Abstract
Increased numbers of mast cells have been reported in explanted human hearts with dilated cardiomyopathy and in animal models of experimentally induced hypertension, myocardial infarction, and chronic volume overload secondary to aortocaval fistula and mitral regurgitation. Accordingly, mast cells have been implicated to have a major role in the pathophysiology of these cardiovascular disorders. In vitro studies have verified that mast cell proteases are capable of activating collagenase, gelatinases and stromelysin. Recent results have shown that with chronic ventricular volume overload, there is an elevation in mast cell density, which is associated with a concomitant increase in matrix metalloproteinase (MMP) activity and extracellular matrix degradation. However, the role of the cardiac mast cell is not one dimensional, with evidence from hypertension and cardiac transplantation studies suggesting that they can also assume a pro-fibrotic phenotype in the heart. These adverse events do not occur in mast cell deficient rodents or when cardiac mast cells are pharmacologically prevented from degranulating. This review is focused on the regulation and dual roles of cardiac mast cells in: (i) activating MMPs and causing myocardial fibrillar collagen degradation and (ii) causing fibrosis in the stressed, injured or diseased heart. Moreover, there is strong evidence that premenopausal female cardioprotection may at least partly be due to gender differences in cardiac mast cells. This too will be addressed.
Keywords: Cardiac mast cells, Myocardial remodelling, Fibrosis, Gender
1. Introduction
Mast cells are derived from precursor cells in the bone marrow, and mature under the influence of the c-kit ligand, stem cell factor with their final phenotype being dependent on the microenvironment in which they reside. While mast cells throughout the body are involved in the pathogenesis of many diseases, the role of cardiac mast cells in diseases of the heart has been understudied despite circumstantial evidence indicating their potential involvement. In addition to the storage of histamine and proteases such as tryptase and chymase in their secretory granules, cardiac mast cells also produce a wide variety of cytokines, growth factors, vasoactive agents and other biologically active mediators that are capable of mediating tissue remodelling. For example, several are capable of activating matrix metalloproteinases (MMPs), which in turn degrade the collagen matrix of the heart. This makes cardiac mast cells key in diseases where MMP-induced remodelling of the heart occurs, such as myocardial infarction and heart failure. Alternatively, cardiac mast cells have also been implicated in the fibrotic remodelling of the heart, such as in hypertension and myocarditis, as well as in the fibrotic and rejection aspects of cardiac transplantation. While the pathologic importance of cardiac mast cells is slowly becoming evident, little is known regarding the mechanisms by which these cells are activated. Furthermore, there appears to be distinct gender differences in mast cell function, which may account, in part, for the cardioprotection afforded to pre-menopausal females. Accordingly, this review will describe the role of cardiac mast cells in a wide range of cardiac pathologies, as well as addressing what is currently known in regard to cardiac mast cell activation and gender differences.
2. Mast cell characteristics
Mast cells are best known for the production of histamine, which is associated with allergic reactions and the subsequent vasodilation that can occur when mast cells are stimulated. However, mast cells are also capable of producing a whole host of growth factors, proteases, cytokines, chemokines and fatty acid metabolites.1 Some of these, such as histamine, chymase, tryptase, phospholipases, and kinins are contained in pre-stored granules that are released upon stimulation. Other products are synthesized de novo including many cytokines (e.g. IL-1, -4, -5, -10), leukotrienes and even nitric oxide.1 As will be discussed in the following sections, some of these mast cell products have been implicated in several cardiac pathologies, however, a great deal of work is required to fully elucidate which cardiac mast cell products are important in mediating disease. Mast cells also have the ability to respond to a wide range of stimuli. Despite this, the activation of cardiac mast cells has been surprisingly understudied with only a few endogenous secretagogues identified. These include endothelin-1 (ET-1), reactive oxygen species, complement factor 5a, several neuropeptides and IL-33. These will be discussed in more detail in a later section. Also detailed in sections to follow is the fact that cardiac mast cell density increases in numerous cardiac pathologies. Surprisingly, little effort has been made to investigate the source(s) of this increase. Nevertheless, potential mechanisms include: (i) recruitment of haematopoietic precursor cells; (ii) maturation of immature resident cells; and (iii) proliferation of resident cells.
2.1. Recruitment of haematopoietic precursor cells
Mast cells are derived from multipotent haematopoietic progenitor cells from bone marrow and do not develop into mast cells until reaching the tissue or organ in which they become resident.2 The search for the specific mast cell precursor cells has been elusive, however, Thy-1loc-Kithi progenitor mastocytes were identified in murine fetal blood and shown to contain cytoplasmic granules and mRNA for mast cell proteases, but did not contain mRNA for FcεRI (IgE receptor).2,3 These cells developed into mast cells, in vitro, but not into other haematopoietic lineages. Lin−Kit+Sca-1−Ly6c−FcεRIα−CD27−β7+T1/ST2+ cells from adult mouse bone marrow also develop into mast cells in culture and can reconstitute as mast cells in mast cell-deficient mice.4 Additionally, a population of cells from mouse spleen, designated as Lin−Kit+FcγRII/IIIhiβ7hi, have been identified as a precursor for both mast cells and basophils, as well as the mast cell precursor CD45+Lin−CD34+β7hiFcεRIαlo in the intestine.5 In humans, mast cell progenitors circulate in the blood as mononuclear leucocytes without granules, and express the surface markers CD13, CD33, CD38, CD34 and Kit.2 As far as we are aware, no studies have investigated whether recruitment of mast cell progenitor cells is the mechanism behind the increase in mast cell density observed in many cardiac pathologies. It seems unlikely that this is a mechanism of increased mast cell density, at least in the initial stages in experimental models, since it has been demonstrated that following depletion of mast cells from the peritoneal cavity in rats, it takes 6 days before mast cell numbers approach control levels and a full 20 days for full restoration to be achieved.6 In the days prior to this point, numbers of new mast cells were low and those present were immature mast cells. It was concluded that these new mast cells were derived from bone marrow-derived progenitor cells since mast cell precursors in the bone marrow decreased dramatically in the days following mast cell depletion. A significant number of these newly recruited cells were also undergoing mitosis.
