Abstract
Aims
The aim of this study was to determine the relative importance of Ca2+ sensitization, ion channels, and intracellular Ca2+ ([Ca2+]i) in the mixed constrictor/relaxation actions of superoxide anion on systemic and pulmonary arteries.
Methods and results
Pulmonary and mesenteric arteries were obtained from rat. Superoxide was generated in arteries and cells with 6-anilino-5,8-quinolinequinone (LY83583). Following pre-constriction with U46619, 10 μmol/L LY83583 caused constriction in pulmonary and relaxation in mesenteric arteries. Both constrictor and relaxant actions of LY83583 were inhibited by superoxide dismutase and catalase. LY83583 caused Rho-kinase-dependent constriction in α-toxin-permeabilized pulmonary but not mesenteric arteries. Phosphorylation of myosin phosphatase-targeting subunit-1 (MYPT-1; as determined by western blot), was enhanced by LY83583 in pulmonary artery only. However, in both artery types, changes in tension were closely correlated with changes in phosphorylation of the 20 kDa myosin light chain as well as changes in [Ca2+]i (as measured with Fura PE-3), with LY83583 causing increases in pulmonary and decreases in mesenteric arteries. When U46619 was replaced by 30 mmol/L K+, all changes in [Ca2+]i were abolished and LY83583 constricted both artery types. The KV channel inhibitor 4-aminopyridine abolished the LY83583-induced relaxation in mesenteric artery without affecting constriction in pulmonary artery. However, LY83583 caused a similar hyperpolarizing shift in the steady-state activation of KV current in isolated smooth muscle cells of both artery types.
Conclusions
Superoxide only causes Rho-kinase-dependent Ca2+ sensitization in pulmonary artery, resulting in constriction, and whilst it opens KV channels in both artery types, this only results in relaxation in mesenteric.
Keywords: Reactive oxygen species, Vascular smooth muscle, Rho-kinase, Intracellular Ca2+, Voltage-gated K+ channel
1. Introduction
Reactive oxygen species (ROS) are important second messenger molecules contributing to normal functioning of multiple cell-signalling pathways.1 However, excessive or dysregulated ROS production or metabolism, termed oxidative stress, promotes enhanced contractility, vascular smooth muscle growth, and inflammation, associated with numerous cardiovascular diseases, including both systemic2 and pulmonary3,4 hypertension.
Acutely, ROS have complex constricting and relaxing actions on the systemic vasculature, depending on the artery size, nature of pre-constriction, amount and type of ROS being generated, and where it is being generated.5–8 The two principal ROS produced in living cells are superoxide anion and its dismutation product hydrogen peroxide, and they have distinct actions on the vasculature. Superoxide inhibits endothelium-dependent relaxation through scavenging of nitric oxide in both pulmonary and systemic arteries.2,3,9 Systemically, hydrogen peroxide may mediate metabolic dilation in the coronary circulation10,11 and may be a candidate endothelium-derived hyperpolarizing factor (EDHF), a major component of endothelium-dependent relaxation in resistance sized arteries,12 acting through opening of K+ channels.5,10,13
For reasons that are not fully understood mechanistically, but may make sense physiologically because of the need to match perfusion to ventilation, the pulmonary circulation responds differently to a number of stimuli, including ROS and hypoxia. In pulmonary arteries (PAs) hydrogen peroxide is more likely to be pro-contractile,14 and EDHF is probably of lesser importance as a component of endothelium-dependent relaxation than it is systemically.15,16 PAs generally constrict in response to hypoxia, whereas systemic arteries generally relax. ROS are also implicated in this constriction, which, as a corollary of chronic obstructive pulmonary disease, can cause pulmonary hypertension (PH), although whether ROS production is increased or decreased during hypoxia in the pulmonary circulation remains controversial.17,18
In addition to the above, both superoxide and hydrogen peroxide contribute to endothelium-independent constriction through activation of multiple protein kinase pathways.19–21 Previously, we showed that both superoxide and hydrogen peroxide directly constricted rat PA, acting predominantly through Rho-kinase22 and protein kinase C (PKC),14 respectively. Hydrogen peroxide also causes Ca2+ release from ryanodine-sensitive stores.14 ROS-mediated Rho-kinase activation is also implicated in chronic hypoxia-induced PH.23 Superoxide does not directly constrict mesenteric or femoral arteries, but causes a concentration-dependent constriction/relaxation response in the presence of agonist pre-constriction.22 In the present study, we set out to determine the nature of the differences in responses of rat pulmonary and mesenteric arteries (MAs) to superoxide, comparing the roles of Ca2+-sensitization, intracellular Ca2+ and voltage-gated K+ channels.
2. Materials and methods
2.1. Reagents and chemicals
Fura PE-3/AM was from Sigma (Poole, UK). L-012, (8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H)dione) was from Wako Chemicals USA, Inc., (USA). Hydrogen peroxide, antioxidant enzymes, LY83583 (6-anilino-5,8-quinolinequinone), paxilline, tetraethylammonium (TEA), 4-aminopyridine, and other pharmacological agents were from Amersham (Bucks, UK), Biomol (Exeter, UK), Calbiochem (Merck Biosciences Nottingham, UK), Fisher (Loughborough, UK), Invitrogen (Paisley, UK), Pierce (Cramlington, UK), or Sigma (Poole, UK).
2.2. Animals and tissues
This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Housing and handling of animals was also in accordance with UK Home Office regulations. Intra-PA (second to third order branches) and MAs (second to fourth order) were obtained from male Wistar rats (200–250 g), killed by pentobarbital injection. Comparably sized coronary and renal arteries were similarly obtained.
