As a consequence of advances in invasive and pharmacological treatment, significantly more patients survive an acute myocardial infarction and enter the chronic phase of infarct healing. Unfortunately, their convalescence is complicated by the development of adverse myocardial remodelling, a pathophysiological condition characterized by dilation and increased sphericity of the infarcted ventricle and by hypertrophy of the non-infarcted myocardium. Post-infarction remodelling is a strong predictor of mortality and arrhythmias and leads to the development of heart failure.1 The pathogenesis of remodelling in the infarcted heart is intertwined with the responses involved in cardiac repair. Loss of cardiomyocytes following infarction initiates an inflammatory reaction that leads to clearance of the wound from dead cells and debris, but also activates pro-apoptotic and matrix-degrading pathways that may exacerbate injury and enhance chamber dilation. Mobilization of fibroblasts provides the cellular substrate necessary to form a supportive scar, but also results in matrix deposition and active interstitial remodelling in the perimysium of the viable myocardium, increasing dysfunction. Deposition and cross-linking of collagen worsens diastolic function, whereas loss of endogenous matrix-derived survival signals, due to active turnover of extracellular matrix proteins and persistent inflammation, may result in cardiomyocyte apoptosis, precipitating further decline in systolic function of the ventricle.2
Beyond their established role in regulation of appetite and metabolism, adipokines have also been implicated in cardiac pathophysiology and in regulation of myocardial injury, inflammation, and repair. In the myocardium, leptin signals through short (Ob-Ra) and long (Ob-Rb) isoforms of the leptin receptor, shown to be closely related to the class I cytokine receptor family. Leptin binding causes conformational changes and triggers reciprocal autophosphorylation of two Janus kinase (JAK) molecules that are associated with the proline-rich cytoplasmic region of leptin receptor. Activated JAK phosphorylates tyrosine residues (Tyr985, Tyr1077, and Tyr1138) of the receptor, providing docking sites for signal transducer and activator of transcription (STAT) proteins that subsequently become tyrosine-phosphorylated by JAK. Phosphorylated STAT molecules dimerize and translocate to the nucleus to activate transcription of target genes. In addition, leptin activates STAT-independent pathways like extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3′-kinase cascades. Evidence suggests that expression of leptin and its receptor is increased in experimental models of myocardial infarction and in the failing human heart. However, because leptin is capable of modulating phenotype and function of all cells associated with cardiac injury and repair, its role in the healing infarct remains poorly understood. Global leptin deficiency results in impaired cardiac structure and function following myocardial infarction. On the other hand, treatment with an anti-leptin neutralizing antibody was shown to mitigate dysfunction following infarction, and exogenous administration of high doses of leptin in infarcted mice resulted in accentuated adverse remodelling.3 Thus, published experimental evidence suggests that leptin may exert both cardioprotective and detrimental effects on the injured heart (Figure 1).
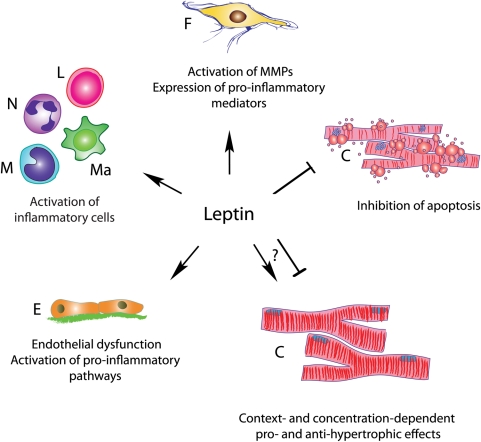
Figure 1.
The cellular effects of leptin. Leptin exerts pleiotropic effects on all cell types involved in cardiac remodelling. F, fibroblast; C, cardiomyocyte; E, endothelium; M, monocyte; Ma, macrophage; N, neutrophil; L, lymphocyte.
McGaffin et al.4 generated a tamoxifen-inducible, cardiomyocyte-specific leptin receptor knockout mouse to dissect cell-specific actions of leptin signalling in the infarcted heart. Selective disruption of leptin signalling in cardiomyocytes resulted in enhanced ventricular remodelling, accentuated hypertrophy, increased cardiomyocyte apoptosis, more intense inflammation, and impaired glucose metabolism in the infarcted ventricle. These defects were associated with impaired activation of STAT-3 and 5′-AMP-activated protein kinase (AMPK). Rescue experiments demonstrated that the protection afforded by leptin was mediated in part through AMPK signalling.