2.2. Maturation of immature resident cells
Maturation and differentiation of resident cardiac mast cells is another possible mechanism of increased mast cell density, and is associated with progressive sulphation of heparin, the formation of mast cell chymase and histamine, and the loss of mitotic activity.7,8 Using differential alcian and safranin staining, four stages of mast cell maturation have been identified as follows: Stage I or immature mast cells are those that appear completely blue following this staining technique; stage II mast cells stain blue (i.e. >60% of the granules), with small amounts of red (i.e. <40% of the granules); stage III mast cells stain predominantly red (i.e. >60% of the granules), with reduced amounts of blue (i.e. <40% of the granules); and stage IV or totally differentiated, mature mast cells appear completely red. In addition to staining differences, Yong et al.8 found the mean diameter of mast cells from adult rat peritoneal washings and cardiac tissue to be smallest in stage I and largest in stage IV. Our data in a model of volume overload indicates that maturation of cardiac mast cells accounts for the increase in mast cell density observed in that model.9 Using the described staining and cell size criteria, we demonstrated a significant decrease in stage II cardiac mast cells, coupled with a significant increase in stage III cells, and no change in the number of stage IV cardiac mast cells relative to sham-operated rats within 24 h of creating volume overload. Thus, the acute rise in cardiac mast cells following volume overload appears to be due to the maturation/differentiation of resident immature cardiac mast cells. Using a cultured tissue slice system, whereby sections of left ventricle from normal rats were incubated in media-containing stem cell factor (20 ng/mL) for 24 h, we have shown that mast cell density increased ∼2-fold (unpublished observation). This demonstrates that mast cell density can increase independently from recruitment of precursor cells because there were no precursors present in the media in which the left ventricular slices were cultured. From our data, it also appears that mast cell degranulation is required in order for mast cell density to increase since mast cell stabilization in volume overloaded animals results in decreased mast cell density.10,11 Similarly, in sham animals mast cells are reduced to below normal levels by mast cell stabilization.10
2.3. Proliferation of resident cells
Using 3H-thymidine uptake, stage I and II (i.e. immature) mast cells have been identified as being capable of mitosis, while stages III and IV were mitotically inactive.8 Thus, in theory it is possible that some proliferation may occur in the population of immature mast cells, but probably not once they have matured. In volume overload, we have shown that proliferation is not a mechanism of increased mast cell density.9
3. Volume overload-induced adverse myocardial remodelling and heart failure
Cardiac mast cell density increases in the left ventricle under conditions of volume overload such as that which occurs with mitral regurgitation.12 To establish a causal relationship between mast cells and adverse myocardial remodelling, we have utilized the aortocaval (AV) fistula model of volume overload. With this model, we first observed that a rapid increase in cardiac mast cell density is concurrent with a significant increase in myocardial MMP-2 activity and 50 and 60% reductions in collagen volume fraction by 3 and 5 day post-fistula, respectively.11 Both the MMP activation and subsequent collagen degradation were prevented with mast cell membrane stabilization with cromolyn sodium (24 mg/kg/day),11 showing a direct causal relationship. We then went on to investigate the role of mast cells in long-term remodelling in response to volume overload, by continuously treating rats with another mast cell stabilizing compound, nedocromil (30 mg/kg/day), for a period of 8 weeks post-fistula.13 Nedocromil attenuated left ventricular hypertrophy (sham, 961 ± 149; AV fistula, 1686 ± 249; AV fistula + nedocromil, 1352 ± 118 mg) and pulmonary oedema, and prevented ventricular dilatation, the increase in compliance, and the decrease in intrinsic contractile function. The collective outcome of this preventive study was a significant decrease in mortality and again provided direct evidence that mast cells are critical to volume overload-induced remodelling. The final approach that we utilized to demonstrate causality between mast cells and adverse myocardial remodelling was the use of mast cell-deficient rats.14 In these animals, hypertrophy was unchanged in comparison to the wild type following 8 weeks of volume overload (1285 ± 248 vs. 1205 ± 78 mg, respectively), however, left ventricular dilatation, determined in vivo by echocardiography, was markedly reduced. MMP-2 activity was not increased and, thus, collagen degradation was prevented at 5 days and 8 weeks post-fistula in mast cell-deficient rats.14 As further confirmation that cardiac mast cells mediate remodelling via activation of MMPs, Chancey et al.,15 using a blood-perfused isolated heart preparation, administered the mast cell secretagogue, compound 48/80, to normal hearts. The resultant mast cell degranulation led to an increase of 126% in MMP activity and a nearly 50% decrease in myocardial collagen volume fraction within 30 min of administration of compound 48/80. A tendency for the left ventricle to dilate was also evident despite a significant histamine-induced myocardial oedema. Thus, using several approaches we have conclusively demonstrated a central role for cardiac mast cells in initiating myocardial remodelling through the activation of MMPs. The fact that mast cell density is also increased in the left ventricle of dogs with experimentally induced mitral regurgitation12 demonstrates that mast cell-mediated myocardial remodelling in response to volume overload is not species or model specific. In these animals, mast cell density was significantly increased at 2 and 4 weeks post-mitral regurgitation (MR), but had returned to normal by 24 weeks. This correlated with an increase in MMP-2 activation and was concurrent with collagen degradation. Chymase activity was also elevated in MR dogs at all time points. Ventricular dilatation was present at 4 weeks post-MR and remained so throughout the duration of the experimental period. An increase at 24 weeks in the left ventricular end diastolic diameter to wall thickness ratio was indicative of an eventual mast cell-mediated inappropriate hypertrophic response of the ventricle to volume overload.16 Together, these studies demonstrate the central role of mast cells in activating MMPs and thereby producing collagen degradation and left ventricular dilatation.
Mast cells store and release several products that are capable of activating MMPs. Studies linking peritoneal and skin mast cells to tissue remodelling have shown that mast cell tryptase can activate interstitial collagenase (MMP-1) and stromelysin (MMP-3) under in vitro conditions.17,18 Gruber et al.19 demonstrated that tryptase was unable to directly activate MMP-1, however, tryptase first cleaves proMMP-3, with active MMP-3 then activating MMP-1. Studies using peritoneal mast cells isolated from mice have shown that chymase can activate MMP-2 and -9.20 Also, mast cells appear to be an important source of TNF-α, which has been shown to activate MMPs.21 In the heart, TNF-α is localized to mast cells under normal conditions.22,23 Histamine may also play a role in myocardial remodelling. In a retrospective and prospective clinical study, the histamine type 2 (H2) receptor antagonist, famotidine (20–40 mg), was found to reduce plasma BNP levels (a marker of left ventricular hypertrophy), left ventricular diameter in diastole and systole, while improving NYHA class.24 Thus, it is likely that multiple pathways mediated by mast cells are involved in the activation of MMPs and subsequent development of heart failure.