2.3. Production and measurement of superoxide
Superoxide was generated within cells and tissues using LY83583.24 We showed previously that this occurs in PASMC using three different measures of ROS production (MitoSOX, DHE, and L-012).22 Block with superoxide dismutase (SOD), but not catalase confirmed superoxide as the principal species produced.22 In the present study, we used L-012 (a luminol derivative, 10 μmol/L) to compare levels of ROS production in PA and MA. Arteries were first incubated with L-012 for 30 min for measurement of a stable baseline before the addition of 1 or 10 μmol/L LY83583. Luminescence was measured with a luminometer (LKB-1251, Bromma, Sweden). Luminescence for each LY83583 concentration in the absence of tissue was subtracted as background, and effects of treatments quantified as fold increases above control.
2.4. Measurement of force, intracellular Ca2+ and α-toxin permeabilization
Isometric tension was measured using a wire myograph with arteries bathed in bicarbonate-buffered physiological salt solution (PSS), at 37°C, pH 7.4, as described previously.25 In order to facilitate characterization of both relaxation and constriction responses to LY83583, arteries were pre-constricted with a concentration of U46619 or KCl that produced robust, sustainable constrictions of 50–75% or 20–50% the size of those produced by 80 mmol/L KCl, respectively. Note: Because several of the other pharmacological agents used also altered constriction amplitude, the concentrations of U46619 required to generate these pre-constrictions varied (10–200 nmol/L). Intracellular Ca2+ ([Ca2+]i) was measured in Fura PE-3 loaded, myograph-mounted arteries. Tension was recorded simultaneously with light emitted by the artery at >510 nm at excitation wavelengths 340 and 380 nm. The ratio of the emission intensities (R340/380) was taken as a measure of [Ca2+]i as described previously.26 For Ca2+-sensitization experiments, myograph-mounted arteries were permeabilized with α-toxin and Ca2+-clamped with 10 mmol/L EGTA, as described previously.25 In order to obtain a comparable Ca2+-induced pre-constriction in PA and MA (i.e. as a per cent of maximum constriction to pCa 4.5), [Ca2+]i was fixed at pCa 6.9 in PA and 6.4 in MA. For assessment of endothelial function in responses to LY83583, myograph-mounted arteries were de-endothelialized by rubbing the lumen with a human hair (verified by loss of relaxation to acetylcholine). Control constrictions revealed no significant effects of vehicle (dimethyl sulphoxide) and no significant run-down (not shown).
2.5. Western blot
For measurement of phosphorylation of myosin phosphatase-targeting subunit-1 (MYPT-1) and the 20 kDa myosin light chain (MLC20), PA or MA were prepared, treated, and homogenized and protein extracted for western blot as described previously.25 Membranes were blocked for 1 h with 5% skimmed milk, probed with primary antibody overnight at 4°C (1:1000–1:5000 in 1% milk), followed by application of horseradish peroxidase-conjugated anti-IgG secondary antibody for 1 h at room temperature (1:5000 in 1% milk). Membranes were first probed with specific anti-phospho- antibodies, stripped for 1 h (Pierce stripping buffer), re-blocked, and re-probed with corresponding anti-‘total’ antibodies. Anti-phospho-MYPT-1 (thr-855) was from Upstate (UK), anti-phospho-MLC20 (ser-19), anti-MLC20, and anti-MYPT-1 were all from Cell Signalling (UK). HRP-conjugated anti-IgG secondary antibodies were from Sigma.
2.6. Electrophysiology
For electrophysiology, freshly isolated pulmonary artery smooth muscle cells (PASMCs) and mesenteric artery smooth muscle cells (MASMCs) were obtained from third to fourth order PA and MA, respectively, by enzymatic dispersion, as described previously.27 Voltage-gated K+ currents (KV) were measured by whole-cell voltage-clamp. Cells were superfused with HEPES-buffered PSS containing 1 μmol/L paxilline and 10 μmol/L glibenclamide in order to inhibit Ca2+-activated and KATP currents, respectively, and dialysed with HEPES-buffered intracellular solution containing 10 mmol/L EGTA, as described previously.27
2.7. Statistical analysis
Data were analysed with SigmaStat (Systat Software, Inc., San Jose, CA, USA). Simple pair-wise comparisons were done by Student's t-test. Multiple comparisons of the effects of treatments against control, of inhibitors against treatments, and of PA against MA, were performed by one-way or two-way ANOVA, with pair-wise post-hoc tests, as appropriate. P < 0.05 was deemed significant. All data are expressed as mean ± SEM.
3. Results
Preliminary experiments showed that, when arteries were pre-constricted with U46619, 10 μmol/L LY83583 caused near-maximal constriction in PA, whereas in MA, it caused near-complete relaxation. Similarly, 10 μmol/L LY83583 also relaxed U46619-pre-constricted renal (79.9 ± 7.4% relaxation, n = 2) and coronary arteries (44.3 ± 13.6% relaxation, n = 5), suggesting that MA is representative of the systemic circulation as a whole. 1 µmol/L LY83583 constricted both PA and MA (as shown previously22). Similar responses were obtained when arteries were pre-constricted with phenylephrine (1 μmol/L) instead of U46619 (not shown). In subsequent experiments, we chose to compare responses between PA and MA in the presence of the NO synthase inhibitor nitro-l-arginine methyl ester (l-NAME, 1 mmol/L) in order to focus on the non-NO-related components of the responses to superoxide. The effects of l-NAME on these responses are presented in Supplementary material online, Figure S1. All constrictor and relaxation responses to LY83583 in both PA and MA were unaffected by disruption of the endothelium (not shown).
