 mg/dL; P < 0.05; n = 10 for each group). Baseline activity prior to infusions was not significantly different between groups (day, aCSF 4 ± 1 counts/min, Ex-4 3 ± 1 counts/min, P > 0.05; Night, aCSF 9 ± 2 counts/min, Ex-4 10 ± 1 counts/min, P > 0.05). Consistent with other reports,27 Ex-4 did not alter activity of the mice at any time of the circadian cycle during the first 24 h of administration (see Supplementary material online, Figure S1A and B). However, Ex-4 did significantly decrease body temperature (see Supplementary material online, Figure S2A and B). Baseline temperature prior to infusions was not significantly different between groups (day, aCSF 36.15 ± 0.08°C, Ex-4 36.12 ± 0.01°C, P > 0.05; Night, aCSF 37.01 ± 0.16°C, Ex-4 37.09 ± 0.06°C, P > 0.05).
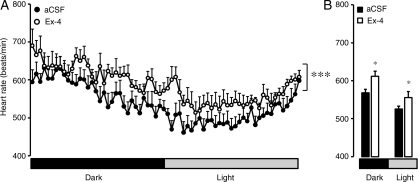
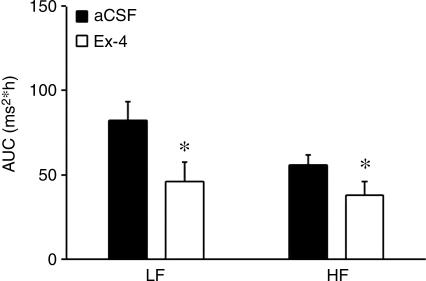
Consistent with other reports,27 centrally administered Ex-4 acutely elevated heart rate (Figure 1A). Baseline heart rates prior to infusions were not significantly different between groups (day, aCSF 531 ± 5 beats/min, Ex-4 526 ± 8 beats/min, P > 0.05; Night aCSF 564 ± 7 beats/min, Ex-4 565 ± 7 beats/min, P > 0.05). Mean heart rate was elevated during both the dark and light cycle of the first day of Ex-4 treatment (Figure 1B). To determine the autonomic effects of Ex-4 treatment, we performed spectral analysis of HRV. In mice, changes in HRV predominantly reflect changes in the parasympathetic nervous system, as both the high frequency (HF) and low frequency (LF) powers are significantly diminished in the presence of atropine.34 Further, because of the vagal predominance of HRV, the LF/HF ratio is not useful for detecting sympatho-vagal balance in mice.35,36 Ex-4 acutely induced a significant decrease in both the LF and HF powers of HRV (Figure 2).
Figure 1.
Acute central administration of Ex-4 increases heart rate. Mice received either aCSF or Ex-4 through an icv cannula. (A) Heart rates were significantly increased on the first day of Ex-4 administration. (B) Average heart rate during dark and light periods. Values are the mean and SE (n = 10 mice in each group). *P < 0.05; ***P < 0.001; two-way ANOVA repeated measures.
Figure 2.
Acute central administration of Ex-4 decreases spectral measures of heart rate variability. Mice received either aCSF or Ex-4 through an icv cannula. Both the LF and HF powers were diminished during Ex-4 administration. Values are the mean and SE (n = 10 mice in each group). *P < 0.05.
3.2. Chronic Ex-4 treatment
To test whether chronic icv administration of Ex-4 alters autonomic nervous system activity, we administered icv Ex-4 (10 ng/h) continuously during a 28 day period. Mice receiving Ex-4 had a substantial decrease in body weight that reached a nadir on treatment day 8 and then slowly returned towards the weight of aCSF-treated control mice at the end of the treatment period (see Supplementary material online, Figure S3A). Chronic icv Ex-4 lowered blood glucose levels during the first 4 days of treatment and glucose levels remained depressed throughout the duration of the treatment period (see Supplementary material online, Figure S3B). Activity of the mice was not significantly altered by chronic Ex-4 administration (see Supplementary material online, Figure S1C and D). However, body temperature was depressed in the initial 2 weeks of Ex-4 administration and returned to the levels of aCSF-treated mice during the last 14 days of the treatment period (see Supplementary material online Figure S2B and C).