4. Myocardial infarction and ischaemic–reperfusion injury
Cardiac mast cell density increases dramatically following myocardial infarction (MI) (1.8 ± 0.3 to 26.3 ± 7.4 cells/mm2).25 Frangogiannis et al.26 demonstrated a striking increase in mast cell numbers during the healing phase post-MI, with maximum accumulation in areas of collagen deposition. Consistent with the concept that mast cells orchestrate inflammation,27 mast cell density was elevated after 7 days of reperfusion following ischaemia in a canine model of MI, with newly recruited macrophages and neutrophils gathered in close proximity to degranulating mast cells.28 Further, mast cells were closely associated with vascular structures after 7 days of reperfusion. In further support of mast cells mediating the inflammatory response, Frangogiannis et al.22 determined that cardiac mast cells were the predominant source of TNF-α in the canine heart in the first few hours following ischaemia-reperfusion. Gilles et al.23 also suggested that this was the case, based on the observation that the mast cell-stabilizing compounds, ketotifen and disodium cromoglycate, prevented the increase in myocardial TNF-α levels following reperfusion. Jaggi et al.29 subjected isolated rat hearts to 30 min of global ischaemia followed by 120 min of reperfusion and used two approaches to investigate the role of cardiac mast cells in ischaemia–reperfusion injury. Initially, they treated the hearts with ketotifen and found that mast cell degranulation and myocardial injury were decreased. Secondly, they degranulated mast cells with compound 48/80 effectively removing mast cell mediators from the heart prior to inducing ischaemia–reperfusion. This too resulted in attenuation of injury. Frangogiannis et al.22 also observed degranulating cardiac mast cells and an ∼2-fold increase in histamine levels in cardiac lymph following ischaemia–reperfusion in the canine heart. Ischaemia–reperfusion studies using histamine receptor antagonists in a canine model of ischaemia–reperfusion revealed that blockade of H2, but not H1, receptors decreased infarct size regardless of whether the H2 antagonist was administered during ischaemia or reperfusion.30 However, this did not lead to functional improvements. The effects of mast cells on the myocardium in response to MI and ischaemia–reperfusion may be secondary to the production of angiotensin (Ang) II since mast cells contain chymase, which is capable of cleaving inactive Ang I to the active Ang II.31 This notion is reinforced by the fact that treatment with an AT1 receptor antagonist had an efficacious effect on mortality post-MI in hamsters, while an ACE inhibitor did not.32 Further, ACE-independent Ang II formation was important for the release of norepinephrine from sympathetic nerves following an ischaemic event in the human heart.33 Also significant amounts of Ang II was mast cell derived following ischaemia–reperfusion in the guinea pig heart34 with this mast cell-derived Ang II being responsible for increased norepinephrine levels and norepinephrine-induced arrhythmias. Mast cell-derived renin is also thought to be important in the formation of myocardial Ang II.34,35
Recent studies have begun to utilize mast cell (c-kit)-deficient mice to attempt to determine the role of mast cells in ischaemia–reperfusion injury and MI. Using a protocol of 30 min ischaemia followed by 6 h of reperfusion in the W/Wv strain of mast cell-deficient mice, Bhattacharya et al.36 found that the amount of viable myocardium was significantly greater in the mast cell (or c-kit)-deficient mice. Using female W/Wv mast cell-deficient mice, Cimini et al.37 reported that at 14 days post-MI, these mice had a greater infarct area and ventricular dilatation, but reduced infarct thickness. However, they discounted the importance of mast cells to myocardial remodelling post-MI due to their small number and concluded that diminished recruitment of myofibroblasts accounted for the impaired healing of the scar. Interestingly, mast cells are known to have a prominent role in regulating myofibroblast function,38 and as described above, although small, the number of mast cells in the normal heart is sufficient to induce MMP activation and collagen degradation when stimulated.15 Ayach et al.39 examined long-term remodelling and cardiac function in male W/Wv mice as well as W/Wv mice reconstituted with bone marrow cells at 35 days post-MI. Their results indicated that W/Wv mice developed larger hearts with more collagen deposition albeit with an increased stroke volume; although they had reduced rates of contraction and relaxation. There was virtually no difference in survival rate between the wild type and W/Wv mice 35 days post-MI. Improvements were observed in all parameters measured post-MI in mast cell-deficient mice reconstituted with bone marrow-derived mast cells. Recently, we investigated the contribution of mast cells to post-MI myocardial remodelling also in the W/Wv mouse at 7 days post-MI (unpublished observations). Converse to the previously mentioned studies, our preliminary results indicate that chamber dilatation is significantly greater in WT hearts compared with W/Wv hearts (126 vs. 73% increase in end diastolic volume, respectively) together with increased wall thinning and an increase collagen deposition in the viable myocardium. While based on the data accumulated thus far, it seems likely that mast cells are important in ischaemia–reperfusion injury, at this stage it is difficult to determine the importance of mast cells in ventricular remodelling post-MI due to the conflicting nature of the results between mouse studies. Further studies are required to shed more light on the subject, and in fact mast cell-deficient mice may not be the ideal model for these studies. These mice suffer from pronounced haematopoietic abnormalities, whereas the mast cell-deficient rat (Ws/Ws) has only a four amino acid deletion associated with the c-kit kinase autophosphorylation site, which means that, because the mutation is highly localized, the only other phenotypic differences in this mutant rat are a deficiency in melanocytes and intestinal interstitial cells of Cajal.40,41
5. Hypertension
Cardiac mast cells have also been implicated in the development of fibrosis in the hypertensive heart. Olivetti et al.42 first described an association between cardiac fibrosis and mast cells when they observed increases in cardiac mast cell density in the right ventricle following pulmonary artery banding in rats; although they did not indicate whether the mast cells were associated with myocyte damage or replacement fibrosis. Panizo et al.43 were the first to investigate mast cells in the left ventricle in response to systemic hypertension. They noted an increase in mast cell density in the left ventricle of spontaneously hypertensive rats (SHR), and that this strongly correlated (r = 0.87) with collagen volume fraction. Subsequently, Shiota et al.44 followed the progression of cardiac mast cell density in the SHR from 1 day through to 20 months of age and observed that density increased dramatically above control levels throughout the lifespan of those animals. The striking increase in mast cell density at 2 weeks of age in the SHR was concurrent with increased levels of stem cell factor and its c-kit receptor, which are known to increase mast cell density.45 Isolated heart studies also showed that cardiac mast cells were a significant source of increased NF-κB and IL-6 expression in the left ventricle of compensated 12 month old SHR. However, these studies stopped short of showing a causal relationship between cardiac mast cells and cardiac fibrosis in the hypertensive heart. We recently provided the first causal evidence when we treated SHR with the mast cell stabilizing compound nedocromil (30 mg/kg/d) and found that fibrosis was completely prevented.46 Searching for mechanisms by which mast cells regulate fibrosis, we found that mast cell stabilization prevented macrophage recruitment and normalized cytokine profiles in the hypertensive heart. Further, incubation of adult cardiac fibroblasts with tryptase led to proliferation and collagen synthesis. Thus, it seems that cardiac mast cells regulate fibrosis via multiple pathways including inflammatory cell recruitment, cytokines and direct effects on fibroblasts. Hara et al.47 focused on the role of mast cells in the progression to heart failure following experimentally induced pressure overload. They followed mast cell-deficient mice over a 15 week period following aortic banding and found that, in contrast to their wild-type counterpart, heart and lung weights were markedly attenuated, ventricular dilatation was prevented and fractional shortening preserved. Therefore, mast cells appear to be involved in various stages of the remodelling process.