3.1. LY83583 generates ROS and mediates both constriction and relaxation via ROS
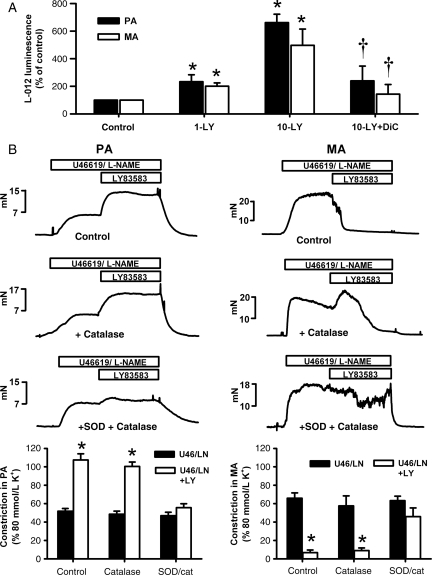
As shown in Figure 1A, ROS production was significantly increased by both 1 and 10 μmol/L LY83583 in both PA and MA, with no significant differences in fold increases above control between the two artery types. ROS generation from 10 μmol/L LY83583 was inhibited by dicoumarol (10 μmol/L), a blocker of the NADPH:quinone oxidoreductase (diaphorase), in both MA and PA, confirming the intracellular origin of LY83583-derived ROS.24 ROS production in MA and PA, and the similarity between the two, was not significantly altered by the presence of l-NAME (data not shown).
Figure 1.
Vascular effects of LY83583 are via generation of reactive oxygen species. (A, bar chart) Effects of LY83583 (1 μmol/L: 1-LY, 10 μmol/L: 10-LY) on L-012 luminescence, taken as a measure of reactive oxygen species production in mesenteric artery (MA, n = 9–10), and pulmonary artery (PA, n = 6–21). *P < 0.05 for LY83583 vs. control (artery without LY83583). †P < 0.05 for dicoumarol (Dic, 10 μmol/L) vs. 10 μmol/L LY83583. (B) Measurement of tension (mN) in PA (left) and MA (right) pre-constricted for 10–15 min with U46619 (range: 100–200 nmol/L) and 1 mmol/L l-NAME before application of 10 μmol/L LY83583 for a further 15 min alone (PA n = 4, MA n = 12), or in the presence of catalase (200 U/mL, PA n = 4, MA n = 7) or superoxide dismutase (SOD) and catalase (200 U/mL each, PA n = 4, MA n = 9). Bar charts: Constriction in PA (left) and MA (right) measured at 15 min before and after application of LY83583, normalized to constriction induced by 80 mmol/L K+. Asterisk denotes significant constriction (PA) or relaxation (MA) in response to LY83583 (P < 0.01).
We confirmed that the actions of LY83583 on PA and MA were via ROS with the use of antioxidant enzymes catalase and SOD. In PA (Figure 1B, left panels), 10 μmol/L LY83583 enhanced U46619/l-NAME-induced constriction. This constriction was not significantly affected by catalase alone, but was almost abolished by the combination of catalase and SOD, and, as shown previously,22 was significantly inhibited by SOD alone (not shown). In MA (Figure 1B, right panels), 10 μmol/L LY83583 caused a near immediate sustained relaxation. In the presence of catalase, there was still a sustained relaxation but this was preceded by a transient constriction (peaking at ∼2.5 min), suggesting that only a part of the relaxation response was due to hydrogen peroxide. In the combined presence of SOD and catalase, however, there was no transient constriction, and the sustained relaxation induced by LY83583 was inhibited. In MA, SOD alone tended to enhance the rate of relaxation induced by 10 μmol/L LY83583 without revealing a transient constriction (not shown), consistent with a pro-relaxant effect of the excess hydrogen peroxide thus generated.13
3.2. Effects of LY83583 on Rho-kinase-dependent Ca2+ sensitization
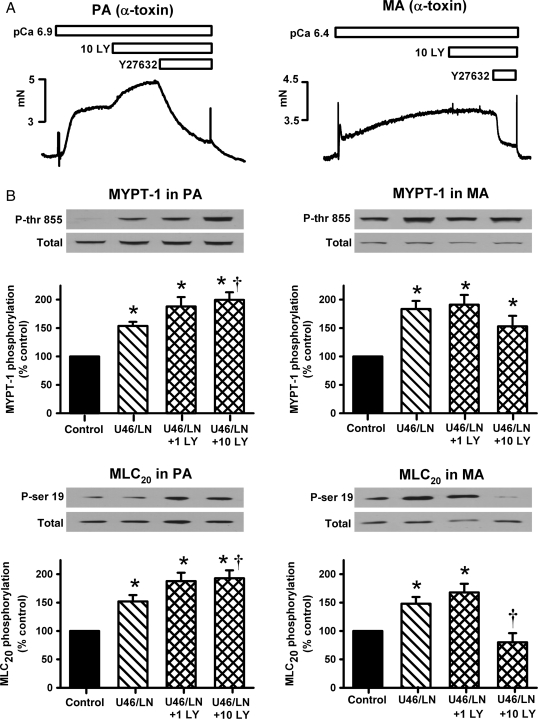
Since we showed previously that superoxide enhances Rho-kinase-mediated Ca2+ sensitization in PA,22 we next examined this pathway in MA and compared it with PA under similar conditions. Arteries were permeabilized with α-toxin and [Ca2+]i clamped at pCa 6.9 (PA) or 6.4 (MA) to induce a small (10–15% of max) pre-constriction. In PA, 10 μmol/L LY83583 caused further constriction, whereas in MA it had no significant effect (Figure 2A). Despite these differences, the Rho-kinase inhibitor Y27632 caused comparable overall inhibition of these constrictions in the two artery types (Figure 2A). This comparison suggests that Rho-kinase is basally active in both artery types, but that LY83583 only influences activity of Rho-kinase in PA. However, when α-toxin permeabilized MA where additionally pre-constricted with U46619/l-NAME, 10 μmol/L LY83583 caused a small but significant relaxation (see Supplementary material online, Figure S2). This relaxation was clearly smaller than that seen in non-permeabilized arteries and was abolished by prior incubation with Y27632 (see Supplementary material online, Figure S2).