The findings provide important insights into the role of leptin-mediated actions following cardiac injury, suggesting potent protective effects against cardiomyocyte apoptosis and hypertrophy via the activation of AMPK. The authors’ current observations are in agreement with previous reports showing anti-hypertrophic effects of leptin,5,6 but are challenged by in vitro evidence demonstrating pro-hypertrophic effects of leptin mediated by MAPK/ERK, RhoA/ROCK, and STAT/JAK pathways7 and by in vivo experiments showing that treatment with a neutralizing leptin receptor antibody attenuated hypertrophy and haemodynamic dysfunction in rats with myocardial infarction.8 Moreover, exogenous administration of high doses of leptin in mice undergoing myocardial infarction protocols resulted in accentuated adverse remodelling.3
What is the possible explanation for the contradictory reports on the role of leptin in the infarcted heart? Although species-specific effects may account for some of the observed differences, the dose- and context-dependent actions of leptin on the various cell types involved in cardiac remodelling may explain the conflicting observations. Effects of leptin in vivo may be dependent on its local concentration: both complete loss and high-level overexpression of leptin may result in hypertrophy and adverse remodelling following myocardial infarction. More importantly, however, the findings may illustrate the significance of cell-specific actions of leptin signalling on the infarcted heart. Cardiac remodelling following infarction is dependent on the extent of cardiomyocyte injury,9 but is also greatly influenced by alterations in the inflammatory and reparative response. Extensive evidence suggests that leptin exerts potent pro-inflammatory actions. Hyperleptinaemia is associated with enhanced systemic inflammation10 and triggers activation of matrix-degrading pathways in the myocardium.9 In vitro, leptin activates leucocytes via integrin up-regulation11 and induces expression of matrix metalloproteinases by cultured cardiac fibroblasts. Leptin-induced accentuation of cardiac inflammation and matrix metabolism would be expected to promote dilative remodelling of the infarcted heart. In animals selectively lacking leptin signalling in cardiomyocytes, any protective effects of leptin on cardiomyocyte survival are abrogated, resulting in unopposed pro-inflammatory and matrix-degrading actions on leucocytes and mesenchymal cells. Thus, leptin-mediated effects on leucocytes and fibroblasts may enhance adverse remodelling, inducing accentuation of inflammation and enhanced proteolytic activity, whereas effects on cardiomyocytes may be primarily protective. The meticulous work by McGaffin et al.4 highlights the complexity and cellular specificity of biological responses and emphasizes the power of conditional manipulation of genes in dissecting the mechanisms involved in cardiac pathophysiological conditions.
Conflict of interest: none declared.
Funding
The authors are supported by NIH R01 HL-76246 and R01 HL-85440 (N.G.F.) and an AHA South Central affiliate post-doctoral award (M.D.).
References
- 1.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 2.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 3.Abe Y, Ono K, Kawamura T, Wada H, Kita T, Shimatsu A, et al. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am J Physiol Heart Circ Physiol. 2007;292:H2387–H2396. doi: 10.1152/ajpheart.00579.2006. [DOI] [PubMed] [Google Scholar]
- 4.McGaffin KR, Witham WG, Yester KA, Romano LC, O'Doherty RM, McTiernan CF, et al. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, et al. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]
- 6.Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 7.Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Leptin as a cardiac hypertrophic factor: a potential target for therapeutics. Trends Cardiovasc Med. 2007;17:206–211. doi: 10.1016/j.tcm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Purdham DM, Rajapurohitam V, Zeidan A, Huang C, Gross GJ, Karmazyn M. A neutralizing leptin receptor antibody mitigates hypertrophy and hemodynamic dysfunction in the postinfarcted rat heart. Am J Physiol Heart Circ Physiol. 2008;295:H441–H446. doi: 10.1152/ajpheart.91537.2007. [DOI] [PubMed] [Google Scholar]
- 9.Schram K, Wong MM, Palanivel R, No EK, Dixon IM, Sweeney G. Increased expression and cell surface localization of MT1-MMP plays a role in stimulation of MMP-2 activity by leptin in neonatal rat cardiac myofibroblasts. J Mol Cell Cardiol. 2008;44:874–881. doi: 10.1016/j.yjmcc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]