6. Cardiac transplantation/rejection
Cardiac mast cells have also been implicated in the fibrosis and rejection that occurs in the transplantated heart. Li et al.48 showed that cardiac mast cells degranulate following cardiac transplantation, and that both the number of mast cells and the extent of degranulation correlate positively with fibrosis (r = 0.63 and 0.73, respectively). In an attempt to further show the importance of mast cells in this fibrotic response, patients were divided into two groups based on the number of mast cells present 2 weeks after transplantation. Those with prominent numbers of mast cells showed a 17% increase in fibrosis by week 3, while the minimal mast cell reaction group showed only a 3.5% increase. Interestingly, patients in the prominent mast cell group also scored higher on the rejection scale. In rat hearts, Zweifel et al.49 found that mast cell density initially decreased at 5 days post-transplantation, which then significantly increased by 16 days post-transplantation.
7. Myocarditis
One of the first reports of disease-related increases in mast cells in the heart was associated with myocarditis.50 While autoimmune myocarditis is recognized as a disease driven by T cells, in particular CD4+ T cells,51 mast cells have also been observed in fibrotic areas in mice with dilated cardiomyopathy following experimentally induced autoimmune myocarditis. Inhibition of acute pathological changes using IL-10 are presumed to be via mast cell inhibition since histamine levels, mast cell density and mast cell size were all reduced by IL-1052. Furthermore, IFN-γ protects against chronic myocarditis by preventing mast cell degranulation and fibrosis.53 In viral myocarditis in mice, induced by infection with coxsackievirus B3, mast cell degranulation was observed within 6 h of infection.54 In mice infected with encephalomyocarditis virus, mast cell density was decreased slightly 5 days after virus inoculation (1.6 ± 0.3 vs. 1.8 ± 0.3 cells/mm2) before subsequently increasing to levels that were significantly greater than normal after 14 days (2.7 ± 0.3 cells/mm2), which coincided temporally with the pattern of fibrosis within these hearts.55 In experimental Chagas' disease, mice infected with Trypanosoma cruzi show increased histamine levels in the heart56 and histological examination revealed that mast cells in these mice appear in areas of fibrosis.57 In the strongest evidence to date, Palaniyandi et al.52 demonstrated an extremely strong correlation between mast cell density and collagen volume fraction (r = 0.946) in hearts from mice with dilated cardiomyopathy following experimentally induced autoimmune myocarditis. Treatment of these mice with a mast cell stabilizing compound reduced mast cell density, fibrosis and TGF-β1.
8. Atherosclerosis/aneurysm
Current evidence has implicated inflammation in atherogenesis and plaque destabilization. Firstly, monocytes and macrophages then lymphocytes were identified as being involved in atherogenesis.58 Now mast cells have also been recognized as composing part of this inflammatory response and have been identified as playing a role in plaque rupture in atherosclerosis. This may occur via several mechanisms. Bot et al.59 provided in vivo evidence that activated mast cells increased vascular leakage and the influx of leucocytes into the plaque, and induced intraplaque haemorrhage causing plaque destabilization with increased risk of rupture. Further, it has been shown that plaque rupture may be brought about by inducing apoptosis of overlying endothelial cells by the secretion of chymase and TNF-α from activated mast cells.60 Another vascular pathology in which mast cells play a pivotal role is aortic abdominal aneurysms. Sun et al.61 used mast cell-deficient mice to demonstrate the importance of mast cells in the development of aortic aneurysms. Further, they reconstituted mast cell-deficient mice with bone marrow mast cells lacking chymase and found that this prevented the development of the aneurysms. This was seemingly through the inhibition of cathepsins and MMP expression.
9. Cardiac mast cell activation
Despite the obvious importance of cardiac mast cells in myocardial remodelling, the factors responsible for initiating mast cell activation are poorly understood, in fact, our understanding of the effects of secretagogues on cardiac mast cells is very limited. This must become a central focus for understanding the myocardial remodelling process.
9.1. Endothelin-1
Cardiac expression of mRNA for both ET-1 and the ETA receptor are increased in volume overload.62 Blockade of the ET-1 receptor prevented MMP activation and attenuated ventricular dilatation in animal models of heart failure,63,64 a response similar to that observed with mast cell stabilization. In fact, Murray et al.65 demonstrated that administration of 20 pg/ml of ET-1 to blood perfused, isolated rat hearts resulted in extensive cardiac mast cell degranulation, MMP-2 activation and collagen degradation. This resulted in moderate ventricular dilatation and was prevented by the mast cell membrane stabilizing compound, nedocromil. The role of ET-1 may also extend beyond activation of cardiac mast cells to include induction of mast cell maturation. Murray et al.66 also showed that the non-selective endothelin receptor antagonist, bosentan, prevented the increase in mast cell density seen in the left ventricle under conditions of volume overload.
9.2. Reactive oxygen species
Surprisingly, little work has been done to investigate the role of reactive oxygen species in activating mast cells. In an interesting study, Calderón-Garcidueñas et al.67 found that degranulated mast cells were associated with scattered foci of mononuclear cells in hearts from dogs in highly polluted cities, while those from less polluted cities showed virtually no abnormalities. Further to this, we have found that incubation of isolated rat cardiac mast cells with Na2SO3 induced a concentration-dependent histamine release.68 Degranulation was prevented and attenuated by the anti-oxidant compounds ebselen and dyphenyleneiodinium, respectively, indicating that cardiac mast cells can degranulate in response to oxidative stress. Furthermore, Gilles et al.23 had shown in ischaemic pre-conditioning studies of the heart, that mast cell-derived TNF-α occurred in response to oxidative stress, indirectly suggesting that cardiac mast cells are responsive to oxidative stress. Masini et al.69 was able to demonstrate this more directly using the superoxide dismutase mimetic, M40403, which prevented mast cell degranulation following reperfusion of the ischaemic rat heart.
9.3. Complement 5a
The complement molecule, C5a, is a known chemotactic factor for mast cells.70 C5a seems to be important in scar formation in MI71 and ischaemia–reperfusion injury.72 Patella et al.73,74 have shown that human cardiac mast cells do degranulate in response to C5a, however, it is not known if activation of cardiac mast cells by C5a is an important factor in myocardial remodelling.
9.4. Neuropeptides
Another group of very important potential cardiac mast cell secretagogues are neuropeptides. These may be especially important because of the prominent role of the nervous system, in cardiac disease and the fact that many mast cells in the heart are spatially located close to nerves.35,75 Despite this, and aside from effects on the vasculature and blood pressure regulation, little attention has been directed toward the role of neuropeptides in myocardial remodelling. While a wide range of neuropeptides have been examined in relation to their effects on mast cells in general, few have been properly evaluated with respect to the cardiac mast cell.