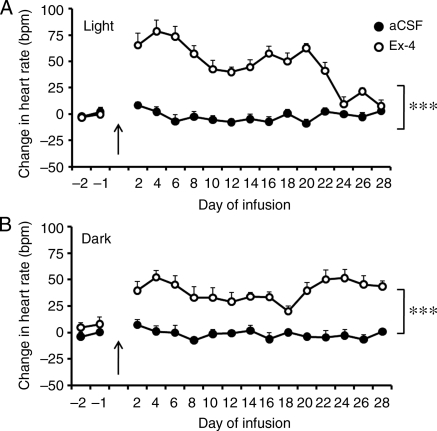
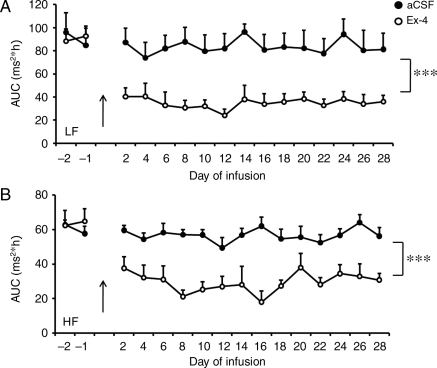
Chronic Ex-4 administration induced a sustained elevation of heart rate over 28 days during both the light and dark cycles (Figure 3A and B). Associated with the higher heart rate was a sustained depression of the LF and HF powers of HRV of mice treated with Ex-4 throughout the 28 day treatment period (Figure 4A and B). This suggests that Ex-4 elevates heart rate by decreasing the parasympathetic modulation of heart rate.
Figure 3.
Chronic central administration of Ex-4 increases heart rate. Mice received either aCSF or Ex-4 through an icv cannula. Ex-4 significantly elevated heart rate during both the light (A) and dark (B) periods. Arrow indicates beginning of infusion with Ex-4. Values are the mean and SE (n = 10 mice in each group). ***P < 0.001; two-way ANOVA with repeated measures.
Figure 4.
Chronic central administration of Ex-4 decreases spectral measures of heart rate variability. Mice received either aCSF or Ex-4 through an icv cannula. Both the LF (A) and HF (B) powers of HRV were significantly depressed. Values are the mean and SE (n = 10 mice in each group). ***P < 0.001; two-way ANOVA with repeated measures.
3.3. Effects of Ex-4 on neurotransmission to cardiac vagal neurons
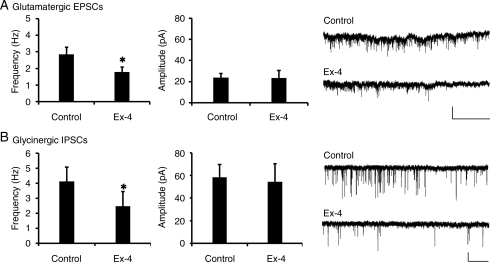
Because HRV was significantly depressed by central Ex-4 administration, and in mice HRV is dominated by parasympathetic activity,34,35 we asked whether neurotransmission to cardiac vagal neurons is altered by Ex-4. Cardiac vagal neurons receive excitatory glutamatergic and inhibitory GABAergic and glycinergic synaptic neurotransmission. Ex-4 (100 nM) significantly depressed glutamatergic EPSC frequency in cardiac vagal neurons from 2.85 ± 0.43 to 1.80 ± 0.21 Hz (Figure 5A; n = 6; P < 0.05). Glutamatergic EPSC amplitude was not altered by Ex-4 (Control 23.66 ± 3.59 pA, Ex-4 25.50 ± 8.49 pA; P > 0.05). All responses were reversible upon washout (EPSC frequency 2.69 ± 0.62 Hz; P > 0.05; EPSC amplitude 19.97 ± 1.74 pA; P > 0.05).
Figure 5.