Figure 2.
Effects of LY83583 on Ca2+-sensitization and phosphorylation of MYPT-1 and MLC20. (A) Measurement of Ca2+-induced tension (mN) in α-toxin permeabilized PA (pCa 6.9, left) and MA (pCa 6.4, right). 15 min applications of 10 μmol/L LY83583 caused further constriction in PA (representative of 24 ± 5% increase, n = 8), but had no effect in MA (representative of n = 11). In both arteries these constrictions were relaxed by the Rho-kinase inhibitor Y27632 (10 μmol/L) (PA 81.6 ± 3.3%, n = 8 and MA 69.2 ± 2.9%, n = 11). (B) Example western blots and summary bar charts showing effects of 1 and 10 μmol/L LY83583 (1 LY or 10 LY) on MYPT-1 and MLC20 phosphorylation in non-permeabilized PA (left) and MA (right), in the presence of 100 nmol/L U46619 and 1 mmol/L l-NAME (U46/LN). Ratio of phospho/total for each blot was calculated and effects of treatments expressed as a percentage change over control (untreated artery). Asterisk denotes significant enhancement vs. control (P < 0.05, PA n = 10–12, MA n = 12). Dagger denotes significant increase (PA) or decrease (MA) in response to LY83583 compared with U46/LN alone (P < 0.05).
To examine further the relationship between Rho-kinase-mediated phosphorylation and constriction in the two artery types, the effects of 1 and 10 μmol/L LY83583 on phosphorylation of MYPT-1 at thr-855 (specifically phosphorylated by Rho-kinase in rat vascular smooth muscle25,28) and MLC20 at ser-19 were determined. This was performed in non-permeabilized MA, in order to establish how closely changes in MYPT-1 phosphorylation were mirrored by changes in MLC20 phosphorylation under conditions where [Ca2+]i was not clamped. As shown in Figure 2B, U46619/l-NAME enhanced phosphorylation of both MYPT-1 and MLC20 in both PA (left panels) and MA (right panels). In PA, the further addition of 1 and 10 μmol/L LY83583 caused further enhancement of phosphorylation of both proteins. In MA, 1 μmol/L LY83583 had no significant effect on either phosphorylation target, whereas 10 μmol/L slightly (but not significantly) reduced MYPT-1 phosphorylation and greatly reduced MLC20 phosphorylation (to below basal), in line with the large relaxations of U46619/l-NAME-pre-constricted MA seen in Figure 1 and see Supplementary material online, Figure S1.
3.3. [Ca2+]i–force relationships in responses to LY83583
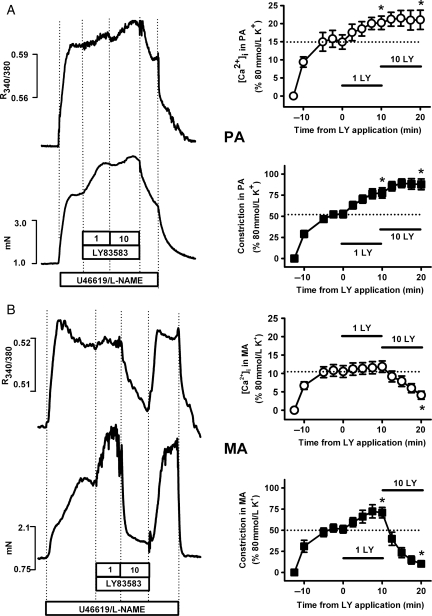
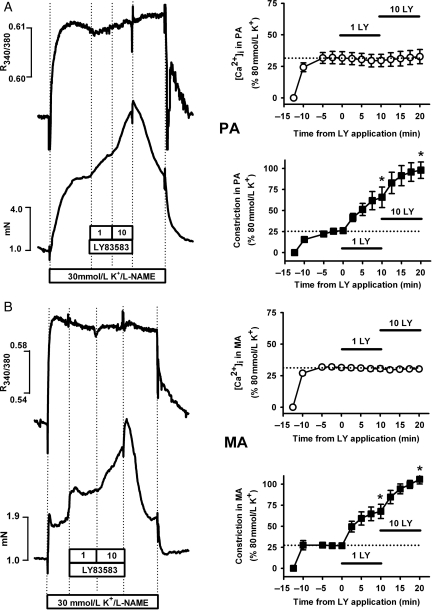
Since LY83583 had limited influence over Ca2+ sensitization and MYPT-1 phosphorylation responses in MA, we next investigated the relationship between constriction/relaxation responses and [Ca2+]i in PA and MA. In PA, when LY83583 was applied cumulatively after pre-constriction with U46619/l-NAME, it caused stepwise increases in both constriction and [Ca2+]i (Figure 3A). In MA, however, 10 μmol/L LY83583 caused a dramatic drop in both constriction and [Ca2+]i, whereas 1 μmol/L LY83583 instead caused constriction and only a small increase in [Ca2+]i (Figure 3B). Changes in contractile force in response to LY83583 therefore broadly match changes in [Ca2+]i both in PA and MA, particularly at the higher LY83583 concentration. To determine whether these changes in [Ca2+]i were related to membrane potential, and therefore perhaps to actions on K+ channels, we next repeated these experiments, but pre-constricting with 30 mmol/L K+ and l-NAME (instead of U46619), in order to partially dampen the driving force for K+ efflux thus preventing hyperpolarization through opening of K+ channels. Under these conditions, both PA (Figure 4A) and MA (Figure 4B) were further constricted in a stepwise fashion by cumulatively applied LY83583, without any further changes in [Ca2+]i in either artery type. Constrictions induced by LY83583 in the presence of 30 mmol/L K+ were not prevented by Y27632 in MA, whereas in PA they were substantially reduced (see Supplementary material online, Figure S3A). In both MA and PA, the constriction remaining in the presence of Y27632 was abolished by the PKC inhibitor Gö6983 (10 μmol/L) (see Supplementary material online, Figure S3B).