Substance P is an 11 amino acid member of the tachykinin family of neuropeptides, which is released predominately from afferent fibres of the sympathetic nervous system acting on neurokinin receptors. In recent years, substance P has become recognized as an important regulator of inflammation.76 In this respect, substance P has been shown to induce degranulation of peritoneal, dural, skin, bladder, and bone-marrow-derived mast cells.77–83 It was believed that cardiac mast cells were unresponsive to substance P,73,74 however, those studies tested cardiac mast cells obtained from transplanted hearts of patients with end-stage cardiac disease. Further, the cardiac mast cell isolation technique used in those studies required numerous washes as well as enzymatic dispersion,73,74,84–87 which damages the cells.85 Using our newly developed isolation technique, we have been able to clearly demonstrate that cardiac mast cells do respond to substance P.88
Neurotensin has been found in nerve fibres associated with the heart, including the coronary vasculature, myocytes, nodal cells, and intra-cardiac ganglia.89 Neurotensin appears to invoke non-cardiac mast cell degranulation,90,91 and while only a couple studies have investigated the effect of neurotensin on cardiac mast cells, the results are convincing. The strongest evidence was presented by Rioux et al.92 who found that infusion of isolated hearts with neurotensin induced a rapid, but transient, release of histamine. This effect was prevented in hearts from rats depleted of histamine by the mast cell secretagogue, compound 48/80. This appears to be a direct effect of neurotensin that is calcium dependent.93 Further evidence by Pang et al.94 demonstrated that cardiac mast cell degranulation, brought about by immobilization-induced stress, could be prevented by neurotensin receptor blockade. While the positive inotropic effects of neurotensin in the heart have been shown to be mediated by the stimulation of norepinephrine release and not histamine or serotonin,95 the effects of neurotensin on cardiac mast cell-mediated myocardial remodelling have not been examined.
9.5. Interleukin-33
IL-33 is a member of the IL-1 family of cytokines and is a functional ligand of the ST2 receptor. Recent interest in arthritis has centred on the potential of IL-33 to regulate mast cell function. Extending this to the heart, we have preliminary studies to indicate that IL-33 can activate isolated rat cardiac mast cells to a level equal to that of the mast cell secretagogue, compound 48/80 (unpublished observations). This may offer an exciting new avenue of investigation in mast cell biology.
10. Gender differences in cardiac mast cells
The prevalence and severity of gender differences in cardiovascular disease has been convincingly identified in human clinical studies,96 however, the underlying mechanisms responsible for the lower incidence of cardiac disease in premenopausal females are poorly understood. Since cardiac mast cells play a central role in mediating the remodelling process through the activation of MMPs leading to the subsequent degradation of the ECM, gender differences in cardiac mast cell activation and function may provide a mechanism for the cardioprotection afforded to pre-menopausal females. However, information regarding gender differences is extremely limited. Isolated hearts from ovariectomized female rats showed a marked increase in MMP-2 activity (133%), a decrease in collagen volume fraction (37%) and ventricular dilatation (15%) when compared with hearts from normal females following stimulation with the mast cell secretagogue, compound 48/80.97 The response seen in the ovariectomized females following mast cell activation is similar to that seen in males following creation of an AV fistula, which we have shown to be mast cell mediated.11,13 Restoration of oestrogen to ovariectomized female rats was able to prevent the changes in MMP-2 activation, collagen volume fraction and ventricular dilatation. A possible explanation for the differences between male and female cardiac mast cells may be that oestrogen prevents the release of mast cell proteases98 or other products such as TNF-α99 as has been shown in non-cardiac mast cells. TNF-α is well established to be critical in remodelling leading to heart failure100–102 and our laboratory has shown that mast cell deficient rats have little myocardial TNF-α at 5 days post-fistula in contrast to the wild type.14 Therefore, the possibility exists that oestrogen is reducing the synthesis of TNF-α by cardiac mast cells as well as other myocardial cells. Preliminary evidence from our laboratory indicates that males have increased levels of TNF-α mRNA following volume overload and similarly ovariectomy of female rats induces an elevation in cardiac TNF-α mRNA. This could be reversed by supplementation with oestrogen.
11. Conclusions
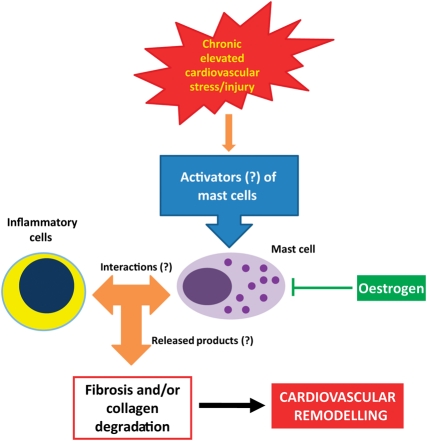
As summarized in Figure 1, the literature presented in this review clearly indicates that cardiac mast cells play a central role in remodelling of the cardiovascular system secondary to sustained elevations in stress or injury. Despite this, our understanding of how mast cells regulate remodelling is limited. While we know that mast cell secretory products activate MMPs, it is not known how they may mediate their pro-fibrotic actions. Several key areas need to become the focus of future investigations, as indicated by question marks in Figure 1, including: (i) the downstream effector molecules by which mast cells induce remodelling; (ii) the interactions of cardiac mast cells with other inflammatory cells; and (iii) the mechanisms responsible for the activation of cardiac mast cells. While mast cell proteases and TNF-α appear to be important products released by cardiac mast cells, many other cytokines and growth factors known to be involved in the remodelling process and recruitment of inflammatory cells may be produced. Although ET-1 is an important activator of cardiac mast cells in vivo, few other secretagogues have been identified and this should become a major area of focus in the future. There is evidence that some neuropeptides, such as substance P, induce cardiac mast cell degranulation, and other neuropeptides also seem likely candidates since mast cells are often found in close proximity to nerves. At this stage there are more questions than answers about the role of cardiac mast cells and many more focused studies are required in order to fully understand the role of these cells in cardiac disease.
Figure 1.
In response to elevations in myocardial stress such as volume or pressure overload, or injury such as lesions of the vasculature or myocardial infarction, a variety of factors are released that have the potential to activate cardiac mast cells. Upon activation, cardiac mast cells release numerous products that may include histamine, chymase, tryptase and TNF-α as well as others that are as yet undetermined. These products can act directly on cardiac fibroblasts, endothelial cells, smooth muscle cells or cardiac myocytes, depending on the pathology involved. In addition to this, cardiac mast cells may also interact with other inflammatory cells such as T cells and macrophages to amplify their effects on remodelling. Oestrogen may protect females from the same degree of adverse cardiovascular remodelling as males by altering the phenotype of cardiac mast cells or by inhibiting their activation.
Conflicts of interest: none declared.
Funding
This work was supported by an American Heart Association Fellowship (0825510E to S.P.L.), Environmental Protection Agency (EPA RD831953 to G.L.B.) and the National Institutes of Health (K99HL093215 to S.P.L., HL62228 and HL073990 to J.S.J.).