Exendin-4 depresses neurotransmission to cardiac vagal neurons. (A) Ex-4 (100 nM) significantly diminished glutamatergic EPSCs in identified cardiac vagal neurons, but did not alter amplitude (middle panel). A representative trace is shown on the right panel. (B) Glycinergic IPSCs were significantly depressed in the presence of Ex-4 (100 nM). The amplitude of glycinergic events was not changed (middle). A representative trace is shown on the right panel. Values are the mean and SE (n = 6 for each group). *P < 0.05; scale bar represents 50 pA/5 s.
Glycinergic neurotransmission to cardiac vagal neurons was also diminished by Ex-4 application. Glycinergic IPSC frequency was significantly decreased from 4.11 ± 0.95 to 2.47 ± 0.74 Hz in the presence of Ex-4 (Figure 5B; n = 6; P < 0.05). Similar to glutamatergic EPSCs, glycinergic IPSC amplitude was not altered by Ex-4 (control 58.31 ± 11.52 pA, Ex-4 54.28 ± 15.96 pA; P > 0.05). All responses were reversible upon washout (IPSC frequency 4.05 ± 1.48 Hz; P > 0.05; IPSC amplitude 58.22 ± 12.81 pA; P > 0.05).
Ex-4 did not alter GABAergic IPSC frequency (n = 7; Control 2.93 ± 0.49 Hz, Ex-4 3.27 ± 0.51 Hz; n = 7; P > 0.05), or amplitude (control 45.39 ± 5.44 Hz, Ex-4 42.77 ± 4.43 Hz; P > 0.05). Further, no change in frequency or amplitude was observed upon washout (IPSC frequency 3.17 ± 0.53 Hz; P > 0.05; IPSC amplitude 39.94 ± 2.31; P > 0.05). These data indicate that Ex-4 depresses both excitatory and inhibitory neurotransmission to cardiac vagal neurons.
4. Discussion
In this study we show that (i) acute and chronic administration of the GLP-1 receptor agonist Ex-4 results in a significant elevation in heart rate, (ii) centrally administered Ex-4 depresses spectral measures of HRV both acutely and chronically, and (iii) Ex-4 inhibits neurotransmission to cardiac vagal neurons in the nucleus ambiguus.
Similar to studies in both anaesthetized and freely moving rodents,10,11,15,27,37 we show that central GLP-1 receptor stimulation elevates heart rate. Acute peripheral or central GLP-1 receptor stimulation increases heart rate and blood pressure.10,11 Our data indicate that both acute (Figure 1) and chronic (Figure 3) central Ex-4 administration increases heart rate during the light and dark cycles. The positive chronotropic effects of central GLP-1 receptor stimulation likely result from changes in parasympathetic activity. GLP-1 receptor agonists activate cells in the adrenal medulla and in sympathetic regulatory nuclei of the brainstem.10 However, propranolol and adrenalectomy block blood pressure increases but not tachycardia following GLP-1 administration, indicating that the elevated heart rate does not result from sympathetic stimulation.38 This suggests that sympathetic stimulation mediates the blood pressure but not heart rate effects of GLP-1. In contrast to sympathetic blockade, bilateral vagotomy prevents tachycardia following icv GLP-1 application in anaesthetized animals, suggesting that inhibition of vagal tone is responsible for elevated heart rate.11 Our data, collected in freely moving unanaesthetized mice, supports and extends the previous findings in anaesthetized animals. Spectral analysis of HRV indicates central GLP-1 receptor signalling significantly depresses parasympathetic modulation of heart rate. Further, electrophysiological recording of cardiac vagal neurons suggests that GLP-1 receptor activation depresses neurotransmission to cardiac vagal neurons. Taken together, our data suggest that central GLP-1 receptor stimulation elevates heart rate by inhibiting vagal modulation of heart rate.