Figure 3.
Effects of LY83583 on relationship between constriction and [Ca2+]i with U46619 pre-constriction. Example traces: Simultaneous measurement of tension (mN) and [Ca2+]i (R340/380) in PA (A) and MA (B). Following pre-constriction with 100 nmol/L U46619 and 1 mmol/L l-NAME, LY83583 was added cumulatively at 10 min intervals, as indicated. Note: constriction and [Ca2+]i responses returned to the pre-constriction level when LY83583 was washed out in the continued presence of U46619/l-NAME. Summary charts: effects of LY83583 on [Ca2+]i (open symbols) and constriction (closed symbols), normalized to responses induced by 80 mmol/L K+, in PA (A, n = 4–5) and MA (B, n = 4). Note: charts do not show effects of washout. Asterisk denotes significant enhancement or reduction of tension or [Ca2+]i compared with the zero time-point (P < 0.05).
Figure 4.
Effects of LY83583 on relationship between constriction and [Ca2+]i with sub-maximal K+ pre-constriction. Example traces: Simultaneous measurement of tension (mN) and [Ca2+]i (R340/380) in PA (A) and MA (B). Following pre-constriction with 30 mmol/L KCl and 1 mmol/L l-NAME, LY83583 was added cumulatively at 10 min intervals, as indicated. Note: constriction and [Ca2+]i responses returned to the pre-constriction level when LY83583 was washed out in the continued presence of U46619/l-NAME. Summary charts: effects of LY83583 on [Ca2+]i (open symbols) and constriction (closed symbols), normalized to responses induced by 80 mmol/L K+ in PA (A, n = 4–6) and MA (B, n = 4–5). Note: charts do not show effects of washout. Asterisk denotes significant enhancement of tension or [Ca2+]i compared with the zero time-point (P < 0.01).
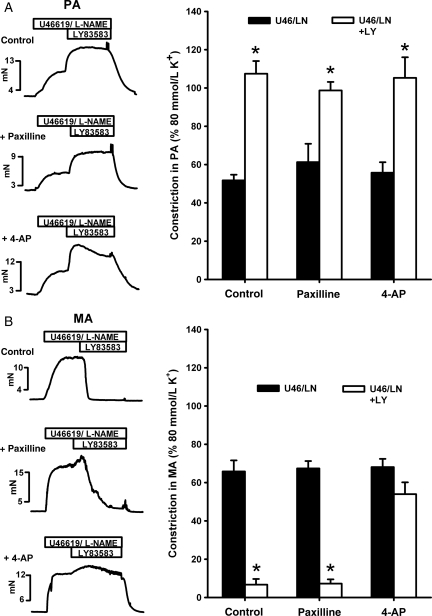
The fact that elevated extracellular [K+] abolished LY83583-induced [Ca2+]i responses (both increases and decreases) indicates that changes in [Ca2+]i were membrane potential-dependent, and that the drop in [Ca2+]i in MA induced by 10 μmol/L LY83583 was possibly related to opening of K+ channels and subsequent hyperpolarization. We tested this latter hypothesis by repeating applications of 10 μmol/L LY83583 to U46619/l-NAME pre-constrictions in PA and MA in the absence or presence of paxilline (1 μmol/L), an inhibitor of Ca2+-activation K+ (BKCa) channels, or 4-aminopyridine (4-AP, 5 mmol/L), an inhibitor of voltage-gated K+ (KV) channels. In PA, neither pre-incubation with paxilline nor with 4-AP had any effect on the sustained constriction (at 15 min) induced by 10 μmol/L LY83583 (Figure 5A). In MA, however, paxilline delayed the relaxation response, which was preceded by a transient constriction (similar to the effect of catalase), whereas 4-AP virtually abolished the relaxation (Figure 5B). Neither paxilline nor 4-AP had any effects on un-pre-constricted PA or MA (not shown). 1 mmol/L TEA had a similar effect on LY83583 responses to that of paxilline (not shown).
Figure 5.
Effects of K+ channel blockers on LY83583-induced constriction and relaxation. Measurements of tension (mN) in PA (A) and MA (B) pre-constricted for 10–15 min with U46619 (range: 10–100 nmol/L) and 1 mmol/L l-NAME. Example traces show effects of 10 μmol/L LY83583 in the absence (control) and presence of BKCa channel blocker paxilline, (1 μmol/L) or KV channel blocker 4-aminopyridine (4-AP, 5 mmol/L). As shown in summary bar charts, significant enhancement of constriction caused by LY83583 in PA (A) was unaffected by either paxilline or 4-AP (*P < 0.05 vs. U46/LN alone, n = 4–6). In contrast, significant relaxation caused by LY83583 in MA (B) was only delayed by paxilline but abolished by 4-AP (*P < 0.01 vs. U46/LN alone, n = 6–12).