References
- 1.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. doi:10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 2.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. doi:10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. doi:10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 4.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. doi:10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci USA. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. doi:10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamur MC, Moreno AN, Mello LF, Souza Junior DA, Campos MR, Pastor MV, et al. Mast cell repopulation of the peritoneal cavity: contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol. 2010;11:32. doi: 10.1186/1471-2172-11-32. doi:10.1186/1471-2172-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combs JW, Lagunoff D, Benditt EP. DIfferentiation and proliferation of embryonic mast cells of the rat. J Cell Biol. 1965;25:577–592. doi: 10.1083/jcb.25.3.577. doi:10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong LC, Watkins S, Wilhelm DL. The mast cell: distribution and maturation in the peritoneal cavity of the adult rat. Pathology. 1975;7:307–318. doi: 10.3109/00313027509081687. doi:10.3109/00313027509081687. [DOI] [PubMed] [Google Scholar]
- 9.Forman MF, Brower GL, Janicki JS. Rat cardiac mast cell maturation and differentiation following acute ventricular volume overload. Inflamm Res. 2006;55:408–415. doi: 10.1007/s00011-006-6016-z. doi:10.1007/s00011-006-6016-z. [DOI] [PubMed] [Google Scholar]
- 10.Murray DB, Levick SP, Brower GL, Janicki JS. Inhibition of matrix metalloproteinase activity prevents increases in myocardial tumor necrosis factor-alpha. J Mol Cell Cardiol. 2010;49:245–250. doi: 10.1016/j.yjmcc.2010.04.005. doi:10.1016/j.yjmcc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2002;283:H518–H525. doi: 10.1152/ajpheart.00218.2000. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JA, Wei CC, Brower GL, Rynders PE, Hankes GH, Dillon AR, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–319. doi: 10.1016/s0022-2828(03)00013-0. doi:10.1016/S0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 13.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Cardiac Fail. 2005;11:548–556. doi: 10.1016/j.cardfail.2005.05.005. doi:10.1016/j.cardfail.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol. 2008;45:56–61. doi: 10.1016/j.yjmcc.2008.04.010. doi:10.1016/j.yjmcc.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chancey AL, Brower GL, Janicki JS. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol Heart Circ Physiol. 2002;282:H2152–H2158. doi: 10.1152/ajpheart.00777.2001. [DOI] [PubMed] [Google Scholar]
- 16.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. doi:10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994;223:171–177. doi: 10.1111/j.1432-1033.1994.tb18980.x. doi:10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Lees M, Newlands GF, Nagase H, Woolley DE. Activation of precursors for matrix metalloproteinases 1 (interstitial collagenase) and 3 (stromelysin) by rat mast-cell proteinases I and II. Biochem J. 1995;305:301–306. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–1662. doi: 10.1172/JCI114344. doi:10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. doi:10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 21.Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine. 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. doi:10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 22.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischaemia/reperfusion. Circulation. 1998;98:699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 23.Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF. Release of TNF-α during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res. 2003;60:608–616. doi: 10.1016/j.cardiores.2003.08.016. doi:10.1016/j.cardiores.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Ogai A, Nakatani S, Hashimura K, Kanzaki H, Komamura K, et al. Impact of blockade of histamine H2 receptors on chronic heart failure revealed by retrospective and prospective randomized studies. J Am Coll Cardiol. 2006;48:1378–1384. doi: 10.1016/j.jacc.2006.05.069. doi:10.1016/j.jacc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 25.Engels W, Reiters PH, Daemen MJ, Smits JF, van der Vusse GJ. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J Pathol. 1995;177:423–429. doi: 10.1002/path.1711770414. doi:10.1002/path.1711770414. [DOI] [PubMed] [Google Scholar]
- 26.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischaemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 27.Kinet JP. The essential role of mast cells in orchestrating inflammation. Immunol Rev. 2007;217:5–7. doi: 10.1111/j.1600-065X.2007.00528.x. doi:10.1111/j.1600-065X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 28.Somasundaram P, Ren G, Nagar H, Kraemer D, Mendoza L, Michael LH, et al. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–111. doi: 10.1002/path.1690. doi:10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaggi AS, Singh M, Sharma A, Singh D, Singh N. Cardioprotective effects of mast cell modulators in ischaemia-reperfusion-induced injury in rats. Methods Find Exp Clin Pharmacol. 2007;29:593–600. doi: 10.1358/mf.2007.29.9.1161005. doi:10.1358/mf.2007.29.9.1161005. [DOI] [PubMed] [Google Scholar]
- 30.Asanuma H, Minamino T, Ogai A, Kim J, Asakura M, Komamura K, et al. Blockade of histamine H2 receptors protects the heart against ischaemia and reperfusion injury in dogs. J Mol Cell Cardiol. 2006;40:666–674. doi: 10.1016/j.yjmcc.2006.02.001. doi:10.1016/j.yjmcc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell α- and β-chymases. Biochimica et Biophysica Acta. 2000;1480:245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 32.Jin D, Takai S, Yamada M, Sakaguchi M, Yao Y, Miyazaki M. Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol. 2001;86:203–214. doi: 10.1254/jjp.86.203. doi:10.1254/jjp.86.203. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama R, Hatta E, Yasuda K, Smith NCE, Levi R. Angiotensin-converting enzyme-independent angiotensin formation in a human model of myocardial ischaemia: modulation of norepinephrine release by angiotensin type 1 and angiotensin type 2 receptors. JPET. 2000;294:248–254. [PubMed] [Google Scholar]
- 34.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischaemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. doi:10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, et al. Mast cells: a unique source of renin. PNAS. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. doi:10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya K, Farwell K, Huang M, Kempuraj D, Donelan J, Papaliodis D, et al. Mast cell deficient W/Wv mice have lower serum IL-6 and less cardiac tissue necrosis than their normal littermates following myocardial ischaemia-reperfusion. Int J Immunopathol Pharmacol. 2007;20:69–74. doi: 10.1177/039463200702000108. [DOI] [PubMed] [Google Scholar]