The Ex-4-induced heart rate elevation observed in our study is likely mediated by medullary GLP-1 receptor signalling. The autonomic effects elicited by GLP-1 are mediated by brainstem receptor signalling, as decerebrated rodents retain autonomic responses to Ex-4.27 GLP-1 receptors are located in several brainstem areas involved in autonomic control of heart rate. The area postrema, which innervates sympathetic preganglionic neurons in the rostral ventrolateral medulla as well as the NTS, expresses GLP-1 receptors and is activated by both peripheral and central GLP-1 administration.7 Further, the NTS expresses GLP-1 binding sites and also produces GLP-1 protein.21,39 However, GLP-1 receptor mRNA is not present in the nucleus ambiguus.39 Consistent with this, our data indicate that Ex-4 alters the frequency, but not amplitude, of neurotransmission to cardiac vagal neurons, suggesting that GLP-1 acts at neurons precedent to cardiac vagal neurons. This suggests that diminished neurotransmission in the presence of GLP-1 likely results from altered activity of neurons that innervate cardiac vagal neurons.
Ex-4 inhibits both excitatory glutamatergic and inhibitory glycinergic neurotransmission to cardiac vagal neurons. These seemingly antagonistic actions of Ex-4 may result from synaptic inputs to cardiac vagal neurons that originate from different physiological reflexes. Stimulation of the NTS elicits a direct glutamatergic pathway to cardiac vagal neurons,20 and likely mediates changes in cardiac vagal neuron activity in the baroreflex. Therefore, Ex-4-induced decreases in excitatory neurotransmission to cardiac vagal neurons may be associated with changes in activity of NTS neurons related to baroreflex function. Conversely, inhibitory neurotransmission to cardiac vagal neurons is strongly tied to respiratory inputs. Respiratory sinus arrhythmia, in which heart rate increases during inspiration to ensure the proper infusion gradient for oxygen in the lungs, is mediated by inhibition of cardiac vagal neurons during inspiration.40 Therefore, Ex-4-induced depression of glycinergic neurotransmission may reflect diminished respiratory-related glycinergic neurotransmission to cardiac vagal neurons in the presence of GLP-1 receptor agonists. Dual inhibition of excitatory and inhibitory neurotransmission to cardiac vagal neurons likely differentially affects their activity based on the combination of active physiological reflex pathways at any given time. Further experiments are required to assess how GLP-1 receptor agonists alter cardiac vagal neuron activity in vivo.
The decreased HRV during central GLP-1 receptor stimulation may be associated with the well-known postprandial increase in heart rate. Food digestion is facilitated by increased parasympathetic activation and decreased sympathetic activation following a meal.41 However, these changes in autonomic tone are heterogeneous; parasympathetic outflow to the gut and pancreas is increased, whereas vagal modulation of heart rate is diminished.41,42 In humans, heart rate increases following a meal, mediated by withdrawal of parasympathetic modulation of the heart (assessed by diminished HRV) and not by sympathetic activation.42 Postprandial changes in vagal outflow are intimately related to GLP-1 signalling. GLP-1 is released after a meal, and central GLP-1 signalling stimulates insulin release and inhibits food intake and gastric emptying through vagally mediated pathways.1,8,43 Our data indicate that GLP-1 receptor signalling increases heart rate, accompanied by diminished HRV. Therefore, parasympathetic responses to GLP-1 receptor activation may also be heterogeneous, resulting in both activation and inhibition of parasympathetic neurons on different target organs. Indeed, vagotomy abolishes the central effects of GLP-1 on gastric emptying,8 as well as on stimulation of the heart rate.11 Our data are consistent with this and suggest that, in addition to increased parasympathetic outflow to the gut, central GLP-1 receptor activation also results in diminished parasympathetic modulation of heart rate.
Several disease states are associated with dysregulation of parasympathetic outflow to the heart. Diabetes is associated with sympathovagal imbalance, hypertension, and coronary artery disease.23,44 Further, patients with type 2 diabetes have a significantly increased risk for myocardial infarction and stroke.25 Parasympathetic withdrawal is associated with ventricular arrhythmias and sudden cardiac death.45 Re-establishment of parasympathetic outflow is associated with increased recovery in ischaemia and reperfusion-induced arrhythmias, as well as myocardial infarction, and restoring proper parasympathetic outflow is suggested as a therapeutic target to reduce mortality and sudden death.46–49 Therefore, the withdrawal of parasympathetic modulation of heart rate following central GLP-1 receptor stimulation may potentially aggravate autonomic imbalance in patients with type 2 diabetes and increase mortality risk. While Ex-4 treatment did not increase heart rate in relatively normotensive patients with type 2 diabetes,13 other studies have reported increases in heart rate with Ex-4.12 Our results suggest that further studies are warranted examining the affects of Ex-4 on autonomic balance in diabetic populations and suggest caution be exercised in administering GLP-1 receptor agonists to at risk populations.