3.4. Effects of LY83583 on voltage-gated K+ currents
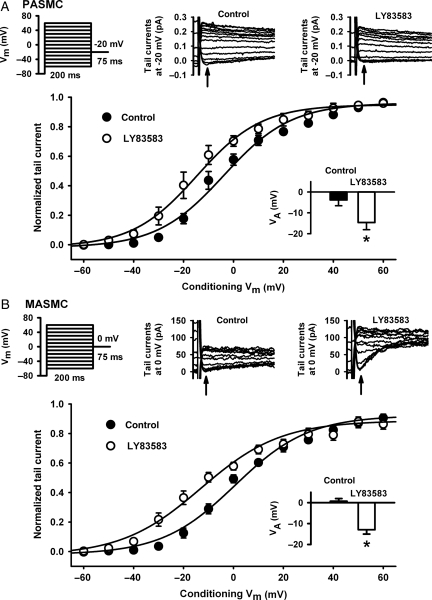
In order to confirm that inhibition of 10 μmol/L LY83583-induced relaxation in MA by 4-AP was through inhibition of KV channels, we examined the effects of LY83583 on KV currents in freshly isolated MASMC by whole-cell voltage-clamp and compared them with PASMC. We examined the steady-state activation characteristic (VA) of the current by applying a range of pre-conditioning potentials and then briefly stepping to an appropriate test potential and measuring the amplitude of the resultant tail current. As shown in Figure 6, LY83583 caused a significant hyperpolarizing shift in VA in both MA and PA, with no significant difference in the size of the shift between the two cell-types (−13.7 ± 2.3 mV in MA and −10.8 ± 1.7 mV in PA).
Figure 6.
Effects of LY83583 on steady-state activation of voltage-gated K+ currents. Voltage protocols: cells serially subjected to a range of preconditioning pulses between −60 and +60 mV, each followed by a short test pulse to −20 mV (A, PASMC) or 0 mV (B, MASMC). Representative traces: tail currents evoked at relevant test potential before (control) and during application of LY83583 (10 μmol/L for 10 min). Note: different test potentials were chosen for ease of measurement of much smaller currents in MASMC. Arrows indicate point at which measurements were taken. Main summary plots: Tail current measurements from PASMC (A, n = 5) and MASMC (B, n = 6), normalized to maximum current amplitude, plotted against conditioning potential and fitted by Boltzmann non-linear regression. Inset bar-charts: VA calculated from Boltzmann fit in PASMC (A, *P < 0.01 vs. control) and MASMC (B, *P < 0.01 vs. control). Note: all currents recorded in the presence of 1 μmol/L paxilline and 10 μmol/L glibenclamide to block BKCa and KATP channels.
3.5. Role of guanylyl cyclase inhibition in responses to LY83583
Since LY83583 may also influence vascular tone through inhibition of guanylyl cyclase, either directly or via ROS,29 we examined responses to LY83583 in PA and MA in the presence of the guanylyl cyclase inhibitor ODQ (10 μmol/L). ODQ greatly enhanced the sensitivity of both artery types to U46619 (not shown), making it exceedingly difficult to match pre-constriction amplitudes to those obtained without ODQ, despite a reduction in U46619 concentration. Nevertheless, when pre-constricted with U46619/l-NAME/ODQ, both 1 and 10 μmol/L LY83583 still caused constriction in PA, and 10 μmol/L still caused relaxation in MA. However, 1 μmol/L was without effect in MA under these conditions (see Supplementary material online, Figure S4; compare with Figure 3).
4. Discussion
Our data show that in the presence of the prostanoid agonist U46619, superoxide (as generated by LY8358324) constricts PA and causes either constriction or relaxation in MA, dependent on concentration. These fundamental differences occur both in the absence or presence of l-NAME, and are largely unaffected by ODQ. This suggests therefore, notwithstanding the importance of NO scavenging and guanylyl cyclase activity to endothelium-dependent relaxation,2,3,9 that there are additional pathways through which ROS can influence vascular tone. MAs are likely to be representative of the systemic circulation as a whole since we also observed superoxide-induced relaxation in agonist pre-constricted coronary and renal arteries.
Ca2+ sensitization is the process through which an increased contractile force can be achieved without a concomitant increase in [Ca2+]i and is thought to be present to varying degrees in all smooth muscles.30 One way in which this occurs is through the MYPT-1 which, when phosphorylated on thr-855, primarily by Rho-kinase, causes inhibition of myosin phosphatase, thus enhancing MLC20 phosphorylation.28,30 In LY83583-stimulated PA, we observe a close correlation between MYPT-1 phosphorylation, MLC20 phosphorylation and Y27632-sensitive constriction, suggestive of Rho-kinase-dependent Ca2+ sensitization. In MA, we observe an apparent dissociation between MYPT-1 phosphorylation and MLC20 phosphorylation (and hence constriction) during responses to superoxide; there was no significant effect of LY83583 on MYPT-1 phosphorylation despite a profound drop in MLC20 phosphorylation and an associated relaxation. Similarly, the rather small relaxation induced by LY83583 in α-toxin permeabilized MA, apparently mediated through inhibition of Rho-kinase (and therefore indicative of a small degree of superoxide-mediated Ca2+-de-sensitization), was not large enough to account for the near complete relaxation observed in non-permeabilized MA. These observed differences between MA and PA are curious when considering that, as far as is known, Rho-kinase is of similar importance to normal and hypertensive systemic blood pressure31,32 as it is to the pulmonary circulation.33,34 Although the importance of Rho-kinase in the mesenteric circulation reportedly diminishes with the size of the artery,31 Ca2+-induced constrictions in α-toxin permeabilized PA and MA were similarly inhibited by the blocker of Rho-kinase, Y27632. These results suggest that either the sensitivity of the Rho-kinase pathway to ROS is less in MA than in PA, perhaps through differences in upstream signalling (e.g. src-family kinases25) or, as discussed below, that ROS-induced vascular tone in MA is more dependent on other forms of Ca2+ sensitization, or on ion channels that influence MLC20 phosphorylation through changes in [Ca2+]i.