- 37.Cimini M, Fazel S, Zhuo S, Xaymardan M, Fujii H, Weisel RD, et al. c-Kit dysfunction impairs myocardial healing after infarction. Circulation. 2007;116:I77–I82. doi: 10.1161/CIRCULATIONAHA.107.708107. [DOI] [PubMed] [Google Scholar]
- 38.Gailit J, Marchese MJ, Kew RR, Gruber BL. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. doi:10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 39.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. doi:10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niwa Y, Kasugai T, Ohno K, Morimoto M, Yamazaki M, Dohmae K, et al. Anemia and mast cell depletion in mutant rats that are homozygous at ‘white spotting (Ws)’ locus. Blood. 1991;78:1936–1941. [PubMed] [Google Scholar]
- 41.Morimoto M, Kasugai T, Tei H, Jippo-Kanemoto T, Kanakura Y, Kitamura Y. Age-dependent amelioration of hypoplastic anemia in Ws/Ws rats with a small deletion at the kinase domain of c-kit. Blood. 1993;82:3315–3320. [PubMed] [Google Scholar]
- 42.Olivetti G, Lagrasta C, Ricci R, Sonnenblick EH, Capasso JM, Anversa P. Long-term pressure-induced cardiac hypertrophy: capillary and mast cell proliferation. Am J Physiol Heart Circ Physiol. 1989;257:H1766–H1772. doi: 10.1152/ajpheart.1989.257.6.H1766. [DOI] [PubMed] [Google Scholar]
- 43.Panizo A, Mindan FJ, Galindo MF, Cenarruzabeitia E, Hernandez M, Diez J. Are mast cells involved in hypertensive heart disease? J Hypertens. 1995;13:1201–1208. doi: 10.1097/00004872-199510000-00015. doi:10.1097/00004872-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Shiota N, Rysa J, Kovanen PT, Ruskoaha H, Kokkonen JO, Lindstedt KA. A role for cardiac mast cells in the pathogenesis of hypertensive heart disease. J Hypertens. 2003;21:1823–1825. doi: 10.1097/00004872-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. doi:10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. doi:10.1161/HYPERTENSIONAHA.108.123158. [DOI] [PubMed] [Google Scholar]
- 47.Hara M, Ono K, Hwang MW, Iwasaki A, Okada M, Nakatani K, et al. Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med. 2002;195:375–381. doi: 10.1084/jem.20002036. doi:10.1084/jem.20002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li QY, Raza-Ahmad A, MacAulay MA, Lalonde LD, Rowden G, Trethewey E, et al. The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts. Transplantation. 1992;53:1047–1051. doi: 10.1097/00007890-199205000-00015. doi:10.1097/00007890-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Zweifel M, Hirsiger H, Matozan K, Welle M, Schaffner T, Mohacsi P. Mast cells in ongoing acute rejection: increase in number and expression of a different phenotype in rat heart transplants. Transplantation. 2002;73:1707–1716. doi: 10.1097/00007890-200206150-00004. doi:10.1097/00007890-200206150-00004. [DOI] [PubMed] [Google Scholar]
- 50.Estensen RD. Eosinophilic myocarditis: a role for mast cells? Arch Pathol Lab Med. 1984;108:358–359. [PubMed] [Google Scholar]
- 51.Afanasyeva M, Georgakopoulos D, Rose NR. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmunity Rev. 2004;3:476–486. doi: 10.1016/j.autrev.2004.08.009. doi:10.1016/j.autrev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Palaniyandi SS, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Involvement of mast cells in the development of fibrosis in rats with postmyocarditis dilated cardiomyopathy. Biol Pharm Bull. 2005;28:2128–2132. doi: 10.1248/bpb.28.2128. [DOI] [PubMed] [Google Scholar]
- 53.Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJL, et al. Interferon-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-β1, interleukin-1β, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, et al. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity. 2004;37:131–145. doi: 10.1080/0891693042000196200. doi:10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 55.Kitaura-Inenaga K, Hara M, Higuchi K, Yamamoto K, Yamaki A, Ono K, et al. Gene expression of cardiac mast cell chymase and tryptase in a murine model of heart failure caused by viral myocarditis. Circ J. 2003;67:881–884. doi: 10.1253/circj.67.881. doi:10.1253/circj.67.881. [DOI] [PubMed] [Google Scholar]
- 56.Pires JG, Milanez MC, Pereira FE. Histamine levels in tissues of Trypanosoma cruzi-infected mice. Agents Actions Suppl. 1992;36:96–98. [PubMed] [Google Scholar]
- 57.Postan M, Correa R, Ferrans VJ, Tarleton RL. In vitro culture of cardiac mast cells from mice experimentally infected with Trypanosoma cruzi. Int Arch Allergy Immunol. 1994;105:251–257. doi: 10.1159/000236765. doi:10.1159/000236765. [DOI] [PubMed] [Google Scholar]
- 58.Libby P, Shi GP. Mast cells as mediators and modulators of atherogenesis. Circulation. 2007;115:2471–2473. doi: 10.1161/CIRCULATIONAHA.107.698480. doi:10.1161/CIRCULATIONAHA.107.698480. [DOI] [PubMed] [Google Scholar]
- 59.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. doi:10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 60.Heikkila HM, Latti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2008;28:309–314. doi: 10.1161/ATVBAHA.107.151340. doi:10.1161/ATVBAHA.107.151340. [DOI] [PubMed] [Google Scholar]
- 61.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. doi:10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown LA, Nunez DJ, Brookes CI, Wilkins MR. Selective increase in endothelin-1 and endothelin A receptor subtype in the hypertrophied myocardium of the aorto-venacaval fistula rat. Cardiovasc Res. 1995;29:768–774. [PubMed] [Google Scholar]
- 63.Fraccarollo D, Bauersachs J, Kellner M, Galuppo P, Ertl G. Cardioprotection by long-term ETA receptor blockade and ACE inhibition in rats with congestive heart failure: mono- versus combination therapy. Cardiovasc Res. 2002;54:85–94. doi: 10.1016/s0008-6363(01)00553-3. doi:10.1016/S0008-6363(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 64.Fraccarollo D, Hu K, Galuppo P, Gaudron P, Ertl G. Chronic endothelin receptor blockade attenuates progressive ventricular dilation and improves cardiac function in rats with myocardial infarction: possible involvement of myocardial endothelin system in ventricular remodeling. Circulation. 1997;96:3963–3973. doi: 10.1161/01.cir.96.11.3963. [DOI] [PubMed] [Google Scholar]
- 65.Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2295–H2299. doi: 10.1152/ajpheart.00048.2004. doi:10.1152/ajpheart.00048.2004. [DOI] [PubMed] [Google Scholar]
- 66.Murray DB, Gardner JD, Brower GL, Janicki JS. Effects of nonselective endothelin-1 receptor antagonism on cardiac mast cell-mediated ventricular remodeling in rats. Am J Physiol Heart Circ Physiol. 2008;294:H1251–H1257. doi: 10.1152/ajpheart.00622.2007. doi:10.1152/ajpheart.00622.2007. [DOI] [PubMed] [Google Scholar]
- 67.Calderon-Garciduenas L, Gambling TM, Acuna H, Garcia R, Osnaya N, Monroy S, et al. Canines as sentinel species for assessing chronic exposures to air pollutants: Part 2. Cardiac pathology. Toxicol Sci. 2001;61:356–367. doi: 10.1093/toxsci/61.2.356. doi:10.1093/toxsci/61.2.356. [DOI] [PubMed] [Google Scholar]
- 68.Melendez GC, Voloshenyuk TG, McLarty JL, Levick SP Brower GL. Oxidative stress mediated cardiac mast cell degranulation. Toxicol Environ Chem. 2010;92:1293–1301. doi:10.1080/02772240903306409. [Google Scholar]