In summary, we report that acute and chronic central GLP-1 receptor stimulation depresses parasympathetic modulation of the heart rate. Further, Ex-4 inhibits neurotransmission to preganglionic parasympathetic cardiac vagal neurons, the predominant autonomic control of heart rate. These data provide a potential mechanism by which central GLP-1 receptor stimulation modulates parasympathetic outflow to the heart and increases heart rate.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH grant HL59895 to D.M. and the Intramural Research Program of the National Institute on Aging.
Supplementary Material
Acknowlegements
The authors would like to thank Dr Nigel Greig for helpful comments on the manuscript.
Conflict of interest: none declared.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. doi:10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt WE, Siegel EG, Creutzfeldt W. Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. doi:10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 3.de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–2270. doi: 10.1007/s00125-008-1149-y. doi:10.1007/s00125-008-1149-y. [DOI] [PubMed] [Google Scholar]
- 4.Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D. Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care. 2003;26:2835–2841. doi: 10.2337/diacare.26.10.2835. doi:10.2337/diacare.26.10.2835. [DOI] [PubMed] [Google Scholar]
- 5.Egan JM, Meneilly GS, Elahi D. Effects of 1-mo bolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab. 2003;284:E1072–1079. doi: 10.1152/ajpendo.00315.2002. [DOI] [PubMed] [Google Scholar]
- 6.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. doi:10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes GM, Browning KN, Tong M, Qualls-Creekmore E, Travagli RA. Vagally mediated effects of glucagon-like peptide 1: in vitro and in vivo gastric actions. J Physiol. 2009;587:4749–4759. doi: 10.1113/jphysiol.2009.175067. doi:10.1113/jphysiol.2009.175067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–966. doi: 10.1124/jpet.300.3.958. doi:10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 12.Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol. 2008;48:1389–1399. doi: 10.1177/0091270008323750. doi:10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- 13.Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. doi: 10.1186/1475-2840-9-6. doi:10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, et al. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R874–R880. doi: 10.1152/ajpregu.00153.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabou C, Campistron G, Marsollier N, Leloup C, Cruciani-Guglielmacci C, Penicaud L, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577–2587. doi: 10.2337/db08-0121. doi:10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. Oxford: Oxford University Press; 1990. [Google Scholar]
- 17.Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in nucleus ambiguus. Am J Physiol. 1996;271:H2609–H2614. doi: 10.1152/ajpheart.1996.271.6.H2609. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Irnaten M, Neff RA, Venkatesan P, Evans C, Loewy AD, et al. Synaptic and neurotransmitter activation of cardiac vagal neurons in the nucleus ambiguus. Ann N Y Acad Sci. 2001;940:237–246. doi: 10.1111/j.1749-6632.2001.tb03680.x. doi:10.1111/j.1749-6632.2001.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 19.Frank JG, Jameson HS, Gorini C, Mendelowitz D. Mapping and identification of GABAergic neurons in transgenic mice projecting to cardiac vagal neurons in the nucleus ambiguus using photo-uncaging. J Neurophysiol. 2009;101:1755–1760. doi: 10.1152/jn.91134.2008. doi:10.1152/jn.91134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neff RA, Mihalevich M, Mendelowitz D. Stimulation of NTS activates NMDA and non-NMDA receptors in rat cardiac vagal neurons in the nucleus ambiguus. Brain Res. 1998;792:277–282. doi: 10.1016/s0006-8993(98)00149-8. doi:10.1016/S0006-8993(98)00149-8. [DOI] [PubMed] [Google Scholar]