The changes in MLC20 phosphorylation induced by 10 μmol/L LY83583 in MA in the presence of U46619 mirrored the changes in arterial tension in non-permeabilized MA, and were matched by similar changes in [Ca2+]i. The close relationship between [Ca2+]i and constriction was abolished by replacing U46619 with 30 mmol/L K+, thus converting the LY83583-induced relaxation in MA to constriction, and crucially abolishing the main differences between MA and PA. This experiment raises several interesting points. First, it clearly suggests that superoxide was lowering [Ca2+]i and inducing relaxation in MA via opening of K+ channels and subsequent hyperpolarization, as shown previously with hydrogen peroxide, which hyperpolarizes mesenteric and coronary arteries predominantly through actions on KV channels,5,10 although BKCa channels may also be affected.35 Our observation that LY83583-induced relaxation was abolished by 4-AP or the combination of SOD and catalase, but only partially blocked by paxilline, TEA, or catalase alone, suggests that both superoxide and hydrogen peroxide contribute to the hyperpolarization, but that the principle K+ channel type acted upon was KV, with at best a minor contribution from BKCa. The lack of effect of ODQ on LY83583-induced relaxation in MA rules out the possibility that this relaxation is via ROS-mediated guanylyl cyclase activation and subsequent PKG-mediation K+ channel opening.
In light of the fact that LY83583 was unable to relax PA, it was somewhat surprising that it caused a similar shift in the steady-state activation of the KV current in PASMC as it did in MASMC. However, the inability of 4-AP to prevent LY83583 from causing constriction in PA also refutes the supposition that ROS differentially modulate KV channel activity in PA (inhibition) and MA (activation). Recently, a direct comparison of KV channel characteristics in the two cell-types suggested that channels of this family are more numerous in PASMC, are more likely to be open in the negative range of membrane potentials, and are therefore more likely to contribute to resting membrane potential in PASMC than in MASMC.27 Although this implies that blocking the KV current would be expected to cause more depolarization and a larger elevation of [Ca2+]i in PA than in MA, it is apparent that this depolarization in itself is not enough to cause constriction, since 4-AP alone, while enhancing agonist-induced constriction, does not directly constrict either PA or MA. Another question arising from this is whether opening KV channels is generally more likely to overcome U46619-induced depolarization and constriction in MA than in PA, and if so, why. An answer to this question may explain why physiological agents that affect membrane potential, such as EDHF, have more of an influence on [Ca2+]i and constriction in systemic compared with PAs.13,15 We previously reported that LY83583 does not elevate [Ca2+]i in PA in the absence of agonist;22 however, in the present study it caused further increases above that induced by U46619. Elevated extracellular [K+] clearly prevented these LY83583-induced increases in [Ca2+]i in PA, suggesting the increase was due to Ca2+ influx rather than release from stores (as shown for hydrogen peroxide14). Non-selective cation channels (NSCC) may be directly ROS sensitive,36 and we showed that agonist-mediated contractions are more dependent on La3+-sensitive NSCC in PA, but more dependent on voltage-gated Ca2+ channels in MA.37 NSCC may therefore contribute to LY83583-induced Ca2+ influx in PA. Either way, this Ca2+ influx, in addition to the enhanced Rho-kinase-mediated Ca2+ sensitization, may be enough to selectively overcome opening of KV channels and cause constriction in PA.
The apparent independence of [Ca2+]i and constriction from KV channel opening in response to superoxide in PA is of relevance to hypoxic pulmonary vasoconstriction (HPV) and chronic hypoxia-induced PH. One proposed mechanism for HPV is that hypoxia causes a reduction in ROS production and this relieves a constitutive redox activation of KV channels, thus causing depolarization and Ca2+ influx.17 Our data, although agreeing that ROS enhance KV current in PA, imply that at least in the presence of a depolarizing and Ca2+-sensitizing agonist, it is irrelevant to constriction. Rather, our results are consistent with the alternative hypothesis that an increased production of mitochondrial ROS during hypoxia causes increases in both [Ca2+]i and Rho-kinase-mediated Ca2+ sensitization which mediate HPV.18,38 This is also relevant to a rat model of chronic hypoxia-induced PH, where a direct link has been made between enhanced superoxide production, Rho-kinase activation and enhanced PA contractility.23
In MA pre-constricted with U46619, 1 μmol/L LY83583 did not elevate MYPT-1 phosphorylation or [Ca2+]i but it nevertheless caused constriction. This constriction was also abolished by prior inhibition of guanylyl cyclase. These results suggest that, at this concentration, LY83583 is constricting MA independently of direct Rho-kinase activation. Futhermore, when pre-constricted with K+ instead of U46619, the resultant Ca2+-independent LY83583-induced constrictions were much less sensitive to Y27632 in MA than in PA, but were blocked by a PKC inhibitor. This all suggests an alternative form of ROS-sensitive Ca2+-sensitization that has a stronger influence over tone in MA than in PA. While this differential involvement of PKC is interesting, particularly since it too is implicated in ROS-mediated constriction,14,19 further analysis of the role of PKC in this constriction is beyond the scope of this study. Furthermore, why similar constrictions did not also occur in α-toxin permeabilized MA and how guanylyl cyclase/protein kinase G may be involved without interaction with Rho-kinase, remain undetermined.