- 69.Masini E, Cuzzocrea S, Mazzon E, Marzocca C, Mannaioni PF, Salvemini D. Protective effects of M40403, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Br J Pharmacol. 2002;136:905–917. doi: 10.1038/sj.bjp.0704774. doi:10.1038/sj.bjp.0704774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, et al. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- 71.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. doi:10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Qin G, Liang G, Li J, Barrington RA, Liu Dx. C5aR-mediated myocardial ischaemia/reperfusion injury. Biochem Biophys Res Comm. 2007;357:446–452. doi: 10.1016/j.bbrc.2007.03.152. doi:10.1016/j.bbrc.2007.03.152. [DOI] [PubMed] [Google Scholar]
- 73.Patella V, de CG, Ciccarelli A, Marino I, Adt M, Marone G. Human heart mast cells: a definitive case of mast cell heterogeneity. Int Arch Allergy Immunol. 1995;106:386–393. doi: 10.1159/000236871. doi:10.1159/000236871. [DOI] [PubMed] [Google Scholar]
- 74.Patella V, Marino I, Lamparter B, Arbustini E, Adt M, Marone G. Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J Immunol. 1995;154:2855–2865. [PubMed] [Google Scholar]
- 75.Arizono N, Matsuda S, Hattori T, Kojima Y, Maeda T, Galli SJ. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62:626–634. [PubMed] [Google Scholar]
- 76.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. doi:10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 77.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunology. 2005;163:92–101. doi: 10.1016/j.jneuroim.2005.02.015. doi:10.1016/j.jneuroim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Shanahan F, Denburg JA, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- 79.Saban R, Gerard NP, Saban MR, Nguyen NB, DeBoer DJ, Wershil BK. Mast cells mediate substance P-induced bladder inflammation through an NK1 receptor-independent mechanism. Am J Physiol Renal Physiol. 2002;283:F616–F629. doi: 10.1152/ajprenal.00096.2002. [DOI] [PubMed] [Google Scholar]
- 80.van der Kleij HPM, Ma D, Redegeld FAM, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by il-4 and stem cell factor. J Immunol. 2003;171:2074–2079. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 81.Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–174. doi: 10.1046/j.1468-2982.1997.1703166.x. doi:10.1046/j.1468-2982.1997.1703166.x. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. doi:10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- 83.Heaney LG, Cross LJ, Stanford CF, Ennis M. Substance P induces histamine release from human pulmonary mast cells. Clin Exp Allergy. 1995;25:179–186. doi: 10.1111/j.1365-2222.1995.tb01024.x. doi:10.1111/j.1365-2222.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 84.Ali H, Pearce FL. Isolation and properties of cardiac and other mast cells from the rat and guinea-pig. Agents Actions. 1985;16:138–140. doi: 10.1007/BF01983121. doi:10.1007/BF01983121. [DOI] [PubMed] [Google Scholar]
- 85.Forman MF, Brower GL, Janicki JS. Spontaneous histamine secretion during isolation of rat cardiac mast cells. Inflamm Res. 2004;53:453–457. doi: 10.1007/s00011-004-1275-z. doi:10.1007/s00011-004-1275-z. [DOI] [PubMed] [Google Scholar]
- 86.Marone G, de Crescenzo G, Adt M, Patella V, Arbustini E, Genovese A. Immunological characterization and functional importance of human heart mast cells. Immunopharmacol. 1995;31:1–18. doi: 10.1016/0162-3109(95)00037-3. doi:10.1016/0162-3109(95)00037-3. [DOI] [PubMed] [Google Scholar]
- 87.Pearce FL, Ali H, Barrett KE, Befus AD, Bienenstock J, Brostoff J, et al. Functional characteristics of mucosal and connective tissue mast cells of man, the rat and other animals. Int Arch Allergy Appl Immunol. 1985;77:274–276. doi: 10.1159/000233808. doi:10.1159/000233808. [DOI] [PubMed] [Google Scholar]
- 88.Morgan LG, Levick SP, Voloshenyuk TG, Murray DB, Forman MF, Brower GL, et al. A novel technique for isolating functional mast cells from the heart. Inflamm Res. 2008;57:1–6. doi: 10.1007/s00011-007-7059-5. [DOI] [PubMed] [Google Scholar]
- 89.Reinecke M, Weihe E, Carraway RE, Leeman SE, Forssmann WG. Localization of neurotensin immunoreactive nerve fibers in the guinea-pig heart: evidence derived by cimmunohistochemistry, radioimmunoassay and chromatography. Neuroscience. 1982;7:1785–1795. doi: 10.1016/0306-4522(82)90036-7. doi:10.1016/0306-4522(82)90036-7. [DOI] [PubMed] [Google Scholar]
- 90.Zhao D, Pothoulakis C. Effects of NT on gastrointestinal motility and secretion, and role in intestinal inflammation. Peptides. 2006;27:2434–2444. doi: 10.1016/j.peptides.2005.12.016. doi:10.1016/j.peptides.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 91.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: a link to neurogenic skin disorders. Brain Behav Immunity. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. doi:10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 92.Rioux F, Kerouac R, Quirion R, St-Pierre S. Mechanisms of the cardiovascular effects of neurotensin. Ann N Y Acad Sci. 1982;400:56–74. doi: 10.1111/j.1749-6632.1982.tb31560.x. doi:10.1111/j.1749-6632.1982.tb31560.x. [DOI] [PubMed] [Google Scholar]
- 93.Rioux F, Kerouac R, St-Pierre S. Characterization of the histamine releasing effect of neurotensin in the rat heart. Peptides. 1985;6:121–125. doi: 10.1016/0196-9781(85)90087-7. doi:10.1016/0196-9781(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 94.Pang X, Alexacos N, Letourneau R, Seretakis D, Gao W, Boucher W, et al. A neurotensin receptor antagonist inhibits acute immobilization stress-induced cardiac mast cell degranulation, a corticotropin-releasing hormone-dependent process. JPET. 1998;287:307–314. [PubMed] [Google Scholar]
- 95.Osadchii O, Norton G, Deftereos D, Badenhorst D, Woodiwiss A. Impact and mechanisms of action of neurotensin on cardiac contractility in the rat left ventricle. Eur J Pharmacol. 2005;520:108–117. doi: 10.1016/j.ejphar.2005.07.014. doi:10.1016/j.ejphar.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 96.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. doi:10.1016/S0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 97.Chancey AL, Gardner JD, Murray DB, Brower GL, Janicki JS. Modulation of cardiac mast cell-mediated extracellular matrix degradation by estrogen. Am J Physiol Heart Circ Physiol. 2005;289:H316–H321. doi: 10.1152/ajpheart.00765.2004. doi:10.1152/ajpheart.00765.2004. [DOI] [PubMed] [Google Scholar]
- 98.Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, et al. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118–G125. doi: 10.1152/ajpgi.00024.2003. doi:10.1152/ajpgi.00024.2003. [DOI] [PubMed] [Google Scholar]
- 99.Kim MS, Chae HJ, Shin TY, Kim HM, Kim HR. Estrogen regulates cytokine release in human mast cells. Immunopharmacol Immunotoxicol. 2001;23:495–504. doi: 10.1081/iph-100108596. doi:10.1081/IPH-100108596. [DOI] [PubMed] [Google Scholar]
- 100.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-α inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. doi:10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. doi:10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 102.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]