- 21.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. doi:10.1016/S0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 22.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. doi:10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15:441–449. doi: 10.1016/j.numecd.2005.06.010. doi:10.1016/j.numecd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Teuscher A, Egger M, Herman JB. Diabetes and hypertension. Blood pressure in clinical diabetic patients and a control population. Arch Intern Med. 1989;149:1942–1945. doi: 10.1001/archinte.149.9.1942. doi:10.1001/archinte.149.9.1942. [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. doi:10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 26.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 27.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. doi:10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol. 2008;93:83–94. doi: 10.1113/expphysiol.2007.040733. doi:10.1113/expphysiol.2007.040733. [DOI] [PubMed] [Google Scholar]
- 29.Mendelowitz D, Kunze DL. Identification and dissociation of cardiovascular neurons from the medulla for patch clamp analysis. Neurosci Lett. 1991;132:217–221. doi: 10.1016/0304-3940(91)90305-d. doi:10.1016/0304-3940(91)90305-D. [DOI] [PubMed] [Google Scholar]
- 30.Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol. 2007;292:G1474–G1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]
- 31.Gaisano GG, Park SJ, Daly DM, Beyak MJ. Glucagon-like peptide-1 inhibits voltage-gated potassium currents in mouse nodose ganglion neurons. Neurogastroenterol Motil. 2010;22:470–479. doi: 10.1111/j.1365-2982.2009.01430.x. e111 doi:10.1111/j.1365-2982.2009.01430.x. [DOI] [PubMed] [Google Scholar]
- 32.Riediger T, Eisele N, Scheel C, Lutz TA. Effects of glucagon-like peptide 1 and oxyntomodulin on neuronal activity of ghrelin-sensitive neurons in the hypothalamic arcuate nucleus. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1061–R1067. doi: 10.1152/ajpregu.00438.2009. [DOI] [PubMed] [Google Scholar]
- 33.Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. doi:10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laude D, Baudrie V, Elghozi JL. Effects of atropine on the time and frequency domain estimates of blood pressure and heart rate variability in mice. Clin Exp Pharmacol Physiol. 2008;35:454–457. doi: 10.1111/j.1440-1681.2008.04895.x. doi:10.1111/j.1440-1681.2008.04895.x. [DOI] [PubMed] [Google Scholar]
- 35.Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2214–R2221. doi: 10.1152/ajpregu.2000.279.6.R2214. [DOI] [PubMed] [Google Scholar]
- 36.Tank J, Jordan J, Diedrich A, Obst M, Plehm R, Luft FC, et al. Clonidine improves spontaneous baroreflex sensitivity in conscious mice through parasympathetic activation. Hypertension. 2004;43:1042–1047. doi: 10.1161/01.HYP.0000125884.49812.72. doi:10.1161/01.HYP.0000125884.49812.72. [DOI] [PubMed] [Google Scholar]
- 37.Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118:33–38. doi: 10.1016/j.regpep.2003.10.025. doi:10.1016/j.regpep.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 38.Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26:1623–1631. doi: 10.1016/j.peptides.2005.02.016. doi:10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. doi:10.1002/(SICI)1096-9861(19990111)403:2<261::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. doi:10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- 41.Robertson D. Primer on the Autonomic Nervous System. London/San Diego: Elsevier; 2004. [Google Scholar]
- 42.Lu CL, Zou X, Orr WC, Chen JD. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci. 1999;44:857–861. doi: 10.1023/a:1026698800742. doi:10.1023/A:1026698800742. [DOI] [PubMed] [Google Scholar]
- 43.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. doi:10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 11:31–39. doi: 10.1007/s11154-010-9131-7. doi:10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hull SS, Jr, Vanoli E, Adamson PB, De Ferrari GM, Foreman RD, Schwartz PJ. Do increases in markers of vagal activity imply protection from sudden death? The case of scopolamine. Circulation. 1995;91:2516–2519. doi: 10.1161/01.cir.91.10.2516. [DOI] [PubMed] [Google Scholar]
- 46.Routledge HC, Chowdhary S, Townend JN. Heart rate variability—a therapeutic target? J Clin Pharm Ther. 2002;27:85–92. doi: 10.1046/j.1365-2710.2002.00404.x. doi:10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- 47.Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. doi:10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- 48.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study . Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 49.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.