In summary, we observed effects of superoxide on both pro-contractile and pro-relaxant pathways in both MA and PA. Rho-kinase Ca2+-sensitization and Ca2+-influx pathways were only significantly activated in PA. An alternative pro-contractile pathway responsive to the lower concentration of LY83583, possibly involving PKC, was more prominent in MA. However, the Kv current was enhanced in both PA and MA. These pathways combine to give a net response to the higher concentration of LY83583 of relaxation in MA and constriction in PA. This suggests a fundamental difference in the way basic signalling pathways interact and which ultimately have a greater influence over MLC20 phosphorylation and tone. A better understanding of these differences may help with the development of anti-hypertensive therapies designed to specifically target the pulmonary or systemic vasculature.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the Wellcome Trust (045232 to V.A.S.) and British Heart Foundation (FS/06/003 to G.A.K.). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Supplementary Material
References
- 1.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, et al. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. doi:10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. doi:10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens. 2010;28:201–212. doi: 10.1097/HJH.0b013e328332bcdb. doi:10.1097/HJH.0b013e328332bcdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, et al. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2008;177:219–226. doi: 10.1164/rccm.200702-264OC. doi:10.1164/rccm.200702-264OC. [DOI] [PubMed] [Google Scholar]
- 5.Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. doi:10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girouard H, de CJ. Acute and chronic effects of free radicals on alpha1-adrenergic-induced vasoconstriction in mesenteric beds of spontaneously hypertensive rats. J Hypertens. 2005;23:807–814. doi: 10.1097/01.hjh.0000163150.43201.ac. doi:10.1097/01.hjh.0000163150.43201.ac. [DOI] [PubMed] [Google Scholar]
- 7.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. doi:10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 8.Pelaez NJ, Braun TR, Paul RJ, Meiss RA, Packer CS. H2O2 mediates Ca2+- and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1185–H1193. doi: 10.1152/ajpheart.2000.279.3.H1185. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Altayo F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther. 2006;316:42–52. doi: 10.1124/jpet.105.088435. doi:10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- 10.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. doi:10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 11.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, et al. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. doi:10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. doi:10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Morikawa K, Fujiki T, Matoba T, Kubota H, Hatanaka M, Takahashi S, et al. Important role of superoxide dismutase in EDHF-mediated responses of human mesenteric arteries. J Cardiovasc Pharmacol. 2004;44:552–556. doi: 10.1097/00005344-200411000-00006. doi:10.1097/00005344-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, et al. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. doi:10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Morio Y, Carter EP, Oka M, McMurtry IF. EDHF-mediated vasodilation involves different mechanisms in normotensive and hypertensive rat lungs. Am J Physiol Heart Circ Physiol. 2003;284:H1762–H1770. doi: 10.1152/ajpheart.00831.2002. [DOI] [PubMed] [Google Scholar]
- 16.Torok J. Histamine-induced relaxation in pulmonary artery of normotensive and hypertensive rats: relative contribution of prostanoids, nitric oxide and hyperpolarization. Physiol Res. 2000;49:107–114. [PubMed] [Google Scholar]
- 17.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. doi:10.1161/01.RES.0000036751.04896.F1. [DOI] [PubMed] [Google Scholar]
- 19.Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. doi:10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. doi:10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- 22.Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A, et al. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca2+ sensitization. Free Radic Biol Med. 2009;46:633–642. doi: 10.1016/j.freeradbiomed.2008.11.015. doi:10.1016/j.freeradbiomed.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. doi:10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa T, Bando A, Tsuchiya K, Abe S, Okamoto M, Kirima K, et al. Enzymatic and nonenzymatic formation of reactive oxygen species from 6-anilino-5,8-quinolinequinone. Biochim Biophys Acta. 2004;1670:19–27. doi: 10.1016/j.bbagen.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Knock GA, Shaifta Y, Snetkov VA, Vowles B, Drndarski S, Ward JP, et al. Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery. Cardiovasc Res. 2008;77:570–579. doi: 10.1093/cvr/cvm073. doi:10.1093/cvr/cvm073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. doi:10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firth AL, Gordienko DV, Yuill KH, Smirnov SV. Cellular localization of mitochondria contributes to Kv channel-mediated regulation of cellular excitability in pulmonary but not mesenteric circulation. Am J Physiol Lung Cell Mol Physiol. 2009;296:L347–L360. doi: 10.1152/ajplung.90341.2008. doi:10.1152/ajplung.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005;389:763–774. doi: 10.1042/BJ20050237. doi:10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontos HA, Wei EP. Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583. Stroke. 1993;24:427–434. doi: 10.1161/01.str.24.3.427. [DOI] [PubMed] [Google Scholar]
- 30.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, Nomura Y. Comparison of inhibitory effects of Y-27632, a Rho kinase inhibitor, in strips of small and large mesenteric arteries from spontaneously hypertensive and normotensive Wistar-Kyoto rats. Hypertens Res. 2003;26:97–106. doi: 10.1291/hypres.26.97. doi:10.1291/hypres.26.97. [DOI] [PubMed] [Google Scholar]
- 32.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–418. doi: 10.1161/01.RES.0000059987.90200.44. doi:10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- 33.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70:174–178. doi: 10.1253/circj.70.174. doi:10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 34.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. doi:10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 35.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 36.Yang XR, Lin MJ, Sham JS. Physiological functions of transient receptor potential channels in pulmonary arterial smooth muscle cells. Adv Exp Med Biol. 2010;661:109–122. doi: 10.1007/978-1-60761-500-2_7. doi:10.1007/978-1-60761-500-2_7. [DOI] [PubMed] [Google Scholar]
- 37.Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, et al. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006;570:53–58. doi: 10.1113/jphysiol.2005.098855. doi:10.1113/jphysiol.2005.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131:5–9. doi: 10.1038/sj.bjp.0703537. doi:10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.